Abstract
CD180 is homologous to TLR4 and regulates TLR4 signaling, yet its function is unclear. We report that injection of anti-CD180 mAb into mice induced rapid Ig production of all classes and subclasses except IgA and IgG2b, with up to 50-fold increases of serum IgG1 and IgG3. IgG production after anti-CD180 injection was not due to reactivation of memory B cells and was retained in T cell-deficient (TCR KO), CD40 KO, IL-4 KO and MyD88 KO mice. Anti-CD180 rapidly increased both transitional and mature B cells, with especially robust increases in transitional B cell number, MZ B cell proliferation, and CD86 but not CD80 expression. In contrast, anti-CD40 induced primarily FO B cell and myeloid expansion with increases in expression of CD80 and CD95 but not CD86. The expansion of splenic B cells was due in part to proliferation and occurred in WT and TCR KO mice whereas T cell expansion occurred in WT but not in B cell-deficient (μMT) mice, indicating a direct role for B cells in CD180 stimulation in vivo. Combinations of anti-CD180 with various MyD88-dependent TLR ligands biased B cell fate as co-injection diminished Ig production but purified B cells exhibited synergistic proliferation. Anti-CD180 had no effect on cytokine production from B cells but increased IL-6, IL-10, and TNF-α production in combination with LPS or CpG. Thus CD180 stimulation induces intrinsic B cell proliferation and differentiation, causing rapid increases in IgG, and integrates MyD88-dependent TLR signals to regulate proliferation, cytokine production, and differentiation.
Introduction
CD180 (RP105) was originally identified as a B cell surface molecule mediating activation and proliferation and was later recognized as a TLR homolog (1, 2). It is a leucine-rich repeat type 1 membrane protein with high extracellular homology to the LPS receptor (2), but unlike TLR4 its expression is restricted to APCs (B cells, macrophages, and dendritic cells (DCs)) (3). CD180 and TLR4 are also similar in that mAbs to these receptors cause B cell proliferation and upregulation of costimulatory molecules (CD86) (4). However, while TLR4 agonists induce only a subset of B cells to proliferate (15%)(5), anti-CD180 activates over 85% of both human and mouse B cells in vitro, causing extensive proliferation.
TLRs recognize conserved microbial components to initiate rapid responses that both prime and skew adaptive immunity (6). Each TLR binds specific ligands via its extracellular domain to initiate dimerization, recruitment of MyD88 and/or TIR-domain-containing adapter-inducing interferon-β (TRIF) intracellular adaptors to the Toll/IL-1-Receptor (TIR) domain, and downstream signaling (7, 8). While each TLR exhibits specificity for agonists with conserved molecular structures, combinations of TLR signals mediate much more robust and specific responses appropriate for fine-tuning against particular pathogens (9, 10). As CD180 is classified as a TLR it is essential to understand its interaction with other TLRs, as multiple TLRs are often required in concert to mediate full physiological function.
In CD180 KO mice B cell responses to LPS are impaired and constitutive serum IgG3 is reduced (11). These data supported a model of CD180 as a required co-receptor for B cell responses to bacterial cell wall components (12), binding LPS and forming heterodimers with TLR4 to enhance signaling. However, while the natural ligand of CD180 is unknown it is not LPS. CD180 does not bind LPS (3) and structural analysis of the CD180 complex revealed that it does not contain the required LPS binding pocket (13) as only four, not six, acyl chains can fit (14). Therefore, while CD180 regulates B cell sensitivity to LPS the mechanism of CD180 support for TLR4 signals remains unknown. Because CD180 lacks the cytoplasmic TIR domain common to all other TLRs, appearing instead to initiate an antigen receptor-like phosphotyrosine and calcium based signaling cascade in B cells (1, 15–17), there is no known point of interaction between the CD180 and TLR pathways. Furthermore, it is unclear how a scaffold of dimerized (18, 19)TIR domains forms with inclusion of CD180 in a heterodimer with TLR4. This led to an opposing model where CD180 forms inactive heterodimers with TLR4 and specifically attenuates LPS responses in myeloid cells, with only artifactual stimulation of B cells (3).
Current literature is confusing as it ascribes opposing functions for CD180, both as a required co-receptor for LPS/stimulator of B cells (11) and also as a specific TLR4 inhibitor in DCs with no physiological effect on B cells (20). While CD180 deficiency has been characterized (3, 11), neither CD180 stimulation in vivo nor the integration of CD180 and TLR signals has been studied (with the single exception of noting increases in CD138+ B cells in spleen sections following anti-CD180 injection (11)). Here we report that anti-CD180 mAb in vivo induces rapid polyclonal B cell expansion and striking Ig production, especially of the IgG1 and IgG3 subclasses. This Ig production is inhibited by co-administration of diverse TLR ligands. In contrast, anti-CD180 synergizes with ligands for all MyD88-dependent TLRs to increase B cell proliferation. While anti-CD180 in combination with TLR signals augmented cytokine production from purified B cells, it does not by itself induce cytokine production. Our data indicate that CD180 signals act directly on B cells to induce strong polyclonal B cell proliferation and Ig production, and that integration of TLR and CD180 signals through MyD88 skews B cells toward proliferation and cytokine production rather than differentiation.
Materials and methods
Mice
WT (C57BL/6), CD40 KO, B cell-deficient (μMT), and T cell-deficient/TCRβ/δKO (TCR KO) mice were from Jackson Laboratory (Bar Harbor, ME) and all other strains were on this background unless noted. TRIFKO spleens were a gift from D. Rawlings (Children’s Research Institute, Seattle, WA). MyD88KO mice and TLR2/4KO spleens were gifts from K. Elkon (University of Washington, Seattle, WA). CD180KO mice were a gift from C. Karp (Children’s Research Foundation, Cincinnati, OH). IL-4 KO mice on a BALB/c background were a gift from S. Ziegler (Benaroya Research Institute, Seattle, WA), and WT BALB/c mice were purchased from the Jackson Laboratory. All mice were sex and age matched and used at six to twelve weeks of age, except for the memory recall studies that utilized 60-week-old mice. All injections were intraperitoneal with a fixed volume of 200 μl in PBS diluent. The University of Washington Institutional Animal Care and Use Committee approved all animal work.
Cell preparation and culture
Spleens were processed by Liberase (Roche, Indianapolis, IN) digestion for DCs or mechanical disruption. Erythrocytes were depleted by Gey’s lysis for total splenocyte preparations. B cells or DCs were isolated by three rounds of enrichment (STEMCELL technologies, Vancouver, BC, Canada) and purity exceeded 99% without expression of activation markers (CD69 or CD86) after 24 hours in unstimulated cultures.
Total splenocytes or purified cells were cultured in complete medium (RPMI-1640 supplemented with 10% fetal calf serum [Hyclone, Logan, UT], 4 mM glutamine, 1 mM pyruvate, 1 × Non-Essential Amino Acids, 100 IU/ml penicillin-streptomycin [Invitrogen, Carlsbad, CA], and 50 uM 2-ME [Sigma-Aldrich, St. Louis, MO]) in the presence of stimuli at a final cell density of 1×106/mL for 64 hours at 37 °C.
ELISA measurement of serum antibody and in vitro cytokine production
Sera were obtained after injection of mice with mAbs and/or TLR agonists. Polystyrene plates were coated with anti-mouse IgG (H+L), or anti-mouse IgM F(ab′)2 with minimal cross-reactivity to rat Ig (Jackson ImmunoResearch, West Grove, PA). After blocking with 4% nonfat dry milk in PBS-Tween, serial dilutions of serum were added. Abs were detected with isotype-specific HRP conjugates (anti-IgG1, anti-IgG2b, and anti-IgG3 from ICL, Newberg, OR; anti-IgM and anti-IgG2c from Southern Biotech, Birmingham, AL) and absorbance was compared with standard curves generated from mouse monoclonal standards (IgG3 from BioLegend, San Diego, CA; IgM from Jackson ImmunoResearch; IgG2c from Southern Biotech; IgG1 and IgG2b standards were purified in our laboratory) for absolute quantitation. No cross-reactions between standards for the IgG subclasses, IgM, or the injected rat IgG2a mAbs were observed. Relative concentrations of serum IgA and IgE were detected, following light chain capture, with anti-IgA and anti-IgE HRP direct conjugates (ICL) and compared to pre-bleed serum values. Total in vitro Ig production was assessed as above after culturing 5×105 splenocytes/ml with the indicated stimuli for 72 hours at 37 °C.
Antigen specific antibody from 4-hydroxy-3-nitro-phenacetyl (NP) conjugated LPS, NP-Ficoll, or NP-chicken gamma-Globulin (CGG) was captured with NP-BSA coated plates (all NP reagents from BioSearch Technologies, Inc., Novato, CA).
IL-6, IL-10, and TNF-α concentrations in 24-hour supernatants from cultures of purified cells were measured by ELISA (DuoSets from R&D Systems, Minneapolis, MN) per the manufacturer’s instructions.
Analysis of lymphocyte subsets and proliferation
Flow cytometry analyses were performed on either a standard FACScan or FACSCanto (Becton Dickinson, Franklin Lakes, NJ). Minimums of 30,000 cells of the final gated population were used for all analyses. Data analysis was performed with FlowJo (Tree Star, Ashland, OR) software. Staining was performed for: CD3, CD24, CD80, and CD95 (Becton Dickinson clones 145-2c11, M1/69, 16-10A1, and Jo2); CD4, CD8β, CD19, CD21, CD23, CD25, and CD69 (BioLegend clones RM4-5, YTS156.7.7, 6D5, 7E9, B3B4, 3C7, and H1.2F3); CD5, CD45R/B220, and CD86 (Clones 53-7.3, RA3-6B2, and GL1 from eBioscience, San Diego, CA). Mouse anti-rat IgG secondary antibody was from Jackson ImmunoResearch. BrdU analysis was performed according to the kit manufacturer’s instructions (Becton Dickinson) following a one hour pulse delivered by i.p. injection on d 3 post anti-CD180 injection.
CFSE (Invitrogen) labeling of cells was performed with a final concentration of 0.8 μM CFSE and 1.6×107 cells/ml in 37°C PBS for four minutes. Proliferation Index was calculated by dividing the geometric MFI for gated live unstimulated singleton B cells by the geometric MFI of equivalently gated cells from the stimulated sample. This measurement simultaneously captures both percent proliferating cells and the average number of divisions per cell. A Proliferation Index of 1 indicates equivalence to unstimulated culture.
Synergy determinations and calculation of the combination index
The Combination Index (CI), a quantitative definition of synergy or antagonism, was calculated by the method of Chou and Talalay (21) through the use of CalcuSyn software (Biosoft, Cambridge, United Kingdom). As the CI method is based on the median effect principle of the mass action law, it is mechanism-independent.
Other antibodies and reagents
The anti-CD180 (RP/14) hybridoma was a gift from K. Miyake (University of Tokyo, Tokyo, Japan) and the rat IgG2a isotype control (9D6) hybridoma was a gift from R. Mittler (Emory University, Atlanta, GA). We used our previously generated hybridoma (1C10, rat IgG2a) to produce anti-CD40 mAb. To ensure equivalence these mAb were sequentially purified on the same protein G column, followed by routine LAL gel-clot (Associates of Cape Cod, East Falmouth, MA) and/or bioassays both alone and in combination with polymyxin B sulfate. LPS (L2143) was from Sigma-Aldrich. Synthetic TLR agonists Pam2CSK4, Pam3CSK4, CL097, and CpG ODN1826 were from InvivoGen (San Diego, CA).
Statistical analyses
Raw data of experimental groups were analyzed either by one-way ANOVA followed by Bonferroni’s Multiple Comparison Test (GraphPadPrism software, version 4.0a for Macintosh, San Diego, CA) or by two-tailed, type two Student’s t-test. Columnar data are represented as mean + standard error (SEM). A value of p < 0.05 was considered to be statistically significant and assigned *, while p < 0.01 and p < 0.001 were assigned ** and ***, respectively.
Results
Anti-CD180 injection induces polyclonal Ig production of multiple isotypes
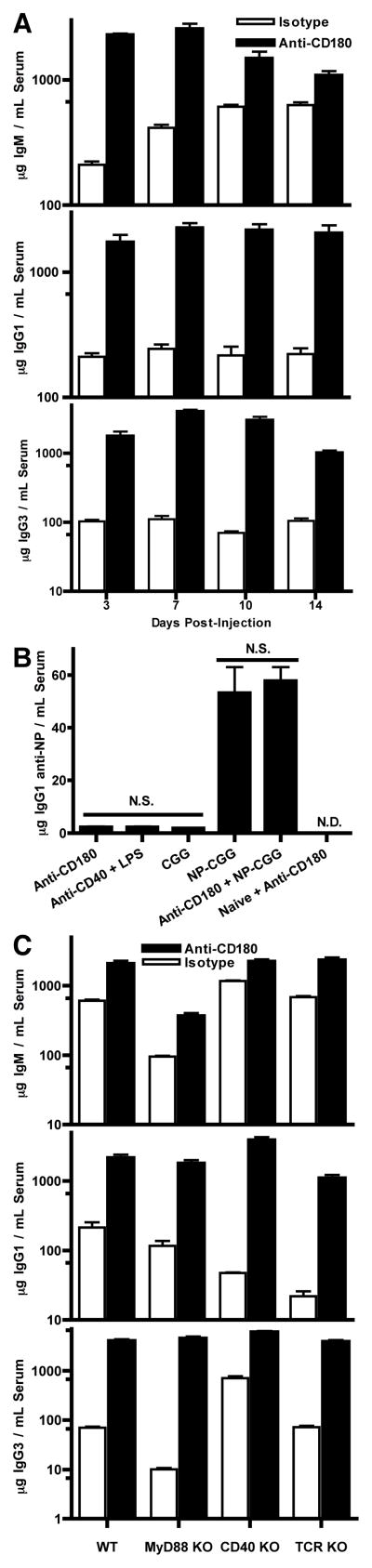
Because CD180 KO mice have low serum concentrations of IgG3 (11), we examined Ig concentrations of WT mice at 3, 7, 10, and 14 d after injection with either anti-CD180 or isotype-matched control mAb (the anti-CD180 antibody is an agonistic rat IgG2a that was not expected to deplete target cells). Dose response assays from 10 μg to 250 μg of anti-CD180 were performed and 100 μg gave a less pronounced effect than 250 μg for both splenic expansion and Ig production (data not shown). All subsequent in vivo assays utilized a 250 μg dose of anti-CD180. At no point did the anti-CD180-injected mice show any evidence of distress, unlike after injection of TLR4 agonists that rapidly induce septic shock.
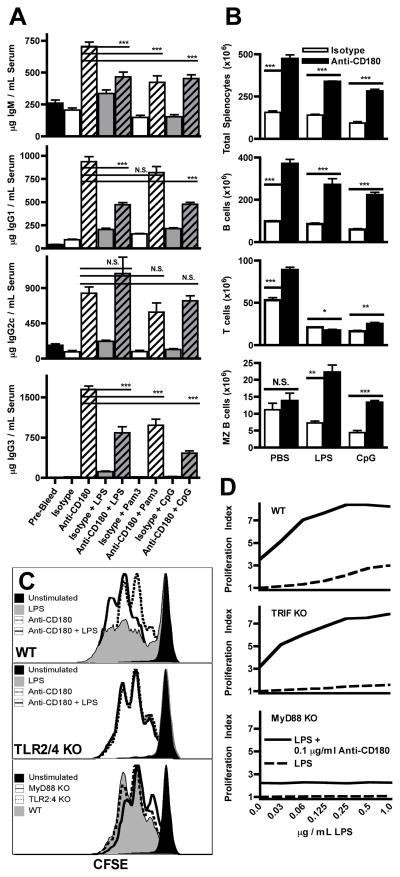
Anti-CD180 alone increased serum Ig concentration of nearly every isotype and subclass by d 3, with increases for IgG1, IgG2c, and IgG3 that were both rapid and also dramatic in magnitude (12, 9.5, and 56-fold average increases at d 10, respectively), while changes in serum concentration of IgM were rapid (11-fold increase at day 3) but transient (2.4-fold increase at day 10) and IgG2b varied with an average of 1.5-fold reductions (Fig. 1A and data not shown). ELISAs for serum IgA and IgE from d 10 bleeds indicate that IgA concentrations were equivalent to pre-injection bleeds in isotype control and anti-CD180 treated mice, while IgE concentrations increased roughly 12-fold in the anti-CD180 group but not the isotype group (Supplementary Fig. 1A).
Figure 1. Anti-CD180 rapidly induces Ig production independently of memory recall, T cell help, or MyD88 signals.
A) WT mice received 250 μg anti-CD180 or isotype control mAb, bled at indicated timepoints, and total serum Ig analyzed by ELISA. B) WT mice were immunized with 50 μg NP-CGG in alum, rested, and challenged with either 250 μg anti-CD180, 100 μg anti-CD40 plus 10 μg LPS, 25 μg uncongugated CGG, 10 μg NP-CGG, or anti-CD180 plus NP-CGG, and bled at d 10 for NP-specific Ig analysis. Non-immunized mice for the naïve group were age matched. C) WT, MyD88KO, CD40 KO, and TCRKO mice were injected, bled at d 10, and total serum Ig analyzed. p value between paired columns < 0.001 unless otherwise noted. Four mice per timepoint, representative of four experiments for each panel.
We examined whether rapid production of IgM and IgG1 after CD180 stimulation was due to reactivation of memory B cells. WT mice were immunized with NP-CGG in alum and rested for 50 weeks before injection of recall stimuli. While recall Ag administration without adjuvant produced robust NP-specific IgG1, neither anti-CD180 nor inflammatory stimuli (LPS plus anti-CD40) induced significant recall compared to unconjugated CGG (Fig. 1B). Addition of anti-CD180 stimulation with Ag did not significantly impact recall IgG1 responses.
Anti-CD180-induced Ig production did not require T cells, CD40, IL-4, or TLR signaling as the increase in IgG concentrations still occurred at d 10 following injection of TCR KO, CD40 KO, IL-4 KO or MyD88 KO mice (Fig. 1C and Supplementary Fig. 1B). IgM production was largely bypassed and IgG production was strikingly delayed in TCR KO mice,indicating a supportive role for T cells despite dispensability for the overall anti-CD180-induced Ig effect (Supplementary Fig. 2).
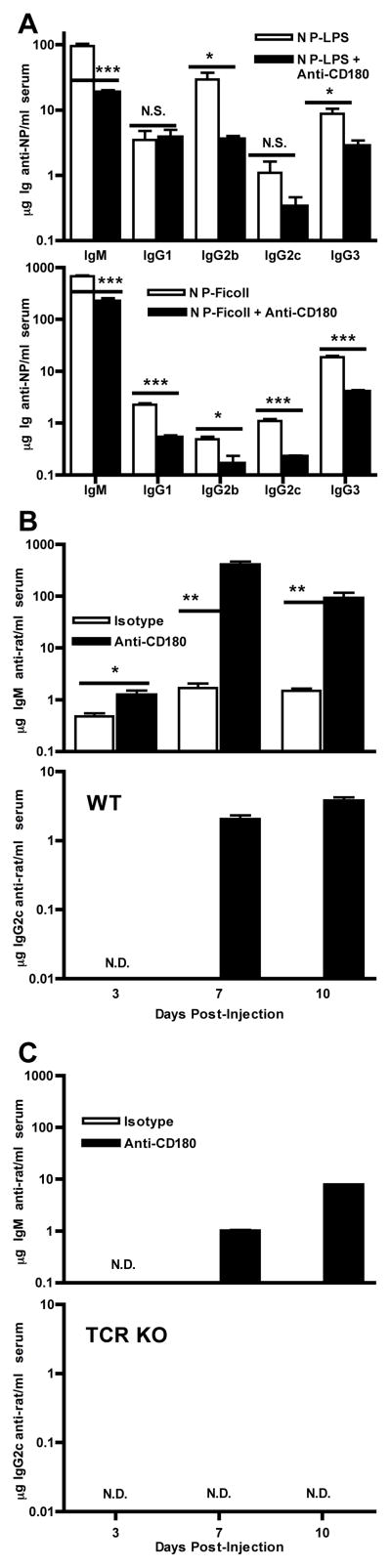
To assess whether anti-CD180-induced Ig is polyclonal or merely an extensive Ag-specific response, we examined antigen-specific responses in combination with CD180 signaling. We measured Ag-specific Ig produced following co-administration of anti-CD180 with T cell-independent (TI) antigens, the Ig produced against the rat IgG2a anti-CD180 mAb itself, and whether autoantibodies developed in anti-CD180 injected mice. Ag-specific antibodies of all isotypes were reduced or unchanged after addition of anti-CD180 mAb to immunization with either the TI-1 Ag NP-LPS or the TI-2 Ag NP-Ficoll (Fig. 2A). Although more anti-rat Ig was generated against the anti-CD180 mAb than the mAb isotype control it is never more than 16% of the total IgM produced. Additionally, production of anti-rat IgM has substantially different kinetics than that of total IgM, with Ag-specific IgM requiring 7 d to peak while total IgM is essentially maximal by d 3 (Fig. 2B). Class-switched anti-rat Ig was predominately of the IgG2c subclass and was not produced against the isotype control mAb. T cell-deficient mice also produced IgM specific for anti-CD180, but not class-switched Ig of any subclass (Fig. 2C). Auto-reactive antibody, as determined by semi-quantitative antinuclear antibody immunoflourescence, did not increase after anti-CD180 injection (data not shown).
Figure 2. T-Independent Type-1 and 2, but not T-Dependent antigen specific antibody, are decreased by co-administration of anti-CD180.
A) WT mice were injected with 1 μg NP-LPS (0.7 NP/LPS) (TI-1), or 20 μg NP-Ficoll (152 NP/Ficoll) in combination with 250 μg anti-CD180 or isotype control mAb, bled on d 10, and serum analyzed for NP-specific antibody. B) WT and C) TCR KO mice were injected with 250 μg anti-CD180 or isotype control mAb, and serum analyzed for anti-rat Ig-specific IgM and IgG2c antibody from d three, seven, and 10 time points. p value between paired columns < 0.001 unless otherwise noted. Four mice per group, representative of four experiments for each panel.
Anti-CD180 injection expands splenic B cells
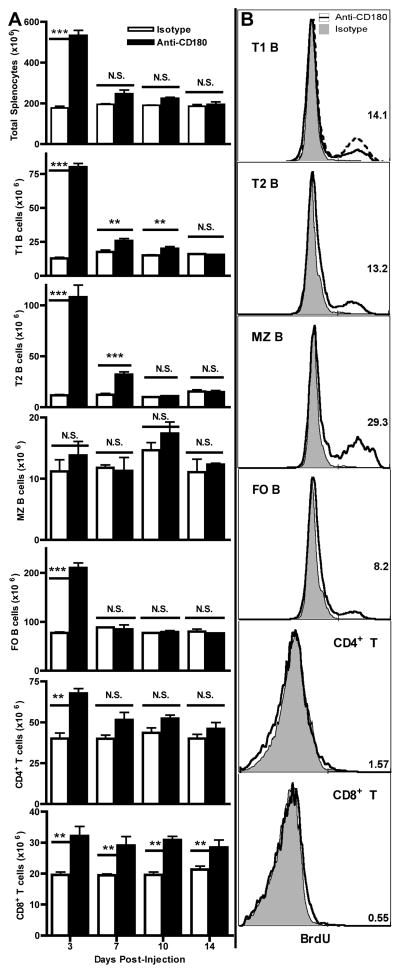
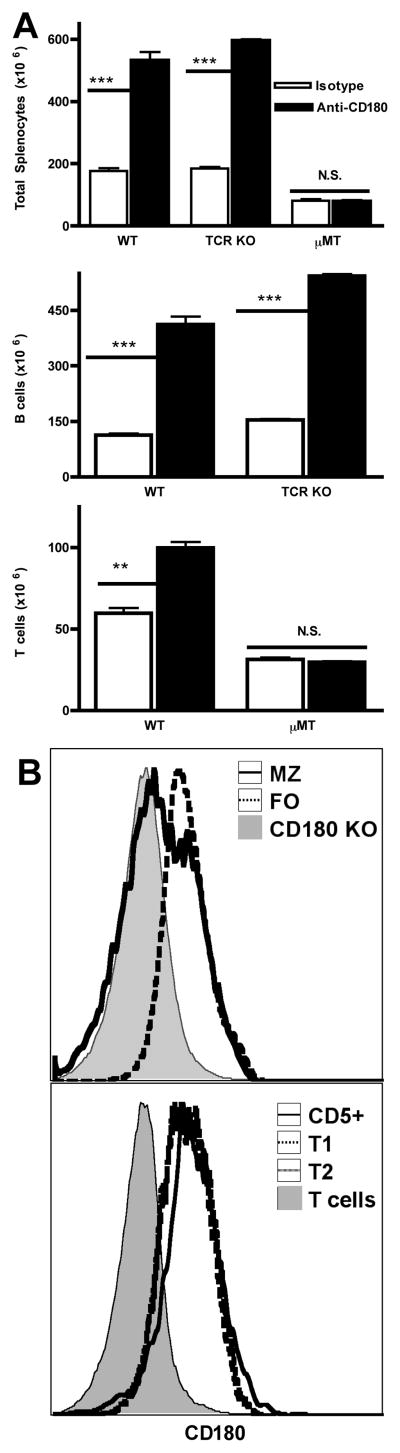
Three days after injection the spleens of anti-CD180-treated mice were enlarged nearly 3-fold compared to control mice (data not shown). Absolute splenic mononuclear cell numbers increased approximately 2.5-fold from controls (Fig. 3A). B cells (CD19+) contributed the majority of the change by expanding 7, 9, and 2.5-fold in transitional 1 (T1), transitional 2 (T2), and follicular (FO) subsets respectively, while the marginal zone (MZ) B cell subset did not change significantly in number (Fig. 3A). To assess whether this was a survival/redistribution effect or if cells were actually induced to proliferate in vivo by anti-CD180, we inoculated mice with BrdU 3 d after anti-CD180 injection and one hour later harvested spleens and quantified BrdU+ cells (Fig. 3B). Anti-CD180 induced significant proliferation in T1, T2, FO and MZ B cells. Although absolute numbers of MZ B cells did not increase after anti-CD180 treatment, MZ B cells proliferated more than the other B cell subsets, even exceeding the BrdU incorporation of the bone marrow cell positive control. Furthermore, T cell numbers also expanded significantly (Fig. 3A), but did not incorporate BrdU.
Figure 3. Anti-CD180 injection expands splenic B cells in vivo by inducing proliferation.
A) Spleens were harvested and cells enumerated 3, 7, 10, or 14 d following injection of 250 μg anti-CD180 or isotype mAb. Total splenocytes were subsetted by standard CD21/23/24 staining for B cell subsets and CD3/4/8β staining for T cell subsets. Three mice per timepoint, representative of three experiments. B) Following a 1 h BrdU pulse on d 3 post injection of anti-CD180 or isotype control, spleens were harvested, stained for BrdU incorporation, and subsetted for analysis. The dashed line in the first panel indicates BrdU uptake in the bone marrow positive control sample (27%). Three mice per timepoint, representative of two experiments.
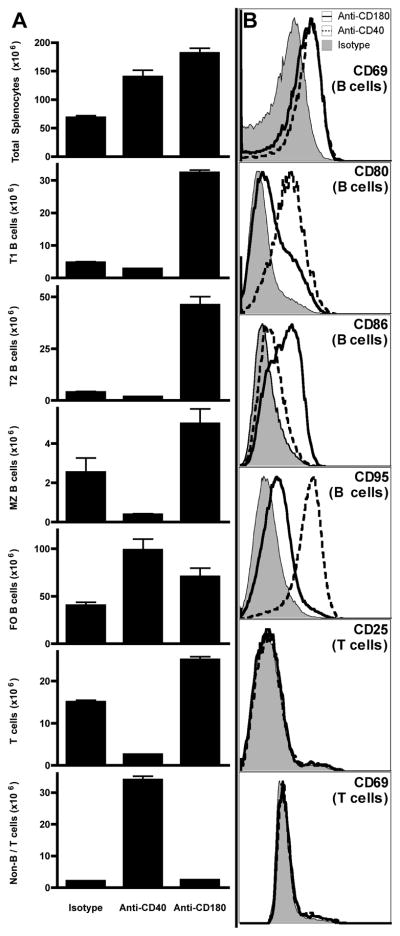
In order to assess possible non-specific effects from the rat IgG2a anti-CD180, we compared anti-CD180 treated mice to mice injected with the same dose of a rat IgG2a anti-CD40 mAb (1C10). While anti-CD40 stimulated B cells as expected, its effects were distinct from those of anti-CD180. Anti-CD40 expanded FO B cells and myeloid cells (Fig. 4A) and induced increases in both CD80 and CD95/FasR expression (Fig. 4B). In contrast, anti-CD180 preferentially induced large increases in numbers of transitional B cells and increases in BrdU uptake in both transitional and MZ B cells, as well as CD86 expression in total CD19+ cell populations, but minimal increases in FO B cells and CD80 or CD95 expression. Both anti-CD180 and CD40 upregulated CD69 on B cells but did not induce upregulation of either CD25 or CD69 on T cells.
Figure 4. Anti-CD180 produces different expansion and activation patterns thn anti-CD40.
A) Spleens were harvested and cells enumerated 3 d following injection of 250 μg IgG2a anti-CD180, anti-CD40, or isotype control mAb. Total splenocytes were subsetted as in Fig. 3A. B) Gated B cells (CD19+) and T cells (CD3+) were analyzed by flow cytometry for expression of common activation markers. Three mice per group; representative of two experiments for each panel.
The lymphoid cell expansion induced after anti-CD180 injection was transient, as cell numbers were lower at d 7 and essentially normal by d 14. The single exception was CD8+ T cells, which remained expanded through d 14. The kinetics of cell expansion paralleled binding of the anti-CD180 antibody, as determined by anti-rat IgG staining ex vivo, which demonstrated maximum binding at d 3, minimal binding at d 7, and undetectable binding on d 14 (data not shown). Expansion of B cells was still evident in TCR KO mice (Fig. 5A) and showed equivalent kinetics (data not shown), indicating that T cells are not required for either expansion or contraction of B cell populations in vivo following anti-CD180 injection. However, T cell expansion is dependent upon B cells, since T cells did not expand in B cell-deficient mice after anti-CD180 treatment. This T cell expansion occurred even though T cells do not express CD180, and thus is an indirect effect requiring B cells. Unlike the B cell expansion, which clearly involves proliferation, T cells did not incorporate significant BrdU despite expanding in number nor display markers of activation. The selective effects of anti-CD180 upon transitional and MZ B cells is also not predicted by the level of CD180 expressed on B cell subsets, as these have at best equivalent CD180 expression with FO B cells yet FO B cells both proliferate and accumulate less extensively (Fig. 3AB, and Fig. 5B).
Figure 5. Anti-CD180 splenic expansion requires B cells but not T cells.
A) WT, T cell-deficient (TCRKO), and B cell-deficient (μMT) mice were injected with 250 μg anti-CD180 or isotype control mAb and splenocytes counted and analyzed at the d 3 timepoint as in Fig. 3A. B) Unstimulated WT cells or those of a CD180 KO (FO B subset) control were stained ex vivo for CD180 expression and subsetted as in Fig. 3A. Three mice per group; representative of two experiments for each panel.
Combinations of TLR and CD180 signals reduce B cell differentiation and enhance proliferation
Due to the known interaction between CD180 and TLR4, we compared Ig production induced by anti-CD180 alone to co-injection with various TLR ligands. Combinations of anti-CD180 and LPS did not augment but instead resulted in decreased or unchanged Ig production (Fig. 6A) resulting in serum concentrations intermediate to anti-CD180 or LPS alone. This effect was also observed with Pam3CSK4 (a TLR2:1 ligand) and CpG (a TLR9 ligand), indicating a general effect of TLR signals rather than a specific interaction between CD180 and TLR4.
Figure 6. TLR signals reduce anti-CD180 induced Ig production but augment proliferation in a MyD88-dependent manner.
A) WT mice were injected with the following TLR agonists in combination with either 250 μg anti-CD180 or isotype control mAb: 1 μg LPS, 2 μg Pam3CSK4, or 10 μg CpG. Sera were obtained at d ten and analyzed by ELISA. Four mice per group, representative of four experiments. B) TLR ligands LPS (10 μg), CpG (25 μg), or an equivalent volume of PBS were co-injected with either anti-CD180 or isotype and splenocytes were analyzed at the d 3 timepoint as in Figure 3A. Three mice per group, representative of four experiments. C) CFSE labeled splenocytes from TLR2/4KO, MyD88 KO, or WT mice were cultured with anti-CD180 (0.2 μg/ml), LPS (0.5 μg/ml), or both. B cells were gated (FSC/SSC, B220+) and CFSE dilution analyzed. B cells from all three genotypes stimulated with anti-CD180 are overlaid for a direct comparison of TLR and MyD88 requirements in CD180 signaling. D) CFSE labeled splenocytes from WT, TRIFKO, or MyD88KO mice were cultured with graded doses of LPS alone or in combination with a constant 0.1 μg/ml dose of anti-CD180. Proliferation Index is graphed against the corresponding LPS concentration. One of three experiments with similar results.
We also injected anti-CD180 in combination with TLR ligands (LPS or CpG) to determine whether these combinations changed how splenic lymphocytes expanded. Compared with anti-CD180 injection alone, mice injected with anti-CD180/TLR agonist combinations showed roughly equivalent B cell expansion (3.5 fold) but had reduced expansion of T cells (Fig. 6B). Despite a lack of MZ B cell expansion with anti-CD180 alone, combinations of CD180 and TLR signals increased splenic MZ B cell populations.
As CD180 KO B cells have diminished proliferative responses to LPS (12), we examined possible reciprocal dependence of CD180 signals on TLR2 and TLR4 as well as the TLR adapter protein MyD88. In WT splenocyte cultures the combination of CD180 and TLR4 stimulation augmented B cell proliferation compared to either stimulus alone, increasing both the percentage of B cells proliferating and the average number of cycles (Fig. 6C). Deficiency of TLR2 and TLR4 had little or no effect on proliferation of B cells in response to anti-CD180, and as expected, TLR2 and TLR4 deficient B cells did not respond to LPS. Similar results were obtained for MyD88 KO B cells. Thus, CD180 and MyD88-dependent TLRs provide distinct, non-redundant, and mutually reinforcing signals for B cell proliferation.
To identify the intersection of CD180 and TLR4 signaling pathways we assayed B cell proliferation with graded doses of LPS, with or without a fixed dose of anti-CD180, in splenocytes from WT, TRIF KO, and MyD88 KO mice (Fig. 6D). Despite minimal proliferation to LPS alone, robust augmentation of anti-CD180 on LPS-induced proliferation was still present in B cells from TRIF KO mice but not from MyD88 KO mice. While MyD88 is not required for CD180 signals to induce B cell proliferation, it is required to mediate the CD180 augmentation of TLR4 signals.
Anti-CD180 synergizes with multiple MyD88-dependent TLR ligands for B cell proliferation
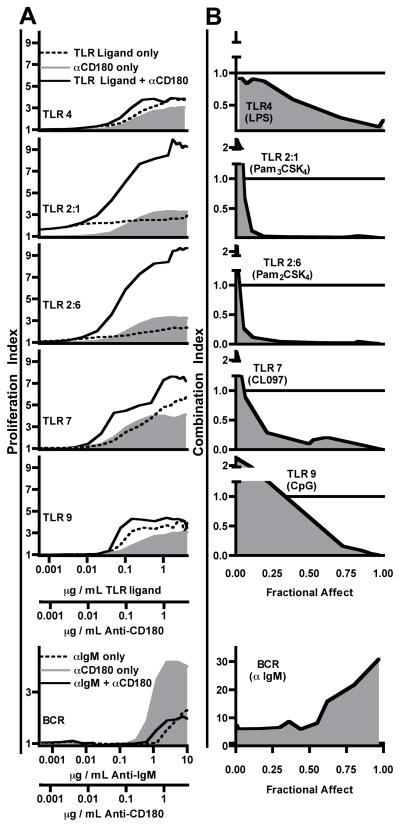
Since Ig production decreased after TLR ligands were co-injected with anti-CD180, we examined the effects of TLR ligands on anti-CD180 induced differentiation and proliferation in quantitative in vitro systems to determine the nature and magnitude of signal interactions. In vitro Ig production experiments reproduced the decreasing trend observed in vivo, with the largest IgG production occurring with anti-CD180 alone, and decreasing as increasing doses of LPS were added to the cultures (Supplementary Fig. 3). To assess proliferative effects from these combinations B cells were isolated and both anti-CD180 and TLR agonists were titrated, first separately (TLR agonist or anti-CD180 alone) and then together (TLR agonist plus anti-CD180) at equivalent dilution ratios. In addition to TLR4 (LPS), the interactions of CD180 with TLR2:1 (Pam3CSK4), TLR2:6 (Pam2CSK4), TLR7 (CL097), TLR9 (CpG ODN1826), and BCR (F(ab’)2 anti-IgM) were also analyzed (Fig. 7A). The proliferation of B cells to combinations of anti-CD180 mAb and TLR agonists was augmented for all combinations. In contrast, antagonism was observed with BCR stimulation.
Figure 7. Anti-CD180 synergizes for proliferation with all TLR ligands that signal through MyD88.
A) Purified (99+ %) WT splenic B cells were stimulated with either TLR agonist alone, anti-CD180 alone, or both in constant ratio combinations. Proliferation Index was calculated for each series and all curves graphed against the corresponding TLR agonist concentrations. The known antagonism of anti-CD180 for anti-IgM-induced proliferation (12) is included for comparison. B) The three Proliferation Index curves were transformed into a single Combination Index (CI) curve as described in “Materials and methods”. Combination Index values of 1 (reference bar) indicate simple additive effect (no interaction), CI values < 1 indicate synergy (greater than additive effect), and CI values > 1 indicate antagonism/inhibition. One of four experiments with similar results.
To extract quantitative information from the titration series of anti-CD180 and TLR (or BCR) interaction, the three separate titration curves were transformed into a single curve (Fig. 7B) by the Combination Index (CI) analysis method (21). The resulting graph displays signal interaction over the entire titration range, with CI = 1 indicating no interaction (mere additive effect), CI < 1 indicating synergy (greater than additive effect), and CI values > 1 indicating antagonism. Despite the previously reported selective relationship between CD180 and TLR4, we demonstrate strong synergy (CI ≪ 1) for all MyD88-dependent TLR agonist combinations with anti-CD180. While CD180 is described as a specific regulator of TLR4, our analyses show significantly greater synergy with ligands of TLR2 or TLR7. Surprisingly, at very low Fractional Affects (relative doses) all combinations other than LPS revealed antagonism. As these experiments used isolated B cells (> 99% pure) the observed interactions are likely intrinsic to B cells, rather than indirect contributions from signaling in rare non-B cells.
Anti-CD180 augments cytokine production by TLRs in isolated B cells
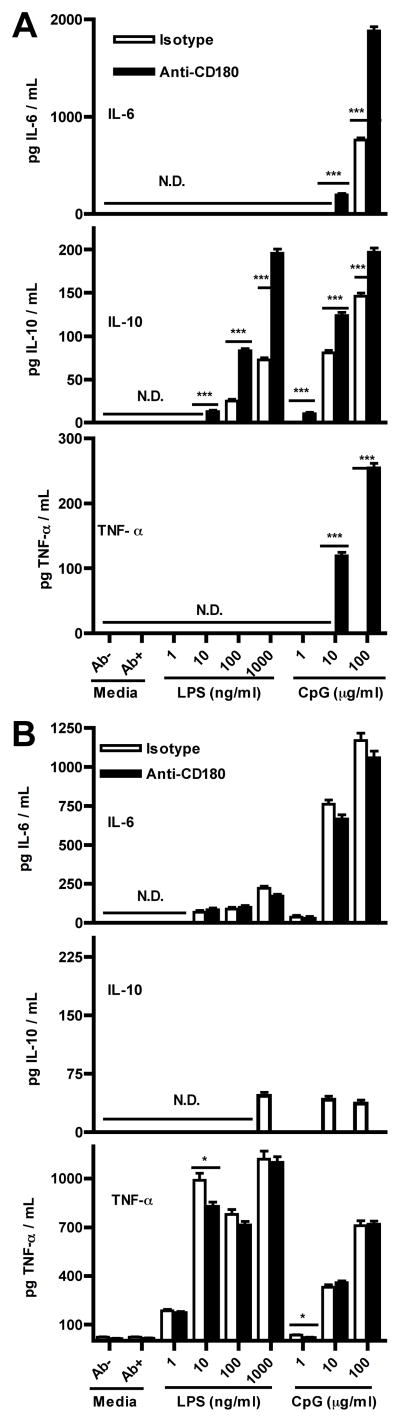
We next examined cytokine production from isolated B cells treated with anti-CD180 or control mAb alone or in combinations with LPS or CpG (Fig. 8A). No production of IL-6, IL-10, or TNF-α was observed with anti-CD180 alone; however there was clear augmentation of cytokine production in combination with TLR ligands. Concentrations of IL-6 and IL-10 were substantially augmented even at LPS concentrations that alone resulted in no effect. Similar augmentation of cytokine production was seen in combinations with CpG and included strongly increased production of TNF-α. Isolated DCs similarly did not produce cytokines after CD180 stimulation alone (Fig. 8B). Rather than the enhancement with CD180/TLR combinations observed in isolated B cells, however, DCs tended to have reduced cytokine production.
Figure 8. Anti-CD180 does not induce cytokine production by B cells but augments induction by TLRs.
A) Purified WT splenic B cells were seeded at 1×106 cells/ml in media with indicated stimulants. Overnight (24 hour) culture supernatants were assayed by ELISA. B) Purified WT splenic DCs were treated as in A. Differences between paired columns are not significant unless otherwise noted. One of two experiments with similar results, all samples run as triplicates.
Discussion
Collectively, our data indicate that CD180 signals induce an extensive and rapid burst of polyclonal proliferation and activation in naïve B cells, proceeding to IgG production within 3 d. CD180 has been implicated in induction of IgG3 antibodies since constitutive serum concentrations of IgG3 in CD180 KO mice are approximately one-tenth that of WT mice (11). Our results broaden this interpretation as anti-CD180 mAb injection caused very rapid and large increases in serum concentrations of multiple Igs, with IgM, IgG1, IgG2c, and IgG3 concentrations each reaching or exceeding 1 mg/ml within 10 d of injection. IgG3 concentrations had the largest change with a > 50-fold increase over basal concentrations. While robust, this response was transient and reminiscent of an extrafollicular response (22) as concentrations of all I sotypes had peaked and begun to decline by d 14. CD138+ B cells in the spleens of anti-CD180 treated mice are significantly increased relative to isotype injected mice in the spleen at day 7, but not detectable in the spleen on day 3, raising the possibility that peritoneal B cells may contribute significantly to the initial production of anti-CD180-induced Ig. (Data not shown.) Further studies are needed to define the initial Ab producing cells in anti-CD180-stimulated mice and the mechanisms involved in their rapid activation.
The anti-CD180-induced Ig is polyclonal and not merely the result of an unexamined Ag-specific response. As exceptionally rapid production of Ag-specific Ig can occur with either TI-1 or TI-2 antigens, and cellular debris may stimulate B cells for these responses, we examined the effect of anti-CD180 on antigen specific responses to NP-conjugated model antigens. The doses of NP-LPS were low and induced little polyclonal Ig but a robust NP-specific response. It is unlikely that cellular debris is stimulating Ig production as Ag-specific antibody was reduced by co-administration of anti-CD180. Also, antinuclear antibody did not increase with anti-CD180 treatment (data not shown). While Ag-specific responses to independent but co-administered Ag decreased, Ig specific for the anti-CD180 mAb itself was increased, though not to more than 16% of the total IgM in the serum following anti-CD180 injection. As the bulk of the IgM and essentially all of the IgG produced by anti-CD180 treatment is neither from memory recall nor specific for concomitantly present antigens, it is likely to be truly polyclonal.
Injection of anti-IgD similarly induces polyclonal B cell activation and production of high serum IgG1 concentrations (23, 24), and is the closest known parallel for the effects of anti-CD180 in vivo. However, there are notable differences between the effects of anti-CD180 and anti-IgD. Anti-IgD induced polyclonal Ig was restricted to IgG1 and IgE isotypes, and required T cell help and specifically IL-4 (25, 26). In contrast, anti-CD180 injection increased all isotypes and subclasses except for IgG2b and IgA, the two prototypic TGF-β induced Ig classes (27) – an effect that did not require the C57BL/6 background, T cells, CD40, IL-4, or MyD88-dependent signaling. As B cell class-switch recombination is thought to require either T cell help or MyD88-dependent TLR/TACI signals (28), anti-CD180-induced Ig production may involve an unrecognized pathway for class-switch induction. Notably, anti-CD180 treatment is remarkable among polyclonal activators by virtue of its profound and rapid induction of diverse Ig classes and subclasses, including those that are normally counter-regulated or poorly induced by polyclonal stimuli. Additionally, while Ig production by anti-IgD required higher order clustering produced by either multiple mAbs or polyclonal sera (23, 25), a single anti-CD180 mAb induces Ig production, suggesting that only dimerization is required. While our data do not support the idea of CD180 signaling via IgD, we cannot rule out the involvement of other BCR signaling components (29). Despite the significant differences between CD180 and IgD as mediators of polyclonal activation, they still may be classified together in that both induce potent effects but have no confirmed function despite their discovery over 20 years ago.
Injection of anti-CD180 mAb resulted in a rapid increase in splenic cellularity; 3 d after injection T1, T2, and FO B cell subsets expanded 7-, 9-, and 2.5-fold, respectively, whereas neither MZ nor CD5+ B cells expanded in number. While these lymphocyte expansions conflate survival and tissue homing effects with proliferation, the uptake of BrdU after a short pulse indicates that B cells were induced to proliferate in vivo and that the expansions are not simply a result of enhanced survival. MZ B cells proliferate the more extensively than any other subset following anti-CD180 injection, displaying even greater BrdU uptake than the bone marrow positive control (29.3% BrdU+ vs. 27.1% BrdU+). As the total number of MZ B cells did not increase in number the fate of the proliferating MZ B cells is unknown. As MZ B cells are the primary source of IgG3, one possibility is that after anti-CD180 treatment MZ B cells become highly activated, proliferate quickly, rapidly produce extrafollicular Ab, and then undergo apoptosis. Further studies are in progress to monitor the fate of the CD180-activated MZ B cells. While T cells do not express CD180 and neither become activated nor proliferate after anti-CD180 stimulation either in vivo or in vitro, their numbers are significantly increased in the spleen following anti-CD180 injection. This suggests that circulating T cells are increased in the spleen by passive mechanisms such as enhanced retention due to an indirect effect mediated by anti-CD180 activated B cells. Regardless of the mechanism, the expansion of T cells in the spleen is abrogated in B cell-deficient μMT mice, indicating that B cells are required for the effect on T cells and not other CD180+ cells (DCs, macrophages). Increases to both B and T cell numbers were transient, approaching normal numbers by d 7 after injection; only CD8+ T cell numbers remained increased through d 14. The function of these persistent CD8+ T cells is unknown, as anti-CD180-induced expansion and contraction of B cells were equivalent in WT and T cell-deficient mice. It is possible that the prevalence of activated B cells is mediating memory T cell reactivation without the presence of cognate antigen.
The effects induced by anti-CD180 injection in vivo are not simply a byproduct of injecting a B cell-binding mAb. Anti-CD40 mAb, another known B cell activator, unlike anti-CD180, which expanded mainly transitional and MZ B cells, induced significant expansion only in FO B cells and myeloid cells (Fig. 4). Additionally, while anti-CD40 increased expression of both CD80 and CD95, classic markers of germinal center formation, anti-CD180 increased neither of these markers, but rather selectively upregulated CD86.
The combined injection of anti-CD180 with LPS, both inducers of polyclonal Ig, did not further increase Ig in serum but instead resulted in a reduction of Ig levels to concentrations intermediate to those seen with either stimulus alone. A similar effect was seen with co-injection of anti-CD180 with either TLR9 or TLR2:1 ligands (CpG or Pam3CSK4). This result was duplicated with an in vitro system, where increasing doses of LPS decreased anti-CD180 induced IgG production in a concentration dependent manner. The suppression of anti-CD180 induced Ig by divergent TLR ligands suggests either a restraining effect from TLR activated non-B cells or an intrinsic negative regulation by TLR signals of CD180 stimulated B cell differentiation. Our data support a model where combinations of CD180 signals and all MyD88-dependent TLR signals drive greater B cell proliferation at the expense of differentiation and Ig production.
Our data regarding B cell proliferation to anti-CD180 and LPS are not consistent with models suggesting CD180 functions by forming heterodimers specifically with TLR4 and regulating the canonical LPS signal (4, 12). Unlike LPS, the B cell proliferative response to anti-CD180 does not require MyD88, TRIF, or TLR4. Indeed, in a series of experiments, the impact of MyD88 deficiency on the anti-CD180 induced proliferation index was minimal while LPS induced proliferation was essentially abrogated. However, CD180 and TLR signals appear to be integrated through MyD88 because the combination of anti-CD180 and LPS signals augments B cell proliferation in TRIF-deficient but not MyD88-deficient B cells. Taken together, these results indicate that CD180 signals augment, but are independent from, those of TLR4. Given these findings, we hypothesized that stimulation of other MyD88-dependent TLRs (e.g. TLR9, TLR7, TLR2:6, and TLR2:1) would also enhance B cell proliferation to CD180 ligation. Indeed, strong augmentation was evident with anti-CD180 and all TLR ligands tested. This effect may not have been detected in previous studies, which used only single concentrations of ligand combinations; saturation concentrations may have resulted in an insignificant augmentation unlike sub-maximal doses. As TLR7 and TLR9 are largely endosomal (30), and not at the cell surface like CD180, our data are not consistent with models of CD180 function requiring direct interactions with TLRs to augment B cell proliferation.
Our analysis allowed the use of the mathematical transformation described by Chou and Talalay (21) to quantify synergy over broad dose ranges. Synergy is highest between anti-CD180 and the TLR2 ligands, followed by TLR7, then by TLR9, with the least synergy between CD180 and TLR4. The analysis also revealed previously unreported antagonism between anti-CD180 and all MyD88-dependent TLR ligands, excluding LPS, at very low doses. Existing models of CD180 as a selectively forming heterodimers with TLR4 predict neither of these patterns, regardless of whether the interaction is stimulatory or inhibitory. Whether CD180 acts as a specific TLR4 “decoy” receptor in B cells, as proposed for DCs (3), or a required co-receptor for a single B cell LPS pathway (12), the effect should impact both the MyD88 and TRIF signaling pathways for LPS and no effect would be expected for other TLRs. Thus, our findings showing that CD180 synergizes with multiple TLR ligands in a MyD88-dependent TRIF-independent manner to enhance proliferation at nearly all dose levels suggest an alternative model where independent CD180 and TLR signals converge in B cells at the level of MyD88.
While anti-CD180 stimulation of purified B cells induced proliferation, it did not induce cytokine production. However, in combination with LPS, anti-CD180 stimulation increased production of IL-10 and IL-6, but not TNF-α, while anti-CD180 plus CpG increased production of all of these cytokines. The IL-10 concentrations were high (>1,000 pg/ml), suggesting that CD180 signals could be involved in development of anti-inflammatory IL-10 secreting B cells (31). Due to the complex effects of IL-10, both suppressing inflammation and activating B cells (32, 33), it is possible that combined CD180/TLR signaling may minimize TLR-induced inflammation while promoting select B cell functions. As with B cells, DCs failed to produce cytokines with anti-CD180 stimulation alone, however unlike B cells TLR-induced cytokine production was not augmented by the combination with anti-CD180. This finding is inconsistent with the inactive dimer model where CD180 suppresses TLR4 sensing of LPS (3); if anti-CD180’s function were simply to sequester CD180 from TLR4 this would lead to an increase in DC sensitivity to LPS rather than the observed minimal decrease. A combination of evidence regarding anti-CD180 treatment - the lack of DC responsiveness, the requirement of B cells for splenic expansion, the production of high serum concentrations of Ig in both WT and T cell-deficient mice, and the proliferation of purified B cells in vitro - together suggest that CD180 stimulation is primarily mediated by, and intrinsic to, B cells. While our data cannot differentiate between a direct stimulatory effect upon B cells or the removal of a repressive signal the fact that anti-CD180 induces rapid expansions in B cell numbers, calcium flux, and enhanced expression of both costimulatory factors and cytokines, we think it is most likely that regardless of mechanism the end effect of anti-CD180 treatment is intrinsic stimulation of B cells.
Our study of CD180 is unique in that use of an agonistic antibody allows us to perform quantitative interaction assays over broad dose ranges and characterize acute responses as opposed to genetic deletion that results in data singular in both dose and kinetics. Taken together, our results suggest that CD180 stimulation plays an important role in B cell proliferation, activation, and differentiation, and that these effects are significantly modulated by integration of MyD88-dependent TLR signals. While it remains to be determined whether the rapidly induced class-switched Ig also involves somatic hypermutation, it appears to be polyclonal. Finally, because anti-CD180 treatment induces immunomodulatory effects (augmenting anti-inflammatory IL-10, blunting Ag-specific responses, and producing polyclonal Ig which may clear apoptotic debris like natural antibody) it may have therapeutic potential in systemic autoimmune diseases; we are currently exploring this possibility in mouse models.
Supplementary Material
Acknowledgments
The authors thank Kevin E. Draves and Amy E. Steadman for excellent laboratory assistance and Rohan Damle for help with synergy analysis calculations. We also wish to thank Craig P. Chappell, Thomas R. Hawn, and Martha Hayden-Ledbetter for helpful discussions.
This work was supported by grants from the National Institutes of Health (grants AI52203 [E.A.C.], AI44257 [E.A.C.], AI85311 [J.A.L.]), Washington State Life Science Discovery Fund 2087750 (E.A.C. and J.A.L.), the Townsend-Henderson Endowment (J.W.C.), and the Public Health Service, National Research Service Award, 2T32 GM007270, from the National Institute of General Medical Sciences (J.W.C.).
Abbreviations used in this article
- CGG
chicken gamma-globulin
- CI
combination index
- DC
dendritic cell
- FO
follicular B cells
- MZ
marginal zone
- NP
4-hydroxy-3-nitro-phenacetyl
- T1
transitional 1 B cell
- T2
transitional 2 B cell
- TI
T cell-independent
- TIR
Toll/IL-1-receptor
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- WT
wildtype
References
- 1.Valentine MA, Clark EA, Shu GL, Norris NA, Ledbetter JA. Antibody to a novel 95-kDa surface glycoprotein on human B cells induces calcium mobilization and B cell activation. J Immunol. 1988;140:4071–4078. [PubMed] [Google Scholar]
- 2.Miyake K, Yamashita Y, Ogata M, Sudo T, Kimoto M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J Immunol. 1995;154:3333–3340. [PubMed] [Google Scholar]
- 3.Divanovic S, Trompette A, Atabani S, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, Kurt-Jones EA, Karp CL. Negative regulation of TLR4 signaling by RP105. Nat Immunol. 2005;6:571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagai Y, Shimazu R, Ogata H, Akashi S, Sudo K, Yamasaki H, Hayashi S, Iwakura Y, Kimoto M, Miyake K. Requirement for MD-1 in cell surface expression of RP105/CD180 and B-cell responsiveness to lipopolysaccharide. Blood. 2002;99:1699–1705. doi: 10.1182/blood.v99.5.1699. [DOI] [PubMed] [Google Scholar]
- 5.Groeneveld PH, Erich T, Kraal G. In vivo effects of LPS on B lymphocyte subpopulations. Migration of marginal zone-lymphocytes and IgD-blast formation in the mouse spleen. Immunobiology. 1985;170:402–411. doi: 10.1016/S0171-2985(85)80064-4. [DOI] [PubMed] [Google Scholar]
- 6.Fedele G, Celestino I, Spensieri F, Frasca L, Nasso M, Watanabe M, Remoli ME, Coccia EM, Altieri F, Ausiello CM. Lipooligosaccharide from Bordetella pertussis induces mature human monocyte-derived dendritic cells and drives a Th2 biased response. Microbes Infect. 2007;9:855–863. doi: 10.1016/j.micinf.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 8.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky JA. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci USA. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagai Y, Kobayashi T, Motoi Y, Ishiguro K, Akashi S, Saitoh S, Kusumoto Y, Kaisho T, Akira S, Matsumoto M, Takatsu K, Miyake K. The Radioprotective 105/MD-1 complex links TLR2 and TLR4/MD-2 in response to microbial membranes. J Immunol. 2005;174:7043–7049. doi: 10.4049/jimmunol.174.11.7043. [DOI] [PubMed] [Google Scholar]
- 12.Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–29. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuneyoshi N, Fukudome K, Kohara J, Tomimasu R, Gauchat JF, Nakatake H, Kimoto M. The functional and structural properties of MD-2 required for lipopolysaccharide binding are absent in MD-1. J Immunol. 2005;174:340–344. doi: 10.4049/jimmunol.174.1.340. [DOI] [PubMed] [Google Scholar]
- 14.Harada H, Ohto U, Satow Y. Crystal structure of mouse MD-1 with endogenous phospholipid bound in its cavity. J Mol Biol. 2010;400:838–846. doi: 10.1016/j.jmb.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 15.Miyake K, Yamashita Y, Hitoshi Y, Takatsu K, Kimoto M. Murine B cell proliferation and protection from apoptosis with an antibody against a 105-kD molecule: unresponsiveness of X-linked immunodeficient B cells. J Exp Med. 1994;180:1217–1224. doi: 10.1084/jem.180.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan VW, Mecklenbrauker I, Su I, Texido G, Leitges M, Carsett R, Lowell CA, Rajewsky K, Miyake K, Tarakhovsky A. The molecular mechanism of B cell activation by Toll-like receptor protein RP-105. J Exp Med. 1998;188:93–101. doi: 10.1084/jem.188.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazawa N, Fujimoto M, Sato S, Miyake K, Asano N, Nagai Y, Takeuchi O, Takeda K, Okochi H, Akira S, Tedder TF, Tamaki K. CD19 regulates innate immunity by the Toll-like receptor RP105 signaling in B lymphocytes. Blood. 2003;102:1374–1380. doi: 10.1182/blood-2002-11-3573. [DOI] [PubMed] [Google Scholar]
- 18.Nunez Miguel R, Wong J, Westoll JF, Brooks HJ, O’Neill LA, Gay NJ, Bryant CE, Monie TP. A dimer of the Toll-like receptor 4 cytoplasmic domain provides a specific scaffold for the recruitment of signaling adaptor proteins. PLoS One. 2007;2:e788. doi: 10.1371/journal.pone.0000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyman T, Stenmark P, Flodin S, Johanson I, Hammarstrom M, Nordlund P. The crystal structure of the human Toll-like receptor 10 cytoplasmic domain reveals a putative signaling dimer. J Biol Chem. 2008;283:11861–11865. doi: 10.1074/jbc.C800001200. [DOI] [PubMed] [Google Scholar]
- 20.Divanovic S, Trompette A, Petinot LK, Allen JL, Flick LM, Belkaid Y, Madan R, Haky JJ, Karp CL. Regulation of TLR4 signaling and the host interface with pathogens and danger: the role of RP105. J Leukoc Biol. 2007;82:265–271. doi: 10.1189/jlb.0107021. [DOI] [PubMed] [Google Scholar]
- 21.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.MacLennan IC, Toellner KM, Cunninham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunological Reviews. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 23.Goroff DK, Holmes JM, Bazin H, Nisol F, Finkelman F. Polyclonal activation of the murine immune system by an antibody to IgD. XI Contribution of membrane IgD cross-linking to the generation of an in vivo polyclonal antibody response. J Immunol. 1991;146:18–25. [PubMed] [Google Scholar]
- 24.Finkelman F, Snapper CM, Mountz JD, Katona IM. Polyclonal activation of the murine immune system by an antibody to IgD. IX Induction of a polyclonal IgE response. J Immunol. 1987;138:2826–2830. [PubMed] [Google Scholar]
- 25.Finkelman F, Scher I, Mond JJ, Kung JT, Metcalf ES. Polyclonal activation of the murine immune system by an antibody to IgD. I Increase in cell size and DNA synthesis. J Immunol. 1982;129:629–637. [PubMed] [Google Scholar]
- 26.Finkelman F, Scher I, Mond JJ, Kessler S, Kung JT, Metcalf ES. Polyclonal activation of the murine immune system by an antibody to IgD. II Generation of polyclonal antibody production and cells with surface IgG. J Immunol. 1982;129:638–646. [PubMed] [Google Scholar]
- 27.Park SR, Seo GY, Choi AJ, Stavnezer J, Kim PH. Analysis of transforming growth factor-beta1-induced Ig germ-line gamma2b transcription and its implication for IgA isotype switching. Eur J Immunol. 2005;35:946–956. doi: 10.1002/eji.200425848. [DOI] [PubMed] [Google Scholar]
- 28.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, Shan M, Xiong H, Bussel JB, Chiu A, Puel A, Reichenbach J, Marodi L, Doffinger R, Vasconcelos J, Issekutz A, Krause J, Davies G, Li X, Grimbacher B, Plebani A, Meffre E, Picard C, Cunningham-Rundles C, Casanova JL, Cerutti A. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rijkers GT, Griffionen AW, Zegers BJ, Cambier JC. Ligation of membrane immunoglobulin leads to inactivation of the signal-transducing ability of membrane immunoglobulin, CD19, CD21, and B-cell gp95. Proc Nat Acad Sci USA. 1990;87:8766–8770. doi: 10.1073/pnas.87.22.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton-Bassiri A, Dillon SB, Cunningham M, Rycyzyn MA, Mills J, Sarisky RT, Mbow ML. Toll-like receptor 9 can be expressed at the cell surface of distinct populations of tonsils and human peripheral blood mononuclear cells. Infect Immun. 2004;72:7202–7211. doi: 10.1128/IAI.72.12.7202-7211.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann NY Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 32.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 33.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin-10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.