Abstract
The overexpression of tissue factor (TF) observed in numerous cancer cells and clinical samples of human cancers makes TF an ideal target for cancer therapy. The purpose of this study is to develop a TF-targeting energized fusion protein hlFVII-LDP-AE, which is composed of a human Factor VII light chain (hlFVII) as the targeting domain conjugated to the cytotoxic antibiotic lidamycin (LDM, LDP-AE) as the effector domain. The potential efficacy of hlFVII-LDP-AE for cancer therapy was tested in vitro by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and colony formation assays and in vivo with a BALB/c nude mouse xenograft model of human liver cancer line HepG2. The inhibitory concentration (IC50) value of hlFVII-LDP-AE varied from 0.15 to 0.64 nM for the various human tumor lines. hlFVII-LDP-AE showed a tumor growth inhibition rate of 90.6% at the dose of 0.6 mg/kg in in vivo animal experiments. The mechanism through which hlFVII-LDP-AE inhibits tumor growth also was determined by Hoechst 33342 staining and Tdt-mediated dUTP nick-end labeling (TUNEL) assay. hlFVII-LDP-AE causes tumor cell death through inducing chromatin condensation and cleavage of genomic DNA. These findings suggest that the hlFVII-LDP-AE protocol is efficacious and tolerated in the mouse model of human liver cancer HepG2 and has clinical applicability for treating cancer patients.
Key words: factor VII, lidamycin, liver cancer, tissue factor
Introduction
Cancer therapy remains a challenge worldwide accounting for its low cure rate. Although antitumor drugs have been developed for many years, their use is limited because of the toxicity to normal tissues.1,2 Therefore, development of cytotoxic drugs that can specifically target tumors with minimal toxicity to normal cells becomes more important, which requires a tumor target and a targeting molecule that can deliver and internalize a cytotoxic molecule.3
Accumulating evidence suggests that the receptor tissue factor (TF) is overexpressed on many cancer cells in solid tumors4–9 and leukemia.10–12 The TF levels of cancer cells are up to 1000-fold greater than those of their normal counterparts. This overexpression is also observed in clinical samples of numerous types of human cancers, with just a few exceptions (e.g., renal cancer).13 Therefore, TF-targeting therapies could be used to eradicate tumor cells.14 TF is also expressed on extravascular cells of several normal tissues and in the adventitial layer of large blood vessel walls in normal condition15,16; however, it is anatomically sequestered from its natural ligand coagulation factor VII (FVII) and from TF-targeting therapeutic agents circulating in blood by the intact semipermeable endothelial layer of normal blood vessels.14 In contrast, because the walls of the tumor vasculature are leaky,17 systemically administered TF-targeting therapeutic agents can thus access TF on tumor cells via the leaky tumor neovasculature.14
As targeting molecule, ligands have many advantages, such as readily available, inexpensive to manufacture, and easy to handle.3 FVII, a ligand that binds to TF with exceptional affinity and specificity,6 is composed of a 20-kDa amino-terminal light chain and a 30-kDa carboxy-terminal heavy chain, which are linked by a disulfide bond.18 The light chain binds to TF, and the heavy chain initiates the blood coagulation pathway.19,20 Several TF-targeting therapeutic agents have been tested for the treatment of cancer. Hu et al. developed the first TF-targeting therapeutics, the antibody-like FVII-targeted Icon (FVII/IgG1 Fc), for cancer immunotherapy in 1999.8 In following years, Icon immunotherapy was tested for the eradication other tumors.6,7,21 Hu et al. also developed two FVII-targeted photodynamic therapeutics for breast cancer by conjugating Sn(IV) chlorine e6 (SnCe6)14 or Verteporfin22 to FVII. To reduce the coagulation activity of FVII-targeted therapeutics, Hu et al. introduced a mutation (K341A) into the FVII protein.6–8 Similarly, Shoji et al. reported the use of an active-site-inactivated recombinant human FVIIa (FFRck-fVIIa) as a carrier for the targeted delivery of a potent synthetic curcumin analog (EF24) to TF-expressing tumor cells.23 In conclusion, FVII used in all the previous FVII-targeted therapeutics was prepared in eukaryotic cells, and its serine protease activity was inactivated by biological or chemical methods. Here, our choice for a targeting molecule was the human Factor VII light chain (hlFVII). As the targeting vehicle, the single-chain hlFVII molecule is significantly smaller than the parental two-chain human FVII molecule, which should provide two therapeutic advantages: facilitating access of the hlFVII-LDP-AE fusion protein to a solid tumor3 and preventing a blood clot that otherwise might occur when the two-chained hFVII molecule binds to TF.20 In addition, the fusion protein hlFVII-LDP in this report was prepared in Escherichia coli.
The choice for a cytotoxic molecule was lidamycin (LDM, also named C-1027), a member of the enediyne antibiotic family derived from Streptomyces globisporus C-1027,24,25 which is cytotoxic for cultured tumor cells and inhibits growth of a panel of human tumor xenografts.26,27 LDM is composed of an apoprotein (LDP, 10,500 Da) and an active enediyne chromophore (AE, 843 Da) in 1:1 stoichiometry.28 AE binds DNA in the minor groove and causes double-strand DNA breaks and tumor cell death, and LDP forms a hydrophobic pocket protecting the AE chromophore.29–31 Intercalation of AE into the hydrophobic pocket of LDP was mainly forced by hydrophobic interaction.32 The two parts of LDM can be dissociated in vitro and reconstituted with restored cytotoxic activity,28,33 which provides a two-step procedure containing DNA recombination and molecule reconstitution for synthesizing novel cytotoxic fusion proteins.28,33
Here we developed an energized fusion protein hlFVII-LDP-AE containing hlFVII as the targeting domain linked to LDM (LDP-AE) as the effector domain. The potential efficacy of hlFVII-LDP-AE for cancer therapy was tested in vitro on four human tumor lines and in vivo in a BALB/c nude mouse xenograft model of human liver cancer line HepG2. The results demonstrate that hlFVII-LDP-AE is cytotoxic for the human tumor lines and strongly inhibits growth of human liver cancer HepG2 xenograft, suggesting that hlFVII-LDP-AE has potential clinical applications.
Materials and Methods
Construction of expression plasmid
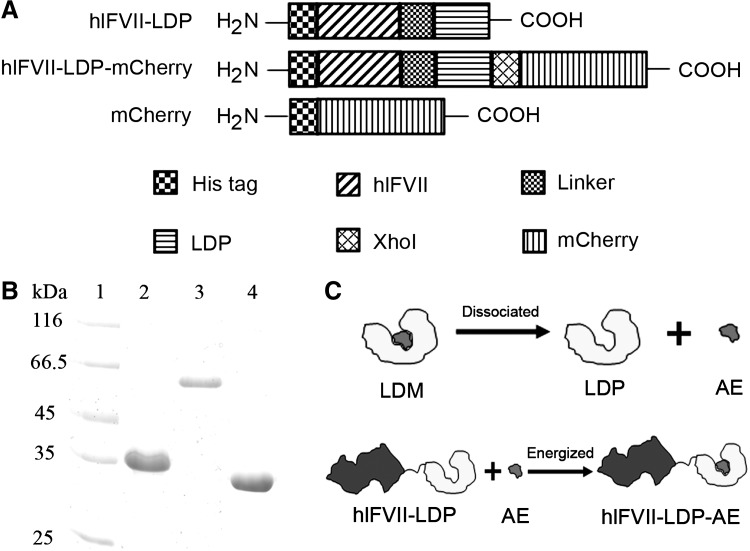
The diagrams of three recombinant proteins in this study are shown in Figure 1A. The expression plasmids of the three recombinant proteins were constructed by conventional molecular cloning techniques. The DNA fragment encoding hlFVII (152 amino acids)34 was cloned from the pCR4-TOPO-hFVII plasmid (FL25053; FulenGen Corp.). The coding sequence for LDP was cloned from the plasmid pET-VH-LDP35 constructed in our laboratory. The DNA fragment encoding mCherry was cloned from the pmCherry-N1 plasmid (Clontech). The expression vector is pET-19b (Novagen).
FIG. 1.
(A) Domain diagram of recombinant proteins hlFVII-LDP, hlFVII-LDP-mCherry, and mCherry. His tag, purifying tag composed of six histidines; hlFVII, the light chain of human factor VII; Linker, a flexible linker composed of triplicate GGGGS. LDP, the apoprotein of lidamycin; mCherry, a monomeric red fluorescent protein. (B) Purified recombinant proteins hlFVII-LDP (lane 2), hlFVII-LDP-mCherry (lane 3), and mCherry (lane 4) were analyzed by SDS-PAGE. (C) Schematic presentations of the dissociation of LDM into AE and LDP and the reconstitution of hlFVII-LDP-AE. LDM, lidamycin; AE, active enediyne; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Expression and purification of recombinant proteins
The recombinant plasmids were transformed into Rosetta-gami B (DE3) pLysS (Novagen) competent cells. A single colony was inoculated into 5 mL of LB medium (pH 7.5) containing 100 μg/mL ampicillin, 34 μg/mL chloramphenicol, 15 μg/mL kanamycin, and 12.5 μg/mL tetracycline and cultured overnight at 37°C. Next day, 1 L of culture medium was inoculated with the overnight culture and incubated with shaking at 37°C until A600=0.6–1. Isopropyl-1-thio-β-d-galactopyranosid was added to the medium to a final concentration of 0.8 mM. After being induced for 6 hours at 37°C, the bacteria were lysed using pulse sonication followed by 60 minutes of centrifugation at 48,400 g. His-tagged recombinant proteins were then purified by affinity chromatography (His Trap HP; GE Healthcare) and ion exchange chromatography (HiTrap Q HP; GE Healthcare) according to the manufacturer's instructions.
Cell culture
Human liver cancer line HepG2, lung cancer line A549, colorectal cancer line HCT-116, and breast cancer line MCF-7 were grown as monolayer in culture plates. Cells were cultured in either Dulbecco's modified Eagle's medium (DMEM) (HepG2, A549, and HCT116) or in Eagle's minimum essential medium (MCF-7) supplemented with 10% fetal bovine serum (Gibco) at 37°C in humidified 5% carbon dioxide (CO2) incubator.
Coimmunoprecipitation assay
hlFVII-LDP was added to medium containing the cultured human liver cancer line HepG2 to a final concentration of 0.5 μM in six-well plates. After culturing for 2 hours, cells were washed twice. Then, 1 mL of ice-cold phosphate-buffered saline (PBS) was added to the wells. Cells were scraped down and collected by centrifuging at 800 g for 5 minutes. Three hundred microliters of ice-cold lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 10 mM KCl, 0.5 mM ethylenediaminetetraacetic acid [EDTA], 1.5 mM MgCl2, 0.5 mM PMSF, 2 mM DTT, 2.5 mM CaCl2, 0.5% NP-40, and 10% glycerol [pH 7.9]) was added to the cells and incubated on ice. The cell suspensions were vortexed one time every 2 minutes for five times. Lysates were centrifuged at 16,000 g in 4°C for 5 minutes. Two hundred microliters of lysate supernatant and 800 μL of ice-cold lysis buffer were added to a 1.5 mL Eppendorf tube. Then, antihuman TF antibody (MAB2339; R&D) was added to the tube to a final concentration 10 μg/mL. The mixture was incubated overnight at 4°C. Complexes were collected with protein A agarose (Invitrogen), and the precipitates were washed three times with ice-cold lysis buffer. Then, proteins were released by boiling in sample buffer and analyzed by Western blot with mouse antihuman FVII antibody (MAB2338; R&D).
Internalization assay
The internalization of hlFVII-LDP assay was performed as described previously36 with some modification. Cells (1×105/well) were seeded in six-well plates and cultured overnight. The next day, the medium was changed to protein transduction medium, which was prepared by mixing the recombinant fusion proteins at a final concentration of 150 nM with normal medium supplemented with 3 mM CaCl2. Cells were incubated for another 12 hours, and then were washed three times with PBS and fixed with 4% paraformaldehyde (PFA) for 10 minutes at room temperature. After three washes, the cells were stained for 5 minutes at room temperature with 4′,6-diamidino-2-phenylindole (Sigma). The cells were observed and photographed with a fluorescence microscopy (OLYMPUS).
Preparation of energized fusion protein hlFVII-LDP-AE
The energized fusion protein hlFVII-LDP-AE was prepared as described previously.37 In brief, the active AE of LDM was separated by C4 column (GE Healthcare) with a 22% acetonitrile in 0.05% trifluoroacetic acid mobile phase. The AE-containing solution was added to hlFVII-LDP/PBS (10 mM, pH 7.0) with the molecular ratio of 3:1, and was incubated at 4°C for 12 hours with rocking. Free AE was removed by a Sephadex G-75 column (GE Healthcare). Assembled energized fusion proteins were confirmed by reverse-phase high-performance liquid chromatography (HPLC) by a Vydac C4 300A column (Grace). Absorbance at 350 nm was measured.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used for measuring the cytotoxicity of energized fusion protein hlFVII-LDP-AE in vitro as described previously.35
Colony formation assay
Cells (500/well) were seeded in a six-well plate and cultured in a humidified 5% CO2 incubator for 24 hours. The next day, the medium was replaced with complete DMEM supplemented with 3 mM CaCl2 and 10−12 M LDM or hlFVII-LDP-AE. After culturing the cells for 2 hours, the medium was replaced with complete DMEM. The cells were cultured for another 10 days. Surviving colonies were stained with p-nitro blue tetrazolium chloride after methanol fixation. The plate was scanned with a scanner (Perfection 4990 Photo; EPSON). The colonies were counted manually.
Chromatin condensation assay
Cells (1×105/well) were seeded in a six-well plate. The next day, the medium was replaced with fresh medium supplemented with 3 mM CaCl2 and 0.50 nM hlFVII-LDP-AE or 0.60 nM LDM. After being incubated for 12 hours, the cells were washed with PBS for three times, and then were stained with the DNA-specific fluorescent dye Hoechst 33342 (10 μg/mL) (Sigma) for 10 minutes at 37°C. The cells were washed with PBS again, and then were observed and photographed with a fluorescence microscopy (OLYMPUS).
Tdt-mediated dUTP nick-end labeling assay
Cells were seeded in 24-well plates. After 24 hours, the medium was replaced with fresh medium supplemented with 3 mM CaCl2 and 0.1 nM hlFVII-LDP-AE or 1 nM LDM and incubated at 37°C. After 12 hours, the cells were washed with PBS for three times and fixed with 4% PFA for 10 minutes. Tdt-mediated dUTP nick-end labeling (TUNEL) was performed by using DeadEnd™ Colorimetric TUNEL System according to the instructions of the manufacturer (Promega). Next, by microscopic analysis the cells were examined for cleavage of genomic DNA.
Efficacy studies in vivo
Female BALB/c nude mice (6 weeks old) were purchased from the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences. The construction of nude mice xenograft model of human liver cancer HepG2 was carried out as described by Guo et al.38 When the tumor size reached about 50 mm3, the mice were divided into six groups (n=8), and five groups were injected i.v. into the tail vein once at day 0 with LDM (0.05 mg/kg, tolerated dose), hlFVII-LDP (30 mg/kg), or hlFVII-LDP-AE (0.15, 0.3, and 0.6 mg/kg) in 200 μL sterile saline. The control group was injected with 200 μL sterile saline. To estimate the volume of developing tumors and the health condition of tumor-bearing mice, the exposed surface of the tumor was measured in two perpendicular directions, and the body weight was recorded. The volume of a tumor was calculated as (width2) (length)/2. At the end of experiment, all mice were weighed and sacrificed, and their tumors were excised. Tumors were weighed, and the mean tumor weight was calculated. The (mean treated tumor weight)/(mean control tumor weight) ×100% is subtracted from 100% to give the tumor growth inhibition (TGI) for each group.39,40
Statistical analysis
Data in all cases are expressed as mean±standard error of the mean. Comparison of mean values between the different treatments was carried out using the two-way independent sample t-test with Microsoft Excel 2003 software. The level of significance was set at p<0.05.
Results
Construction and preparation of fusion proteins
Recombinant DNA encoding proteins hlFVII-LDP, hlFVII-LDP-mCherry, and mCherry were cloned and inserted into pET-19b expression vectors. After induction and purification by Ni2+ affinity chromatography and ion exchange chromatography, the fusion proteins were detected by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1B). The energized fusion protein hlFVII-LDP-AE was prepared by molecular reconstitution with AE and hlFVII-LDP (Fig. 1C). Data from reverse-phase HPLC showed that the AE molecule integrated into hlFVII-LDP successfully, and the purity of hlFVII-LDP-AE was 92.78% (data not shown).
Receptor binding and internalization of hlFVII-LDP
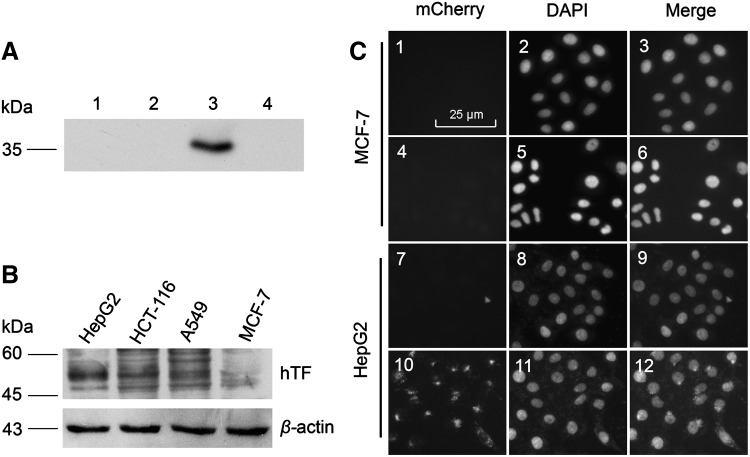
The receptor-binding activity of hlFVII-LDP was analyzed by coimmunoprecipitation assays. As shown in Figure 2A, hlFVII-LDP appears as a component of the immunoprecipitation complex with human TF expressed in HepG2 cells (Fig. 2A, lane 3) that was confirmed to express TF with a high level (Fig. 2B); however, preimmune serum (Fig. 2A, lane 4) did not precipitate hlFVII-LDP protein, and no signal was observed in two other negative controls in which hlFVII-LDP was substituted with bovine serum albumin (Fig. 2A, lane 1) or the cells were substituted with the human breast cancer line MCF-7 (Fig. 2A, lane 2) expressing low level TF (Fig. 2B).41
FIG. 2.
Binding and internalization of hlFVII-LDP. (A) The binding specificity of hlFVII-LDP to TF expressed on HepG2 cells was verified by coimmunoprecipitation assay. Total protein extracted from HepG2 cells (lanes 1, 3, and 4) and MCF-7 cells (lane 2) incubated with bovine serum albumin (lane 1) or hlFVII-LDP (lanes 2–4) was incubated with either preimmune serum (lane 4) or an antihuman TF monoclonal antibody (lanes 1–3). The complexes formed were collected with Protein A agarose and were analyzed by SDS-PAGE and Western blot with antihuman FVII monoclonal antibody. (B) Expression of human TF on different cancer cell lines analyzed by Western blot with antihuman TF monoclonal antibody. (C) Internalization assay of hlFVII-LDP by hlFVII-LDP-mCherry. Cells in 1–3 and 7–9 were treated with mCherry, and cells in 4–6 and 10–12 were treated with hlFVII-LDP-mCherry. Magnification,×400. TF, tissue factor; hTF, human tissue factor.
To further confirm whether hlFVII-LDP could be internalized by TF-expressing tumor cells, we constructed the mCherry-tagged hlFVII-LDP (hlFVII-LDP-mCherry). The mCherry tag was used as a reporter. Free mCherry was used as a negative control. As shown in Figure 2C, the fluorescence of hlFVII-LDP-mCherry was observed both on the membrane surface and in the cytoplasm of HepG2 cells (Fig. 2C, 10–l2). However, no fluorescence could be detected in the mCherry group of the HepG2 cells (Fig. 2C, 7–9) and in the MCF-7 cells incubated with mCherry (Fig. 2C, 1–3) or hlFVII-LDP-mCherry (Fig. 2C, 4–6). These results show that hlFVII-LDP can bind to TF expressed on HepG2 cells specifically and can be internalized into the cytoplasm.
Efficacy of energized fusion protein hlFVII-LDP-AE in vitro
The cytotoxicity of hlFVII-LDP, hlFVII-LDP-AE, and LDM was measured on four human tumor lines (HepG2, HCT-116, A549, and MCF-7) by MTT assay (see Table 1). As shown by the half maximal inhibitory concentration (IC50) values, all three proteins show potent cytotoxicity to all kinds of cancer cells. Further comparisons showed that the antitumor effects of hlFVII-LDP-AE were much more potent (17–77-fold) than those of hlFVII-LDP. However, hlFVII-LDP-AE did not show more potent antitumor effects than those of free LDM. We presumed that LDM is a kind of cytotoxic drug, so a long-termed incubation of drugs with cultured cells for 72 hours, a general procedure in MTT assay, should be unhelpful for comparing hlFVII-LDP-AE with LDM for the specific targeting.
Table 1.
Determined IC50 Values on Different Cancer Cell Lines by MTT Assay
| |
IC50 (nM) |
|||
|---|---|---|---|---|
| Groups | HepG2 | HCT-116 | A549 | MCF-7 |
| LDM | 0.60 | 0.94 | 0.14 | 0.60 |
| hlFVII-LDP | 17.76 | 33.23 | 2.58 | 11.85 |
| hlFVII-LDP-AE | 0.50 | 0.43 | 0.15 | 0.64 |
IC, inhibitory concentration; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; LDM, lidamycin; hlFVII, human Factor VII light chain; LDP, the apoprotein of lidamycin; AE, active enediyne.
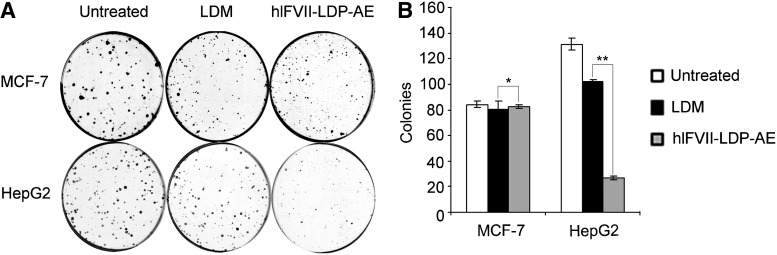
To further determine the targeting effect of hlFVII, colony formation assays were carried out. As shown in Figure 3, the growth inhibition effects of hlFVII-LDP-AE and free LDM did not show a significant difference for MCF-7 cells. In contrast, hlFVII-LDP-AE of HepG2 cells resulted in a significant reduction of colony formation compared with HepG2 cells treated with free LDM. These results clearly indicate that the targeting domain hlFVII increased the TF-selective cell death activity of LDM.
FIG. 3.
The inhibitory effects of hlFVII-LDP-AE on cell colony formation in HepG2 and MCF-7. (A) Inhibitory effects of hlFVII-LDP-AE on cell colony formation in HepG2 and MCF-7. (B) Representative dishes by colony-formation assay. Columns, mean of three determinations; bars, SEM. Results shown are representative of three independent experiments. *p=0.97; **p=0.001. SEM, standard error of the mean.
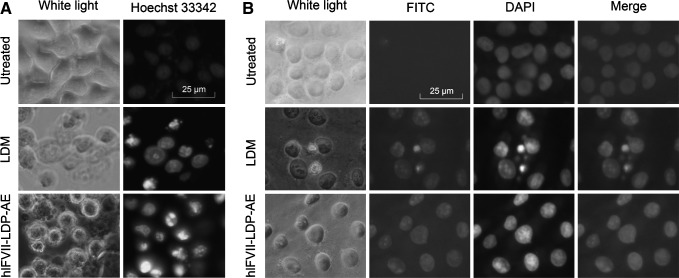
hlFVII-LDP-AE induced cancer cell death by causing chromatin condensation and cleavage of genomic DNA
As shown in Figure 4A, chromatin condensations were clearly observed in the cells treated with hlFVII-LDP-AE or the free LDM. Further, TUNEL assay showed that hlFVII-LDP-AE could induce cleavage of genomic DNA (Fig. 4B). These data suggest that the energized fusion protein hlFVII-LDP-AE causes tumor cell death through inducing chromatin condensation and cleavage of genomic DNA as the mechanism of LDM.42
FIG. 4.
Chromatin condensation and cleavage of genomic DNA in human liver cancer HepG2 cells induced by hlFVII-LDP-AE were determined by staining with the fluorescent dye Hoechst 33342 and TUNEL assay, respectively. (A) Chromatin condensation assay. (B) TUNEL assay. Magnification, ×400. TUNEL, Tdt-mediated dUTP nick-end labeling.
Therapeutic efficacy of energized fusion proteins hlFVII-LDP-AE in vivo
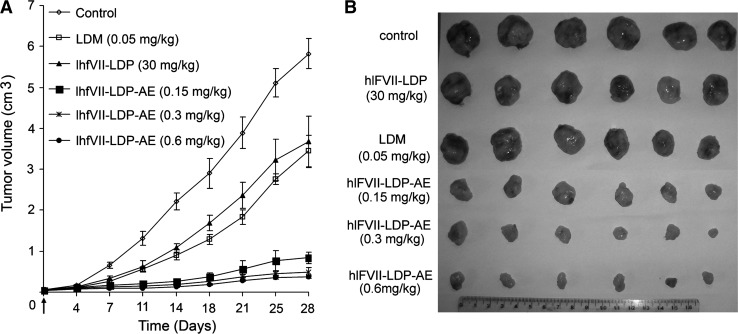
The antitumor effects of hlFVII-LDP-AE in vivo were tested in a nude mouse xenograft model of human liver cancer using the HepG2 cell line. As shown in Figure 5A, HepG2 xenografts were growing rapidly in control group in 28-day duration of experiment. At the end of experiment, the tumors were excised (Fig. 5B). As evaluated on day 28 (see Table 2), the fusion protein hlFVII-LDP (30 mg/kg) showed moderate therapeutic efficacy with 24.9% TGI; free LDM (0.05 mg/kg, tolerated dose) showed 36.9% TGI; whereas, hlFVII-LDP-AE at 0.15, 0.3, and 0.6 mg/kg greatly increased the antitumor efficacies with 82.3%, 88.5%, and 90.6% TGI, respectively. Further, the TGI of hlFVII-LDP-AE at 0.15, 0.3, and 0.6 mg/kg showed statistically significant differences compared with that of LDM at 0.05 mg/kg (p<0.01) and that of hlFVII-LDP at 30 mg/kg (p<0.01). Body weight was monitored as a systemic toxicity indicator of the drug administered. As shown in Table 2, the weight loss resulting from the hlFVII-LDP-AE treatment at the termination of the experiment at different doses did not exceed 10% of the pretreatment weight. Thus, the dosage of hlFVII-LDP-AE was tolerated.38
FIG. 5.
The growth inhibition of hlFVII-LDP-AE on HepG2 xenografts in nude mice. (A) The tumor growth curves in 28-day duration of experiments. Data are expressed as mean tumor volume±SEM (n=6). (B) Dissected tumors at the end of the experiments.
Table 2.
The Growth Inhibition of Human Liver Cancer HepG2 Xenograft in Athymic Mice
| Groups | Dosage (mg/kg) | No. of mice (begin/end) | Body weight changea(g) | Tumor weight (mean±SEM) (g) | % TGIb |
|---|---|---|---|---|---|
| Control | – | 6/6 | +4.6 | 5.31±0.41 | – |
| LDM | 0.05 | 6/6 | +1.33 | 3.35±0.33 | 36.9c |
| hlFVII-LDP | 30 | 6/6 | +4.07 | 3.99±0.19 | 24.9c |
| hlFVII-LDP-AE | 0.15 | 6/6 | −0.4 | 0.94±0.11 | 82.3d,e,f |
| hlFVII-LDP-AE | 0.3 | 6/6 | −0.55 | 0.61±0.15 | 88.5d,e,f |
| hlFVII-LDP-AE | 0.6 | 6/6 | −0.93 | 0.50±0.02 | 90.6d,e,f |
Body weight was monitored as a systemic toxicity indicator of the drug administered.
The % TGI was calculated using the final mean excised tumor weight for each treatment group rather than the final tumor weights estimated by dimensional measurement.
p<0.05 compared with control.
p<0.01 compared with control.
p<0.01 compared with LDM group.
p<0.01 compared with hlFVII-LDP group.
SEM, standard error of the mean; TGI, tumor growth inhibition.
Discussion
In this study, we developed an effective and selective hlFVII-targeted LDM (hlFVII-LDP-AE) for liver cancer HepG2 therapy. To the best of our knowledge, there are no published articles reporting that the light chain of FVII has been used as a targeting vehicle for the development of a targeted therapeutic agent.
We show that the hlFVII-LDP fusion protein prepared in E. coli can selectively bind to human TF-expressing cancer cells (Fig. 2A, lane 3). Further, mCherry-tagged hlFVII-LDP can be internalized into cells (Fig. 2C, 10–12). In this experiment, we also observed that some of the mCherry-tagged fusion proteins gathered near the nucleus (Fig. 2C, 10–12). Why the fusion protein could be directed to the periphery of the nucleus needs further study. Because LDM induces cell death mainly by AE, which binds DNA in the minor groove and causes double-strand DNA breaks,29 the fact that LDM is directed to cytoplasm and the periphery of the nucleus facilitates its function.
A possible caveat is that preventing blood clots might promote bleeding. However, no bleeding was detected in the mice treated with hlFVII-LDP-AE, which is consistent with similar results from another study in which mice were treated with an Icon molecule containing a two-chained hFVII targeting domain that had been mutated to prevent blood clotting.7
In conclusion, we report for the first time an hlFVII-targeted LDM (hlFVII-LDP-AE) that selectively and effectively killed cultured tumor cells in vitro and significantly inhibited the tumor growth of human liver cancer in vivo. As TF is expressed broadly in different types of tumors, including solid tumors4–8 and leukemia,11 hlFVII-LDP-AE has a broad therapeutic potential for liver and other cancers.10–12
Acknowledgments
This work was supported by grants from “Significant new drugs development” Science and Technology Major Projects of China (No. 2009ZX09103-698) and National Natural Science Foundation of China (No. 31000579), duration: 2011.1–2013.12.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Geney R. Chen J. Ojima I. Recent advances in the new generation taxane anticancer agents. Med Chem. 2005;1:125. doi: 10.2174/1573406053175292. [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka T. Okamoto T. Ichinose Y, et al. Methodological aspects of current problems in target-based anticancer drug development. Int J Clin Oncol. 2006;11:167. doi: 10.1007/s10147-006-0580-7. [DOI] [PubMed] [Google Scholar]
- 3.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 4.Callander NS. Varki N. Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70:1194. doi: 10.1002/1097-0142(19920901)70:5<1194::aid-cncr2820700528>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Contrino J. Hair G. Kreutzer DL, et al. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z. Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc Natl Acad Sci U S A. 2001;98:12180. doi: 10.1073/pnas.201420298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Z. Garen A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc Natl Acad Sci U S A. 2000;97:9221. doi: 10.1073/pnas.97.16.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z. Sun Y. Garen A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc Natl Acad Sci U S A. 1999;96:8161. doi: 10.1073/pnas.96.14.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoji M. Hancock WW. Abe K, et al. Activation of coagulation and angiogenesis in cancer: Immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152:399. [PMC free article] [PubMed] [Google Scholar]
- 10.Andoh K. Kubota T. Takada M, et al. Tissue factor activity in leukemia cells. Special reference to disseminated intravascular coagulation. Cancer. 1987;59:748. doi: 10.1002/1097-0142(19870215)59:4<748::aid-cncr2820590414>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Bauer KA. Conway EM. Bach R, et al. Tissue factor gene expression in acute myeloblastic leukemia. Thromb Res. 1989;56:425. doi: 10.1016/0049-3848(89)90255-7. [DOI] [PubMed] [Google Scholar]
- 12.Hair GA. Padula S. Zeff R, et al. Tissue factor expression in human leukemic cells. Leuk Res. 1996;20:1. doi: 10.1016/0145-2126(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 13.Rak J. Milsom C. Magnus N, et al. Tissue factor in tumour progression. Best Pract Res Clin Haematol. 2009;22:71. doi: 10.1016/j.beha.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z. Rao B. Chen S, et al. Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer. Breast Cancer Res Treat. 2011;126:589. doi: 10.1007/s10549-010-0957-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake TA. Morrissey JH. Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox JN. Smith KM. Schwartz SM, et al. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senger DR. Galli SJ. Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 18.Nemerson Y. Tissue factor and hemostasis. Blood. 1988;71:1. [PubMed] [Google Scholar]
- 19.Ruf W. Kalnik MW. Lund-Hansen T, et al. Characterization of factor VII association with tissue factor in solution. High and low affinity calcium binding sites in factor VII contribute to functionally distinct interactions. J Biol Chem. 1991;266:15719. [PubMed] [Google Scholar]
- 20.Toomey JR. Smith KJ. Stafford DW. Localization of the human tissue factor recognition determinant of human factor VIIa. J Biol Chem. 1991;266:19198. [PubMed] [Google Scholar]
- 21.Tang Y. Borgstrom P. Maynard J, et al. Mapping of angiogenic markers for targeting of vectors to tumor vascular endothelial cells. Cancer Gene Ther. 2007;14:346. doi: 10.1038/sj.cgt.7701030. [DOI] [PubMed] [Google Scholar]
- 22.Hu Z. Rao B. Chen S, et al. Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice. BMC Cancer. 2010;10:235. doi: 10.1186/1471-2407-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoji M. Sun A. Kisiel W, et al. Targeting tissue factor-expressing tumor angiogenesis and tumors with EF24 conjugated to factor VIIa. J Drug Target. 2008;16:185. doi: 10.1080/10611860801890093. [DOI] [PubMed] [Google Scholar]
- 24.Hu JL. Xue YC. Xie MY, et al. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J Antibiot (Tokyo) 1988;41:1575. doi: 10.7164/antibiotics.41.1575. [DOI] [PubMed] [Google Scholar]
- 25.Otani T. Minami Y. Marunaka T, et al. A new macromolecular antitumor antibiotic, C-1027. II. Isolation and physico-chemical properties. J Antibiot (Tokyo) 1988;41:1580. doi: 10.7164/antibiotics.41.1580. [DOI] [PubMed] [Google Scholar]
- 26.Zhen YS. Ming XY. Yu B, et al. A new macromolecular antitumor antibiotic, C-1027. III. Antitumor activity. J Antibiot (Tokyo) 1989;42:1294. doi: 10.7164/antibiotics.42.1294. [DOI] [PubMed] [Google Scholar]
- 27.Xu YJ. Li DD. Zhen YS. Mode of action of C-1027, a new macromolecular antitumor antibiotic with highly potent cytotoxicity, on human hepatoma BEL-7402 cells. Cancer Chemother Pharmacol. 1990;27:41. doi: 10.1007/BF00689274. [DOI] [PubMed] [Google Scholar]
- 28.Shao RG. Zhen YS. Enediyne anticancer antibiotic lidamycin: Chemistry, biology and pharmacology. Anticancer Agents Med Chem. 2008;8:123. doi: 10.2174/187152008783497055. [DOI] [PubMed] [Google Scholar]
- 29.Xu YJ. Zhen YS. Goldberg IH. C1027 chromophore, a potent new enediyne antitumor antibiotic, induces sequence-specific double-strand DNA cleavage. Biochemistry. 1994;33:5947. doi: 10.1021/bi00185a036. [DOI] [PubMed] [Google Scholar]
- 30.McHugh MM. Gawron LS. Matsui S, et al. The antitumor enediyne C-1027 alters cell cycle progression and induces chromosomal aberrations and telomere dysfunction. Cancer Res. 2005;65:5344. doi: 10.1158/0008-5472.CAN-05-0015. [DOI] [PubMed] [Google Scholar]
- 31.Jiang B. Li DD. Zhen YS. Induction of apoptosis by enediyne antitumor antibiotic C1027 in HL-60 human promyelocytic leukemia cells. Biochem Biophys Res Commun. 1995;208:238. doi: 10.1006/bbrc.1995.1329. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T. Fukuda-Ishisaka S. Hirama M, et al. Solution structures of C-1027 apoprotein and its complex with the aromatized chromophore. J Mol Biol. 2001;309:267. doi: 10.1006/jmbi.2001.4621. [DOI] [PubMed] [Google Scholar]
- 33.Xin C. Ye S. Ming Y, et al. Efficient inhibition of B-cell lymphoma xenografts with a novel recombinant fusion protein: Anti-CD20Fab-LDM. Gene Ther. 2010;17:1234. doi: 10.1038/gt.2010.76. [DOI] [PubMed] [Google Scholar]
- 34.Hagen FS. Gray CL. O'Hara P, et al. Characterization of a cDNA coding for human factor VII. Proc Natl Acad Sci U S A. 1986;83:2412. doi: 10.1073/pnas.83.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao QF. Liu XY. Shang BY, et al. An enediyne-energized single-domain antibody-containing fusion protein shows potent antitumor activity. Anticancer Drugs. 2007;18:127. doi: 10.1097/CAD.0b013e3280112779. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H. Wu S. Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong G. Zhang S. Li Y, et al. A tandem scFv-based fusion protein and its enediyne-energized analogue show intensified therapeutic efficacy against lung carcinoma xenograft in athymic mice. Cancer Lett. 2010;295:124. doi: 10.1016/j.canlet.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Guo Xf. Zhu Xf. Shang Y, et al. A bispecific enediyne-energized fusion protein containing ligand-based and antibody-based oligopeptides against epidermal growth factor receptor and human epidermal growth factor receptor 2 shows potent antitumor activity. Clin Cancer Res. 2010;16:2085. doi: 10.1158/1078-0432.CCR-09-2699. [DOI] [PubMed] [Google Scholar]
- 39.Williams JI. Weitman S. Gonzalez CM, et al. Squalamine treatment of human tumors in nu/nu mice enhances platinum-based chemotherapies. Clin Cancer Res. 2001;7:724. [PubMed] [Google Scholar]
- 40.Weitman S. Marty J. Jolivet J, et al. The new dioxolane, (-)-2′-deoxy-3′-oxacytidine (BCH-4556, troxacitabine), has activity against pancreatic human tumor xenografts. Clin Cancer Res. 2000;6:1574. [PubMed] [Google Scholar]
- 41.Collier ME. Li C. Ettelaie C. Influence of exogenous tissue factor on estrogen receptor alpha expression in breast cancer cells: Involvement of beta1-integrin, PAR2, and mitogen-activated protein kinase activation. Mol Cancer Res. 2008;6:1807. doi: 10.1158/1541-7786.MCR-08-0109. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z. Non-caspase-mediated apoptosis contributes to the potent cytotoxicity of the enediyne antibiotic lidamycin toward human tumor cells. Biochem Pharmacol. 2003;65:1767. doi: 10.1016/s0006-2952(03)00117-5. [DOI] [PubMed] [Google Scholar]