Abstract
Background
Associative learning is required for face-name association and is impaired in alcoholism, but the cognitive processes and brain structural components underlying this deficit remain unclear. It is also unknown whether prompting alcoholics to implement a deep level of processing during face-name encoding would enhance performance.
Methods
Abstinent alcoholics and controls performed a levels-of-processing face-name learning task. Participants indicated whether the face was that of an honest person (deep encoding) or that of a man (shallow encoding). Retrieval was examined using an associative (face-name) recognition task and a single-item (face or name only) recognition task. Participants also underwent a 3T structural MRI.
Results
Compared with controls, alcoholics had poorer associative and single-item recognition, each impaired to the same extent. Level of processing at encoding had little effect on recognition performance but affected reaction time. Correlations with brain volumes were generally modest and based primarily on reaction time in alcoholics, where the deeper the processing at encoding, the more restricted the correlations with brain volumes. In alcoholics, longer control task reaction times correlated modestly with volumes across several anterior to posterior brain regions; shallow encoding correlated with calcarine and striatal volumes; deep encoding correlated with precuneus and parietal volumes; associative recognition RT correlated with cerebellar volumes. In controls, poorer associative recognition with deep encoding correlated significantly with smaller volumes of frontal and striatal structures.
Conclusions
Despite prompting, alcoholics did not take advantage of encoding memoranda at a deep level to enhance face-name recognition accuracy. Nonetheless, conditions of deeper encoding resulted in faster reaction times and more specific relations with regional brain volumes than did shallow encoding. The normal relation between associative recognition and corticostriatal volumes was not present in alcoholics. Rather, their speeded reaction time occurred at the expense of accuracy and was related most robustly to cerebellar volumes.
Keywords: alcoholism, associative learning, memory, structural MRI, depth of processing
Introduction
Alcoholism can disrupt memory functioning even in the absence of the profound amnesia of alcoholic Korsakoff’s syndrome (Oscar-Berman and Pulaski 1997; Sullivan et al. 1997; Pitel et al. 2007a). Early studies of uncomplicated alcoholics reported impaired associative memory, as used in remembering face-name association (Becker et al. 1983; Schaeffer and Parsons 1987), but the cognitive and brain mechanisms underlying this deficit were only assumed.
Given that alcoholics have preserved abilities to process non-emotional faces (Maurage et al. 2007; Foisy et al. 2007), it is unlikely that face-name learning deficit is related to fundamental ability to discern faces. On the contrary, compromised long-term memory for visual and verbal information (Beatty et al. 1995; Fama et al. 2004; Le Berre et al. 2010) may hamper face-name associative learning that could lead to deficits of both associative (face-name association) and single-item (single face or name) learning. Additional difficulties in creating new face-name associations arising from affected abilities in binding or integrating multimodal information (de Rosa et al. 2004; Pitel et al. 2007a) could result in disproportionate impairment of associative learning compared with single-item learning in alcoholics. The two last statements can be tested by comparing performances on associative and single-item learning tasks.
The involvement of multiple cognitive components in face-name association increases the likelihood that such learning depends on the integrity of several brain regions such as the lateral temporal lobe, including the fusiform gyrus and the fusiform face area for face perception and processing (Kanwisher et al. 1997); the medial temporal lobe, and more particularly the hippocampus, for episodic memory (Dickerson and Eichenbaum 2010; Milner, 1958); the prefrontal cortex (Fletcher and Henson 2001) with the ventrolateral prefrontal cortex being involved in selecting relevant item information; and the dorsolateral prefrontal cortex in supporting long-term memory formation by building associations among items that are active in memory (Blumenfeld and Ranganath 2006). Anatomically, chronic alcoholism affects multiple relevant brain structures involved in associative memory, such as frontal and temporal cortices (Sullivan et al. 1995; Makris et al. 2008; Harper 2009; Cardenas et al. 2007, 2011; Fein et al. 2002). The first goal of the present study was to investigate and compare in alcoholism both processes of face-name association learning and single-item face and name learning. Also, we aimed herein to identify the relations between these cognitive processes and the structural integrity of their respective brain substrates.
Finally, we examined whether prompting alcoholics to implement a deep level-of-processing at encoding would enhance memory performance and compensate for potential altered, self-initiated encoding strategies (Pitel et al. 2007b). According to the depth of processing model (Craik and Lockhart 1972), memory performance is enhanced when subjects actively engage in a deep level of processing, enabling semantic evaluation of material in contrast to shallow encoding operations, drawing attention to physical properties at memoranda encoding. Marinkovic et al. (2009) examined the depth-of-processing effect on face and word encoding and recognition in controls and alcoholics and found that, relative to shallow processing, deep processing resulted in better memory performance and evoked stronger functional activation in the prefrontal cortex. Therefore, we examined whether prompting alcoholics to implement a deep level of processing during face-name encoding would result in better memory performance than after a shallow encoding. Because all participants had undergone magnetic resonance imaging (MRI) scanning, we tested whether associative memory performance and differential depth of processing effects were related to volumes of brain regions thought to underlie each process.
Material and Methods
Subjects
The two groups comprised 10 alcoholics and 10 controls. The alcoholics (8 men, 2 women) were recruited from community treatment centers, outpatient clinics, and hospitals. Controls (5 men, 5 women) were recruited from the local community. All participants were administered the Structured Clinical Interview for DSM-IV-TR (SCID; First et al, 1998) by a clinical psychologist or research nurse who undergo regular calibration sessions; all diagnostic decisions were made in conference consensus meetings, and ties were broken by a third research clinician. Alcohol dependence was determined by DSM-IV criteria. Exclusion factors were lifetime DSM-IV-TR criteria for other Axis I diagnoses, including schizophrenia, bipolar disorder, attention deficit hyperactivity disorder, and posttraumatic stress disorder; mood disorder other than bipolar was not exclusive if the depression or anxiety onset had postdated the alcoholism onset. Nonalcoholic drug use or abuse over the last year was not permitted. Although toxicology screening was not performed, all subjects completed breathalyzer testing at the beginning of a morning and afternoon testing sessions. Subjects were excluded if the breathalyzer exceeded 0.0. The diagnostic interview also made inquiries regarding history of medical disorders. Participants with pathology or taking medication that might affect cognitive functions or with medically uncontrolled hypertension or diabetes were excluded as were those who tested positive for HIV infection; because of our ongoing HIV study, all alcoholics and most controls underwent laboratory testing for HIV infection, and none was positive. All participants provided written informed consent and received a modest stipend for study participationLifetime alcohol consumption was estimated using a modification (Pfefferbaum et al. 1988) of a semi-structured, time-line interview (Skinner and Sheu 1982). Drinks of each type of alcoholic beverage were standardized to units containing approximately 13.6g of alcohol and summed over the lifetime.
Controls and alcoholics were matched on age. Although the alcoholics had fewer years of education than the controls, the groups did not differ statistically in general intelligence, estimated with the National Adult Reading Test (Nelson 1982). Lifetime alcohol consumption was 30-fold higher in the alcoholic than the control group (Table 1).
Table 1.
Demographic characteristics of the control and alcoholic groups (mean±SD)
|
|
|||
|---|---|---|---|
| Control group N=10 |
Alcoholic group N=10 |
p-value | |
| Men, women | 5, 5 | 8, 2 | |
| Age (years) | 37.10 ± 10.53 | 39.20 ± 10.48 | 0.66 |
| Education (years) | 14.00 ± 0.82 | 11.90 ± 1.20 | <0.01 |
| NART IQ | 110.88 ± 9.57 | 102.89 ± 8.39 | 0.65 |
| Lifetime alcohol consumption (kg) | 46.78 ± 54.45 | 1384.53 ± 917.95 | <0.01 |
| Length of sobriety (days) | NA | 263.00 ± 339.34 | NA |
Behavioral Paradigm
The task involved learning of unfamiliar faces arbitrarily paired with fictional last names. We selected 120 color face photographs with neutral expression from a database (Minear and Park 2004): 30 for the encoding (15 shallow, 15 deep), 30 for the control task (no name associated), and 60 for the recognition task (distractors). Through a database, http://www.census.gov/genealogy/www/data/2000surnames/index.html, 90 surnames were selected from the 1000 most frequent last names in the U.S.: 15 for shallow encoding, 15 for deep condition, and 60 for the name recognition task (distractors). Stimuli were presented by E-Prime® software (Psychology Software Tools Inc., Pittsburgh, PA).
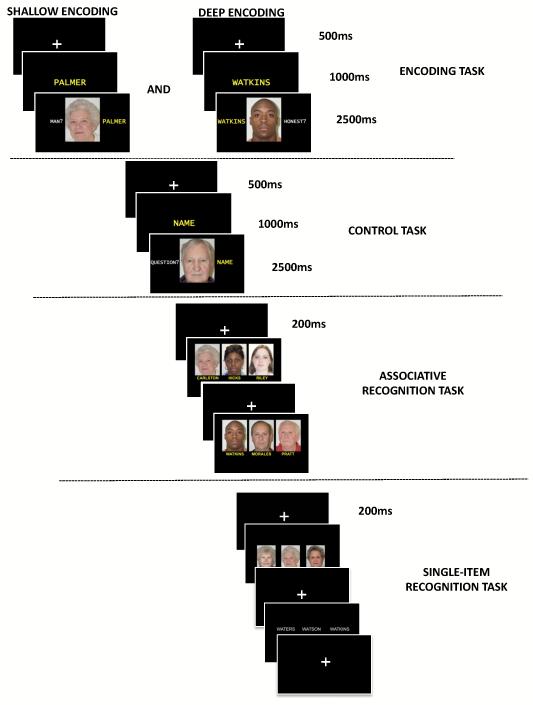
Four tasks were performed twice to avoid floor effects (Figure 1). Subjects used a keyboard to enter responses as quickly and as accurately as possible. There was no time limit for the recognition tasks, but subjects were instructed to answer as quickly and as accurately as possible. Analyses included accuracy and reaction time (RT). All the participants practiced until the experimenter (ALP) was sure they had fully understood the instructions.
Figure 1.
Behavioral paradigm. The protocol included two learning sessions. Each session consisted of an encoding task, a control task, and two recognition tasks. During the encoding task, subjects had 1) to remember which name was associated with which face, and 2) to indicate whether the face represented an honest person (deep encoding) or whether it was that of a man (shallow encoding). During the control task, subjects had to press a key as quickly as possible each time a face was presented. During the associative recognition task subjects had to indicate which of the three face-name associations presented on the screen was correctly paired. The fourth task consisted of single-item recognition, during which subjects saw either three faces or three names (one target and two distractors) and decided which of the three had been presented during the encoding task.
The first task (encoding task) entailed face-name association encoding during which subjects remembered which name was associated with which face. While memorizing the face-name association, subjects had to indicate whether the face represented an honest person [deep encoding (Bower and Karlin 1974)] or whether it was that of a man (shallow encoding). Each name was presented first for 1000ms. Then, the face-name association was presented for 2500ms during which subjects had to silently memorize the association and process it according to the encoding condition. The interstimulus interval was 500ms. The order of the encoding conditions was counterbalanced. The second task (control task) was a reaction time task that used the same procedure as the encoding task. Instead of providing a unique name for each face, the different last names were replaced by the word “NAME,” thereby eliminating association learning. During this task, subjects had to press a key as quickly as possible each time a face was presented. The third task involved associative recognition, during which three face-name associations were presented with only one correct pairing. The fourth task consisted of randomized single-item recognition, during which subjects saw either three faces or three names (one target and two distractors) and decided which of the three had been presented during the encoding task. The distractors were chosen to be close to the target: same gender, age and ethnicity for faces, and two identical first letters for last names.
MRI Data Acquisition
Anatomical MRI data were acquired on a General Electric 3T clinical system with a volumetric SPoiled Gradient Recalled (SPGR) sequence (124, 1.25-mm-thick slices; skip=0 mm; TR=6.5 ms; TE=1.54 ms; flip angle=15°; matrix=256×256). A dual-echo fast spin-echo (FSE) sequences (62, 2.5mm thick slices; skip=0mm; TR=8585 ms; TE=17/102 ms; matrix=256×192) was also acquired for brain extraction and automated fluid-tissue delineation. A clinical neuroradiologist read all structural studies to identify space-occupying lesions or other dysmorphology. Additional review of images identified images with quality too poor for quantification. MRI data from all participants were determined to be usable and were included in the analysis. The scanning procedure was conducted within 4 months of the behavioral testing in controls and within 2 months in alcoholics.
Region-of-interest (ROI) analysis
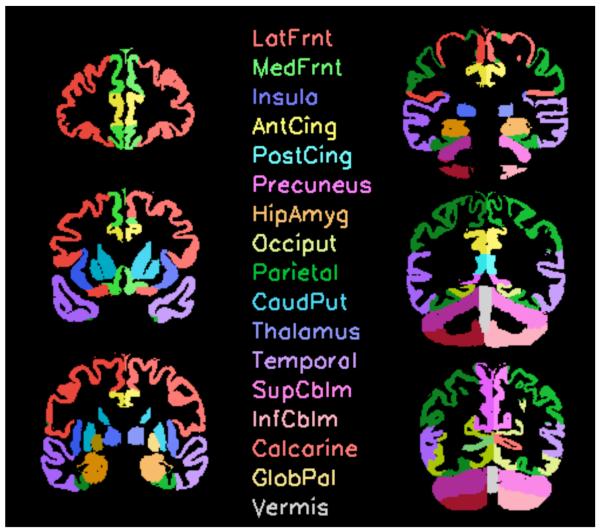
A parcellated template was first created using the SRI24 brain atlas (Rohlfing et al. 2010; http://nitrc.org/projects/sri24/). The atlas was semi-automatically parcellated using an already published description of 17 (16 bilateral + vermis) anatomical brain regions (Pfefferbaum et al. 2010) (Figure 2). Structural volumes were estimated using the following protocol for each subject: 1) Intensity bias field correction was performed separately on the SPGR and early-echo FSE image. The early-echo FSE bias field was also applied to the late-echo FSE image. 2) The bias-corrected early-echo FSE image was registered (http://nitrc.org/projects/sri24/) to the bias-corrected SPGR image. 3) The bias-corrected SPGR and early-echo and late-echo FSE images were then each passed independently through the FSL Brain Extraction Tool (BET) (Smith 2002) to extract the brain and exclude dura, skull, scalp, and other non-brain tissue. The final brain mask for the SPGR data was then created from the three separate, co-registered channel brain masks using majority voting (i.e., a pixel was labeled “brain” if two out of the three input masks labeled it as such). 4) The brain-extracted SPGR data were registered to the SPGR channel of the SRI24 atlas. 5) All atlas ROIs were reformatted to subject SPGR image coordinate space and resolution. 6) A three-compartment segmentation [cerebrospinal fluid (CSF), gray matter, and white matter] map was created with FSL-FAST (Zhang et al. 2001) and applied to the brain-extracted SPGR image. 7) Then, gray matter, white matter, and CSF volume of each cortical ROI were determined. For subcortical and medial temporal lobe ROIs and for the cerebellum, the whole local tissue volume (GM+WM) was used in the analyses because tissue type conspicuity limitations precluded accurate separation of GM and WM.
Figure 2.
Representation of the 16 bilateral regions-of-interest used to derive brain volumes (Pfefferbaum et al. 2010). The color-coded parcellated images start from the anterior (top left column) downwards 3 slices to the top right to the bottom right column (most posterior slice).
Statistical Analysis
To measure the effect of different levels of processing at encoding, we compared reaction time (RT) in the control condition and two encoding conditions in the two groups with an analysis of variance (ANOVA, 2 groups x 3 levels of processing). Then, ANOVA compared between-groups recognition performance according to type of stimulus and level of processing at encoding: 2-group (controls vs. alcoholics) × 2-stimulus (association vs. single-item) × 2-depth-of-processing at encoding (shallow vs. deep) in recognition performance (accuracy and RT). Correlation analyses were conducted between drinking history variables and performance in the alcoholic group.
One-tailed nonparametric correlations (Spearman’s Rho) were carried out between performance obtained in the control, encoding and recognition tasks, and gray matter volume in the parcellated brain for each group separately. We used a p-value <0.05 and indicated correlations that reached a more restrictive p-value <0.003 (Bonferroni corrected for 17 directional comparisons). We then conducted analyses of covariance (ANCOVAs), with performance as dependent variables (accuracy or reaction time), the group as an independent variable, and regional brain volume as covariates. To reduce the number of analyses, we conducted ANCOVAs only when there was a significant performance - brain volume correlation (p<0.05). The group by covariate interaction tested for whether there were significant between-group differences in performance - brain volume associations. Then, follow-up analyses examined between-groups differences in volumes of the brain regions shown to correlate with performance. Finally, correlation analyses were conducted between drinking history variables and regional brain volumes in the alcoholic group.
Results
Behavioral Performance
RT in the Control and Encoding Tasks
The 2-groups x 3-levels-of-processing ANOVA on RT data revealed a significant effect of processing level [F(2,53)=41.40; p<0.001] but neither a group effect [F(1,53)=0.003; p=0.95] nor an interaction [F(2,53)=0.08; p=0.93]. Post-hoc t-tests indicated the expected step-wise effect, with the shortest RT for the control task [reaction-time task], longer RT for shallow encoding (‘Is the face that of a man?’), and the longest RT for deep encoding (‘Does the face look honest?’) (p<0.01; Figure 3A).
Figure 3.
Behavioral result. A: Reaction time in the control and encoding tasks; B: Accuracy in the associative and single-item recognition tasks; C: Reaction time in the associative and single-item recognition tasks
Accuracy and RT in the Recognition Tasks
Even though there was a between-group difference in education, this variable was not correlated with recognition performance and was, therefore, not included as a covariate in the subsequent analyses. The ANOVA based on accuracy scores showed that alcoholics performed below controls [F(1,72)=11.54; p=0.001] and that associative recognition scores were lower than single-item recognition scores [F(1,72)=2.54; p<0.001]. Neither the depth-of-processing effect [F(1,72)=1.69; p=0.20] nor the group-by-depth of processing interaction [F(1,72)=0.03; p=0.87] was significant (Figure 3B).
For RT, the ANOVA indicated an effect of stimulus type [F(1,72)=30.25; p<0.001] with RT for associative recognition longer than that for single-item recognition. There was no effect of group [F(1,72)=0.75; p=0.39], depth of processing at encoding [F(1,72)=0.51; p=0.48], or group-by-depth of processing interaction [F(1,72)=0.02; p=0.90] (Figure 3C).
Brain structure and function
Relation with performance on the control and encoding tasks
In the alcoholic group, longer reaction times on the control task correlated with smaller volumes in the lateral and medial frontal cortex, temporal and parietal cortices, anterior and middle cingulate gyrus, insula, caudate and putamen, globus pallidus and inferior cerebellum. On the shallow encoding task, longer reaction times correlated with smaller volumes in the medial frontal and temporal cortices, calcarine gyrus, insula, caudate and putamen, and globus pallidus (Figure 4A). On the deep encoding task, longer reaction times correlated with smaller volumes restricted to the precuneus and parietal cortex. No significant relation was found between regional brain volumes or reaction times on the control and encoding tasks in the control group. All results are reported in Table 2.
Figure 4.
Relations between gray matter volume in the caudate-putamen nuclei and A) reaction time on the shallow encoding task in the alcoholic group; and B) accuracy on the associative recognition task after deep encoding in the control group.
Table 2.
Relationships between performance on the control, encoding and recognition tasks and regional brain volume
| Control group | Alcoholic group | ||
|---|---|---|---|
| CONTROL TASK | RT | NA | Lateral frontal cortex (Rho=−0.56, p=0.04) |
| Medial frontal cortex (Rho=−0.60, p=0.03) | |||
| Ant/Mid cingulate gyrus (Rho=−0.75, p=0.005) | |||
| Temporal cortex (Rho=−0.59, p=0.035) | |||
| Parietal cortex (Rho=−0.68, p=0.01) | |||
| Insula (Rho=−0.63, p=0.02) | |||
| Caudate/Putamen (Rho=−0.56, p=0.04) | |||
| Globus pallidus (Rho=−0.65, p=0.02) | |||
| Inferior cerebellum (Rho=−0.57, p=0.04) | |||
| ENCODING TASK | |||
| Shallow encoding | RT | NA | Medial frontal cortex (Rho=−0.63, p=0.02) |
| Temporal cortex (Rho=−0.59, p=0.035) | |||
| Insula (Rho=−0.67, p=0.015) | |||
| Calcarine gyrus (Rho=−0.79, p=0.003)* | |||
| Caudate/Putamen (Rho=−0.84, p=0.001)* | |||
| Globus pallidus (Rho=−0.57, p=0.04) | |||
| Deep encoding | RT | NA | Precuneus (Rho=−0.73, p=0.005) |
| ASSOCIATIVE RECOGNITION TASK | Parietal cortex (Rho=−0.73, p=0.005) | ||
| Shallow encoding | CA | Thalamus (Rho=0.63, p=0.025) | NA |
| RT | NA | NA | |
| Deep encoding | CA | Lateral frontal cortex (Rho=0.80, p=0.0025)* | NA |
| Medial frontal cortex (Rho=0.76, p=0.005) | |||
| Temporal cortex (Rho=0.57, p=0.04) | |||
| Precuneus (Rho=0.55, p=0.045) | |||
| Parietal cortex (Rho=0.55, p=0.045) | |||
| Insula (Rho=0.70, p=0.02) | |||
| Caudate/Putamen (Rho=0.82, p=0.0015) * | |||
| Globus pallidus (Rho=0.61, p=0.025) | |||
| RT | NA | Inferior cerebellum (Rho=−0.75, p=0.005) | |
| Superior cerebellum (Rho=−0.64, p=0.025) | |||
| SINGLE-ITEM RECOGNITION TASK | |||
| Shallow encoding | CA | NA | Precuneus (Rho=0.60, p=0.03) |
| Superior cerebellum(Rho=0.55, p=0.045) | |||
| NA | NA | ||
| Deep encoding | CA | NA | NA |
| RT | NA | NA |
CA: accuracy; RT: reaction time
: Correlation also significant at p<0.003 (Bonferroni corrected)
Significant group by regional brain volume interactions (p<0.05) are in bold
Relation with performance on the associative recognition task
In the alcoholic group, shorter reaction times on the associative recognition task after deep encoding correlated with larger volume in the superior and inferior cerebellum (Table 2). No other significant relation emerged between regional brain volumes and performance on the associative recognition task in this group.
In the control group, lower scores on the associative recognition task after shallow encoding correlated with smaller volume in the thalami. Lower scores on the associative recognition task after deep encoding correlated with smaller volumes in the lateral and medial frontal cortices, temporal cortex, precuneus, parietal cortex, insula, and caudate and putamen (Table 2 and Figure 4B).
Relation with performance on the single-item recognition task
In the alcoholic group, higher scores on the single-item recognition task after shallow encoding were correlated with larger gray matter volume in the precuneus and superior cerebellum (Table 2). There was no significant relation between performance on the single-item recognition task and regional brain volumes in the control group.
Between-group differences in performance – regional brain volume associations
The ANCOVAs indicated significant group by covariate (regional brain volume) interactions for the control task when the volumes of the medial frontal cortex, anterior/middle cingulate gyrus, temporal and parietal cortices, caudate and putamen, and globus pallidus were used as covariates. The group by covariate interaction was also significant for the associative recognition task after shallow encoding when the thalamus volume was used as a covariate, and for the associative recognition task after deep encoding when the volume of the caudate/putamen was used as a covariate (Table 2).
Between-group differences in regional brain volume and relation to alcohol history
There was no between-group difference in regional brain volumes highlighted in the correlational analyses (all p values > 0.05). Further, there was no significant relation between drinking history variables and regional brain volumes in the alcoholic group (all p values > 0.05).
Discussion
This investigation of face-name association learning in alcoholics and controls revealed that, first, compared with controls, these alcoholics had poorer associative and single-item recognition but the two recognition tasks were impaired to the same extent. Second, even though the depth of processing at encoding had little effect on performance, the relations between performance and regional brain volumes differed depending on the encoding condition and the group. Reaction time at encoding correlated with brain volumes only in alcoholics, and the deeper the processing at encoding the more specific the set of correlations. Third, performances on the associative and single-item recognition tasks were associated with different regional brain volumes in the two groups. In alcoholics, performance on both recognition tasks correlated with cerebellar volumes, whereas in controls memory scores on the association recognition task after deep encoding were mainly related to corticostriatal volumes.
Associative Versus Single-Item Learning in Alcoholics
Consistent with earlier studies (Becker et al. 1983; Schaeffer and Parsons 1987), the alcoholics performed more poorly than controls on the associative recognition task. Yet, the alcoholics had preserved performance on the control and encoding tasks (Maurage et al. 2007; Foisy et al. 2007), indicating that their deficit of face-name learning was unlikely to be related to impaired face processing. Given that associative learning involves both the binding and retention of multimodal information, we expected alcoholics to be even more impaired on the associative recognition task than on the single-item recognition task. Performance on associative recognition was lower than for single-item recognition in both groups but, surprisingly, the associative recognition was no more difficult for alcoholics than controls. This finding suggests that impaired face-name learning in alcoholics may not be related to their failure to form new associations (Pitel et al. 2007a; de Rosa et al. 2004). Rather, our results suggest that alcoholism affects associative memory to the same extent as other memory components, including visual and verbal memory (Beatty et al. 1995; Fama et al. 2004; Le Berre et al. 2010; Sullivan et al. 2000).
Analysis of reaction time data extended our understanding of the learning strategies used by alcoholics. Here, alcoholics favored speed over accuracy, resulting in impaired accuracy but preserved speed (cf., Pfefferbaum et al. 1987; Sullivan et al. 2002). Indeed, alcoholics answered as quickly as controls on the recognition tasks. This finding indicates alcoholism-related differences in speed-accuracy ratios.
Depth-of-Processing Effect at Encoding
The levels-of-processing model predicts that memory performance is enhanced when subjects actively engage in deep rather than shallow encoding operations (Craik and Lockhart 1972). Contrary to the results of Marinkovic et al. (2009), our results did not show a depth-of-processing effect on recognition performance for either group, although it was present for reaction time at encoding. The discrepancy between those findings and ours may result from the difference in the nature of the tasks, i.e., single-item (face or word only; Marinkovic et al. 2009) vs. associative (face-name association herein) learning. Our depth-of-processing effect on recognition may have been diminished because subjects had to focus on associating names and faces in addition to processing faces, thereby drawing on deep processing for efficient performance whatever the apparent level of processing. Yet, these findings suggest that prompting alcoholics to implement learning strategies involving deeper processing may promote compensation for inefficient self-initiated learning strategies.
Further, in the alcoholic group, the reaction time measures at encoding, which reflect a depth-of-processing effect, were correlates of regional brain volumes. Reaction time on the deep encoding task was correlated solely with volume in parietal regions, which have been proposed to be involved in encoding processes (Uncapher and Wagner 2009). Reaction times for shallow encoding were related to volumes in a much larger corticostriatal constellation of volumes (Alexander et al. 1986), which involves regions implicated in gender judgment of faces (calcarine region; Joassin et al. 2001), emotional processing of faces (insula; Jehna et al. 2011), episodic memory functioning (frontal and temporal cortices; Blumenfeld and Ranganath 2006; Cheung and Chan, 2003), and learning (striatum; Graybiel, 1995; Devan et al. 2011). Performance on the control task, during which only basic perceptual processes were required, was correlated with volumes in an even larger cortical, subcortical and cerebellar network. Reaction time on the shallow and deep encoding tasks, therefore, correlated with regional brain volumes in non-overlapping regions-of-interest; here, the deeper the processing level at encoding, the more selective the correlations. The most striking between-group difference in performance – brain volume association concerned the control task, which was related to regional volume in more regions in alcoholics than controls. These findings suggest that even though alcoholics had preserved reaction times on this basic reaction time task, performance was related to a larger brain network.
Brain correlates of single-item and associative recognition
Results on the recognition tasks were not related to the same brain regions in controls and alcoholics. In alcoholics, both associative and single-item recognition were related to cerebellar volumes. The contribution of the cerebellum in cognition (including memory; Schmahmann, 2010; Marvel and Desmond 2010) is now firmly established, and functional MRI studies have provided evidence for its role in augmenting performance and compensating for functional deficits attributable to frontal cortical disruption in alcoholics (Desmond et al., 2003; Parks et al., 2003; Sullivan et al., 2003). In controls, accuracy on the associative recognition task was correlated with volumes involving the cerebral cortex, thalamus and striatum (Albin et al. 1989, Blandini et al. 2000), whereas performance on the single-item recognition task was not associated with regional brain volumes. In alcoholics, the absence of relations between associative recognition performance and corticostriatal volumes may underlie the observed face-name learning impairment.
In summary, associative recognition is a high-order cognitive function, which requires the proficient operation of a constellation of component processes and brain regions. Despite prompting alcoholics to encode memoranda at a deep level, which resulted in more specific relations with regional brain volumes than shallow encoding, alcoholics did not take advantage of this strategy and thus in this group, both single-item and associative recognition were impaired. The comparison of associative and single-item learning suggests that this face-name learning deficit is unlikely to result from specific impairment in binding information’s or integration of the association. Further, the normal relation of associative recognition performance to corticostriatal substrates was not present in these alcoholics. Alcoholics’ memory results were related to volumes in the cerebellum, which has been shown to play an important role in cognitive processes in alcoholism. These observations and conclusion require replication with larger samples to extend the generalization of these preliminary results. The present study provides indirect information about the structural brain substrates underlying face-name association learning deficits in alcoholism. Thus, this analysis does not provide information on brain activity while performing the task, which would require functional imaging. Nonetheless, the relations observed herein provide a basis for hypothesis testing with other imaging modalities.
Acknowledgments
This work was supported by grants from the U.S. NIAAA (AA010723, AA017168, AA017923) and NIBIB (EB008381).
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical manual of Mental Disorders. American Psychiatric Association; Washington: 1994. [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend. 1995;37:247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Becker JT, Butters N, Hermann A, D’Angelo N. Learning to associate names and faces. Impaired acquisition on an ecologically relevant memory task by male alcoholics. J Nerv Ment Dis. 1983;171:617–623. [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH, Karlin MB. Depth of processing pictures of faces and recognition memory. Journal of Experimental Psychology. 1974;103:751–757. [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain Morphology at Entry into Treatment for Alcohol Dependence Is Related to Relapse Propensity. Biol Psychiatry. 2011 May 19; doi: 10.1016/j.biopsych.2011.04.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MC, Chan AS. Memory impairment in humans after bilateral damage to lateral temporal neocortex. Neuroreport. 2003;14:371–374. doi: 10.1097/00001756-200303030-00015. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: A framework for memory reearch. Journal of Verbal Lerning & Memory Behavior. 1972;11:671–684. [Google Scholar]
- De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan EV. The human basal forebrain integrates the old and the new. Neuron. 2004;41:825–837. doi: 10.1016/s0896-6273(04)00080-7. [DOI] [PubMed] [Google Scholar]
- De Rosa E, Sullivan EV. Enhanced release from proactive interference in nonamnesic alcoholic individuals: implications for impaired associative binding. Neuropsychology. 2003;17:469–481. doi: 10.1037/0894-4105.17.3.469. [DOI] [PubMed] [Google Scholar]
- Devan BD, Hong NS, McDonald RJ. Parallel associative processing in the dorsal striatum: Segregation of stimulus-response and cognitive control subregions. Neurobiol Learn Mem. 2011 doi: 10.1016/j.nlm.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naïve alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Petiau C, et al. Impaired emotional facial expression recognition in alcoholics: are these deficits specific to emotional cues? Psychiatry Res. 2007;150:33–41. doi: 10.1016/j.psychres.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- Jehna M, Neuper C, Ischebeck A, et al. The functional correlates of face perception and recognition of emotional facial expressions as evidenced by fMRI. Brain Res. 2011;1393:73–83. doi: 10.1016/j.brainres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Joassin F, Maurage P, Campanella S. The neural network sustaining the crossmodal processing of human gender from faces and voices: an fMRI study. Neuroimage. 2011;54:1654–1661. doi: 10.1016/j.neuroimage.2010.08.073. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Pinon K, Vabret F, et al. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcohol Clin Exp Res. 2010;34:1888–1898. doi: 10.1111/j.1530-0277.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev. 2010;20:271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Philippot P, Verbanck P, et al. Is the P300 deficit in alcoholism associated with early visual impairments (P100, N170)? An oddball paradigm. Clin Neurophysiol. 2007;118:633–644. doi: 10.1016/j.clinph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Milner B. Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis. 1958;36:244–257. [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 2004;36:630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Nelson Publishing Company; Windsor, Canada: 1982. [Google Scholar]
- Oscar-Berman M, Pulaski JL. Association learning and recognition memory in alcoholic Korsakoff patients. Neuropsychology. 1997;11:282–289. doi: 10.1037//0894-4105.11.2.282. [DOI] [PubMed] [Google Scholar]
- Parks MH, Morgan VL, Pickens DR, et al. Brain fMRI activation associated with self-paced finger tapping in chronic alcohol-dependent patients. Alcohol Clin Exp Res. 2003;27:704–711. doi: 10.1097/01.ALC.0000062759.14944.CF. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Volumetric cerebral perfusion imaging in healthy adults: regional distribution, laterality, and repeatability of pulsed continuous arterial spin labeling (PCASL) Psychiatry Res. 2010;30:266–273. doi: 10.1016/j.pscychresns.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin Exp Res. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Ford JM. Late event-related potential changes in alcoholics. Alcohol. 4:275–281. doi: 10.1016/0741-8329(87)90023-1. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, et al. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol Clin Exp Res. 2007a;31:1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Witkowski T, Vabret F, et al. Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcohol Clin Exp Res. 2007b;31:238–248. doi: 10.1111/j.1530-0277.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Hum Brain Mapp. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer KW, Parsons OA. Learning impairment in alcoholics using an ecologically relevant test. J Nerv Ment Dis. 1987;175:213–218. doi: 10.1097/00005053-198704000-00004. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Desmond JE, Lim KO, Pfefferbaum A. Speed and efficiency but not accuracy or timing deficits of limb movements in alcoholic men and women. Alcohol Clin Exp Res. 2002;26:705–713. [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SH, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A. Patterns of content, contextual, and working memory impairments in schizophrenia and nonamnesic alcoholism. Neuropsychology. 1997;11:195–206. doi: 10.1037//0894-4105.11.2.195. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]