Abstract
Nanomedicine has focused on targeted neurotrophic gene delivery to the brain as a strategy to stop and reverse neurodegeneration in Parkinson’s disease. Because of improved transfection ability, synthetic nanocarriers have become candidates for neurotrophic therapy. Neurotensin (NTS)-polyplex is a “Trojan horse” synthetic nanocarrier system that enters dopaminergic neurons through NTS receptor internalization to deliver a genetic cargo. The success of preclinical studies with different neurotrophic genes supports the possibility of using NTS-polyplex in nanomedicine. In this review, we describe the mechanism of NTS-polyplex transfection. We discuss the concept that an effective neurotrophic therapy requires a simultaneous effect on the axon terminals and soma of the remaining dopaminergic neurons. We also discuss the future of this strategy for the treatment of Parkinson’s disease.
Keywords: Neurorestoration, neuroprotection, neurodegeneration, regeneration, survival
Parkinson’s Disease (PD) results from a progressive loss of dopaminergic neurons in the substantia nigra with concurrent gliosis in the midbrain.1 These early-stage events in PD result in dopamine depletion in the caudoputamen, with the lateral nigral projections to the putamen being the most affected.2 As the disease advances, positron emission tomography shows a progressive loss of dopamine storage in striatal, cingulate and frontal brain regions.3
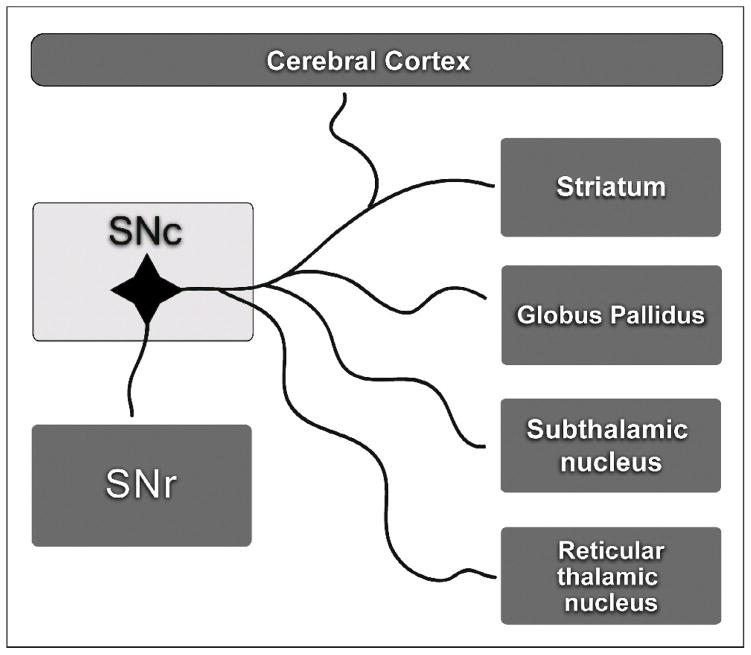
The earliest and most striking physical disabilities resulting from these changes in the basal ganglia are motor impairments. These include paucity and slowness of movement (akinesia, bradykinesia), muscle stiffness (rigidity), tremor at rest, and postural instability. An increasing number of clinical reports suggest that several nonmotor symptoms of PD, such as sleep disorders, autonomic dysfunction, neuroendocrinal problems, and neuropsychiatric symptoms (anxiety, apathy, dementia, cognitive dysfunction, and depression) also result in part from dopaminergic impairment.4 These nonmotor symptoms of PD are thought to be caused by reduced dopamine neurotransmission in more than one area of the cerebral cortex, diencephalon, and hypothalamus (Figure 1). In the rat, individual dopaminergic neurons of the substantia nigra profusely branch out to innervate several brain structures, including the striatum, the globus pallidus, the subthalamic nucleus, the substantia nigra reticulata, the reticular thalamic nucleus and the cerebral cortex.5–12 Such extensive axonal divergence may explain in part why loss of dopamine neurons results in reduced dopaminergic transmission in multiple structures and generates the multiple motor and nonmotor symptoms of PD (Figure 1). Consequently, an effective therapy for PD should aim to stop dopamine neuron degeneration and repair not only the nigrostriatal pathway but also the projections of nigral dopaminergic neurons in multiple target areas.
Figure 1.
Diagram showing that the branching of dopaminergic axons originating in the SNc is quite extensive, innervating several nuclei of the brain. The lesion of dopaminergic neuron cell bodies can thus reduce dopamine transmission in multiple structures, thus causing the motor and nonmotor manifestations of Parkinson’s disease.
The progressive character of dopaminergic neurodegeneration and the availability of new methods allowing early diagnosis have encouraged the use of neurotrophic therapy for PD.13,14 The residual dopaminergic neurons and terminals are a significant substrate on which neurotrophic factors can act to promote cell survival and axonal repair. Differently from the use of neurotrophic proteins, which require repeated injections or continuous infusion using pumps, gene therapy has the advantage of providing sustained production of the protein following a single administration of its gene.15 Thus, gene therapy for PD aims to insert a foreign neurotrophic gene (transgene) within the cells of a brain region, where the released transgenic protein can simultaneously reach the somatodendritic and terminal compartments of nigral dopaminergic neurons to slow down their progressive cell death and restore their functional connectivity with target nuclei.
Today, a majority of gene therapy protocols for PD use viral vectors to insert neurotrophic transgenes into cells of either the striatum or the substantia nigra.16 These two approaches have yielded different results in experimental animals depending on the time of injection, the neurotrophic factor, and the viral vector used. In general, transduction of striatal cells leads to sprouting and regrowth into the area of transgenic neurotrophic factor expression, along with a varying level of functional recovery. In contrast, injection of the vector into the substantia nigra protects the nigral cell bodies and causes extensive local sprouting of tyrosine hydroxylase (TH)-positive fibers, but they are unable to functionally reinnervate the striatum.17 To date, only one gene-therapy approach for PD has been tested in the putamen of human patients and involved the use of neurturin (NRTN), a member of the glial cell-line-derived neurotrophic-factor (GDNF) family ligands.18 Notable time-dependent improvements in a variety of clinical ratings were reported in patients in a phase I trial of adeno-associated virus (AAV)-based NRTN-gene therapy.19 Data from a Phase II trial in 58 patients with advanced PD showed a clinically modest benefit after 18 months of treatment [Press release on 27 May 2009 by Ceregene Inc.]. The trial’s sponsor also reported clear evidence of NRTN expression in the targeted putamen but no evidence for transport of this protein to the cell bodies of the degenerating dopaminergic neurons. Scarcity of the nigrostriatal terminals surviving in advanced PD might limit the retrograde transport of the AAV vector and-or NRTN to the nigral cell bodies. This is a limiting factor for all forms of neurotrophic gene therapy for PD, especially considering the extensive loss of divergent innervation of multiple structures following degeneration of dopaminergic neurons.
NTS-polyplex is the first nonviral system for gene delivery with a proven ability to transfect dopamine neurons of the substantia nigra in vitro and in vivo (Figure 2).20–23 Previous studies in the 6-hydroxydopamine (6-OHDA) rat model show that the NTS-polyplex transfection of a neurotrophic gene into the substantia nigra of rats after the neurotoxin injection produces biochemical, anatomical, and behavioral recovery from Parkinsonism.24–26 These findings have raised the interest of using NTS-polyplex in the neurotrophic therapy for Parkinson’s disease (PD), thus making it important to analyze its transfection mechanism and its possible usefulness in neurotrophic therapy for PD. In this review, we propose the hypothesis that the NTSR1-mediated endocytosis is the major route of the NTS-polyplex internalization and of the consequent transgene expression in vitro and in vivo (Figure 3).20,22,27 Because of its mechanism of entry and control of transgene expression by a promoter derived from a dopamine cell-specific gene, the NTS-polyplex has become an efficient, cell-specific, and sustained strategy for transgene expression in dopaminergic neurons. Based on this feature, we also propose that the NTS-polyplex transfection of a neurotrophic gene into the residual dopaminergic neurons of PD patients will be able to provide a simultaneous effect on the cell bodies and terminals of those neurons. The expressed neurotrophic protein will be released by cell bodies and, after its anterograde axonal transport, by axon terminals. Finally, we discuss the limitations, perspectives, and possibilities of the NTS-polyplex nanoparticle system in a neurotrophic therapy for PD and other neurodegenerative diseases of the central nervous system.
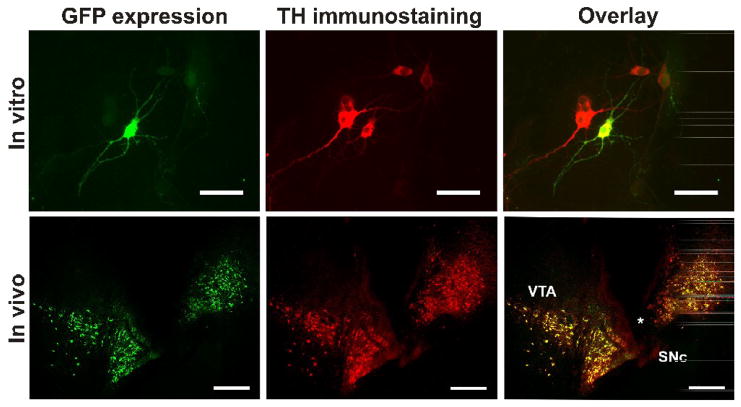
Figure 2.
Ability of NTS-polyplex to transfect dopaminergic neurons in vitro and in vivo. Top panel. Confocal micrographs showing the GFP expression in cultured dopaminergic neurons after transfection with the plasmid pEGFP-N1. NTS-polyplex was formed at 1:833:36 molar ratio (pEGFP-N1, 6 nM: PK, 5 μM: NTS-vector, 216 nM) in a mixture of Neurobasal medium and B27, serum free, and applied immediately to 2-day-old primary cultures. After 6-h exposure, the transfection medium was replaced by fresh culture medium and cells were incubated for an additional 72 h, as described previously. 150 Cells were subjected to double immunofluorescence against GFP and TH. The primary antibodies were a rabbit polyclonal antibody to GFP (Abcam; Cambridge MA, USA) and a mouse monoclonal antibody to TH (Sigma-Aldrich, St. Louis, MO, USA). The secondary antibodies were an Alexa 488 chicken anti-rabbit (Molecular Probes Inc., Eugene, OR, USA) and a donkey anti-mouse TRITC (Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA). Calibration bars = 50 μm. Bottom panel. Confocal micrographs showing the GFP expression in dopaminergic neurons of the substantia nigra after the local injection of the NTS-polyplex with pDAT-EGFP. Calibration bars = 200 μm. Micrographs of panel B were reproduced from Arango-Rodriguez et al. (2006).
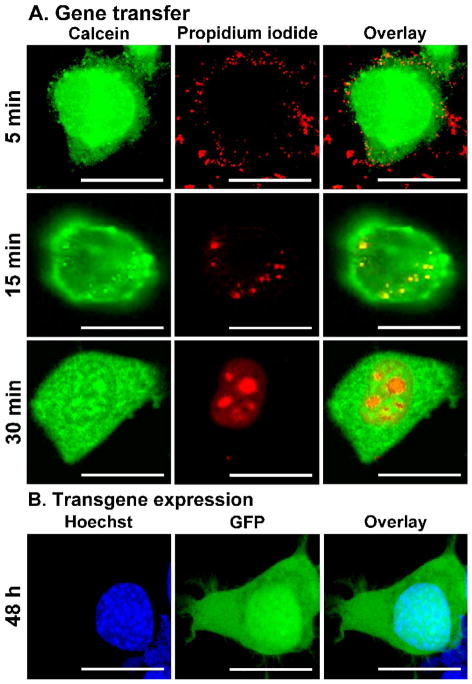
Figure 3.
Schematic illustration of the sequence of NTS-polyplex-mediated gene transfection documented by confocal microscopy in neuroblastoma N1E-115 cells. A. NTS-polyplex harboring a propidium iodide-labeled plasmid DNA was used for transfection and calcein was used to delimit the cell area. The micrographs of the first row were taken 5 min after incubation with propidium iodide-labeled NTS-polyplex in the presence of 0.45 M sucrose to block receptor-mediated endocytosis 58. The incubation time with 0.45 M sucrose was 30 min.22,27,45 B. GFP expression in N1E-115 cells counterstained with Hoechst, a nuclear staining. The microphotographs were taken at the times after transfection shown at the left margins. Calibration bars = 20 μm. The transfection efficiency is shown in figures 6 and 7 (in vitro) and figures 2 and 9 (in vivo). The first and third panels were reproduced from Navarro-Quiroga et al. (2002).
NTS-polyplex-mediated gene transfer
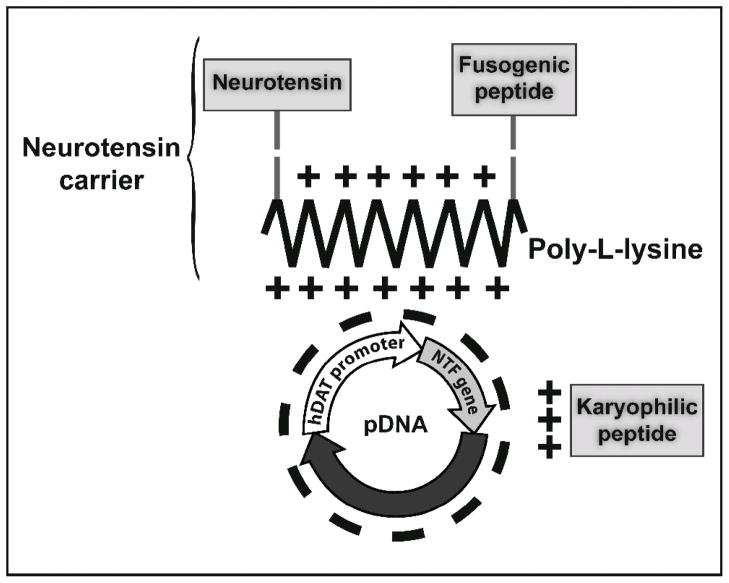
Gene-therapy protocols for PD have not paid much attention to nonviral vectors for neurotrophic gene delivery. One of the reasons may be that many consider such vectors as not being able to provide sustained gene expression in the brain. 28 NTS-polyplex was the first nonviral system that showed long-lasting transgene expression in dopaminergic neurons of the substantia nigra in the rat brain.20–22 NTS-polyplex consists of nanoparticles (Figure 4, D) resulting from the electrostatic binding of the conjugate NTS–poly-L-lysine (NTS carrier) to plasmid DNA (pDNA). The NTS carrier takes advantage of the endocytosis of NTS with its high-affinity receptor (NTSR1) to transfer the pDNA into dopamine neurons.20,21 In addition, the NTS-polyplex incorporates a fusogenic peptide (FP) and a karyophilic peptide (KP) of viral origin that enhance transfection efficiency (Figure 5). The FP is also cross-linked with the poly-lysine moiety; its role is to rescue NTS-polyplex from endosomes before the enzymatic degradation and extreme acidity become prevalent22,29 (Figure 5). The KP is a nuclear localization signal that is electrostatically bound to the pDNA to promote its import to the cell nucleus.22,30
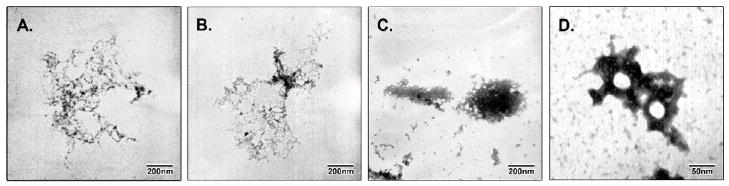
Figure 4.
Electron microscopy analysis of the sequential steps of the formation of the NTS-polyplex nanoparticles with pEGFP-N1. The micrographs show the natural form of 6 nM pDNA alone (A), the initial condensation of pDNA caused by the addition of 6 μM KP (B), the condensation of the KP-pDNA complex in the presence of 1% FBS (C), and the toroidal condensation of the KP-pDNA complex caused by the addition of the NTS-vector at the optimum molar ratio (1:34) to form the NTS-polyplex nanoparticles (D). Micrographs were reproduced from Arango-Rodriguez et al. (2006).
Figure 5.
Scheme of the NTS-polyplex components. The neurotensin carrier is the conjugate of neurotensin and fusogenic peptide with poly-L-lysine. The karyophilic peptide is electrostatically bound to a plasmid DNA (pDNA) to form the KP-pDNA complex, which is bound to the neurotensin carrier to form the NTS-polyplex. The pDNA encompasses the gene of interest under control of a tissue-specific promoter; for instance, hDAT (human dopamine transporter), a promoter specific for dopaminergic neurons. NTS = pyroGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH. Modified hemagglutinin HA2 FP = Gly-Leu-Phe-Glu-Ala-Ile-Ala-Glu-Phe-Ile-Glu-Gly-Gly-Trp-Glu-Gly-Leu-Ile-Glu-Gly-Cys-Ala-Lys-Lys-Lys-OH. SV40 Vp1 NLS = Met-Ala-Pro-Thr-Lys-Arg-Lys-Gly-Ser-Cys-Pro-Gly-Ala-Ala-Pro-Asn-Lys-Pro-Lys-OH.
Plasmid DNA condensation
Condensation of pDNA into a small, compact structure, is the first decisive step for gene transfer mediated by cationic polymers.31 This process is directed by electrical forces resulting in spontaneous formation of polyplexes when an equimolar ratio between the cationic polymer and the plasmid DNA is achieved.32 The resulting particles are toroid structures or rods with a size of 50 to 100 nm.
Several cationic polymers have been used to compact both ribonucleotides and deoxyribonucleotides (polyanionic) because of their electrostatic bonding properties. The reacting group of polymers includes primary, secondary, tertiary, and quaternary amines or other positively charged molecules such as amidines.33 To date, the cationic polymers mostly used in gene delivery are spermidine, protamine, polyhistidine, polyarginine, and polylysine.34 Poly-L-lysine is an attractive molecule to use in human gene-therapy because it has been tested as a coadjuvant for prolonged release of certain drugs with proven clinical efficacy and good biosafety results. Its clinical use relies on its exceptional solubility, absence of acute toxicity, and inability to cause immunogenicity with repeated doses.35 The epsilon-amino groups of poly-L-lysine are positively charged at physiological pH, which represents a particularly advantageous chemical property. This charge allows poly-l-lysine to bind the negatively charged pDNA to form the polyplex under physiological conditions. In addition, the epsilon-amino groups are good acceptors of molecules active in receptor-meditated endocytosis, such as proteins, peptides and sugars, which are incorporated into poly-L-lysine by using chemical crosslinkers, nonenzymatic glycosylation, or avidin-biotin technique.34,36
A polyplex nanoparticle based on polylysine comprises various pDNA molecules covered by quite a few polymeric chains, whose structure, size, and solubility depend on the following factors: 1) the ratio of electrostatic charges of pDNA and poly-L-lysine, 2) the size and ramification grade of poly-L-lysine, 3) the physical and chemical conditions used for polyplex formation, and 4) the sequence used to mix the reactants during polyplex formation.21 The pDNAconcentration is also a factor influencing polyplex solubility; concentrations less than 20 μg/mL are required to form a soluble complex. Interestingly, the polyplex nanoparticle size is independent of the pDNA size.37
An electron-microscopy study showed that the electrostatic binding of the NTS-poly-L-lysine conjugate (the NTS-carrier) to a pDNA produces mostly toroidal nanoparticles of NTS-polyplex with an average diameter of 100 nm (Figure 4, D).21 This study also showed that both the KP (6 μM) and fetal bovine serum (1%) caused partial condensation of pDNA (6 nM), which is further compacted to a toroid structure by the addition of the NTS-carrier (Figure 4, A–D).21 This precondensation may be caused by the interaction between the negatively charged pDNA, the positively charged KP, and the cationic proteins of the serum. The addition of serum is known to stabilize the structure of the neutral polyplex at physiological salt concentrations, thus avoiding the rapid formation of large aggregates,38 which are generally ineffective gene-delivery agents and can even be dangerous because of embolization of the particles in the lung.39 In the process of NTS-polyplex formation, serum is more effective if added before the NTS-vector.21 The subsequent addition of the NTS-carrier produces neutral nanoparticles (Martinez-Fong et al., unpublished results) that are unable to react with the electrically charged serum protein, thus avoiding the formation of large aggregates and embolization when injected intravenously. The absence of circulatory problems when NTS-polyplex is repeatedly administered through the blood stream was recently shown in an animal model of cancer gene-therapy.40
In summary, the NTS-polyplex nanoparticles must fulfill two conditions to cause efficient transfection; an adequate condensation of pDNA into a toroid structure and a sufficient concentration of these structures at an optimum molar ratio.21 Because the cellular entry of nanomaterials depends on electrical charge, particle size, and particle shape 41, further characterization of the NTS-polyplex nanoparticles should be accomplished using different techniques, such as Rx-analysis, atomic force microscopy, scanning electron microscopy, and dynamic light scattering to determine size and z-potential. The knowledge of these characteristics will be useful in the formulation of the NTS-polyplex for clinical application in PD.
Receptor-mediated endocytosis
In mammalian cells, clathrin-coated pits and vesicles provide a well-defined pathway for internalizing extracellular material, ligands and plasma membrane. In this process, called receptor-mediated endocytosis, macromolecules bind to complementary, transmembrane receptor proteins, accumulate in coated pits and then enter the cell as receptor-macromolecule complexes in clathrin-coated vesicles.42 The tridecapeptide NTS in the NTS-polyplex is the natural ligand of three receptors, NTSR1, NTSR2, and NTSR3, which internalize NTS upon ligand activation in cells of the human and rodent brain.43,44 Of these three NTS-receptors, results from internalization and expression assays showed that NTSR1-mediated endocytosis is the major route of NTS-polyplex internalization and of the consequent transgene expression in vitro (Figures 6 and 7) and in vivo.20,27,45 N1E-115 and human colonic-adenocarcinoma HT-29 cells were selected as a gene-therapy model in vitro because they express NTSR146 but not NTSR2.47 They were also used to validate the ability of NTSR antagonists or endocytosis blockers to prevent fluorescent-NTS-polyplex uptake and reporter-gene expression.22,27 This strategy, validated in vitro, was used to demonstrate the specificity of transfection in vivo.20,22 In the brain, the highest density of NTSR1 RNA and binding sites have been found in the ventral mesencephalon, followed by hypothalamus, prefrontal cortex, striatum, and cerebellum.47–51 Of the two mesencephalic nuclei rich in NTSR1, the substantia nigra was selected for NTS-polyplex transfection because its dopaminergic population possesses the highest density of NTSR1 binding sites in the brain.52,53 The internalization and expression studies yielded similar results in vitro and in vivo, thus supporting the participation of NTSR1 in NTS-polyplex transfection.
Figure 6.
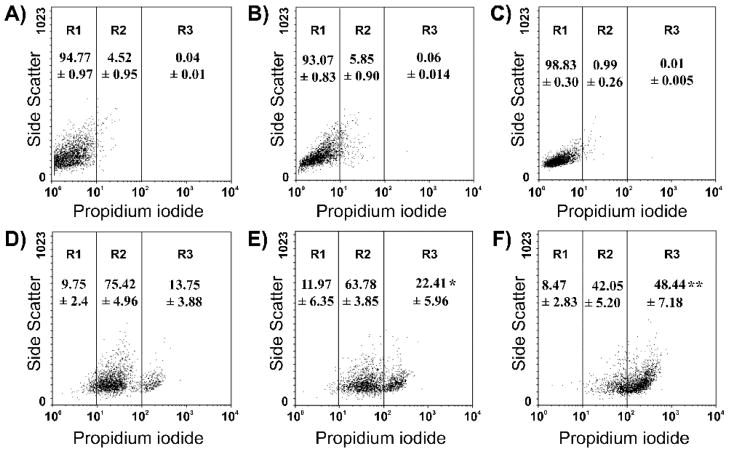
Flow cytometry analysis showing the contribution of fusogenic and karyophilic peptides to the improvement of NTS-polyplex internalization. The plasmid was labeled with propidium iodide. (A) Basal fluorescence in N1E-115 cells.(B) Blockade by 100 nM SR-48692 of fusogenic-karyophilic-NTS-polyplex internalization in N1E-115 cells. (C) Failure of fusogenic-karyophilic-NTS-polyplex to internalize in COS7 cells, which lack NTSR1. (D) Internalization of karyophilic-NTS-polyplex in N1E-115 cells.(E) Internalization of fusogenic-NTS-polyplex in N1E-115 cells.(F) Internalization of fusogenic-karyophilic-NTS-polyplex in N1E-115 cells. Populations of 104 cells emitting red fluorescence of propidium iodide were distributed in three arbitrary regions (R1, R2 and R3) according to their fluorescence intensity. *, Significantly different from R3 in (D); **, significantly different from R3 in (E). The figure was reproduced from Navarro-Quiroga et al. (2002).
Figure 7.
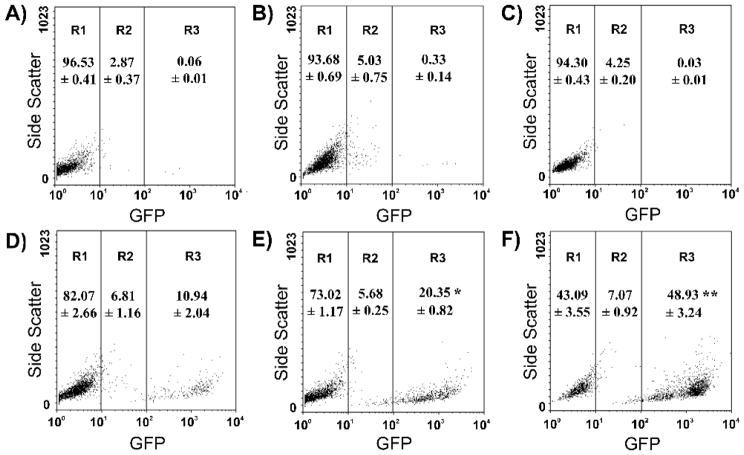
Flow cytometry analysis showing the contribution of fusogenic and karyophilic peptides to the improvement of NTS-polyplex-mediated gene expression. pGreen Lantern 1 was delivered by different polypplexes. (A) Basal fluorescence in N1E-115 cells. (B) Lack of GFP expression in N1E-115 cells exposed to the fusogenic-karyophilic-NTS-polyplex in the presence of 100 nM SR-48692. (C) Lack of GFP expression in COS7 exposed to fusogenic-karyophilic-NTS-polyplex. (D) GFP expression in N1E-115 cells transfected by karyophilic-NTS-polyplex. (E) GFP expression in N1E-115 cells transfected by karyophilic-NTS-polyplex fusogenic-NTS-polyplex. (F) GFP expression in N1E-115 cells transfected by karyophilic-NTS-polyplex fusogenic-karyophilic-NTS-polyplex. Populations of 104 cells emitting the GFP fluorescence were distributed in three arbitrary regions (R1, R2 and R3) according to their fluorescence intensity. *, Significantly different from R3 in (D); **, significantly different from R3 in (E). The figure was reproduced from Navarro-Quiroga et al. (2002).
In internalization studies, either calcein or TH immunofluorescence were used when using confocal microscopy to delimit the cell area and locate the NTS-polyplex harboring a propidium iodide-labeled plasmid DNA within the cell. Confocal microscopy showed the fluorescence of propidium-iodide within calcein-stained cells in vitro (Figure 3)22,27,45 and in TH-stained neurons 20,22 thus suggesting the intracellular presence of NTS-polyplex. Interestingly, the kinetics of NTSR1 to internalize the NTS-polyplex were similar to that of NTS or NTS agonist internalization in cultured neurons from the brain of mouse and rat embryos and cell lines.54 In those cells, the radioactive ligand and the GFP- or epitope-tagged receptor were rapidly removed from the cell surface and clearly located within cytoplasmic vesicles during the first 15 to 30 min.54–56 In TH-positive nigral neurons, nuclear propidium iodine signal was detected in the cell 4 h after local injection of NTS-polyplex.20 The blockade of pDNA uptake by either an excess of NTS or the NTSR1 antagonist SR-4869257 confirmed both in vitro and in vivo that NTS-polyplex resulted from NTSR1 internalization.20,22,27 The absence of similar uptake under conditions where clathrin-coated pit formation was blocked by hypertonic sucrose58 also provided direct support for the idea that receptor-mediated endocytosis is the mechanism used by the NTS-polyplex to internalize in cells.20,22,27 Accordingly, the transfection of reporter genes (green fluorescent protein and chloramphenicol acetyl transferase) using the NTS-polyplex led to transgene expression only in NTSR1-bearing cell lines and nigral dopaminergic neurons. Expression was also absent in cell lines lacking NTSR1, such as COS-7 and L-929 cells.20,22,27
Similar to NTSR1, NTSR2 is also a G-protein-coupled receptor 59,60 which internalizes after activation by agonists.61 However, no transgene expression was seen when NTS-polyplex was injected into the ansiform lobule of the cerebellum, a region rich in NTSR2.48,62 In addition, astrocytes of the substantia nigra, known to express NTSR2,49 were unable to internalize the NTS-polyplex and express reporter genes.20,22 Internalization and expression assays in primary cultures of substantia nigra glial cells confirmed that glial NTSR2 does not mediate NTS-polyplex transfection.20 These cells show only membrane binding of the NTS-polyplex, which is blocked by 1 μM levocabastine, a competitive antagonist of NTSR2.47,59
NTSR3 is a single transmembrane-domain receptor, which is 100% homologous to gp95-sortilin,63 mainly localized in the trans Golgi-network, and poorly expressed at the plasma membrane.64 Because NTSR1 and NTSR3 are able to form a complex to internalize NTS in HT29 cells,65 the participation of the NTSR3 in NTS-polyplex endocytosis cannot be ruled out. It would be worthwhile to explore this issue when selective pharmacological ligands for NTSR3 become available.
Quantitative studies on the NTS-polyplex components determined that a functional NTS-polyplex provides sufficient NTS to activate NTSR1-mediated endocytosis in vitro and in vivo. Using [125I]NTS, it was determined that an efficient NTS carrier contains one molecule of NTS and two molecules of poly-L-lysine.21,22 Based on the equimolar ratio between pDNA and the NTS carrier, those authors determined that the concentration of NTS provided by a functional NTS-polyplex depends on the plasmid.21 For instance, the optimal NTS concentrations were 253 nM for pEGFP-N1 and 745 nM for pDAT-EGFP.21 These NTS concentrations are at least 50 times higher than the affinity constant (Kd) of NTS binding to NTSR1 in N1E-115 cells (5 nM)66 and dopamine neurons (0.3 nM),67 thus assuring maximal activation of NTSR1-mediated endocytosis.55
NTSR1-mediated retrograde transport of NTS from axonal terminals to nigral cell bodies could be another route for the NTS-polyplex transfection of dopaminergic neurons that can be useful in experimental studies and future clinical trials. This retrograde transport has been extensively documented in the nigrostriatal and mesolimbic pathways and it is possible that it occurs in the collaterals innervating the other targets of dopaminergic neurons.68 Taking advantage of this route of NTS transport toward nigral dopaminergic neurons, it is feasible to transfect nigral neurons after injection of NTS-polyplex into the striatum.21 The involvement of NTSR1 in the uptake of NTS-polyplex and in subsequent expression of the reporter gene was confirmed by the absence of transgene expression in experiments using either NTS-polyplex in the presence of the selective NTS-receptor antagonist SR-4869257 or an untargeted polyplex (this contains all the molecular components of NTS-polyplex except NTS).21
Endosomal escape
Shuttling through the endosome is a natural port of access to the cell cytoplasm. Cells use endosomes to incorporate extracellular material needed for normal physiological functions, signal transduction and cell signaling through phagocytosis and regulated endocytosis.69 Endocytosis can be triggered by activating cell-surface receptors that are clustered at plasma membrane invaginations and further enclosed into membrane vesicles.42 This mechanism has been used by viruses that activate cell-surface receptors leading to the formation of endosomes.70 In an effort to fight these noxious intruders, cells have created a natural barrier to respond to the threat of exogenous bioparticles. The response is a natural defense that uses acidification of the endosome and pH-activated proteases, lipases and nucleases to denature incoming particles.71 Itis well known that acidification triggers denaturation of proteins and nucleic acids by changing their secondary and tertiary structure. However, several viruses have evolved strategies to overcome this cellular defense mechanism: for example, some pH-sensitive viral envelope proteins change their tertiary structure when the pH reaches a certain acidic point inside the endosome. 72 The proteins thus adopt a new tertiary structure that is rich in α-helix, imparting a lipophilic property that disrupts the endoplasmic membrane.73,74 The result of this viral peptide-mediated endoplasmic membrane fusion is the escape of the virus to the cell cytoplasm.72
Similar to viruses, the first limiting factor of nonviral vectors is their inactivation in acidic endosomal vesicles75 and their degradation in the lysosomal compartment.71,76 To overcome the first barrier, diverse approaches have been successfully used together with receptor-mediated gene-transfer systems, including hepatectomy-induced liver regeneration after the injection of a asialoglycoprotein-polyplex,77 replication-defective adenovirus to cause disruption of the DNA-containing endosomes,78 histidylation of polylysine to destabilize the endosome membrane,79 and chloroquine to neutralize the acidic pH of the endosomes.80
A 22 amino acids peptide (GLFEAIAEFIEGGWEGLIEGCA) from the amino-terminus of the influenza virus hemagglutinin HA2, capable of fusing with the endocytotic vesicle lipid bilayer has been isolated and characterized.29,81 The addition of this FP to the culture media of hepatoma cells significantly increased gene expression when the cells were transfected via receptor-mediated endocytosis.81 Based on this report, a synthetic replica of this FP was cross-linked to poly-L-lysine of NTS-carrier to provide more effective, targeted gene delivery in vivo(Figure 5).22 This conjugate has considerable benefits because it combines activation of NTSR1-mediated endocytosis and a mechanism for escape from endosomes. Quantitative studies on NTS-polyplex components using [125I]NTS and [3H]-FP determined that a molecule of NTS-carrier contains one molecule of NTS, four molecules of FP, and two molecules of poly-L-lysine.21 Quantitative techniques demonstrated that the presence of the FP in the NTS-vector improved both pDNA internalization and the subsequent expression of the reporter gene in vitro and in vivo by more than 300%.22 This improvement might have resulted from an increased amount of exogenous DNA in the cytoplasm after endosomal membrane disruption by the FP (Figure 7). Remarkably, the NTS-polyplex keeps its specificity despite the addition of FP, as demonstrated by the absence of gene transfer in NTSR1-lacking COS7 cells and in N1E-115 cells incubated with SR-48692 to block NTSR1-mediated endocytosis. These results further confirm that the FP is inactive at neutral pH such as that of the extracellular medium (Figures 6 and 7).22
Dissociation or precipitation of the NTS-polyplex might occur because of the acidic pH to which it is exposed during its passage through the endosome prior to reaching the nucleus. However, electrophoresis analysis in a pH gradient showed that the presence of the FP in the NTS-polyplex contributes positively to its integrity and stability at pH 6.0.21 Mechanistic studies have shown that, at neutral pH, FP exists in a non-fusogenic state, but upon exposure to low pH, an alpha-helix conformation of the structure occurs to expose a fusogenic activity.74 It is possible that this mechanism is conserved in the FP of NTS-polyplex and that this peptide changes conformation at acidic pH and destabilizes the endosomal membranes thus resulting in an increased cytoplasmic gene delivery.
In summary, the results analyzed above clearly establish that the incorporation of the FP into the NTS-polyplex is an efficient strategy to improve the efficiency of gene transfer in vivo.21,22 However, the route of the NTS-polyplex through the intracellular compartments is not completely characterized. In addition, other techniques should be used to confirm and give details about the intracellular trafficking; for details see reference # 41. After endosomal escape of the NTS-polyplex, the precious DNA “cargo” must traverse the cytoplasm to reach the nucleus where it will be transcribed into mRNA of a therapeutic peptide.
Nuclear targeting
The nuclear membrane is endowed with receptors that function as check points to limit access to the nucleus. To pass through the nuclear membrane and activate nuclear receptors, proteins or RNA need appropriate conformations or “access codes”, a sophisticated security mechanism that viruses have evolved to bypass. The mechanisms responsible for nuclear import of viral cargos and the insertion of viral DNA in the host genome can be exploited by gene-therapy approaches. However, the insertion of viral DNA can activate proto-oncogenes or cause mutations, which might result in cancer. To improve transfection efficiency without activating proto-oncogenes, nonviral vectors must be designed to take advantage of nuclear-import but not viral DNA insertion mechanisms.82
Once the virus reaches the cytoplasm of the infected cell, some viral proteins possessing karyophilic determinants, also known as the nuclear-localization signal (NLS), import the virus genome and functional viral proteins to the nucleus of the host cell.83 In 1984, Kalderon and coworkers characterized a nuclear-targeting signal in the simian virus 40 (SV40) large-T antigen and described the first nuclear-import system.84 Since then, several NLS and pathways for nuclear transport have been described, of which the classical nuclear-import pathway is the best characterized.83 The NLS of the SV40 major capsid-protein Vp1 is responsible for nuclear targeting of Vp1 and virions.85 Further analysis of Vp1 NLS has shown that a mutant19-amino acids long (MAPTKRKGSCPGAAPNKPK) peptide has potent nuclear-import activity.30 To increase transfection, this viral peptide was integrated in NTS-polyplex. This strategy intends to introduce exogenous DNA into the cell nucleus and force cells to transcribe, translate and put to work transgene products without affecting normal cell function.
The SV40 Vp1 NLS (KP) was selected because of its potent import activity and the simplicity of incorporating it into the NTS-polyplex.22 The presence of four lysines in the peptidic backbone of KP allows it to bind electrostatically to plasmid DNA before the addition of the NTS-vector to form the NTS-polyplex22 (Figure 4, B). Evidence of the incorporation of KP into the pDNA and of pDNA condensation was provided by transmission electron microscopy and agarose-gel electrophoresis.21 The electrostatic interaction between the KP and pDNA caused retardation in the mobility of the pDNA in an agarose-gel electrophoresis confirming the formation of a KP-pDNA complex.21
The incorporation of viral peptide in the NTS-polyplex produced an efficient and durable expression of transgenes in cultured cells and in vivo in the rat (Figure 2). Quantitative analysis showed that the presence of only the KP in the NTS-polyplex (lacking the FP) increases transgene expression in vitro by approximately 50%, which increases to > 600% when the NTS-polyplex contains both the FP and KP (Figures 6 and 7).22 These data strongly suggest that the KP remains bound to pDNA after the addition of the NTS-vector, even though it contains polylysine. This suggestion is further supported by the finding that a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus.86 In addition, there is evidence in the gene therapy literature that NLS mediated gene transfer still works after irreversible chemical linkage of the NLS-peptide to cDNA. 87,88
Similarly, the combined use of the endosomal neutralizing agent chloroquine and of the SV40 large-tumor-antigen NLS improves the transfection efficiency of transferrin-polyplex.80,89 The successful use of strategies to escape from the endosome or to inhibit endosomal acidity strongly supports the idea that the major barrier to receptor-mediated gene-transfer systems is acidification of endosomal vesicles.90 Because of its capacity for cell entry, endosomal escape and nuclear import, the NTS-polyplex thus has the ability to provide efficient and durable transgene expression without the requirement for complementary treatments such as chloroquine, which increases the transfection efficiency by preventing endosome acidification.91
The use of a synthetic peptide in the NTS-polyplex has considerable benefits over the use of mutated or chemically modified viruses. It results insmaller complexes and considering the lack of toxicity or of any potential infectivity, the technique holds great promise for therapeutic intervention, especially for PD. The most striking benefit of merging the viral strategy with the transfection specificity of NTS-polyplex is the ability to achieve durable transgene expression in dopaminergic neurons of the substantia nigra. The injection of the NTS-polyplex into the substantia nigra of rats produces strong transgene expression in large numbers of dopaminergic neurons but not in nearby glial cells (Figures 2 and 9).21–24 The blockade of NTSR1 binding site by NTS-antagonists and the ineffectiveness of an untargeted polyplex (lacking NTS) clearly demonstrated that NTSR1-mediated endocytosis plays a key role in gene transfer in vivo.21,22 Transgene expression has been detected for up to two months (the end of the study) after NTS-polyplex injection and the observation of high levels of transgene expression at the study end point suggests that it can endure even longer.22–24
Figure 9.
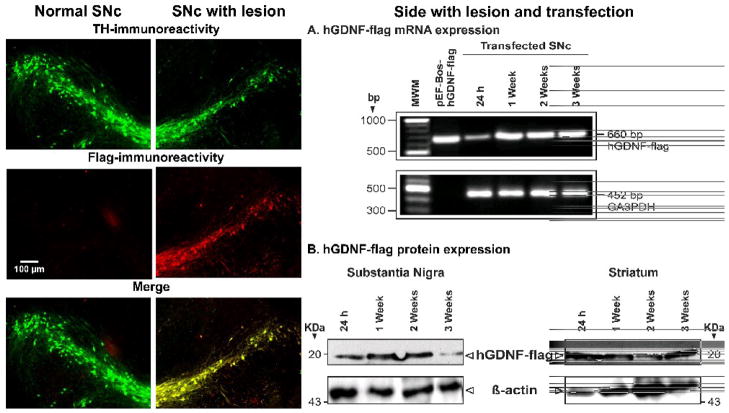
The overexpression of hGDFN gene in dopaminergic neurons of the substantia nigra leads to the presence of hGDFN protein in the striatum, suggesting the axonal transport of the transgenic protein to the other nuclei innervated by nigral dopaminergic neurons. The micrographs of double immunofluorescence against TH and flag show hGDNF-flag expression in dopamine neurons of the substantia nigra of hemiparkinsonian rats at 3 weeks following intranigral transfection of pEF-BoshGDNF-flag using NTS-polyplex. The calibration bar in the left middle panel is valid for all other panels. A) The representative RT-PCR assays shows the time course of hGDNF-flag gene expression in the substantia nigra of hemiparkinsonian rats. B) The western blot assay shows the presence of hGDNF-flag protein in the substantia nigra and striatum. The figures were reproduced from Gonzalez-Barrios et al. (2006).
In summary, although the involvement of the NTSR1 receptor in the NTS-polyplex transfection is solidly demonstrated in vitro and in vivo, the route of the NTS-polyplex through the intracellular compartments and the import mechanism into the cell nucleus remain to be clarified by using different techniques; for details see reference # 41. In addition, the identification of the nuclear compartment where the transgene is lodged and the molecular mechanisms of transgene transcription and regulation are still unknown.
Tissue-specific promoters
A high degree of dopamine cell-specificity of transgene expression has been achieved with the NTS-polyplex approach, based on the distribution of NTSR1integral to the transfection process and the ability to target NTS-polyplex injections into the immediate vicinity of dopaminergic neurons or their terminals (see above). It might be possible to further enhance cell-specificity of expression by placing the transgene under the control of a promoter derived from a dopamine cell-specific gene (Figure 5). The dopamine transporter (DAT) is a plasma membrane protein that functions to clear released dopamine from the extracellular space, thereby controlling the amplitude and duration of dopamine signaling.92 The DAT gene is most robustly expressed within substantia nigra dopamine neurons (those cells most impacted in PD), with a specificity of cell expression unrivaled by any other genes associated with the dopaminergic phenotype.93 Recently, an NTS-polyplex containing the BDNF-flag gene under control of the previously characterized94 human DAT promoter sequence, was injected into the striatum, a tissue known to contain high levels of NTSR1.48 As expected, the human DAT promoter prevented transgene expression in these striatal cells.21 In contrast, BDNF-flag expression was detected in dopaminergic neurons of the substantia nigra due to NTSR1-mediated retrograde transport of the transgene from striatal terminals. The retrograde transport of NTS by NTSR1 is well-characterized in experimental animals68 and represents an alternative route of transfection of nigral dopaminergic neurons. In addition, the use of tissue-specific promoters might prolong NTS-polyplex-mediated gene expression because DAT promoter activity is not expected to be transcriptionally silenced by methylation, as is the case for viral promoters such as CMV.95 In the future, it might be possible to modify the DNA sequence, or pharmacologically manipulate the functional activity of tissue-specific promoters to provide even more refined control of transgene expression.
Neurotrophic therapy
Traditionally, the term neurotrophic factors (NTFs) has referred to a group of secreted proteins that regulate the life and death of specific sets of neuronal subpopulations during development. The role of NTFs is well-established in CNS development. In contrast, the function of NTFs in the survival, maintenance and plasticity of the mature CNS is less clear. Nevertheless, evidence in the adult brain showing that NTFs promote neuronal regeneration following diverse types of injury has encouraged their use in the treatment of neurodegenerative disease and neural trauma.
The recent discovery and characterization of two new evolutionarily-conserved NTFs, cerebral dopamine-neurotrophic factor (CDNF) and mesencephalic astrocyte-derived neurotrophic factor (MANF), has augmented the families of NTFs to four groups.96,97 The modern classification98 includes:
the neurotrophins: Nerve growth factor (NGF), BDNF, neurotrophin-3 (NT-3) and NT-499
GDNF family ligands (GFLs): GDNF, neurturin (NRTN), artemin (ARTN) and persephin (PSPN)18,100
neuropoietic cytokines (also referred as the interleukin-6 (IL-6) family)101 and
NTFs act on neurons through transmembrane receptors with intrinsic tyrosine kinase activity or via other receptor-associated kinases.100 The receptors for CDNF and MANF are presently unknown.98
Dopaminergic neurons of the substantia nigra respond specifically to GDNF, NRTN and BDNF, through GFRα1, GFRα2, and trkB, their respective high-affinity receptors.103–107 These receptors are located on the cell body and axon terminals of dopaminergic neurons.104,105,108,109 GDNF can also activate RET via GFRα2 and NRTN via GFRα.110,111 GDNF can also activate completely different receptors, such as the neural cell-adhesion molecule and syndecan glycoproteins.112 A large variety of in vitro studies have demonstrated that BDNF, NRTN or GDNF play important roles in growth, differentiation and survival of dopaminergic neurons107,113–115, though the role of these NTFs in vivo is not completely understood. GDNF is expressed at low levels in the adult brain, but is indispensable for adult catecholaminergic neuron survival, as shown by the occurrence of massive neuronal death when the GDNF gene is turned off in a conditional knockout mouse model.116 This finding suggests that if a decrease of GDNF expression occurs in PD, it could contribute to cell death.117 The role of BDNF in mature dopaminergic neurons is still controversial. Interestingly, BDNF is expressed by dopaminergic neurons.106,118 Reduced expression of BDNF within the substantia nigra accompanies the deterioration of dopaminergic neurons in PD patients.119 However, this association is not solidly supported by the results of experiments carried out in BDNF knockout mice. Though a study shows a 23% reduction of these cells at postnatal day 21 in Wnt1-Cre mice,120 other reports showed that chronic deficits in BDNF alone do not affect survival or function of dopaminergic nigrostriatal neurons during aging, as revealed by the study of the BDNF−/− mice.121
The potential utility of GDNF, NRTN and BDNF in promoting the protection and survival of dopaminergic neurons and axonal regeneration is well-established in different animal models of PD.115,122,123 This question has been studied at length following either intracerebral infusion of a purified recombinant protein or viral gene delivery. Acute or continuous infusions of these NTFs produce robust neuroprotective effects in both rodent and primate models of PD.124–126 However, the short half-life of the recombinant protein because of enzymatic degradation in situ has encouraged the development of other techniques. Among those described in the literature, thus far, viral vector-mediated gene transfer is undoubtedly the most powerful technique to provide increased levels of transgenic growth factors in situ. In laboratory animals, viral vectors can be injected locally in the striatum, in the substantia nigra, or at both locations, either before (preventive) or after (restorative) the administration of the neurotoxic agent. Although an extensive review of the data is beyond the scope of the present review, suffice it to say that in all gene therapy models, viral overexpression of GDNF, NRTN or BDNF have proven effective to prevent the loss nigrostriatal dopaminergic neurons (for reviews, see references# 15,17,127). The success of preclinical studies with infusion protocols and viral gene therapy led to their use in PD patients in an attempt to reduce dopamine neuron degeneration.128
To date, only mechanically injected GDNF and viral-delivered NRTN have been explored in clinical trials to cure PD. Whereas they have demonstrated relative safety, they have been clinically disappointing.19,128,129 The negative findings might have resulted from the fact that only advanced stage PD patients were used for these studies. Because NTFs require that significant dopaminergic innervation remains, earlier-stage PD patients are likely to be the ideal candidates for neurotrophic therapy. Another possibility for the negative results in clinical trials is that the GDNF and NRTN were only supplied within the striatum, without any expression in the cell bodies of dopamine neurons or in their extensive collaterals in other structures. We propose that there is a strong rationale for targeting gene transfer into dopaminergic neurons in the substantia nigra. To evaluate the possibility that the proposed rationale is correct, the NTS-polyplex was used to transfect a neurotrophic gene into dopaminergic neurons of a rat model of PD, as detailed below.24
NTS-polyplex-gene therapy model
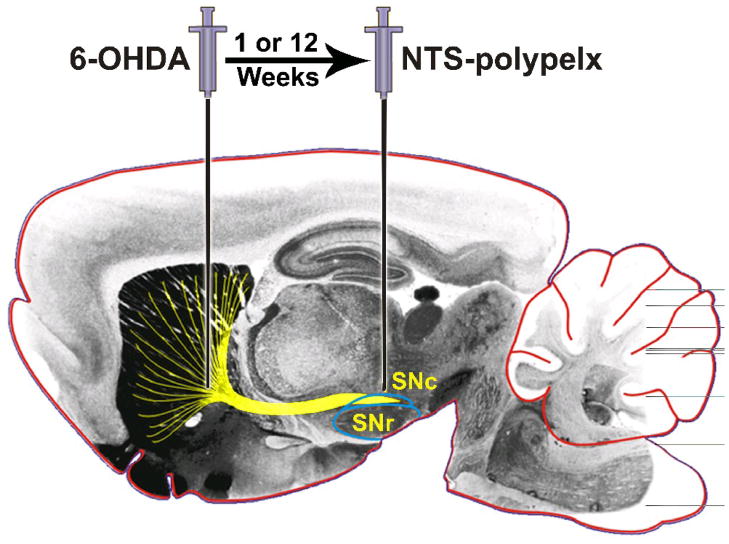
The 6-OHDA hemiparkinsonian rat model is a useful model to test neurotrophic gene transfection of dopaminergic neurons with the NTS-polyplex (Figure 8). In addition to progressive dopaminergic degeneration, such an animal model of PD avoids additional, mechanical injury of neurons in the substantia nigra. Furthermore, the massive striatal dopamine deficiency causes detectable drug-induced circling behavior at day 7 after 6-OHDA injection.24 To date, a single dose of NTS-polyplex in the proximity of substantia nigra is the only route of transfection that has been evaluated (Figure 8). A volume of 1 μL of NTS-polyplex dissolved in Dulbecco’s modified Eagle medium was injected at a flow rate of 0.1 μL/min. The dosing of NTS-polyplex components was established on the basis of molar ratios determined by retardation and retention microassays by 0.2% agarose gel electrophoresis. 21,22,45 For instance, the molar ratios for NTS-polyplex containing pEF-Bos-hGDNF were determined to be 30 nM pDNA: 20 μM KP: 300 nM neurotensin-carrier (Figure 5). The total amount of pDNA in 1 μL injected was 118 ng, which is significantly lower than the limiting concentration (20 μg/mL) required to form a soluble complex. 37
Figure 8.
Sites of the 6-OHDA lesion and NTS-polyplex transfection. 6-OHDA was injected in the striatum at the coordinates: anteroposterior (AP) +7.7 mm from interaural line; mediolateral (ML) + 4.0 mm from interparietal suture and dorsoventral (DV) −5.4 mm from dura mater. One or twelve weeks after 6-OHDA injection, different NTS-polyplexes were injected into the ipsilateral substantia nigra at the coordinates AP + 2.5 mm from interaural line, ML + 2.0 mm from midline and DV −6.7 from dura mater. The coordinates were adapted from the Paxinos atlas for rats weighing 220 g. 24
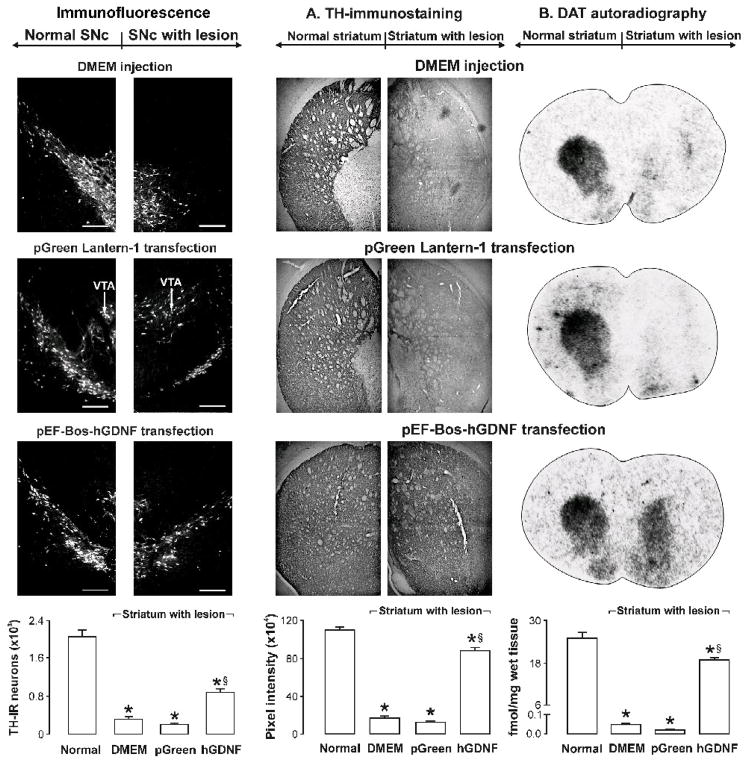
Three independent techniques (reverse transcriptase-polymerase chain reaction, immunofluorescence, and western blot) showed that the human GDNF-flag was expressed in the substantia nigra in response to NTS-polyplex transfection (Figure 9).24 A strong correlation between the reduction of motor impairment and the increase in dopamine content in the substantia nigra and striatum was established. This result suggests that the effectiveness of neurotrophic therapy depends on the recovery of neurotransmission at the level of cell bodies and terminals, which was attained by the intranigral transfection.24 Moreover, neuron survival in the substantia nigra and striatal reinnervation were also demonstrated, resulting from the presence of human GDNF-flag protein in those nuclei (Figure 10).24 The presence of human GDNF-flag protein in the striatum (Figure 9, B) strongly suggests the occurrence of both anterograde transport of the human GDNF-flag and the release of this factor at the level of the neuronal cell body and axon terminals in target structures. All these results support the contention that transfection of the neurotrophic gene into dopaminergic neurons is the correct strategy to simultaneously affect their soma and terminals. Preliminary results of NTS-polyplex-mediated transfection of either NRTN-V5-HIS or BDNF-flag in the 6-OHDA-rat model show neurotrophic and behavioral effects similar to those of the hGDNF-flag transfection.25,26 Interestingly, NTS-polyplex transfection of NRTN-V5-HIS in a different, chronic model of hemiparkinsonism significantly reduced motor impairment and produced a restorative effect on the dopaminergic nigrostriatal system. In that later study, NTS-polyplex-mediated transfection of NRTN-V5-HIS was performed on day 90 after the 6-OHDA injection, with the behavioral test and TH-immunohistochemistry performed one month after the transfection;26 this model thus has face validity as a model for assessing treatment of more advanced stage PD.
Figure 10.
pEF-Bos-hGDNF transfection in the substantia nigra promotes survival in the nigral dopaminergic neurons and reinnervation of the striatum in hemiparkinsonian rats. The figures were reproduced from Gonzalez-Barrios et al. (2006).
Together, this evidence suggests that the transfection of neurotrophic genes into dopamine neurons of the substantia nigra is key to achieve simultaneous neurotrophic effects on the remaining soma and terminals. Under such conditions, the expression of GDNF, NRTN and BDNF in the cell body of dopaminergic neurons can be accompanied by localization to axon terminals following anterograde transport.130 NTFs can then be released from both the soma and axon terminals to exert simultaneous autocrine and paracrine effects on neuronal survival and axon regeneration. Because a single dopaminergic neuron sends extensive collaterals to multiple nuclei in the brain (Figure 1),5 it is expected that transgenic NTFs will also be present and act upon all of the target structures. This approach is likely to be more effective than the administration of viral vectors or infusions into the caudo-putamen nucleus since this nucleus is much larger than the substantia nigra and several administrations should be required to cover most of the striatal volume and ensure uptake of the NTF by the remaining dopaminergic terminals. Although in principle, striatal expression of NTFs can lead to sufficient supply of these factors to the soma of dopaminergic neurons, this strategy is unlikely to affect the numerous targets of dopamine neurons.
The large extent of dopaminergic denervation and the advanced stage of PD when the therapy is initiated are likely to be important limiting factors for the success of neurotrophic gene-therapy for PD. For an effective gene therapy, we suggest that it may be important to transfect neurotrophic genes into dopaminergic neurons in an early stage of PD in order to take advantage of sufficient substrate for the action of the expressed NTFs.
Towards clinical use of NTS-polyplex
To date, a single injection of the NTS-polyplex in the proximity of the substantia nigra is the only route of neurotrophic gene transfection that has been evaluated in the 6-OHDA hemiparkinsonian-rat model.24–26 Though studies on the potential neurotoxicity of the NTS-polyplex in the rat have not been done, the results from the local transfection of neurotrophic genes suggest that this approach might also produce attenuation of neuroinflammation caused by 6-OHDA and the surgical procedure.24–26 In that case, the local administration of the NTS-polyplex in the neurotrophic gene therapy for PD would be a safe and beneficial procedure. In support of this suggestion, clinical trials have shown that neurotrophic factor infusion or injections of NRTN-AAV into the brain have been relatively safe.19,129 Further support is provided because PD is the only neurodegenerative disorder routinely treated with neurosurgery.128
The NTS-polyplex can also deliver a neurotrophic gene from the striatum into nigral dopaminergic neurons through the NTSR1-mediated retrograde transport of NTS.21 This result suggests that the injection of the NTS-polyplex into the striatum might be another route to transfect dopaminergic neurons in the treatment for PD patients. However, the striatal injection of the NTS-polyplex has two possible disadvantages in comparison to its nigral injection. The first disadvantage is that the striatum is a much larger nucleus than the substantia nigra in humans. This implies that a large dose of NTS-polyplex or repeated injections of small dose in different sites should be made to cover the whole of the striatum, thus assuring an efficient transfection of the nigral dopaminergic neurons. The second possible disadvantage is the decrease in the NTSR1 density because of the scarcity of dopaminergic axon terminals in the striatum of PD patients that might limit the retrograde transport of the NTS-polyplex to the nigral cell bodies. This is a limiting factor for all forms of neurotrophic gene therapy for PD, especially because of the extensive loss of divergent innervation of multiple structures after degeneration of dopaminergic neurons.
Recently, the NTS-polyplex harboring a suicide gene has been intravenously injected in repeated doses to kill malignant cells in a model of metastatic neuroblastoma developed by subcutaneous allograft of N1E-115 cells in nude mice.40 This therapeutic approach was based on the finding that neuroblastoma cells overexpress NTSR1.46 This work showed that the NTS-polyplex was able to reach and transfect only tumorous cells and in a lesser extension the NTSR1-expressing cells of the intestinal tract after the injection into the blood stream.40 However, no transgene expression was seen in the brain suggesting that the NTS-polyplex is unable to cross the blood brain barrier (BBB), possibly caused by the inability of the NTS to penetrate the BBB. Though the BBB hindrance protects the brain from the NTS-polyplex-mediated transfection of cell-death-promoting genes, the BBB is a limitation in the targeted neurotrophic gene delivery to the brain through the blood stream. Two possible strategies have been considered to overcome the BBB. The first strategy is to replace the NTS as ligand of the NTS-polyplex by its synthetic analogue NT69L, which resists degradation by plasma enzymes, binds NTSR1 with higher affinity than NTS, and is able to cross the BBB.131 The second invasive strategy involves the systemic administration of the NTS-polyplex in conjunction with transient BBB disruption using vasoactive amines such as bradykinin, histamine, and the synthetic bradykinin analog RMP-7 (receptor-mediated permeabilizer).132 Other routes of the NTS-polyplex to bypass the BBB might be the intraventricular or intranasal administration, which have been effective in drug delivery into the brain.132
Limitations, perspectives, and possibilities of the NTS-polyplex for PD therapy
The NTS-polyplex appears to have a promising future in the neurotrophic gene therapy for PD. However, some limitations unrelated to the nonviral gene technology but with the nature of the disease have raised concerns about the future of this therapeutic approach. First, neuron loss silently accumulates before clinical signs become evident.1 Symptoms develop when approximately 50% of dopaminergic neurons are still present.15 Therefore, neurotrophic therapy should start in the early stage of PD to retain sufficient dopamine neurons that are compromised but not dead to halt the advance of neurodegeneration. However, the pharmacological treatment at this stage of PD is still effective and therefore rules out gene therapy as a first-elective approach. Although PD is a gradually developing disease and the potential window of treatment appears to be large, the clinical trials of neurotrophic gene therapy using viral vectors in advanced PD are not conclusive. The other major issue that confronts neurotrophic gene therapy is that it fails to address the etiology and the complexity of PD.1,4 Though the neurotrophic gene transfection into the compromised dopaminergic neurons might lead to repopulation and restoration of multiple brain circuits in many brain regions (those innervated by those nigral neurons), it fails to repair the genetic alteration that causes Parkinson’s disease.133 Recently, interference RNA technology has been proposed for PD therapy because the over-expression of various proteins is known to kill the nigral dopaminergic neurons in animal models and in familial forms of PD. 41 Similarly to viral vectors, the NTS-polyplex might be used in the delivery of interference RNA into dopaminergic neurons to knock down the altered proteins, such as α-synuclein or LRRK2 (protein leucine-rich repeat kinase 2).133,134
Notwithstanding the limitations that all gene vectors suffer, the NTS-polyplex nanoparticle system seems to have broad perspectives for a clinical use for PD. By taking advantage of the potential of the NTS-polyplex to codeliver two genes into the same cell,21 this system might be used to deliver a neurotrophic gene and interference RNA. Hypothetically, this approach might yield a neurotrophic effect and decrease in the mutated protein levels. Another possible combined gene therapy using the NTS-polyplex might be with a neurotrophic gene and one of the genes coding for a Redox enzyme such catalase.135 This treatment would be intended to attenuate neuroinflammation and increase neuroprotection in patients with PD. In summary, a combined gene therapy for PD can be formulated not only with different neurotrophic genes but also with a great variety of genetic approaches, as mentioned above, well beyond the limit of imagination. However, from a theoretical point of view the NTS-polyplex nanoparticles have the limitation to incorporate a drug in addition to the transgene. This incorporation would exceed the precise stoichiometry to assemble the five components of the NTS-polyplex into functional nanoparticles for gene delivery.
Potential use of neurotrophic therapy in other neurodegenerative diseases
An attractive characteristic of the NTS-polyplex that makes it a promising candidate for PD gene-therapy is its specificity through NTSR1-mediated endocytosis mechanism to shuttle the transgene into surviving dopamine neurons. Other neurons of the central nervous system (CNS), such as those of the dopaminergic ventral tegmental area and of the cholinergic basal-forebrain also express NTSR1,67,136,137 so they are other putative targets for gene delivery via the NTS-polyplex. For example, NTS-polyplex transfection of neurotrophic factors into basal-forebrain cholinergic neurons could be useful to prevent neurodegeneration in Alzheimer’s disease.138,139 Two gene therapy approaches for Alzheimer’s disease that have been previously successful with viral vectors could perhaps be adapted to take advantage of NTS-polyplex transfection. The first approach aims to increase NGF concentration in order to promote survival, regeneration and protection of basal-forebrain cholinergic neurons.139 The second approach is overexpression of the CREB-binding protein to overcome the interference of amyloid-β accumulation, which plays a primary role in the cognitive deficits of AD.140
Although the potential of neurotrophic gene-therapy is very extensive, the absence of NTSR1 in degenerating neurons limits the use of NTS-polyplex in other pathologies of the central and peripheral nervous system.
Benefits and limitations of NTS-polyplex system in comparison to viral carriers
Polyplexes have the advantage over viral gene transfer of being non-immunogenic, easy to produce and not oncogenic. Although the immunological and safety evaluation of the NTS-polyplex has not been accomplished, published evidence, both direct and indirect, supports the biosafety of NTS-polyplex-mediated transfection in vitro and in vivo. A recent study reports that NTS-polyplex-mediated transfection does not affect cell viability in vitro.21 In all of the studies performed in vivo, the NTS-polyplex has been shown not to cause more damage to brain parenchyma than that caused by mechanical microinjection by itself.20–22,24–26 Moreover, repeated intravenous injections of NTS-polyplex for targeted gene-delivery of a suicide gene in an animal model of neuroblastoma lead to an antitumoral effect without signs of tissue damage in other organs.40 This evidence agrees with the demonstrated biosafety of lactoferrin-polyplex, that has been successfully used in animal models of PD.141,142 In contrast, non-degradable polyplexes, such as those based on high molecular weight polyethylenimines, are able to cause cytotoxicity as a result from their poor clearance.143 These later kinds of polyplexes have limited use in gene therapy despite their high transfection efficiency in vitro and in vivo.
To date, only lactoferrin-polyplex and NTS-polyplex systems have been explored to deliver the GDNF gene delivery in parkinsoninan rats.24,144 The efficacy of those systems in promoting functional recovery in the rat 6-HODA lesion model is similar to that of viral carriers. 145,146 However, clinical trials with such nanoparticles will be required to confirm the usefulness of this strategy for the treatment of PD.
Lack of regulation of gene expression is a current limitation for all current protocols in neurotrophic therapy. The uncontrolled and robust expression of a neurotrophic protein might lead to undesirable side effects such as aberrant innervation of the regenerating dopaminergic fibers 147 and decrease of dopamine synthesis.148 Furthermore, neurotrophins, for instance, could reach levels high enough to stimulate the p75 receptor, thus leading healthy and recovering neurons to apoptosis.149 The use of inducible promoters in conjunction with NTS-polyplex thus needs to be explored.
Conclusions
Neurotrophic gene-therapy continues to be at the forefront of PD research because it holds the promise of stopping neurodegeneration, reversing neurological damage and maintaining the integrity of divergent projections of nigral dopaminergic-neurons. All neurotrophic gene-therapy approaches are effective and relatively safe in rodent and primate models of PD, although some of them, in particular those with GDNF, produce undesirable behavioral effects resulting from aberrant innervation of the regenerating dopaminergic fibers147 and decrease of dopamine synthesis.148 Although the results of clinical trials with recombinant GDNF-protein infusion or NRTN gene-therapy have not been particularly positive, they show that these approaches are safe in humans. We believe that the modest clinical benefit might be related to the selection of putamen cells as the source of NTFs. From this region, the diffusion of NTFs confronts physical and biochemical obstacles to reach the cell bodies and the other innervation targets of dopaminergic neurons. In particular, the long distance that separates the other targets from the putamen in the human brain and enzymatic degradation of NTFs could be the main causes of the poor neurotrophic effect. We propose that transfection of neurotrophic genes in dopaminergic neurons might be a much more effective strategy to obtain a neurotrophic effect simultaneously in the cell body and terminal regions of dopaminergic neurons. The NTS-polyplex has the ability to transfect dopaminergic neurons via NTSR1-mediated endocytosis at the cell body or via retrograde transport of NTSR1. Nigral injection of the NTS-polyplex with neurotrophic genes (GDNF, NRTN or BDNF) has been effective in the treatment of acute and chronic experimental hemiparkinsonism in the rat. The preliminary data suggesting adequate biosafety of this approach anticipates the safe use of the NTS-polyplex in clinical trials of neurotrophic gene-therapy for PD in the near future. Meanwhile, evaluation of the NTS-polyplex in Parkinsonian nonhuman primates represents the next important test of this promising nonviral gene-transfer system.
Acknowledgments
Funding sources: This work was supported by the ConsejoNacional de Ciencia y Tecnología de México Grant # 83229 (D.M-F.), InstitutoCientífico Pfizer (D.M-F.), Canadian Institutes of Health Research Grants OPD-79574, MOP-49591 and MOP-106556 (L.E.T.), Parkinson Society Canada (L.E.T.), and National Institutes of Health Grant DA006470 (M.J.B.). M.L.A-R., N.G.H-C, and D. R-C were recipients of scholarships from the ConsejoNacional de Ciencia y Tecnología de México.
List of abbreviations
- AAV
adeno-associated virus
- ARTN
artemin
- BBB
blood brain barrier
- BDNF
brain-derived neurotrophic factor
- sCDNF
cerebral dopamine-neurotrophic factor
- CMV
cytomegalovirus
- CNS
central nervous system
- DAT
dopamine transporter
- FP
fusogenic peptide
- GDNF
glial cell-line-derived neurotrophic factor
- GFLs
glial cell-line-derived neurotrophic factor family ligands
- GFP
green fluorescence protein
- GFR
high-affinity receptors for glial cell-line-derived neurotrophic factor family ligands
- IL
interleukin
- KP
karyophilic peptide
- MANF
mesencephalic astrocyte-derived neurotrophic factor
- NGF
Nerve growth factor
- NLS
nuclear-location signal
- NRTN
neurturin
- NT
neurotrophin
- NTF
neurotrophic factor
- NTS
neurotensin
- NTSR
neurotensin receptor
- 6-OHDA
6-hydroxydopamine
- PD
Parkinson’s disease
- pDNA
plasmid deoxyribonucleic acid
- PSPN
persephin
- SV40
simian virus 40
- TH
tyrosine hydroxylase
- Trk
tyrosine kinase receptor
Footnotes
Financial Disclosure: The authors have no financial, personal or other relationships with other people or organizations within five years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–74. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 3.Brooks DJ. PET studies on the function of dopamine in health and Parkinson’s disease. Ann N Y Acad Sci. 2003;991:22–35. doi: 10.1111/j.1749-6632.2003.tb07460.x. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 5.Anaya-Martinez V, Martinez-Marcos A, Martinez-Fong D, Aceves J, Erlij D. Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: implications for abnormal motor behavior. Neuroscience. 2006;143:477–86. doi: 10.1016/j.neuroscience.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Debeir T, Ginestet L, Francois C, Laurens S, Martel JC, Chopin P, et al. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp Neurol. 2005;193:444–54. doi: 10.1016/j.expneurol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Prensa L, Parent A. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci. 2001;21:7247–60. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floran B, Aceves J, Sierra A, Martinez-Fong D. Activation of D1 dopamine receptors stimulates the release of GABA in the basal ganglia of the rat. Neurosci Lett. 1990;116:136–40. doi: 10.1016/0304-3940(90)90399-t. [DOI] [PubMed] [Google Scholar]
- 9.Rosales MG, Flores G, Hernandez S, Martinez-Fong D, Aceves J. Activation of subthalamic neurons produces NMDA receptor-mediated dendritic dopamine release in substantia nigra pars reticulata: a microdialysis study in the rat. Brain Res. 1994;645:335–7. doi: 10.1016/0006-8993(94)91669-1. [DOI] [PubMed] [Google Scholar]
- 10.Flores G, Liang JJ, Sierra A, Martinez-Fong D, Quirion R, Aceves J, et al. Expression of dopamine receptors in the subthalamic nucleus of the rat: characterization using reverse transcriptase-polymerase chain reaction and autoradiography. Neuroscience. 1999;91:549–56. doi: 10.1016/s0306-4522(98)00633-2. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Fong D, Rosales MG, Gongora-Alfaro JL, Hernandez S, Aceves J. NMDA receptor mediates dopamine release in the striatum of unanesthetized rats as measured by brain microdialysis. Brain Res. 1992;595:309–15. doi: 10.1016/0006-8993(92)91065-m. [DOI] [PubMed] [Google Scholar]
- 12.Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 13.Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfler C, et al. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol. 2011;258:1254–60. doi: 10.1007/s00415-011-5915-2. [DOI] [PubMed] [Google Scholar]
- 14.Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66:1460–8. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorklund T, Kirik D. Scientific rationale for the development of gene therapy strategies for Parkinson’s disease. Biochim Biophys Acta. 2009;1792:703–13. doi: 10.1016/j.bbadis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Ulusoy A, Bjorklund T, Hermening S, Kirik D. In vivo gene delivery for development of mammalian models for Parkinson’s disease. Exp Neurol. 2008;209:89–100. doi: 10.1016/j.expneurol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- 18.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 19.Marks WJ, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–8. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Maya I, Navarro-Quiroga I, Meraz-Rios MA, Aceves J, Martinez-Fong D. In vivo gene transfer to dopamine neurons of rat substantia nigra via the high-affinity neurotensin receptor. Mol Med. 2001;7:186–92. [PMC free article] [PubMed] [Google Scholar]
- 21.Arango-Rodriguez ML, Navarro-Quiroga I, Gonzalez-Barrios JA, Martinez-Arguelles DB, Bannon MJ, Kouri J, et al. Biophysical characteristics of neurotensin polyplex for in vitro and in vivo gene transfection. Biochim Biophys Acta. 2006;1760:1009–20. doi: 10.1016/j.bbagen.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Navarro-Quiroga I, Gonzalez-Barrios JA, Barron-Moreno F, Gonzalez-Bernal V, Martinez-Arguelles DB, Martinez-Fong D. Improved neurotensin-vector-mediated gene transfer by the coupling of hemagglutinin HA2 fusogenic peptide and Vp1 SV40 nuclear localization signal. Brain Res Mol Brain Res. 2002;105:86–97. doi: 10.1016/s0169-328x(02)00396-0. [DOI] [PubMed] [Google Scholar]
- 23.Orozco-Barrios CE, Battaglia-Hsu SF, Arango-Rodriguez ML, Ayala-Davila J, Chery C, Alberto JM, et al. Vitamin B12-impaired metabolism produces apoptosis and Parkinson phenotype in rats expressing the transcobalamin-oleosin chimera in substantia nigra. PLoS One. 2009;4:e8268. doi: 10.1371/journal.pone.0008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Barrios JA, Lindahl M, Bannon MJ, Anaya-Martinez V, Flores G, Navarro-Quiroga I, et al. Neurotensin polyplex as an efficient carrier for delivering the human GDNF gene into nigral dopamine neurons of hemiparkinsonian rats. Mol Ther. 2006;14:857–65. doi: 10.1016/j.ymthe.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Chan N, Zamudio S, Escobedo L, De la Cruz F, Gongora-Alfaro JL, Bannon MJ, et al. BDNF-Flag Gene Transfer by NT-Polyplex to Nigral Dopaminergic Neurons Causes Morphological and Functional Recovery from Hemiparkinsonism in the Rat. Mol Ther. 2008;16(974) [Google Scholar]
- 26.Reyes-Corona D, Escobedo L, Ayala-Davila J, Orozco-Barrios CE, Arango-Rodriguez ML, Martinez-Fong D. Program No. 643.7/U4.2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. NT-Polyplex-mediated neurturin delivery to dopaminergic neurons of hemiparkinsonian rats: A new approach in the repertoire of neurotrophic therapy. Online. 2009. [Google Scholar]
- 27.Martinez-Fong D, Navarro-Quiroga I, Ochoa I, Alvarez-Maya I, Meraz MA, Luna J, et al. Neurotensin-SPDP-poly-L-lysine conjugate: a nonviral vector for targeted gene delivery to neural cells. Brain Res Mol Brain Res. 1999;69:249–62. doi: 10.1016/s0169-328x(99)00114-x. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez CA, Rice KG. Engineered nanoscaled polyplex gene delivery systems. Mol Pharm. 2009;6:1277–89. doi: 10.1021/mp900033j. [DOI] [PubMed] [Google Scholar]
- 29.Esbjorner EK, Oglecka K, Lincoln P, Graslund A, Norden B. Membrane binding of pH-sensitive influenza fusion peptides. positioning, configuration, and induced leakage in a lipid vesicle model. Biochemistry. 2007;46:13490–504. doi: 10.1021/bi701075y. [DOI] [PubMed] [Google Scholar]
- 30.Ishii N, Minami N, Chen EY, Medina AL, Chico MM, Kasamatsu H. Analysis of a nuclear localization signal of simian virus 40 major capsid protein Vp1. J Virol. 1996;1996:1317–22. doi: 10.1128/jvi.70.2.1317-1322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saccardo P, Villaverde A, Gonzalez-Montalban N. Peptide-mediated DNA condensation for non-viral gene therapy. Biotechnol Adv. 2009;27:432–8. doi: 10.1016/j.biotechadv.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Hud NV, Vilfan ID. Toroidal DNA condensates: unraveling the fine structure and the role of nucleation in determining size. Annu Rev Biophys Biomol Struct. 2005;34:295–318. doi: 10.1146/annurev.biophys.34.040204.144500. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Yang YL, Wang C, Yao Y, Ma YZ, Hou S, et al. Polymeric effects on DNA condensation by cationic polymers observed by atomic force microscopy. Colloids Surf B Biointerfaces. 2010;75:230–8. doi: 10.1016/j.colsurfb.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 34.Midoux P, Pichon C, Yaouanc JJ, Jaffres PA. Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br J Pharmacol. 2009;157:166–78. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekrami HM, Shen WC. Carbamylation decreases the cytotoxicity but not the drug-carrier properties of polylysines. J Drug Target. 1995;2:469–75. doi: 10.3109/10611869509015916. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Fong D, Mullersman JE, Purchio AF, rmendariz-Borunda J, Martinez-Hernandez A. Nonenzymatic glycosylation of poly-L-lysine: a new tool for targeted gene delivery. Hepatology. 1994;20:1602–8. doi: 10.1002/hep.1840200633. [DOI] [PubMed] [Google Scholar]
- 37.Wagner E, Ogris M, Zauner W. Polylysine-based transfection systems utilizing receptor-mediated delivery. Adv Drug Deliv Rev. 1998;30:97–113. doi: 10.1016/s0169-409x(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 38.Cotten M, Wagner E. Non-viral approaches to gene therapy. Curr Opin Biotechnol. 1993;4:705–10. doi: 10.1016/0958-1669(93)90053-y. [DOI] [PubMed] [Google Scholar]
- 39.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 40.Rubio-Zapata HA, Rembao-Bojorquez JD, Arango-Rodriguez ML, Dupouy S, Forgez P, Martinez-Fong D. NT-polyplex: a new tool for therapeutic gene delivery to neuroblastoma tumors. Cancer Gene Ther. 2009;16:573–84. doi: 10.1038/cgt.2009.1. [DOI] [PubMed] [Google Scholar]
- 41.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–95. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–96. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 43.Mazella J, Vincent JP. Internalization and recycling properties of neurotensin receptors. Peptides. 2006;27:2488–92. doi: 10.1016/j.peptides.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Souaze F, Forgez P. Molecular and cellular regulation of neurotensin receptor under acute and chronic agonist stimulation. Peptides. 2006;27:2493–501. doi: 10.1016/j.peptides.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Fong D, Navarro-Quiroga I. Synthesis of a non-viral vector for gene transfer via the high-affinity neurotensin receptor. Brain Res Brain Res Protoc. 2000;6:13–24. doi: 10.1016/s1385-299x(00)00032-5. [DOI] [PubMed] [Google Scholar]
- 46.Amar S, Kitabgi P, Vincent JP. Stimulation of inositol phosphate production by neurotensin in neuroblastoma N1E115 cells: implication of GTP-binding proteins and relationship with the cyclic GMP response. J Neurochem. 1987;49:999–1006. doi: 10.1111/j.1471-4159.1987.tb09986.x. [DOI] [PubMed] [Google Scholar]
- 47.Kitabgi P, Rostene W, Dussaillant M, Schotte A, Laduron PM, Vincent JP. Two populations of neurotensin binding sites in murine brain: discrimination by the antihistamine levocabastine reveals markedly different radioautographic distribution. Eur J Pharmacol. 1987;140:285–93. doi: 10.1016/0014-2999(87)90285-8. [DOI] [PubMed] [Google Scholar]
- 48.Mendez M, Souaze F, Nagano M, Kelly PA, Rostene W, Forgez P. High affinity neurotensin receptor mRNA distribution in rat brain and peripheral tissues. Analysis by quantitative RT-PCR. J Mol Neurosci. 1997;9:93–102. doi: 10.1007/BF02736853. [DOI] [PubMed] [Google Scholar]
- 49.Nouel D, Faure MP, StPierre JA, Alonso R, Quirion R, Beaudet A. Differential binding profile and internalization process of neurotensin via neuronal and glial receptors. J Neurosci. 1997;17:1795–803. doi: 10.1523/JNEUROSCI.17-05-01795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quirion R, Welner S, Gauthier S, Bedard P. Neurotensin receptor binding sites in monkey and human brain: autoradiographic distribution and effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment. Synapse. 1987;1:559–66. doi: 10.1002/syn.890010608. [DOI] [PubMed] [Google Scholar]
- 51.Szigethy E, Beaudet A. Correspondence between high affinity 125I-neurotensin binding sites and dopaminergic neurons in the rat substantia nigra and ventral tegmental area: a combined radioautographic and immunohistochemical light microscopic study. J Comp Neurol. 1989;279:128–37. doi: 10.1002/cne.902790111. [DOI] [PubMed] [Google Scholar]
- 52.Palacios JM, Kuhar MJ. Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature. 1981;294:587–9. doi: 10.1038/294587a0. [DOI] [PubMed] [Google Scholar]
- 53.Sadoul JL, Checler F, Kitabgi P, Rostene W, Javoy-Agid F, Vincent JP. Loss of high affinity neurotensin receptors in substantia nigra from parkinsonian subjects. Biochem Biophys Res Commun. 1984;125:395–404. doi: 10.1016/s0006-291x(84)80381-2. [DOI] [PubMed] [Google Scholar]
- 54.Mazella J, Leonard K, Chabry J, Kitabgi P, Vincent JP, Beaudet A. Binding and internalization of iodinated neurotensin in neuronal cultures from embryonic mouse brain. Brain Res. 1991;564:249–55. doi: 10.1016/0006-8993(91)91460-i. [DOI] [PubMed] [Google Scholar]
- 55.Toy-Miou-Leong M, Cortes CL, Beaudet A, Rostene W, Forgez P. Receptor trafficking via the perinuclear recycling compartment accompanied by cell division is necessary for permanent neurotensin cell sensitization and leads to chronic mitogen-activated protein kinase activation. J Biol Chem. 2004;279:12636–46. doi: 10.1074/jbc.M303384200. [DOI] [PubMed] [Google Scholar]
- 56.Vanisberg MA, Maloteaux JM, Octave JN, Laduron PM. Rapid agonist-induced decrease of neurotensin receptors from the cell surface in rat cultured neurons. Biochem Pharmacol. 1991;42:2265–74. doi: 10.1016/0006-2952(91)90229-x. [DOI] [PubMed] [Google Scholar]
- 57.Gully D, Canton M, Boigegrain R, Jeanjean F, Molimard JC, Poncelet M, et al. Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc Natl Acad Sci U S A. 1993;90:65–9. doi: 10.1073/pnas.90.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci. 1996;16:5613–20. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vita N, Laurent P, Lefort S, Chalon P, Dumont X, Kaghad M, et al. Cloning and expression of a complementary DNA encoding a high affinity human neurotensin receptor. FEBS Lett. 1993;317:139–42. doi: 10.1016/0014-5793(93)81509-x. [DOI] [PubMed] [Google Scholar]
- 61.Gendron L, Perron A, Payet MD, Gallo-Payet N, Sarret P, Beaudet A. Low-affinity neurotensin receptor (NTS2) signaling: internalization-dependent activation of extracellular signal-regulated kinases 1/2. Mol Pharmacol. 2004;66:1421–30. doi: 10.1124/mol.104.002303. [DOI] [PubMed] [Google Scholar]
- 62.Lepee-Lorgeoux I, Betancur C, Rostene W, Pelaprat D. Differential ontogenetic patterns of levocabastine-sensitive neurotensin NT2 receptors and of NT1 receptors in the rat brain revealed by in situ hybridization. Brain Res Dev Brain Res. 1999;113:115–31. doi: 10.1016/s0165-3806(99)00009-7. [DOI] [PubMed] [Google Scholar]
- 63.Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–6. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem. 1999;274:8832–6. doi: 10.1074/jbc.274.13.8832. [DOI] [PubMed] [Google Scholar]
- 65.Martin S, Navarro V, Vincent JP, Mazella J. Neurotensin receptor-1 and -3 complex modulates the cellular signaling of neurotensin in the HT29 cell line. Gastroenterology. 2002;123:1135–43. doi: 10.1053/gast.2002.36000. [DOI] [PubMed] [Google Scholar]
- 66.Cusack B, Stanton T, Richelson E. Developmental Regulation of Neurotensin Receptor Expression and Function in Murine Neuroblastoma Clone N1E-115. Eur J Pharmacol. 1991;206:339–42. doi: 10.1016/0922-4106(91)90119-3. [DOI] [PubMed] [Google Scholar]
- 67.Boudin H, Pelaprat D, Rostene W, Pickel VM, Beaudet A. Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. J Neurosci. 1998;18:8473–84. doi: 10.1523/JNEUROSCI.18-20-08473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laduron PM. Functional consequences of retrograde axonal transport of receptor-bound neurotensin. Trends Pharmacol Sci. 1995;16:338–43. doi: 10.1016/s0165-6147(00)89067-7. [DOI] [PubMed] [Google Scholar]
- 69.Sorkin A, von ZM. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–22. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medina-Kauwe LK. “Alternative” endocytic mechanisms exploited by pathogens: new avenues for therapeutic delivery? Adv Drug Deliv Rev. 2007;59:798–809. doi: 10.1016/j.addr.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Authier F, Posner BI, Bergeron JJ. Endosomal proteolysis of internalized proteins. FEBS Lett. 1996;389:55–60. doi: 10.1016/0014-5793(96)00368-7. [DOI] [PubMed] [Google Scholar]
- 72.White J, Kielian M, Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983;16:151–95. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- 73.Casali M, Banta S, Zambonelli C, Megeed Z, Yarmush ML. Site-directed mutagenesis of the hinge peptide from the hemagglutinin protein: enhancement of the pH-responsive conformational change. Protein Eng Des Sel. 2008;21:395–404. doi: 10.1093/protein/gzn018. [DOI] [PubMed] [Google Scholar]
- 74.Swalley SE, Baker BM, Calder LJ, Harrison SC, Skehel JJ, Wiley DC. Full-length influenza hemagglutinin HA(2) refolds into the trimeric low-pH-induced conformation. Biochemistry. 2004;43:5902–11. doi: 10.1021/bi049807k. [DOI] [PubMed] [Google Scholar]
- 75.Minchin RF, Yang S. Endosomal disruptors in non-viral gene delivery. Expert Opin Drug Deliv. 2010;7:331–9. doi: 10.1517/17425240903512931. [DOI] [PubMed] [Google Scholar]
- 76.Howell DP, Krieser RJ, Eastman A, Barry MA. Deoxyribonuclease II is a lysosomal barrier to transfection. Mol Ther. 2003;8:957–63. doi: 10.1016/j.ymthe.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Wu CH, Wilson JM, Wu GY. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J Biol Chem. 1989;264:16985–7. [PubMed] [Google Scholar]
- 78.Cristiano RJ, Smith LC, Kay MA, Brinkley BR, Woo SL. Hepatic gene therapy: efficient gene delivery and expression in primary hepatocytes utilizing a conjugated adenovirus-DNA complex. Proc Natl Acad Sci U S A. 1993;90:11548–52. doi: 10.1073/pnas.90.24.11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug Chem. 1999;10:406–11. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 80.Chan CK, Jans DA. Enhancement of polylysine-mediated transferrinfection by nuclear localization sequences: polylysine does not function as a nuclear localization sequence. Hum Gene Ther. 1999;10:1695–702. doi: 10.1089/10430349950017699. [DOI] [PubMed] [Google Scholar]
- 81.Midoux P, Mendes C, Legrand A, Raimond J, Mayer R, Monsigny M, et al. Specific gene transfer mediated by lactosylated poly-L-lysine into hepatoma cells. Nucleic Acids Res. 1993;21:871–8. doi: 10.1093/nar/21.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chowdhury EH. Nuclear targeting of viral and non-viral DNA. Expert Opin Drug Deliv. 2009;6:697–703. doi: 10.1517/17425240903025744. [DOI] [PubMed] [Google Scholar]
- 83.Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J Biol Chem. 2007;282:5101–5. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalderon D, Richardson WD, Markham AF, Smith AE. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984;311:33–8. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 85.Ishii N, Nakanishi A, Yamada M, Macalalad MH, Kasamatsu H. Functional Complementation of Nuclear Targeting-Defective Mutants of Simian-Virus-40 Structural Proteins. J Virol. 1994;68:8209–16. doi: 10.1128/jvi.68.12.8209-8216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci U S A. 1999;96:91–6. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van der AM, Koning G, van der GJ, d’Oliveira C, Oosting R, Hennink WE, et al. Covalent attachment of an NLS-peptide to linear dna does not enhance transfection efficiency of cationic polymer based gene delivery systems. J Control Release. 2005;101:395–7. [PubMed] [Google Scholar]
- 88.Roulon T, Helene C, Escude C. Coupling of a targeting peptide to plasmid DNA using a new type of padlock oligonucleotide. Bioconjug Chem. 2002;13:1134–9. doi: 10.1021/bc025551o. [DOI] [PubMed] [Google Scholar]
- 89.Chan CK, Senden T, Jans DA. Supramolecular structure and nuclear targeting efficiency determine the enhancement of transfection by modified polylysines. Gene Ther. 2000;7:1690–7. doi: 10.1038/sj.gt.3301275. [DOI] [PubMed] [Google Scholar]
- 90.Reddy JA, Low PS. Enhanced folate receptor mediated gene therapy using a novel pH-sensitive lipid formulation. J Control Release. 2000;64:27–37. doi: 10.1016/s0168-3659(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 91.Guy J, Drabek D, Antoniou M. Delivery of DNA into mammalian cells by receptor-mediated endocytosis and gene therapy. Mol Biotechnol. 1995;3:237–48. doi: 10.1007/BF02789334. [DOI] [PubMed] [Google Scholar]
- 92.Bannon MJ. The dopamine transporter: role in neurotoxicity and human disease. Toxicol Appl Pharmacol. 2005;204:355–60. doi: 10.1016/j.taap.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Bannon MJ, Whitty CJ. Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology. 1997;48:969–77. doi: 10.1212/wnl.48.4.969. [DOI] [PubMed] [Google Scholar]
- 94.Sacchetti P, Brownschidle LA, Granneman JG, Bannon MJ. Characterization of the 5′-flanking region of the human dopamine transporter gene. Brain Res Mol Brain Res. 1999;74:167–74. doi: 10.1016/s0169-328x(99)00275-2. [DOI] [PubMed] [Google Scholar]
- 95.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 96.Lindholm P, Voutilainen MH, Lauren J, Peranen J, Leppanen VM, Andressoo JO, et al. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007;448:73–7. doi: 10.1038/nature05957. [DOI] [PubMed] [Google Scholar]
- 97.Lindholm P, Peranen J, Andressoo JO, Kalkkinen N, Kokaia Z, Lindvall O, et al. MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci. 2008;39:356–71. doi: 10.1016/j.mcn.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 98.Lindholm P, Saarma M. Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol. 2010;70:360–71. doi: 10.1002/dneu.20760. [DOI] [PubMed] [Google Scholar]
- 99.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 100.Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8:221–32. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- 102.Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, et al. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173–88. doi: 10.1385/jmn:20:2:173. [DOI] [PubMed] [Google Scholar]
- 103.Yoong LF, Wan G, Too HP. GDNF-induced cell signaling and neurite outgrowths are differentially mediated by GFRalpha1 isoforms. Mol Cell Neurosci. 2009;41:464–73. doi: 10.1016/j.mcn.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 104.Sarabi A, Hoffer BJ, Olson L, Morales M. GFRalpha-1 mRNA in dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area. J Comp Neurol. 2001;441:106–17. doi: 10.1002/cne.1400. [DOI] [PubMed] [Google Scholar]
- 105.Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, et al. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–50. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 106.Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–34. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- 107.Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–80. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 108.Kozlowski DA, Miljan EA, Bremer EG, Harrod CG, Gerin C, Connor B, et al. Quantitative analyses of GFRalpha-1 and GFRalpha-2 mRNAs and tyrosine hydroxylase protein in the nigrostriatal system reveal bilateral compensatory changes following unilateral 6-OHDA lesions in the rat. Brain Res. 2004;1016:170–81. doi: 10.1016/j.brainres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Numan S, Seroogy KB. Increased expression of trkB mRNA in rat caudate--putamen following 6-OHDA lesions of the nigrostriatal pathway. Eur J Neurosci. 1997;9:489–95. doi: 10.1111/j.1460-9568.1997.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 110.Wang LC, Shih A, Hongo J, Devaux B, Hynes M. Broad specificity of GDNF family receptors GFRalpha1 and GFRalpha2 for GDNF and NTN in neurons and transfected cells. J Neurosci Res. 2000;61:1–9. doi: 10.1002/1097-4547(20000701)61:1<1::AID-JNR1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 111.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, et al. Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci U S A. 1997;94:7018–23. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–62. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 113.Knusel B, Winslow JW, Rosenthal A, Burton LE, Seid DP, Nikolics K, et al. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991;88:961–5. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meyer M, Johansen J, Gramsbergen JB, Johansen TE, Zimmer J. Improved survival of embryonic porcine dopaminergic neurons in coculture with a conditionally immortalized GDNF-producing hippocampal cell line. Exp Neurol. 2000;164:82–93. doi: 10.1006/exnr.2000.7419. [DOI] [PubMed] [Google Scholar]
- 115.Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci. 1998;18:4929–37. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–61. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 117.Hunot S, Bernard V, Faucheux B, Boissiere F, Leguern E, Brana C, et al. Glial cell line-derived neurotrophic factor (GDNF) gene expression in the human brain: a post mortem in situ hybridization study with special reference to Parkinson’s disease. J Neural Transm. 1996;103:1043–52. doi: 10.1007/BF01291789. [DOI] [PubMed] [Google Scholar]
- 118.Gall CM, Gold SJ, Isackson PJ, Seroogy KB. Brain-derived neurotrophic factor and neurotrophin-3 mRNAs are expressed in ventral midbrain regions containing dopaminergic neurons. Mol Cell Neurosci. 1992;3:56–63. doi: 10.1016/1044-7431(92)90009-q. [DOI] [PubMed] [Google Scholar]
- 119.Parain K, Murer MG, Yan Q, Faucheux B, Agid Y, Hirsch E, et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport. 1999;10:557–61. doi: 10.1097/00001756-199902250-00021. [DOI] [PubMed] [Google Scholar]
- 120.Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–9. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baker SA, Stanford LE, Brown RE, Hagg T. Maturation but not survival of dopaminergic nigrostriatal neurons is affected in developing and aging BDNF-deficient mice. Brain Res. 2005;1039:177–88. doi: 10.1016/j.brainres.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 122.Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–41. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 123.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–9. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 124.Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- 125.Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Bjorklund A. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson’s disease after administration into the striatum or the lateral ventricle. Eur J Neurosci. 1999;11:1554–66. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- 126.Mufson EJ, Kroin JS, Liu YT, Sobreviela T, Penn RD, Miller JA, et al. Intrastriatal and intraventricular infusion of brain-derived neurotrophic factor in the cynomologous monkey: distribution, retrograde transport and co-localization with substantia nigra dopamine-containing neurons. Neuroscience. 1996;71:179–91. doi: 10.1016/0306-4522(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 127.Bartus RT, Herzog CD, Bishop K, Ostrove JM, Tuszynski M, Kordower JH, et al. Issues regarding gene therapy products for Parkinson’s disease: the development of CERE-120 (AAV-NTN) as one reference point. Parkinsonism Relat Disord. 2007;13 (Suppl 3):S469–S477. doi: 10.1016/S1353-8020(08)70052-X. [DOI] [PubMed] [Google Scholar]
- 128.Kaplitt MG. Parkinson disease: Another player in gene therapy for Parkinson disease. Nat Rev Neurol. 2010;6:7–8. doi: 10.1038/nrneurol.2009.214. [DOI] [PubMed] [Google Scholar]
- 129.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–66. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 130.von Bartheld CS, Wang X, Butowt R. Anterograde axonal transport, transcytosis, and recycling of neurotrophic factors: the concept of trophic currencies in neural networks. Mol Neurobiol. 2001;24:1–28. doi: 10.1385/MN:24:1-3:001. [DOI] [PubMed] [Google Scholar]
- 131.Boules M, Fredrickson P, Richelson E. Current topics: brain penetrating neurotensin analog. Life Sci. 2003;73:2785–92. doi: 10.1016/s0024-3205(03)00674-x. [DOI] [PubMed] [Google Scholar]
- 132.Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6:252–73. [PubMed] [Google Scholar]
- 133.Manfredsson FP, Lewin AS, Mandel RJ. RNA knockdown as a potential therapeutic strategy in Parkinson’s disease. Gene Ther. 2006;13:517–24. doi: 10.1038/sj.gt.3302669. [DOI] [PubMed] [Google Scholar]
- 134.Habig K, Walter M, Poths S, Riess O, Bonin M. RNA interference of LRRK2-microarray expression analysis of a Parkinson’s disease key player. Neurogenetics. 2008;9:83–94. doi: 10.1007/s10048-007-0114-0. [DOI] [PubMed] [Google Scholar]
- 135.Haney MJ, Zhao Y, Li S, Higginbotham SM, Booth SL, Han HY, et al. Cell-mediated transfer of catalase nanoparticles from macrophages to brain endothelial, glial and neuronal cells. Nanomedicine (Lond) 2011;6:1215–30. doi: 10.2217/nnm.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 137.Szigethy E, Leonard K, Beaudet A. Ultrastructural localization of [125I]neurotensin binding sites to cholinergic neurons of the rat nucleus basalis magnocellularis. Neuroscience. 1990;36:377–91. doi: 10.1016/0306-4522(90)90433-5. [DOI] [PubMed] [Google Scholar]
- 138.Cattaneo A, Capsoni S, Paoletti F. Towards non invasive nerve growth factor therapies for Alzheimer’s disease. J Alzheimers Dis. 2008;15:255–83. doi: 10.3233/jad-2008-15210. [DOI] [PubMed] [Google Scholar]
- 139.Mandel RJ. CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer’s disease. Curr Opin Mol Ther. 2010;12:240–7. [PubMed] [Google Scholar]
- 140.Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:22687–92. doi: 10.1073/pnas.1012851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang R, Ke W, Han L, Liu Y, Shao K, Jiang C, et al. Lactoferrin-modified nanoparticles could mediate efficient gene delivery to the brain in vivo. Brain Res Bull. 2010;81:600–4. doi: 10.1016/j.brainresbull.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 142.Huang R, Ke W, Han L, Liu Y, Shao K, Ye L, et al. Brain-targeting mechanisms of lactoferrin-modified DNA-loaded nanoparticles. J Cereb Blood Flow Metab. 2009;29:1914–23. doi: 10.1038/jcbfm.2009.104. [DOI] [PubMed] [Google Scholar]
- 143.Halama A, Kulinski M, Librowski T, Lochynski S. Polymer-based non-viral gene delivery as a concept for the treatment of cancer. Pharmacol Rep. 2009;61:993–9. doi: 10.1016/s1734-1140(09)70160-4. [DOI] [PubMed] [Google Scholar]
- 144.Huang R, Ke W, Liu Y, Wu D, Feng L, Jiang C, et al. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J Neurol Sci. 2010;290:123–30. doi: 10.1016/j.jns.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 145.Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, et al. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson’s disease. Gene Ther. 2002;9:381–9. doi: 10.1038/sj.gt.3301682. [DOI] [PubMed] [Google Scholar]
- 146.Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 147.Georgievska B, Kirik D, Bjorklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp Neurol. 2002;177:461–74. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- 148.Sajadi A, Bauer M, Thony B, Aebischer P. Long-term glial cell line-derived neurotrophic factor overexpression in the intact nigrostriatal system in rats leads to a decrease of dopamine and increase of tetrahydrobiopterin production. J Neurochem. 2005;93:1482–6. doi: 10.1111/j.1471-4159.2005.03139.x. [DOI] [PubMed] [Google Scholar]
- 149.Kenchappa RS, Tep C, Korade Z, Urra S, Bronfman FC, Yoon SO, et al. p75 neurotrophin receptor-mediated apoptosis in sympathetic neurons involves a biphasic activation of JNK and up-regulation of tumor necrosis factor-alpha-converting enzyme/ADAM17. J Biol Chem. 2010;285:20358–68. doi: 10.1074/jbc.M109.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fasano C, Thibault D, Trudeau LE. Culture of postnatal mesencephalic dopamine neurons on an astrocyte monolayer. Curr Protoc Neurosci. 2008;Chapter 3(Unit) doi: 10.1002/0471142301.ns0321s44. [DOI] [PubMed] [Google Scholar]