Abstract
Aims
Examine the association of person-specific trajectories of withdrawal symptoms of urge-to-smoke, negative affect, physical symptoms, and hunger during the first 7 days after smoking cessation with abstinence at end of treatment (EOT) and 6 months.
Design
Hierarchical Linear Modeling (HLM) was used to model person-specific trajectory parameters (level, slope, curvature and volatility) for withdrawal symptoms.
Setting
University-based smoking cessation trials.
Participants
Treatment seeking smokers in clinical trials of transdermal nicotine versus nicotine spray (n=514) and bupropion versus placebo (n=421)
Measurements
Self-reported withdrawal symptoms for 7 days after the planned quit date, and 7 day point prevalence and continuous abstinence at EOT and 6 months.
Findings
In regressions that included trajectory parameters for one group of withdrawal symptoms, both urge-to-smoke and negative affect were predictive of abstinence while physical symptoms and hunger were generally not predictive. In stepwise regressions that included the complete set of trajectory parameters across withdrawal symptoms (for urge-to-smoke, negative affect, physical symptoms, and hunger), with a single exception, only the trajectory parameters for urge-to-smoke were predictive. Area under the Receiver Operator Characteristic curve was 0.594 for covariates alone, and 0.670 for covariates plus urge-to-smoke trajectory parameters.
Conclusion
Among a number of different withdrawal symptoms (urge-to-smoke, negative affect, physical symptoms, and hunger) urge-to-smoke trajectory parameters (level, slope, and volatility) over the first 7 days of smoking cessation show the strongest prediction of both short and long term relapse. Other withdrawal symptoms increase the predictive ability by negligible amounts.
Keywords: smoking cessation, withdrawal symptoms, urge-to-smoke, negative affect, physical symptoms, hunger, nicotine replacement therapy, bupropion
INTRODUCTION
Previous research by Piasecki et al. [1–3] provided evidence of substantial between-person variability in total withdrawal symptom severity following smoking cessation. The level, slope, and volatility of the total symptom trajectory were highly predictive of subsequent relapse among smokers randomized to receive treatment for nicotine dependence [3]. Javitz et al. [4] extended those results to urge-to-smoke (e.g., craving) symptoms in an independent sample of clinical trial participants undergoing treatment for smoking cessation.
Because withdrawal symptoms are multidimensional and consist of several types of symptoms in addition to urge-to-smoke [5–8] this study was conducted to determine (1) the extent to which features of other withdrawal symptoms (negative affect, physical symptoms, and hunger) are predictive of subsequent relapse over and above the predictive ability of demographic, smoking history and treatment characteristics, and (2) whether they add incremental value in predicting relapse over that provided by urge-to-smoke trajectory features. This study extends Javitz et al. [4] by examining the absolute predictive value of three other withdrawal symptom clusters (negative affect, hunger, and other physical symptoms) and their predictive value relative to urge-to-smoke.
METHODS
Subjects
Data for this study were obtained from the same clinical trials as in Javitz et al [4]. One study was an open-label clinical trial of treatment-seeking smokers who were randomly assigned to receive 8 weeks of transdermal nicotine therapy or nicotine spray plus seven sessions of standardized behavioral group counseling [9,10]. Nicotine replacement therapy (NRT) was initiated on the morning of the target quit date (TQD) after 2 weeks of counseling. The second study was a double-blind clinical trial of treatment-seeking smokers who were randomly assigned to receive 10 weeks of either active or placebo bupropion plus seven sessions of behavioral counseling [10,11]. Bupropion was delivered at the standard therapeutic dose. Bupropion study participants were instructed to quit smoking 2 weeks after initiating medication and counseling. In both trials, participants included smokers who were 18 years of age or older, reported smoking 10 or more cigarettes a day for the prior year, and provided informed consent for genotyping and treatment. Overall, 270 individuals received placebo, 298 nicotine spray, 302 NRT patch, and 285 bupropion. All individuals with no more than 1 missing withdrawal symptom score on days 1 through 7 after the TQD were included in this analysis (n=200, 239, 275, and 223, respectively).
Measures
Self-report data on point prevalence and continuous abstinence smoking status were obtained at EOT (8 weeks post-TQD) and at 6-month follow-up. Participants who self-reported complete abstinence (not even a puff of a cigarette) for at least 7 days prior to the assessment were asked to complete an in-person visit for biochemical verification of abstinence, using exhaled carbon monoxide in the first trial and saliva cotinine testing in the second trial. Baseline assessments included demographics and smoking history (see Table 1).
Table 1.
Descriptive statistics for treatment arms
| Variable | Placebo | Bupropion | Nicotine Spray |
Transdermal Nicotine |
p-value+ | N with trajectory |
N enrolled |
|---|---|---|---|---|---|---|---|
| Number in treatment arm | 270 | 285 | 298 | 302 | 1155 | ||
| Scale scores on >= 6 days | 198 | 223 | 239 | 275 | 935 | ||
| Percent with Scale Scores | 73.3 | 78.2 | 80.2 | 91.1 | |||
| Demographics | |||||||
| Age M (SD) | 45.7 (10.8) | 45.0 (11.6) | 45.4 (10.2) | 46.0 (11.0) | 0.739 | 45.5 (10.9) | 45.3 (10.9) |
| Gender (% female) | 52.0 | 58.3 | 56.1 | 51.3 | 0.367 | 54.3 | 55.2 |
| BMI at baseline M (SD) | 26.9 (5.5) | 26.8 (4.9) | 28.3 (6.1) | 27.7 (5.7) | 0.016* | 27.5 (5.6) | 27.4 (5.6) |
| CESD at baseline M (SD) | 11.7 (8.0) | 12.5 (8.6) | 11.9 (8.6) | 11.8 (8.7) | 0.019* | 12.0 (8.5) | 12.0 (8.5) |
| Race (%) | |||||||
| Hispanic | 4.0 | 1.4 | 1.7 | 2.6 | 0.290 | 2.4 | 2.3 |
| African American | 11.6 | 13.1 | 24.7 | 27.7 | <0.001*** | 20.0 | 21.3 |
| Asian | 2.5 | 1.4 | 0.0 | 2.2 | 0.057 | 1.5 | 1.6 |
| Caucasian | 80.3 | 83.3 | 69.9 | 64.2 | < 0.001*** | 73.6 | 72.3 |
| Other | 1.5 | 0.9 | 3.8 | 3.3 | 0.138 | 2.5 | 2.6 |
| Smoking history | |||||||
| CPDM (SD) | 21.3 (10.3) | 20.2 (7.8) | 22.3 (9.5) | 21.5 (10.3) | 0.158 | 21.3 (9.6) | 21.4 (9.7) |
| FTNDM (SD) | 5.0 (2.2) | 4.9 (2.0) | 5.5 (2.2) | 5.4 (2.1) | 0.005** | 5.2 (2.1) | 5.4 (2.1) |
| 7 day point prevalence (%) | |||||||
| EOT | 24.8 | 40.4 | 38.9 | 38.9 | 0.002** | 36.3 | 30.9 |
| 6 months | 19.2 | 31.4 | 24.7 | 21.1 | 0.015* | 24.1 | 20.5 |
| Continuously abstinent (%) | |||||||
| EOT | 19.9 | 34.2 | 29.8 | 28.3 | 0.012* | 28.4 | 24.4 |
| 6 months | 13.3 | 25.7 | 20.6 | 18.2 | 0.013* | 19.6 | 16.7 |
p-values are for tests of equality in means or proportions across the four study arms.
p < 0.05;
p < 0.01;
p < 0.001
Whereas in Javitz et al [4] only the urge-to-smoke was analyzed, the current study includes additional withdrawal symptom groupings. Nineteen of the daily withdrawal symptoms self-reported at baseline and for 7 consecutive days following the target quit date (TQD) were grouped into 4 scales based on the literature and previously published analyses [5,6,12–14]. The urge-to-smoke scale was the average response to questions about "craving for cigarettes" and "urge to smoke". The negative affect scale was the average of 6 questions about irritability, difficulty concentrating, restlessness, impatience, anxiousness/tension, and depression. The physical symptoms scale was the average of 9 questions about nausea, tremors, increased heart rate, general physical complaints, headache, gastrointestinal disturbance, insomnia, drowsiness, and fatigue. The hunger scale was the average of 2 questions about increased hunger and increased eating. The minimum item-rest correlation and Cronbach's alpha for each scale are as follows: urges to smoke (0.68, 0.81), negative affect (0.50, 0.85), physical symptoms (0.33, 0.74) and hunger (0.82, 0.90).
For each question, symptoms within the past day were reported on a 4 point scale (not present, mild, moderate, or severe). Participants were given 7 copies of the withdrawal assessment questionnaire and were instructed to complete the items at the end of each day for the first week after quitting. At baseline, participants were also asked to record their symptoms for the previous week using the same scale.
Statistical Methods
For each of the 4 symptom scales as dependent measures, HLM was used to estimate the coefficients for variables representing level (at day 4), slope, and curvature (centered at day 4) of the scale scores, person-to-person variability (i.e., random effects) in those parameters, and the covariance between random effects. A person-specific volatility parameter was estimated as the root mean square difference between the observed and predicted withdrawal symptom scale scores. Trajectories were calculated regardless of whether participants maintained complete abstinence during the first week of the quit attempt.
Logistic regression was conducted with abstinence as the dependent variable and trajectory parameters as the independent variables to determine the extent to which the trajectory parameters were statistically significant. Regressions were conducted both with and without baseline covariates of age, gender, body mass index (BMI) African American race, cigarettes per day (CPD), Fagerström score (FTND), the Center for Epidemiologic Studies (CESD) depression scale, and pharmacotherapy assignment (bupropion, transdermal nicotine, nicotine spray, or placebo bupropion). Regressions also included the baseline measurement of the symptom scale in the prior week. The area under the receiver operator characteristic (ROC) curves were calculated to quantify the extent to which covariates alone, covariates in combination with trajectory parameters for each withdrawal symptom scale, and covariates in combination with two sets of trajectory parameters could predict abstinence.
RESULTS
Table 1 shows that there were statistically significant differences between study groups in the proportion who were African American, and baseline BMI, CESD and FTND, due primarily to differences between populations in the two randomized studies. Since the objective of this study was to examine person-specific trajectories rather than to compare the outcomes of the different trials, we did not adjust for these differences in the calculation of the trajectory parameters. The 935 individuals in this analysis and the entire cohort of 1,155 enrollees have similar demographic and smoking history values. Continuous and 7-day point prevalence abstinence outcomes are higher for this analysis than for the full cohort, because this analysis excluded 218 individuals without symptom data, almost all of whom had dropped out and were classified as relapsers in the full cohort intent-to-treat analysis.
Trajectory Parameters
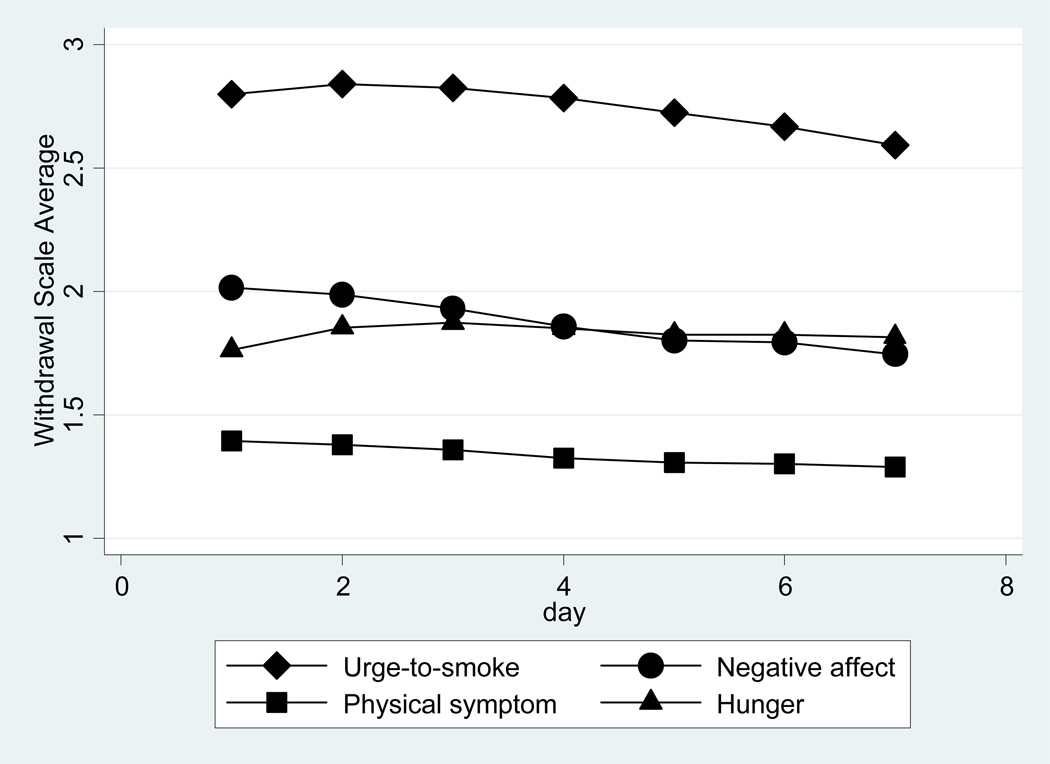
Figure 1 shows the average withdrawal symptom trajectories for the four withdrawal symptoms scales across the 7 day period. Trajectories show modest changes across time when averaged across all individuals and treatments; however, there is substantial person-to-person variability. The standard deviation of the urge-to-smoke score varies from 0.84 to 0.89 across days; the ranges for negative affect, hunger, and physical symptoms are 0.68 to 0.72, 0.84 to 0.87, and 0.36 to 0.41, respectively. Table 2 shows that the standard deviation of the urge-to-smoke level is 0.70 (i.e., about 30% of individuals were more than 0.70 away from the average level of 2.8 on an urge-to-smoke scale ranging from 1 to 4).
Figure 1. Average withdrawal symptom trajectories.
Table 2.
Withdrawal symptom subtype trajectory parameter descriptive statistics
| Trajectory parameters (Mean and Standard Deviation) | ||||
|---|---|---|---|---|
| Withdrawal symptom clusters |
Level | Slope | Curvature | Volatility |
| Urge-to-smoke | 2.784 (.698)bcd | −.0378 (.0997)bcd | −.00919 (.0270)bc | .208 (.203)bcd |
| Negative affect | 1.866 (.577)ac | −.0473 (.0763)acd | .00254 (.0226)ad | .121 (.160)ac |
| Physical symptoms | 1.355 (.339)abd | −.0914 (.0487)abd | .00154 (.0169)ad | .026 (.047)abd |
| Hunger | 1.859 (.767)ac | .0019 (.0882)abc | −.00743 (.0215)bc | .133 (.174)ac |
mean of the column parameter is statistically different than urge-to-smoke at p < 0.05;
mean of the column parameter is statistically different than negative affect at p < 0.05;
mean of the column parameter is statistically different than physical symptoms at p < 0.05,
mean of the column parameter is statistically different than hunger at p < 0.05
Table 2 shows the average trajectory parameters and their standard deviations across individuals for each withdrawal symptom scale. Person-to-person variability is substantial. For example, the between-person variability in the slope of the urge-to-smoke trajectory implies that the slope for the 5% of individuals with the largest (smallest) slopes will be approximately 1.645 × 0.0997 = 0.164 greater (less) than the average slope. Across 7 days these differences in slopes result in an additional increase or decrease in urge-to-smoke of 1 response unit (on 1 to 4 scale). While between-person variability is substantial across all withdrawal symptom subtypes and parameters, variability in the urge-to-smoke trajectory parameters tend to be as large or larger than variability in the other scales (except for variability in the hunger level).
Table 3 shows the correlations of trajectory parameters within and across symptom groups. Within each withdrawal symptom cluster, the correlations of trajectory slope and curvature and curvature and level range from −.20 to −.54. For withdrawal symptom scales other than urge-to-smoke, correlations between level and volatility range from 0.28 to 0.42. Correlations of physical symptom and hunger scale trajectory features with the corresponding urge-to-smoke trajectory features are relatively modest (≤0.30), while those of negative affect trajectory features are moderately large (0.33 to 0.58).
Table 3.
Correlations among trajectory parameters within and across symptom groups
| Urge to Smoke | Negative Affect | Physical Symptom | Hunger | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | Parameter | slope | curvature | level | volatility | slope | curvature | level | volatility | slope | curvature | level | volatility | slope | curvature | level | volatility |
| Urge to Smoke | slope | 1.00 | |||||||||||||||
| Urge to Smoke | curvature | −0.20 | 1.00 | ||||||||||||||
| Urge to Smoke | level | 0.14 | −0.35 | 1.00 | |||||||||||||
| Urge to Smoke | volatility | 0.04 | −0.11 | −0.01 | 1.00 | ||||||||||||
| Negative Affect | slope | 0.50 | −0.13 | −0.03 | 0.04 | 1.00 | |||||||||||
| Negative Affect | curvature | −0.17 | 0.49 | −0.16 | −0.02 | −0.39 | 1.00 | ||||||||||
| Negative Affect | level | 0.08 | −0.16 | 0.58 | −0.06 | 0.04 | −0.31 | 1.00 | |||||||||
| Negative Affect | volatility | 0.06 | −0.04 | 0.13 | 0.33 | 0.00 | −0.03 | 0.28 | 1.00 | ||||||||
| Physical Symptom | slope | 0.23 | −0.06 | −0.02 | −0.01 | 0.44 | −0.16 | −0.04 | −0.02 | 1.00 | |||||||
| Physical Symptom | curvature | −0.09 | 0.21 | −0.07 | −0.03 | −0.23 | 0.40 | −0.14 | −0.03 | −0.47 | 1.00 | ||||||
| Physical Symptom | level | 0.02 | −0.09 | 0.29 | 0.02 | 0.05 | −0.15 | 0.53 | 0.15 | −0.02 | −0.32 | 1.00 | |||||
| Physical Symptom | volatility | −0.01 | −0.05 | 0.08 | 0.21 | −0.02 | −0.02 | 0.19 | 0.30 | −0.17 | −0.14 | 0.42 | 1.00 | ||||
| Hunger | slope | 0.17 | −0.02 | 0.08 | −0.01 | 0.17 | −0.11 | 0.06 | 0.00 | 0.15 | −0.12 | 0.04 | −0.01 | 1.00 | |||
| Hunger | curvature | −0.06 | 0.15 | −0.14 | −0.01 | −0.09 | 0.18 | −0.13 | −0.02 | −0.06 | 0.15 | −0.11 | −0.06 | −0.53 | 1.00 | ||
| Hunger | level | 0.04 | −0.12 | 0.30 | −0.05 | 0.00 | −0.10 | 0.34 | 0.08 | −0.05 | −0.02 | 0.30 | 0.10 | 0.19 | −0.47 | 1.00 | |
| Hunger | volatility | 0.01 | −0.07 | 0.08 | 0.18 | −0.08 | −0.05 | 0.08 | 0.19 | −0.05 | −0.03 | 0.08 | 0.18 | 0.02 | −0.17 | 0.32 | 1.00 |
*,†,‡,§,∥,¶
Table 4 presents the odds ratios for logistic regressions where the dependent variables are 7-day point prevalence abstinence (PPA) and continuous abstinence at EOT and 6 months. Each regression includes the four trajectory parameters for that withdrawal symptom scale, a baseline measure of that scale obtained approximately two weeks prior to the planned quite date, demographic characteristics (African American, gender, CESD, BMI), smoking quantity and dependence (CPD and FTND), and pharmacotherapy (bupropion, placebo, NRT spray, or transdermal NRT). Odds ratios in this table are expressed as per standard deviation change in the trajectory parameters.
Table 4.
Odds ratios for logistic regressions of trajectory parameters on abstinence measures† in regressions including covariates‡
| Withdrawal symptom subtype trajectory parameters |
Abstinence outcomes | |||
|---|---|---|---|---|
| EOT | 6 months | |||
| Point prevalence |
Continuous abstinence |
Point prevalence |
Continuous abstinence |
|
| Urge-to-smoke | ||||
| Level | 0.6378*** | 0.5787*** | 0.6938*** | 0.6355*** |
| Slope | 0.7467*** | 0.7669*** | 0.8482 | 0.7443** |
| Curvature | 0.7881** | 0.8857 | 0.8657 | 0.9293 |
| Volatility | 1.0362 | 0.7885** | 0.8628 | 0.8128* |
| Negative Affect | ||||
| Level | 0.7533** | 0.7987* | 0.7767* | 0.8023* |
| Slope | 0.7923** | 0.8485 | 0.9082 | 0.9279 |
| Curvature | 0.8010** | 0.8677 | 0.8990 | 0.9329 |
| Volatility | 0.9826 | 0.9642 | 1.1129 | 1.0584 |
| Physical Symptoms | ||||
| Level | 0.9280 | 0.8850 | 0.9006 | 0.9124 |
| Slope | 0.8206* | 1.0166 | 0.9046 | 0.9933 |
| Curvature | 0.8223* | 0.8928 | 0.9319 | 0.8724 |
| Volatility | 0.9131 | 0.9784 | 1.0912 | 1.0275 |
| Hunger | ||||
| Level | 1.0642 | 1.1176 | 0.9287 | 1.0295 |
| Slope | 1.1797* | 1.1038 | 1.0823 | 1.0881 |
| Curvature | 1.0328 | 1.0584 | 0.9728 | 0.9967 |
| Volatility | 1.0015 | 1.0485 | 1.1455 | 1.1072 |
Odds ratios are expressed per standard deviation change in the trajectory parameters
Covariates were age, gender, African American race, indicator for type of pharmaceutical used, baseline cigarettes per day, FTND, BMI, CESD depression score, and the baseline value for the withdrawal symptom group.
p < 0.05;
p < 0.01;
p < 0.001
Prediction of abstinence
EOT— Both urge-to-smoke and negative affect trajectory features were highly predictive of PPA at EOT. The pattern of significant odds ratios was generally similar for urge-to-smoke features in the prediction of PPA and continuous abstinence whereas the consistency of the prediction pattern for negative affect features across the two outcomes at EOT was not as evident (negative affect level being the only consistently associated feature).
In contrast, to the 6 statistically significant associations for urge-to-smoke, there were only two significant associations between physical symptom features and PPA at EOT and only one for the hunger scale. None of the trajectory features for these two symptom clusters were associated with continuous abstinence at EOT. Compared to urge-to-smoke and negative affect, the trajectory parameters for these two symptom subtypes have relatively little predictive ability at EOT.
6 months—In general, the predictive power of trajectory features declined at 6 months. Urge-to-smoke and negative affect levels were both consistently associated with PPA and continuous abstinence outcomes at 6 months. Interestingly, urge-to-smoke slope and volatility were also associated with continuous abstinence at 6 months. Physical symptom and hunger trajectory parameters showed no evidence of being associated with 6-month outcomes.
Table 5 presents the odds ratios for the trajectory parameters when covariates and all 16 trajectory parameters are included in stepwise regressions with backwards elimination. Only odds ratios with p values less than 0.20 are shown. Urge-to-smoke trajectory features remained significantly associated with outcomes at EOT (level, slope, and volatility) and at 6 months (level, slope, and curvature). There was only a single other statistically significant trajectory parameter (curvature for physical symptoms). Regression results were unaffected by the exclusion of baseline CESD and baseline values for negative affect and urge-to-smoke.
Table 5.
Odds ratios† for logistic regressions of trajectory parameters on abstinence measures‡ in stepwise regressions including all trajectory parameters and covariates§
| Withdrawal symptom cluster trajectory parameters |
Abstinence outcomes | |||
|---|---|---|---|---|
| EOT | 6 months | |||
| Point Prevalence |
Continuous abstinence |
Point Prevalence |
Continuous abstinence |
|
| Urge-to-smoke | ||||
| Level | 0.6347*** | 0.5488*** | 0.6873*** | 0.6312*** |
| Slope | 0.7585*** | 0.7837** | 0.8457* | 0.7405*** |
| Curvature | 0.7988** | 0.8674 | ||
| Volatility | 0.8135* | 0.7774* | ||
| Negative Affect | ||||
| Level | 1.1463 | |||
| Slope | 0.8675 | |||
| Curvature | 0.8640 | |||
| Volatility | 1.1306 | 1.1493 | ||
| Physical Symptoms | ||||
| Level | ||||
| Slope | 0.8658 | 1.1486 | ||
| Curvature | 0.8692 | 0.8446* | ||
| Volatility | 1.1104 | |||
| Hunger | ||||
| Level | ||||
| Slope | ||||
| Curvature | ||||
| Volatility | ||||
Odds ratios are shown if the level of significance is 0.20 or less
Odds ratios are expressed per standard deviation change in the trajectory parameters
Covariates were age, gender, African American race, indicator for type of pharmaceutical used, and baseline cigarettes pre day, Fagerstrom index, BMI, CESD depression score, and the baseline value for the withdrawal symptom group.
p < 0.05;
p < 0.01;
p < 0.001
Table 6 presents the area under the ROC curve, denoted AUC, which measures the proportion of correctly classified abstinent individuals using various combinations of covariates and trajectory parameters at EOT and at 6-months. The first four rows show AUC values for various combinations of predictor variables. For example, the first row (row A) displays that when only covariates are used for classification of outcomes, the AUC varies from 0.58 (PPA at 6 months) to 0.61 (continuous abstinence at 6 months) with an average of 0.59. The AUC using covariates and urge-to-smoke scores ranged from 0.63 to 0.70 with an average of 0.67 (Row B), covariates and negative affect scores ranged from 0.60 to 0.62 with an average of 0.61 (Row C), and covariates, urge-to-smoke, and negative affect scores from 0.64 to 0.70 (Row D).
Table 6.
Receiver-operator characteristic AUC† values for predicting abstinence using trajectory parameters and covariates‡
| Combinations of covariates and withdraw al symptom group parameters used in AUC calculation |
Abstinence outcomes | Row Average |
|||
|---|---|---|---|---|---|
| EOT | 6 months | ||||
| Point prevalence |
Continuous abstinence |
Point prevalence |
Continuous abstinence |
||
| A. Covariates only | 0.589 | 0.600 | 0.580 | 0.607 | 0.594 |
| B. Covariates and urge-to-smoke | 0.662 | 0.696 | 0.630 | 0.690 | 0.670 |
| C. Covariates and negative affect | 0.618 | 0.618 | 0.595 | 0.621 | 0.613 |
| D. Covariates, urge-to-smoke, and negative affect |
0.668 | 0.700 | 0.636 | 0.696 | 0.675 |
| E. Incremental AUC from adding urge-to- smoke to covariates only (row B- row A) |
0.073*** | 0.096*** | 0.050* | 0.083*** | 0.076 |
| F. Incremental A U C from adding negative affect to covariates only (row C - row A) |
0.029* | 0.018 | 0.015 | 0.014 | 0.019 |
| G. Incremental AUC from adding urge-to- smoke to covariates and negative affect (row D - row C) |
0.050*** | 0.082*** | 0.041* | 0.075*** | 0.062 |
| H. Incremental AUC from adding negative affect to covariates and urge- to-smoke (row D - row B) |
0.006 | 0.004 | 0.006 | 0.006 | 0.005 |
AUC: Area under the curve
Covariates include age, gender, African American race, indicator for type of pharmaceutical used, baseline cigarettes per day, Fagerstrom index, BMI, and the CESD depression score. Each set withdrawal symptom group parameters consists of the level, slope, curvature, and volatility of the trajectory and the baseline value for the withdrawal symptom group.
If the covariates are restricted to FTND and CESD then the ROC values for point prevalence at EOT, continuous abstinence at EOT, point prevalence at 6 months, and continuous abstinence at 6 months are 0.520, 0.534, 0.524, and 0.527, respectively.
p < 0.05;
p < 0.01;
p < 0.001
The last four rows show how much the AUC values increase when various parameters are added to the predictors in rows A–D. For example, adding urge-to-smoke scores to the covariates (row E) increases the average AUC value by 0.08. Adding the negative affect scores to the covariates (row F) increases the AUC value by a modest 0.02. Negative affect has very little additive value if the regression equation already includes covariates and urge-to-smoke; in that case (row H), the AUC value increases by an average of 0.01. Adding urge-to-smoke to other predictors (rows E and G) always results in a statistically significant increase in AUC values. In contrast, adding negative affect to covariates resulted in only a single instance of a statistical significant increase (PPA at EOT; row F), and when added to urge-to-smoke never resulted in incremental statistical significance.
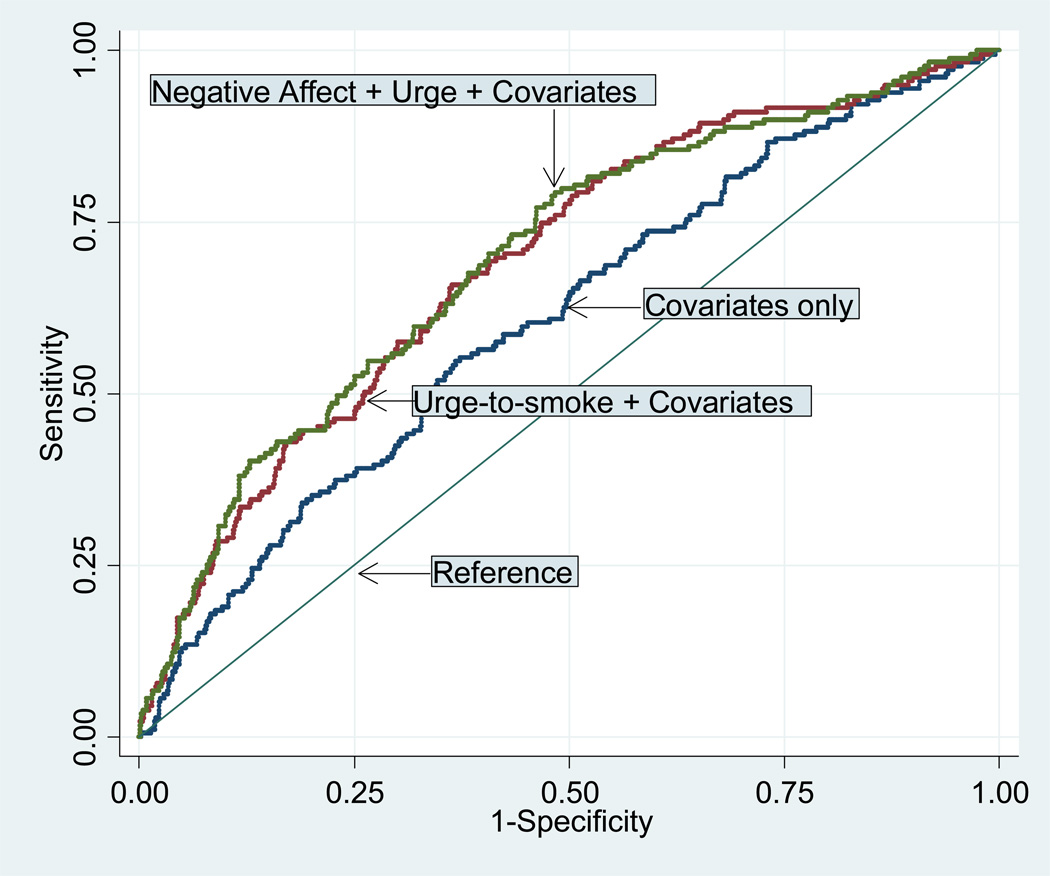
Figure 2 presents the ROC curves for predicting continuous abstinence at 6 months using (1) covariates only, (2) covariates and urge-to-smoke trajectory parameters, and (3) covariates, urge-to-smoke and negative affect trajectory parameters. Increasing distance of the ROC curve from the reference line indicates improvement in correct classification of the abstinence outcome over that expected by chance alone. We note that the curve using only covariates and the urge-to-smoke trajectory parameters is almost identical to the uppermost curve resulting from the addition of negative affect to the classification equation. A similar near overlap would occur for other abstinence outcomes.
Figure 2. Receiver operator characteristic curves for continuous abstinence at 6 months.
Covariates were gender, African American race, indicator for type of pharmaceutical used, and baseline cigarettes per day, FTND, BMI, and CESD depression score. In addition, the ROC for urge-to-smoke included the baseline urge-to-smoke score, and the ROC for combined negative affect and urge-to-smoke included baseline scores for both urge-to-smoke and negative affect.
DISCUSSION
On average, the severity of urge-to-smoke was higher on each of the seven days of assessment than were the negative affect, physical symptom, and hunger ratings, consistent with findings from another study [6].
In addition to that seen previously for urge-to-smoke [4,6] and negative affect [6], novel findings from the present study include the presence of substantial between-person variability in the trajectory features for physical symptoms and hunger. Average trajectory slopes were negative (declining over time) for urge-to-smoke, negative affect, and physical symptoms but positive (increasing over time) for hunger. There appears to be differences across the symptom subtypes with respect to the nature of curvature with ("u" shaped) convexity being apparent for negative affect and physical symptoms and ("n" shaped) concavity present for urge-to-smoke and hunger. Urge-to-smoke volatility is greater than that of the other three symptom subtypes; by comparison, average volatility was an order of magnitude less for physical symptoms. Comparatively high volatility in urge-to-smoke is consistent with the presence of multiple environmental cues that elicit craving as hypothesized previously [1,2,15,16].
Trajectory features were variably associated with relapse risk at each of the follow-up time points. Increasing symptom slope was associated with increased risk for relapse at EOT for urge-to-smoke, negative affect, and physical symptoms while an opposite association was observed for the slope of hunger symptoms (increasing levels of hunger associated with decreased risk for relapse, an association that has been noted previously [7]). None of the symptom slopes was associated with 7-day smoking outcome at 6 months (although urge-to-smoke slope was associated with continuous abstinence at 6 months) suggesting a generalized diminution of effect over time for this trajectory feature.
The magnitude of the association of trajectory features with outcomes (see Table 4) appear to be strongest for urge-to-smoke at both EOT and at 6 months. The associations seen between negative affect features and PPA at EOT were much more impressive than associations predicting 6-month outcomes. Associations between trajectory features and 6-month outcomes were nonexistent for physical symptoms and hunger. Thus, with the exception of urge-to-smoke, withdrawal symptom trajectory features lose their predictive power for longer term outcomes.
As noted in this paper and by others, withdrawal symptoms are multidimensional [5,7,8] and withdrawal symptom dimensions and their trajectory features are significantly intercorrelated [4–8,17–19]. Thus, an examination of the predictive utility of any one symptom subtype must take into account this pre-existing covariance structure. As far as we know, the present paper is the first to utilize a stepwise procedure to determine the predictive utility of 16 symptom trajectory features in conjunction with covariates. The results in Tables 5 and 6 and Figure 2 reveal that the pre-eminent predictive utility with respect to abstinence outcomes lies with the urge-to-smoke trajectory features to the exclusion of almost all other trajectory features including those for negative affect. This finding is consistent with some authors who place an emphasis on the importance of craving in relapse [8,15,20–22] and less supportive of other authors who place an equal or greater emphasis on negative affect as a motivational influence to resume smoking [6,19,23]. The present analysis reveals that adding negative affect trajectory features to urge-to-smoke has only minimal effect on the prediction of the likelihood of relapse in treatment seeking smokers.
A recent multivariate analysis by Piper et al (2011) [6] of real-time withdrawal symptom data in 1,504 adult smokers enrolled in a cessation clinical trial found that pre- to post-cessation increases in both mean craving and negative affect scores each contributed to the prediction of 8-week point prevalence abstinence. We attempted to confirm the Piper et al. results by calculating pre- to post quit change scores of average levels for both the urge-to-smoke and negative affect scales used in the present analysis. In separate multivariate models in our analysis, both urge-to-smoke and negative affect change scores were strongly associated with end-of-treatment abstinence after adjustment for type of pharmacotherapy (placebo vs monotherapy). However, when both change scores were included in the same regression model, the change in urge-to-smoke level remained as significant (p < .001) while that for change in negative affect did not (p = 0.311). We conclude, therefore, that unlike results seen in Piper et al., in our study negative affect does not retain its predictive importance when in the presence of urge-to-smoke.
The present analysis was designed to examine the predictive utility of various symptom subtypes with the goal being the identification of the best set of trajectory features for prediction of short- and long-term smoking cessation outcomes. Remaining unanswered is the possibility that negative affect mediates the relationship between craving and relapse as some have previously suggested [6,19], or that craving is a mediator for negative affect (i.e., negative affect increases craving) as well as craving having an independent effect on relapse.
These results suggest that the further investigation of pharmacogenetic and environmental correlates of withdrawal should utilize the urge-to-smoke trajectory features as the primary phenotypes of interest since the goal of such research is to identify potential drug targets to reduce risk for subsequent relapse. While the identification of specific genetic factors associated with withdrawal in clinical trial participants is in the early stage, recent investigations suggest several candidate genes of high interest including the beta 2 nicotinic cholinergic receptor (CHRNB2) [24], the alpha 5, alpha 3, beta 4 nicotinic cholinergic receptor cluster (CHRNA5-A3-B4) [25], the serotonin transporter (5-HTT)-linked polymorphic region (5-HTTLPR) and dopamine D2 receptor (DRD2) [23] and galanin receptor 1 (GALR1) [26].
Study Limitations
Study limitations include: 1) possible bias due to the exclusion of individuals with 2 or more missing symptom scores out of 7 possible scores (potentially resulting in differential exclusion rates for individuals experiencing early and severe negative affect, urge-to-smoke, or other symptoms); 2) possible bias due to study site differences in the two medication studies; 3) limitation of the follow-up assessment to 7 days (potentially resulting in an underestimation of the effect for symptoms that take longer to influence clinical outcomes than urge-to-smoke); and 4) reliance upon self-reported withdrawal symptoms collected at a point at least 1 day removed from the occurrence of the symptom (possibly inducing recall bias). Dropout rates for treatment groups range from 8.9% to 26.7%. To the extent to which the causes for dropout include negative affect (or other withdrawal symptoms), our analysis will have underestimated the importance of those symptoms. We note that a relatively short observation period does have the advantage of increasing the feasibility of using trajectory information for phenotyping or clinical decision-making, and decreasing the likelihood that observed trajectories are artifacts of relapses.
A previous paper by McCarthy et al. [21] utilized electronic diary methodology to determine negative affect withdrawal symptoms and craving before and 3 weeks after cessation in 70 smokers assigned to active or placebo patch condition. As in the previous study that relied on recall, substantial heterogeneity across quitters was observed for elevation, slope, and variability of withdrawal and craving. This suggests that trajectory features seen in the present study may be robust to the method of assessment, recall or real time collection.
Conclusions
This analysis extends those of Javitz et al [4] on urge-to-smoke trajectories, and those of Piasecki and colleagues [1–3] (based on a measure of total withdrawal symptoms measured over 56 days) to different types of withdrawal symptoms for predicting different measures of abstinence at EOT and 6 months. Physical symptoms and hunger had little predictive ability. Negative affect had modest predictive ability and increased AUC relative to covariates alone only by an average of 0.014, with the largest increment of 0.024 occurring for PPA at EOT. Urge-to-smoke increased AUC relative to using covariates alone by an average of 0.061 across all of the abstinence measures, and by the largest amounts (0.076 and 0.087) for the two continuous abstinence measures. Negative affect did not result in a statistically significant increment in AUC when added to regressions that included both covariates and urge-to-smoke trajectory parameters. Thus it appears that the among the different withdrawal symptoms examined, urge-to-smoke has the largest effect on abstinence, and that other withdrawal symptoms increase the predictive ability by negligible amounts over that available with covariates and urge-to-smoke trajectory parameters.
WHAT THIS RESEARCH ADDS.
This study examined the ability of the trajectory parameters of different groups of withdrawal symptoms (negative affect, physical symptom, hunger, and urge-to-smoke) to predict abstinence in two clinical trials, controlling for demographics and smoking phenotypes. Urge-to-smoke had the largest ability to predict abstinence. Other withdrawal symptoms increased the predictive ability by negligible amounts over that available with urge-to-smoke trajectory parameters.
Acknowledgements
We acknowledge the helpful contributions of TTURC staff members especially Angela Pinto and Susan Ware.
SOURCE OF FUNDING AND DECLARATION OF INTEREST
This research was supported by grants U01 DA020830 from NIDA, NCI, NHGRI, and NIGMS, P50CA143187, and R01CA63562. Nicotine nasal spray (Nicotrol) was provided by Pharmacia and Upjohn, Helsingborg, Sweden. GlaxoSmithKline provided study medication for the bupropion clinical trial, but had no role in the study design, interpretation, or funding. Dr. Swan was an advisor to Pfizer and received an honorarium for a one-day meeting in 2008. Dr. Lerman has been a paid consultant and has conducted research sponsored by Pfizer, Astra Zeneca, and GlaxoSmithKline. Dr. Javitz has conducted research sponsored by Pfizer, SmithKline Beecham, CV Therapeutics, Biogen, Berlex Laboratories, Johnson & Johnson, Ciba-Geigy, Angiotech, Merck & Co., Eli Lilly and the Pharmaceutical Researchers and Manufacturers Association.
Footnotes
CLINICAL REGISTRATION
The clinical trial registration numbers for these clinical trials are NCT00326781 (study of NRT patch versus spray) and NCT00326755 (study of bupropion versus placebo)
REFERENCES
- 1.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003;112(1):3–13. [PubMed] [Google Scholar]
- 2.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112(1):14–27. [PubMed] [Google Scholar]
- 3.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Exp Clin Psychopharmacol. 2003;11(4):276–285. doi: 10.1037/1064-1297.11.4.276. [DOI] [PubMed] [Google Scholar]
- 4.Javitz HS, Swan GE, Lerman C. The dynamics of the urge-to-smoke following smoking cessation via pharmacotherapy. Addiction. 2011 May 11; doi: 10.1111/j.1360-0443.2011.03495.x. [Epub ahead of print] 2011. [DOI] [PubMed] [Google Scholar]
- 5.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol (Berl) 1999;7:354–371. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 6.Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Curr Med Res Opin. 2005;21(5):749–760. doi: 10.1185/030079905X43712. [DOI] [PubMed] [Google Scholar]
- 8.Etter JF, Hughes JR. A comparison of the psychometric properties of three cigarette withdrawal scales. Addiction. 2006;101(3):362–372. doi: 10.1111/j.1360-0443.2005.01289.x. [DOI] [PubMed] [Google Scholar]
- 9.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Lerman C, Jepson C, Wileyto PE, Epstein LH, Rukstalis M, Patterson F, et al. Role of Functional Genetic Variation in the Dopamine D2 Receptor (DRD2) in Response to Bupropion and Nicotine Replacement Therapy for Tobacco Dependence: Results of Two Randomized Clinical Trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- 11.Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84(4):320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 12.Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 13.Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiori MC, et al. Smoking withdrawal dynamics in unaided quitters. J. Abnorm Psychol. 2000;109(1):74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- 14.Javitz HS, Brigham J, Lessov-Schlaggar CN, Krasnow RE, Swan GE. Association of tabacco dependence and quit attempt duration with Rasch-modeled withdrawal sensitivity using retrospective measures. Addiction. 2009;104(6):1027–1035. doi: 10.1111/j.1360-0443.2009.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skinner MD, Aubin HJ. Craving's place in addiction theory: contributions of the major models. Neurosci Biobehav Rev. 2010;34(4):606–623. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson SG, Shiffman SJ. The relevance and treatment of cue-induced cravings in tobacco dependence. J. Subst Abuse Treat. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- 18.Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. In: P. Clayton Rivers and Richard Dienstbier, editor. Nebraska Symposium on Motivation: Vol 34. Alcohol and Addictive Behavior. Lincoln: University of Nebraska Press; 1986. pp. 257–323. [PubMed] [Google Scholar]
- 19.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 20.Shiffman SJ, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, et al. A day at a time, predicting smoking lapse from daily urge. J. Abnorm Psychol. 1997;106(1):104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- 22.Shiffman SJ, Kirchner TR. Cigarette-by-cigarette satisfaction during ad libitum smoking. J. Abnorm Psychol. 2009;118(2):348–359. doi: 10.1037/a0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert DG, Zuo Y, Rabinovich NE, Riise H, Needham R, Huggenvik JI. Neurotransmission-related genetic polymorphisms, negative affectivity traits, and gender predict tobacco abstinence symptoms across 44 days with and without nicotine patch. J Abnorm Psychol. 2009;118(2):322–334. doi: 10.1037/a0015382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti DV, Lee W, Li D, Liu J, Van Den Berg D, Thomas PD, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17(18):2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker TB, Weiss RB, Bolt DM, von Niederhausern A, Fiore MC, Dunn DM, et al. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11(7):785–796. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lori A, Tang Y, O'Malley S, Picciotto MR, Wu R, Conneely KN, et al. The galanin receptor 1 gene associates with tobacco craving in smokers seeking cessation treatment. Neuropsychopharmacology. 2011;36(7):1412–1420. doi: 10.1038/npp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]