Abstract
The thin filaments of differentiated smooth muscle cells are composed of actin and tropomyosin isoforms and numerous ancillary actin-binding proteins that assemble together into distinct thin filament classes. These different filament classes are segregated in smooth muscle cells into structurally and functionally separated contractile and cytoskeletal cellular domains. Typically, thin filaments in smooth muscle cells have been considered to be relatively stable structures like those in striated cells. However, recent efforts have shown that smooth muscle thin filaments indeed are dynamic and that remodeling of the actin cytoskeleton, in particular, regulates smooth muscle function. Thus, the cytoskeleton of differentiated smooth muscle cells appears to function midway between that of less dynamic striated muscle cells and that of very plastic proliferative cells such as fibroblasts. Michael and Kate Bárány keenly followed and participated in some of these studies, consistent with their broad interest in actin function and smooth muscle mechanisms. As a way of honoring the memory of these two pioneer members of the muscle research community, we review data on distribution and remodeling of thin filaments in smooth muscle cells, one of the many research topics that intrigued them.
Keywords: Actin, cytoskeleton, contraction, smooth muscle, tropomyosin
Introduction
Michael Bárány invited both of us to write articles for the monograph on the “Biochemistry of Smooth Muscle Contraction” that he edited and published in 1996. The following is a sequel to these articles and dedicated to the memory of Michael and his wife and colleague, Kate Bárány. But first some personal reflections:
As a first year graduate student, one of us (WL) attended the New York Heart Association Symposium entitled “The Contractile Process” in December 1966, where Michael was set to present and discuss what many consider his most seminal series of experiments that showed that the speed of contraction is related to the ATPase activity of the myosin (ATPase Activity of Myosin Correlated with Speed of Muscle Shortening. J. Gen. Physiol. 50, 197–216 (1967)). The symposium was held in the ballroom at the Biltmore Hotel in Manhattan (an elegant old hotel later demolished in 1981). Midway through the conference, a fire broke out in the kitchen and the exhaust vents filled the ballroom with smoke. I and the rest of the audience exited the scene while John Pringle continued to deliver his talk. “After the smoke cleared,” the program of the symposium was adjusted and the results of Michael’s work had to wait until the publication of the symposium’s proceedings in 1967 (an issue of the Journal of General Physiology still worth reading). Needless to say, the symposium left a lasting memory. Listening to the oral presentations and later reading the symposium papers were among the reasons that I decided to become a muscle researcher. In fact, the Bárány paper is quoted in my successful application for a Muscular Dystrophy Association Postdoctoral Fellowship that gave me the opportunity to enter the field. Though under less spectacular circumstances, KGM’s first encounter with Michael was equally memorable. Michael had invited KGM, as a beginning independent scientist, to speak in a FASEB symposium, just one example of his expression of strong support of women as scientists. His introduction was delivered in his own inimitable manner and parts of it remain a puzzle to this day as it included the comment that “women scientists are doubly important since, without them, there would be no men scientists.” Michael and Kate were always certain to visit and evaluate our poster presentations at conferences, and then discuss our career progress and offer encouragement; they invariably were among the first to send us postcards requesting reprints of our new publications, an ancient practice that now seems quaint.
Our respective articles in the 1996 Bárány monograph focused on related themes, suggesting that the differentiated, contractile, smooth muscle cell is not simply an assembly of contractile filaments surrounded by an excitable membrane, but rather is subdivided into different functional domains composed of different components responding to the effects of translocating signaling proteins (Lehman et al. 1996; Khalil and Morgan 1996). We have since learned that the functional cytoarchitecture of smooth muscle cells is in fact subdivided into domains, and that the actin cytoskeleton, in particular, is capable of remodeling as part of its normal response to physiological changes.
In contrast to skeletal and cardiac muscle fibers, smooth muscle cells are capable of undergoing dramatic shape changes during contraction. When maximally activated in vivo, smooth muscles can contract to roughly half their initial length, whereas striated muscles normally shorten by no more than 20% of their resting length (Widmaier et al. 2011). The cortical cytoskeletal elements now known to be present in smooth muscle cells must therefore accommodate these changes. Any such remodeling of the cortical cytoskeleton may additionally facilitate maintenance of tension by tonic smooth muscles; however, the relationship between cytoskeletal dynamics and contractile output has been a longstanding puzzle in this field (Gunst and Zhang 2008). While it is well-established that dynamic remodeling of the actin cytoskeleton is controlled by various actin-binding proteins in non-muscle cells and in migratory “dedifferentiated” smooth muscle cells, less emphasis has been devoted to characterizing the cytoskeleton of differentiated smooth muscle cells. Nevertheless, it is likely that actin-binding proteins control transient reorganization of the differentiated smooth muscle cell cortical cytoskeleton as well (cf. Gunst and Zhang 2008; Kim et al. 2008a,b).
Smooth Muscle Contractile and Cytoskeletal Actin Can Be Distinguished
At first glance, actin filaments in smooth muscle appear to have a disordered distribution. Rather than displaying the regular, almost crystalline, pattern of interdigitating thick and thin filaments found in skeletal muscle, filaments in smooth muscle cells are not in register and have the appearance of being interspersed throughout the cell. However, it has been suggested that there is a detectable alignment of “mini-sarcomeres” along the long axis of the spindle shaped cells (Kargacin et al. 1989; Herrera et al. 2005). Thin filaments insert, directly or indirectly, into α-actinin-rich “dense-bodies” (the analog of skeletal muscle Z-lines) scattered about the cytoplasm, and into dense “adhesion plaques” at the cell membrane (the smooth muscle equivalent of focal adhesions). However, careful immunocytological analysis indicates a remarkable degree of actin cytoskeletal organization in differentiated vascular, visceral and airway smooth muscle cells.
Four actin isoforms are known to be present in smooth muscle. The smooth muscle alpha and smooth muscle gamma isoforms are separate gene products and are generally referred to as “contractile” isoforms, with alpha smooth muscle actin predominating in vascular smooth muscle and gamma smooth muscle actin predominating in gastrointestinal smooth muscle (Fatigati and Murphy 1984; Kim et al. 2008b). The two “cytoplasmic”, i.e. cytoskeletal “non-muscle” actin isoforms present in all smooth muscles are the beta and gamma isoforms, also separate gene products (Fatigati and Murphy 1984; Drew and Murphy 1991; Kim et al. 2008b).
Although initial studies with early generation antibodies suggested that actin isoforms are not segregated within smooth muscle cells (Drew and Murphy, 1991), several more recent studies have now shown that actin isoforms do segregate into filament classes with distinct localization patterns (Fürst et al. 1986; Small et al. 1986; North et al. 1994a,b; Mabuchi et al. 1996; Parker et al. 1994, 1998). Care must be taken, however, to associate published findings with the type of smooth muscle used in each study.
Avian gizzard has been a commonly used source of smooth muscle tissue, particularly for biochemical studies because of its large mass. Two clearly different types of gizzard actin filament classes are defined by differential immunoprecipitation (Lehman et al. 1987; Lehman, 1991), one that is more associated with myosin and caldesmon and another with “cytoskeletal” domains devoid of thick filaments (North et al. 1944a, b; Lehman et al. 1987; Lehman 1991). The initial model first proposed by North et al. (1994a,b) for the arrangement of cytoskeletal versus contractile actin was based on immunofluorescence colocalizations and immuno-electron microscopy of ultrathin sections of gizzard smooth muscle tissue. While the essence of model, viz. segregation of actin filament domains, is consistent with current findings on mammalian vascular smooth muscle (see below), it is based on gizzard smooth muscle morphology and may not be strictly comparable to other smooth muscle cell types. In addition, the North model was compromised by lack of actin isoform-specific antibodies available at the time (other than an anti- -actin antibody) and by uncertainty about the contractile state of the muscle tissue examined. Moreover, the model envisioned that the contractile filaments directly insert into adhesion plaques and that the non-muscle actin and intermediate filament cytoskeleton form a static scaffold to maintain a constant cell structure. As we will discuss, we now realize that smooth muscle cytoskeletal filaments remodel dynamically and it is possible that contractile filaments may do so as well.
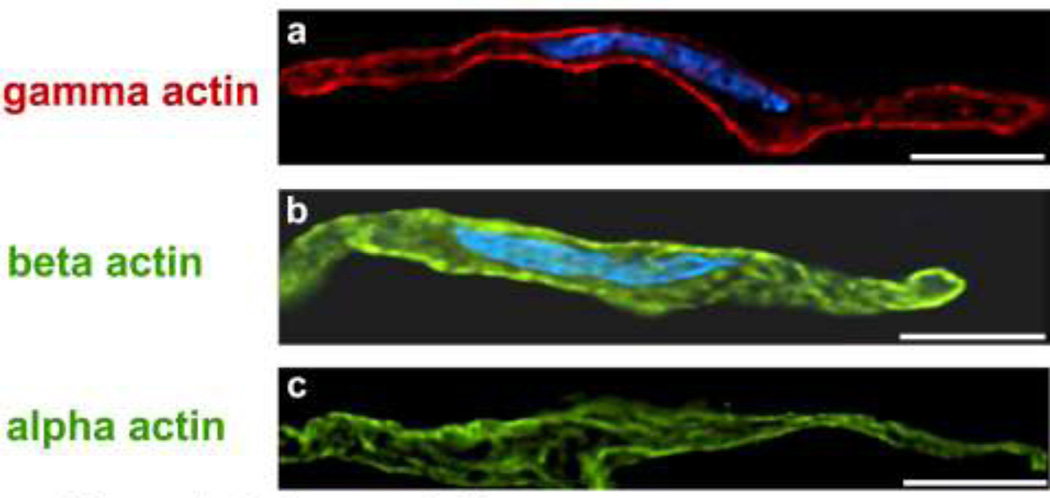
Imaging of non-muscle β-actin in mammalian vascular smooth muscle has shown it to co-distribute with alpha-actinin, i.e. in dense bodies at the ends of contractile filaments (Parker et al. 1998). Here β-actin may form cytoplasmic ribs linking adjacent dense bodies to each other (North et al. 1994b). Beta-actin also localizes in punctae at the cell cortex (Fig. 1b) in mammalian vascular smooth muscle (Parker et al. 1998) where it may again bridge adhesion plaque proteins to other cytoskeletal structures including the intermediate filament lattice and the dense bodies (North et al. 1994a). Recently, cytoplasmic gamma actin has also been visualized in contractile vascular smooth muscle cells where it is concentrated in the cortex of the cell (Fig. 1a), in contrast to the alpha smooth muscle actin in the contractile filaments that reptate along the length of the cell (Fig. 1c). Hence, if both contractile and cytoskeletal actin insert into dense-bodies and adhesion plaques, then both dense bodies and adhesion plaques could act as dual-purpose anchorage sites for the filament arrays. Alternatively, contractile filament-generated forces might trigger changes in cytoskeletal structure during cellular shape alterations.
Figure 1.
Deconvolution microscopy of freshly dissociated aortic smooth muscle cells, center sections to illustrate differences in actin isoform distribution. A. Gamma cytoplasmic actin (red), primarily located in cell cortex (mouse monoclonal antibody, Chaponnier lab). B. Nucleus is co-stained with DAPI (blue). Beta actin (green) located in punctae at cell cortex and in cell interior (monoclonal mouse antibody, Sigma (Sigma-Aldrich Corp., St. Louis, MO). Nucleus again is co-stained with DAPI (blue).C. Alpha smooth muscle actin (green) in filamentous structures that twist through the length of the cell (mouse monoclonal antibody, Sigma). Calibration bar, 10 microns, all panels. Reproduced with permission, from Gallant et al. (2011).
The smooth muscle cortical cytoskeleton is a dynamic cellular feature
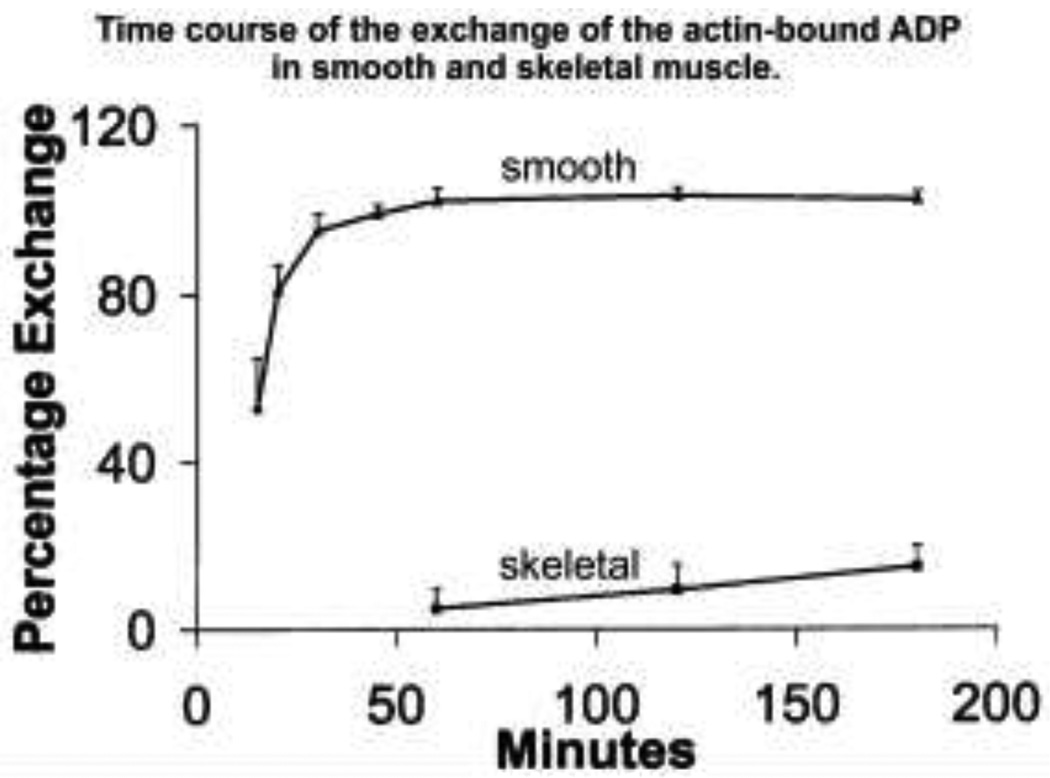
It has become increasingly apparent that the smooth cortical actin cytoskeleton is a dynamic structure which, in turn, is essential for regulation of vital cellular functions as diverse as force transduction, cell shape changes, stiffness and adhesion. The gamma cytoplasmic actin in vascular muscle, primarily localized to the cortex, is reported to be the population of actin most subject to reversible G-actin to Factin transitions in response to vasoconstrictors, reflecting changes in polymerization/depolymerization (Kim et al. 2008b, 2010b). Additionally, agonist-activated actin polymerization both in vascular and airway smooth muscle has been shown to correlate with an increase in the magnitude of contractile force generated (Mehta and Gunst 1999; Jones et al. 1999; Gunst and Zhang 2008; Kim et al. 2008a,b, 2010a,b; of note, parallel work by the Báránys showed that the exchange of actin-bound nucleotide in smooth muscle is considerably more rapid than in skeletal muscle (Fig. 2 from Bárány et al. 2001)). In vascular smooth muscle, agonist-induced changes in F/G actin ratios were first demonstrated both at the tissue level with differential centrifugation and at the single cell level with a fluorescent phalloidin/DNase I imaging method on freshly dissociated contractile cells (Kim et al. 2008b). Relatively little is known regarding the detailed molecular pathways by which polymerization/depolymerization is regulated in smooth muscle, but recent studies have shown that both contractility and actin remodeling in vascular smooth muscle requires the actin elongation factor VASP (Kim et al.. 2010b) and in airway smooth muscle both pathways require the actin branching factor N-WASP (Zhang et al. 2005). Whether both factors are required for normal actin dynamics in different smooth muscle types remains to be proven.
Figure 2.
Time course of the exchange of the actin-bound ADP in smooth and skeletal muscle. The comparatively high exchange of bound nucleotide on smooth muscle actin indicates relatively dynamic G/F-actin depolymerization/polymerization. Reproduced with permission, from Bárány et al. (2001).
Possible associations of the smooth muscle cortical cytoskeleton with plasma membrane complexes
Cortical actin filament association at the plasma membrane can be divided into subdomains in smooth muscle cells. Typical vinculin-rich focal adhesions (also called adhesion or dense plaques) alternate with membrane invaginations, or caveolae, rich in dystrophin and dystroglycans (North et al. 1993), components normally considered in a skeletal muscle context, as well as alternating with ion channels and pumps (Moore et al. 1993, 2004). Of particular note, the local regions surrounding both the adhesion plaque and the caveolae are replete with signaling scaffolds, kinases and phosphatases and thus may respond to extracellular stimuli associated with cortical remodeling (Li et al. 2007, 2009; Kim et al. 2008a, b, 2010a).
Much attention has been focused on the role of dystrophin in skeletal muscle and the effects of dystrophin-deficiencies that cause muscular dystrophy. In contrast, less information is available on the distinct dystrophin isoform that is present in significant amounts in smooth muscle (Straub et al. 1999; Halayko and Stelmack, 2005; Hoffman et al. 1988), even though it is known that muscular dystrophies are associated with digestive and vascular disorders (Cohn and Campbell, 2000). The superstructure of this part of the cortical actin and intermediate filament cytoskeleton in smooth muscle, including the corresponding connections to the dystrophin/dystroglycan complex, dense plaques and caveolae may be analogous to the “costamere” assembly described in striated muscle (Ervasti 2003). Costameres in striated muscle are thought to be a functional assembly that connects sarcomeres to the cell membrane. Disruption of a smooth muscle costameric structure in muscular dystrophy may lead to significant but poorly characterized dysfunction.
Whether smooth muscle “costameres” are mechanically coupled to dense plaque complexes and are part of a larger superstructure is unknown. It is generally acknowledged that smooth muscle cells, lacking the tendons of skeletal muscle cells, transmit contractile force through the dense plaques to the extracellular matrix, which, in turn acts as an “intra-muscular tendon.” One possibility is that in smooth muscle thin filament-linked dystrophin at caveolae serves as a transient structural shock-absorber, as in skeletal muscle, while other thin filament associated complexes at focal adhesions bear, transmit and/or sense contractile force.
Actin-binding proteins in smooth muscle
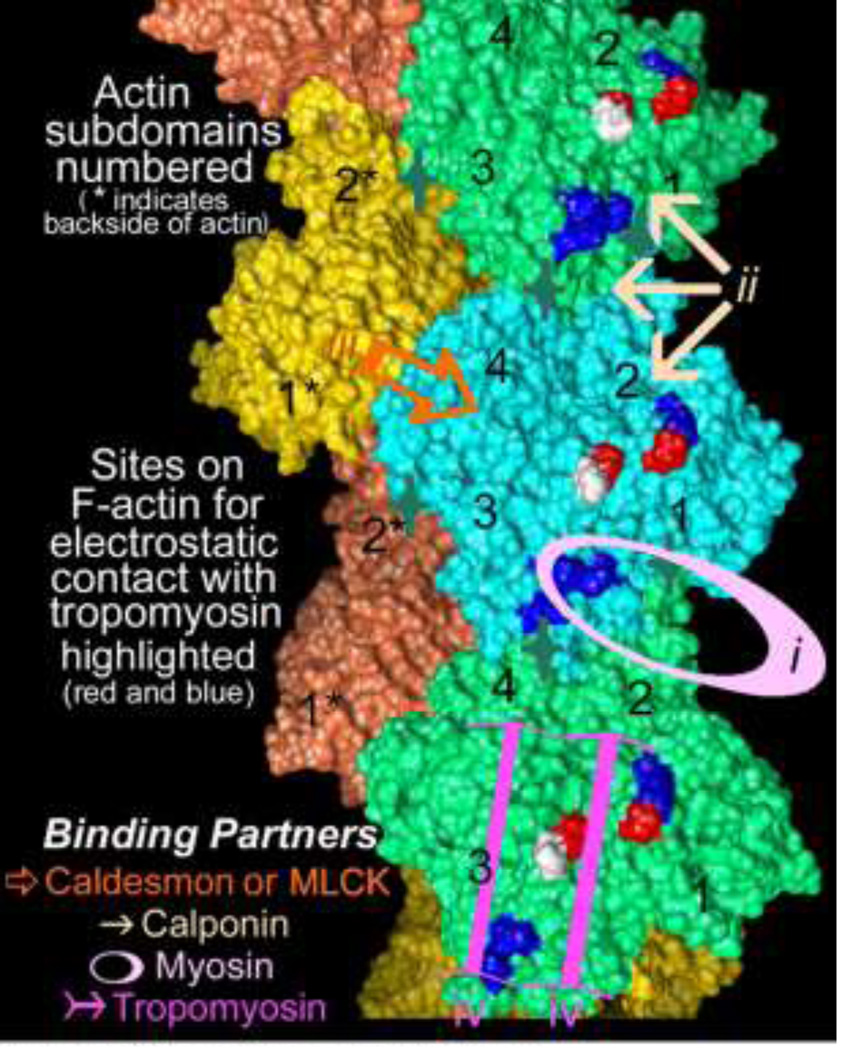
A large number of actin-binding proteins associate with smooth muscle actin filaments. As in non-muscle cell systems, these proteins no doubt govern filament polymerization/depolymerization, filament mechanical properties, crosslinking into bundles and/or formation of networks of supermolecular structures and, as is well known, interaction with myosin motors. A partial list of such smooth muscle actin-associated proteins includes -actinin, Arp2/3, caldesmon, calponin, cofilin, dystrophin, filamin, fascin, fimbrin, leiomodin, myosin, myosin light-chain kinase, myosin light-chain phosphatase (possibly via p116Rip or Par4), tropomyosin, VASP, and WASP. A “parking problem” on filaments might therefore result in restricting binding associations on actin, particularly given the limited binding surface on actin. This consideration has been partially addressed by EM reconstruction, mapping the F-actin surface associated with smooth muscle tropomyosin (Hodgkinson et al. 1997a, b; Lehman et al. 2000, 2009), the actin-binding domain of smooth muscle myosin light chain kinase (Hatch et al. 2001), fulllength and expressed caldesmon and its actin-binding fragments (Vibert et al. 1993; Hodgkinson et al. 1997b; Lehman et al. 1997; Foster et al. 2004), full-length calponin (Hodgkinson et al. 1997b; Galkin et al. 2006), and engineered constructs representing paired calponin-homology (CH) domains derived from diverse actin-binding partners such as α-actinin (McGough et al. 1994, 2003), dystrophin (Sutherland-Smith et al. 2003), fimbrin (Hanein et al. 1997, 1998; Galkin et al. 2008) and plectin (Galkin et al. 2008). These and other studies suggest that many actin-binding proteins bind to consensus “hot-spots” on actin (Fig. 3). In certain cases, they would be expected to compete with each other for binding; for example, competition between caldesmon and calponin correlates well with results of straightforward co-sedimentation assays of the two on F-actin (Makuch et al. 1991). In addition, the ubiquitous presence of one or another tropomyosin isoform in animal cells and its extensive and supple interactions on the surface of thin filaments make it a good candidate to regulate protein binding and hence act as the parking attendant on actin filaments in smooth muscle and other cells.
Figure 3.
The topology of the F-actin filament (Oda et al. 2009) and the regions of the actin surface targeted by major smooth muscle actin binding proteins including (i.) myosin, indicated by pink oval, (ii) calponin, indicated by three tan arrows, (iii) caldesmon, indicated gold open arrow, iv. tropomyosin, indicated by magenta arrows as well as by red and blue charged residues on actin; actin subdomains numbered. Sites of actin monomer-monomer interaction (Dominguez and Holmes 2011), acted on by capping, severing and sequestering proteins, are indicated by dark-green four-pointed stars.
Tropomyosin is the thin filament’s “parking attendant”
Tropomyosin is widely distributed throughout the animal kingdom and is associated with actin in virtually all cells. Elongated tropomyosin molecules bind to each other in an end-to-end fashion to form an axially continuous strand with each tropomyosin spanning seven actin molecules (~38 nm) (Brown and Cohen 2004). On average, tropomyosin has an inherent shape that almost perfectly matches the contours of the F-actin filament surface (Li et al. 2010). Tropomyosin in isolation (and on F-actin) has a very high persistence length (~10 lengths of the molecule) and therefore is quite stiff (ibid). As different tropomyosin isoforms are capable of localizing on the F-actin surface specifically to different azimuthal positions, the notion of positional specificity of any particular tropomyosin isoform gating access of actin-binding proteins actin has gained traction (Lehman et al. 2000, 2009; Gunning et al. 2008; Holmes and Lehman 2008). In thoroughly characterized striated muscles, tropomyosin alternates between discrete azimuthal positions on actin under the control of troponin and Ca2+. Depending on its position, tropomyosin then profoundly affects myosin binding, actomyosin ATPase, and contractile function (Poole et al. 2006).
Whereas smooth muscles are primarily regulated by Ca2+-calmodulin–induced activation of myosinphosphorylation and are devoid of troponin (Stull et al. 1991; Somlyo and Somlyo 2003), their actin filaments contain a full complement of tropomyosin (Marston and Lehman 1985). To date, no smooth muscle proteins, analogous to striated muscle troponin, have been shown to function in a strict Ca2+-dependent manner, reversibly binding Ca2+ like troponin and thereby constraining and releasing tropomyosin from a “blocked” configuration on actin. Yet, full activation of actin-myosin interaction in smooth muscles, as in all other muscles, requires a myosin-induced movement of tropomyosin to a completely open state of the thin filament (Lehman et al. 2000; Bacchiocchi et al. 2002, 2004). The latter movement, i.e. myosin facilitating its own binding by moving tropomyosin, thus is cooperative and results in an “explosive” switching-on of muscle during activation. Hence, despite the absence of troponin, tropomyosin in smooth muscles will affect both enzymatic function and contractile force production (Lehman et al. 2000). Tropomyosin not only saturates smooth muscle contractile thin filaments but is also abundant on cytoskeletal filaments as well (Lehman et al. 1987). Here again tropomyosin is likely to influence the binding of cytoskeletal actin binding proteins, and such proteins may reciprocally affect the binding, mechanical, and enzymatic effects of tropomyosin. In fact, caldesmon and calponin alter tropomyosin position on actin and thereby modulate the availability of other open binding sites on F-actin (Vibert et al. 1993; Hodgkinson et al. 1997a,b; Lehman et al. 1997). Whether or not Ca2+-independent, ERK-mediated caldesmon-phosphorylation and subsequent dis-inhibition of contractile activity as in myometrial smooth muscle affects thin filament activation of myosin by way of further modulating tropomyosin on actin is not known.
Tropomyosin distribution and gatekeeping in smooth muscle cells
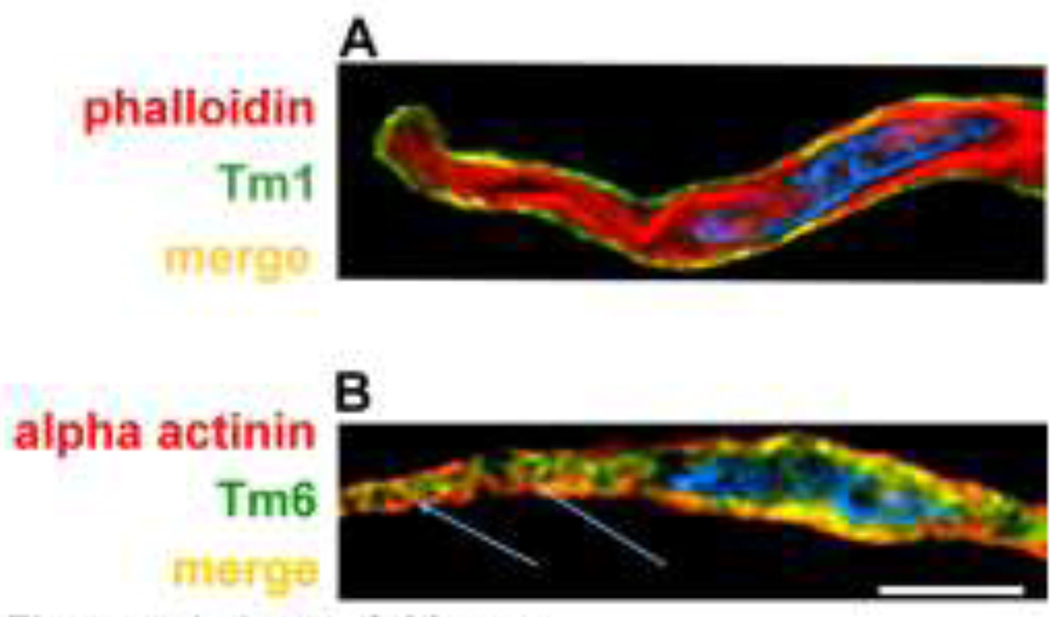
A growing body of literature indicates that, like actin, the various tropomyosin isoforms segregate into different functional domains in non-muscle cells (Gunning et al. 2008), and by analogy the same blueprint is expected for smooth muscle cells. Given that tropomyosin species are known to occupy different azimuthal positions on actin, they therefore will gate association of actin-binding proteins in a pattern that also will be cell-domain specific. Most of our knowledge of smooth muscle tropomyosin function has come from protein chemistry of chicken gizzard muscle. This work indicates that gizzard tropomyosin primarily consists of heterodimers of -smooth muscle tropomyosin (also called Tm6) and -smooth muscle tropomyosin (Tm1), with the two chains present on contractile thin filaments in equimolar amounts and possibly in similar ratio on cytoskeletal filaments as well (Jancsó and Graceffa 1991). However, in marked contrast, systematic screening of mammalian aorta smooth muscle with panels of antibodies has demonstrated the presence of at least 5 isoforms present at the protein level in varying amounts. In fact, in aorta muscle none of the isoforms occur in quantities close to equimolar amounts expected of a heterodimeric tropomyosin. The most abundant isoform is Tm6, present in 5 to 10 times molar excess over is Tm1; in turn, these two isoforms each occur in far greater amount than “non-muscle” Tm5NM1, Tm2 and Tm4 (Gallant et al. 2011). Moreover, Tm6 and Tm1 are differentially localized in smooth muscle cells, with Tm6 present in the contractile filament domain and Tm1 largely in the cell cortex. Figure 4a shows a freshly dissociated differentiated aortic cell colabeled to identify Tm1 (green), primarily restricted to the cortex of the cell, in contrast to the bulk of contractile filaments labeled in red with phalloidin. In contrast, in Figure 4b, Tm6 (green) is localized along the contractile filaments which are extending toward the (red) alpha-actinin-containing adhesion plaques and dense bodies. Immunoprecipitation studies confirm the association of cortical - non-muscle actin with Tm1 and its relative lack of association with Tm6 (Gallant et al. 2011).
Figure 4.
Deconvolution microscopy of freshly dissociated aortic smooth muscle cells, center sections to illustrate differences in distribution of alpha smooth muscle and beta “non-muscle” tropomyosin. A. Merged view of cell with co-labeling of Tm1 (monoclonal antibody, from lab of J.J. Lin, green) and actin (phalloidin, red) to identify the bulk of the contractile filaments. Nucleus is stained with DAPI (blue). Overlapping structures are yellow. Reproduced with permission, from Gallant et al. (2011). B. Co-labeling of Tm6 (alpha2a sheep polyclonal, Millipore (EMD-Millipore USA, Billerica, MA), green) and alpha-actinin (mouse monoclonal, Sigma, red) to identify dense bodies and adhesion plaques. Calibration bar, 10 microns, applies to both panels.
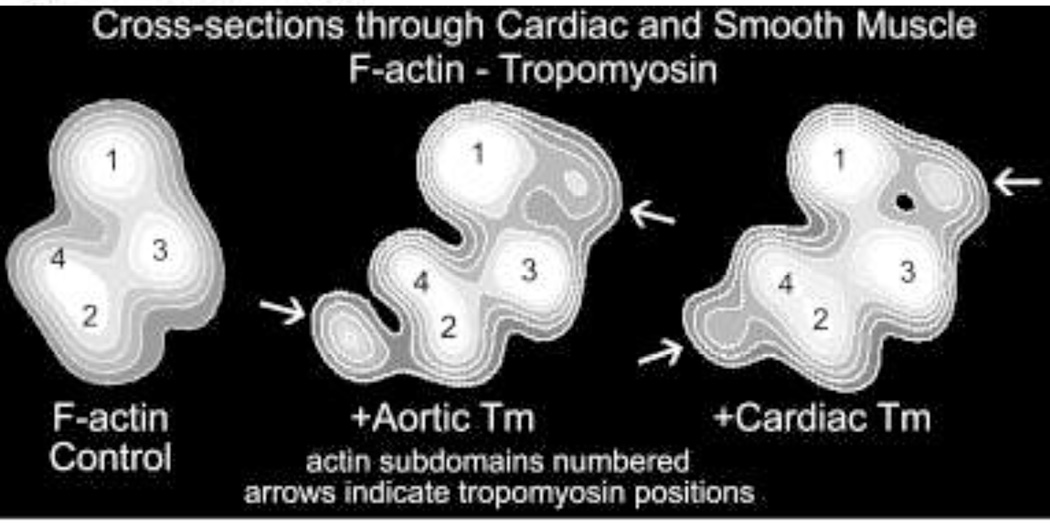
We now also know that both aortic and gizzard smooth muscle bulk tropomyosin(s) localize onto different positions on the F-actin filament from the ones taken by cardiac tropomyosin on F-actin (Lehman et al. 2000, 2009) (Fig. 5). Thus, at this level of organization too, the smooth muscle tropomyosin isoform would be expected to have different gatekeeping function than its striated muscle counterpart. Moreover, smooth muscle tropomyosin attaches to F-actin more tightly than does cardiac tropomyosin, possibly to exert greater gating activity (Sousa et al. 2010). Complete elucidation of the impact of tropomyosin on smooth muscle contractile and cytoskeletal function promises to be very informative.
Figure 5.
Comparison of cardiac and smooth muscle tropomyosin equilibrium binding positions on actin. Cross-sections of reconstructions of F-actin combined with either aorta smooth muscle or with cardiac muscle tropomyosin as indicated. Note the closer association of the smooth muscle tropomyosin density with the actin outer domain (i.e. subdomains 1 and 2), pointed out by white arrows. Actin subdomains numbered. Reproduced with permission, from Lehman et al. (2009).
Further support for a gatekeeper role of tropomyosin comes from in vitro and in vivo experiments, which show that tropomyosin in an isoform specific manner does restrict the actin-binding of many actin-binding proteins in non-muscle cells (Ishikawa et al. 1989, 1998; Blanchoin et al. 2001; Kuhn and Bamburg 2008; Skau et al. 2011). For example tropomyosin blocks the binding of a diverse set of actin binding proteins including fascin, fimbrin, cofilin and Arp2/3, which, in one or another case, must influence actin filament bundling, severing and branching. Thus, if the smooth muscle tropomyosin isoforms are each localized to separate cellular compartments as in non-muscle cells, they then would be expected to specify and control local smooth muscle cell architecture and function. Tropomyosin acting as a gatekeeper may, for example, stabilize actin against the action of proteins like N-WASP/ARP/2/3 or cofillin and hence prevent remodeling of such filaments. In contrast, two actin bundling proteins, plastin (also called fimbrin) and fascin have been shown to compete with tropomyosin for actin binding in other systems (Ishikawa et al. 1998; Skau et al. 2011). Such action in distinct domains in smooth muscle cells could create tropomyosin-bare zones on cortical actin filaments, explaining their dynamic nature. Thus, in the more dynamic cortical cytoskeleton domain, there may be a population of actin filaments bare of certain tropomyosin isoforms but perhaps containing fascin and/or fimbrin as well as calponin. Of particular note, fascin is best known for its association with podosomes, organelles that are uncharacteristic of differentiated smooth muscle cells. However, increased expression of fascin is a hallmark of vascular smooth muscle pathologies, including increased aortic stiffness, smooth muscle cell invasiveness and atherosclerosis (Durier et al. 2003).
Is non-muscle myosin II is necessary for normal smooth muscle cell contractility, cortical tension and plasticity?
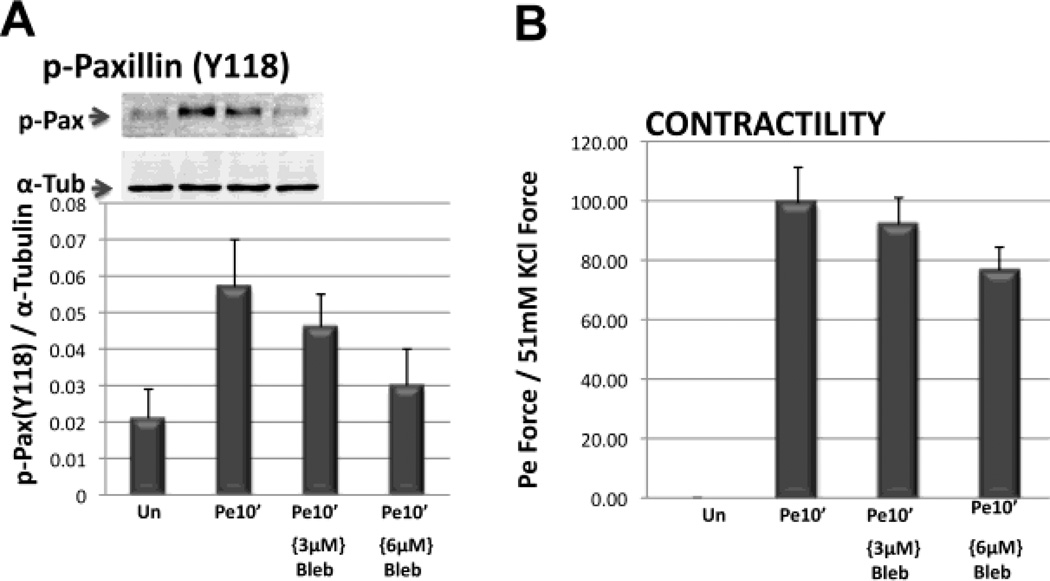
It has recently become clear that in non-muscle cells “non-muscle” myosin-II (NMII) activation is necessary for the growth and maturation of new adhesion plaques, although the mechanisms are not well defined (Kuo et al. 2011). As mentioned above, adhesion plaques are linked to the actin cytoskeleton and also are linked to the extracellular matrix via integrins. Although a role for NMII has been suggested in vascular smooth muscle (Yuen et al. 2009), the precise mechanism involved is unknown. In contrast, vascular smooth muscle contractile filaments are thought to be largely NMII-free but contain mainly the smooth muscle isoform of “muscle” myosin II (SMII). Recently, we have obtained data (Fig. 6) consistent with the concept that adhesion plaques “mature” or remodel in differentiated smooth muscle cells in response to phenylephrine-based alpha-adrenergic induced force development. As NMII function is very sensitive to the myosin inhibitor blebbistatin (IC50 2–5 M) whereas SMII is quite insensitive (IC50 80 M) (Limouze et al. 2004), we tested the effect of blebbistatin on both aorta contractility and phenylephrine-induced tyrosine-phosphorylation of the adhesion plaque protein paxillin. We found that 3 to 6 M blebbistatin inhibits both parameters (Fig. 6) and actually brought paxillin Y118 phosphorylation, a marker of adhesion plaque remodeling, down to unstimulated levels. These results point strongly to a role for non-muscle myosin in the regulation of cortical adhesion plaque connections and their role in facilitating contractile filament-induced force transmission.
Figure 6.
Blebbistatin inhibits vascular smooth muscle adhesion plaque phosphorylation and contractility. A. Densitometry of immunoblots of quick-frozen aortic homogenates. Top panel illustrates a typical blot for phospho-paxillin at residue Y118 for one experiment. Lower panel shows average p-Paxillin Y118 densitometry from 3 experiments, each normalized to tubulin densitometry from the same sample. B. Parallel measurement of tissues that were stretched to optimal length in the absence or in the presence of phenylephrine (PE), for 10 minutes, with or without the indicated concentrations of blebbistatin and then quick-frozen. PE is an alpha-adrenergic agonist used here to induce an increase in contractile force. Blebbistatin is an inhibitor of NMII.
Perspective
In our contributions to the Bárány monograph on the “Biochemistry of Smooth Muscle Contraction,” we stated that the “understanding of smooth muscle contractility and its regulation has frequently lagged behind that of skeletal and cardiac muscle” but “that a wealth of new information awaits us” (Lehman et al. 1996). We anticipated that by applying advances in digital imaging techniques (Khalil and Morgan, 1996) and the tools of structural biology (Lehman et al. 1996) significant progress would lead to a better understanding of fundamental smooth muscle biology. Some of this progress, particularly as it relates to our own past and current efforts, has been outlined here.
Concluding comments
Michael Bárány began his career in science as a student of the Nobel laureate Albert Szent-Györgyi and his associate Brunó Ferenc Straub, who had first purified actin from muscle (Straub 1942) (and who later became President of Hungary near the end of the 1980s). Many of Michael’s last studies were on the biochemistry of smooth muscle contraction. We hope that our own short communication here on smooth muscle actin filaments brings together some aspects of these subjects forged by Michael and Kate Bárány. We look forward to further developments in the understanding of smooth muscle mechanisms to continue the Bárány legacy.
Acknowledgments
We are grateful for support from NIH grant PO1-HL86655. We thank the Chaponnier (Université de Genève), Gunning (University of New South Wales) and Lin (University of Iowa) laboratories for providing antibodies.
Contributor Information
William Lehman, Department of Physiology and Biophysics, Boston University School of Medicine, 72 East Concord Street, Boston, MA 02118 USA, wlehman@bu.edu.
Kathleen G. Morgan, Health Sciences Department, Boston University Sargent College, 635 Commonwealth Avenue, Boston, MA 02215 USA, kmorgan@bu.edu
References
- Bacchiocchi C, Graceffa P, Lehrer SS. Myosin-induced movement of alphaalpha, alphabeta, and betabeta smooth muscle tropomyosin on actin observed by multisite FRET. Biophys J. 2004;86:2295–2307. doi: 10.1016/S0006-3495(04)74287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchiocchi C, Lehrer SS. Ca(2+)-induced movement of tropomyosin in skeletal muscle thin filaments observed by multi-site FRET. Biophys J. 2002;82:1524–1536. doi: 10.1016/S0006-3495(02)75505-7. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. Activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50:197–216. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M, Barron JT, Gu L, Bárány K. Exchange of the actin-bound nucleotide in intact arterial smooth muscle. J Biol Chem. 2001;276:48398–48403. doi: 10.1074/jbc.M106227200. [DOI] [PubMed] [Google Scholar]
- Blanchoin L, Pollard TD, Hitchcock-DeGregori SE. Inhibition of the Arp2/3 complex nucleated actin polymerization and branch formation by tropomyosin. Curr Biol. 2001;11:1300–1304. doi: 10.1016/s0960-9822(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Brown JH, Cohen C. Regulation of muscle contraction by tropomyosin and troponin: how structure illuminates function. Adv Protein Chem. 2005;71:121–159. doi: 10.1016/S0065-3233(04)71004-9. [DOI] [PubMed] [Google Scholar]
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Dominguez R, Holmes KC. Actin structure and function. Ann Rev Biophys. 2011;40:169–186. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew JS, Moos C, Murphy RA. Localization of isoactins in isolated smooth muscle thin filaments by double gold immunolabeling. Am J Physiol. 1991;260:C1332–C1340. doi: 10.1152/ajpcell.1991.260.6.C1332. [DOI] [PubMed] [Google Scholar]
- Durier S, Fassot C, Laurent S, Boutouyrie P, Couetil JP, Fine E, et al. Physiological genomics of human arteries: quantitative relationship between gene expression and arterial stiffness. Circulation. 2003;108:1845–1851. doi: 10.1161/01.CIR.0000091407.86925.7A. [DOI] [PubMed] [Google Scholar]
- Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- Fatigati V, Murphy RA. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem. 1984;259:1438–14388. [PubMed] [Google Scholar]
- Foster DB, Huang R, Hatch V, Craig R, Graceffa P, Lehman W, et al. Modes of caldesmon binding to actin: sites of caldesmon contact and modulation of interactions by phosphorylation. J Biol Chem. 2004;279:53387–53394. doi: 10.1074/jbc.M410109200. [DOI] [PubMed] [Google Scholar]
- Fürst DO, Cross RA, De Mey J, Small JV. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J. 1986;5:51–257. doi: 10.1002/j.1460-2075.1986.tb04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin VE, Orlova A, Cherepanova O, Lebart MC, Egelman EH. High-resolution cryo-EM structure of the F-actin-fimbrin/plastin ABD2 complex. Proc Natl Acad Sci U S A. 2008;105:1494–1498. doi: 10.1073/pnas.0708667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin VE, Orlova A, Fattoum A, Walsh MP, Egelman EH. The CH-domain of calponin does not determine the modes of calponin binding to F-actin. J Mol Biol. 2006;59:478–485. doi: 10.1016/j.jmb.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Gallant C, Appel S, Graceffa P, Leavis PC, Lin JJ, Gunning PW, et al. Tropomyosin variants describe distinct functional subcellular domains in differentiated vascular smooth muscle cells. Am J Physiol Cell Physiol. 2011;300:C1356–C1365. doi: 10.1152/ajpcell.00450.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning PW, O’Neill G, Hardemann EC. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol Rev. 2008;88:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halayko AJ, Stelmack GL. The association of caveolae, actin, and the dystrophin-glycoprotein complex: a role in smooth muscle phenotype and function? Can J Physiol Pharmacol. 2005;83:877–891. doi: 10.1139/y05-107. [DOI] [PubMed] [Google Scholar]
- Hanein D, Matsudaira P, DeRosier DJ. Evidence for a conformational change in actin induced by fimbrin (N375) binding. J Cell Biol. 1997;139:387–396. doi: 10.1083/jcb.139.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanein D, Volkmann N, Goldsmith S, Michon AM, Lehman W, Craig R, et al. An atomic model of fimbrin binding to F-actin and its implications for filament crosslinking and regulation. Nat Struct Biol. 1998;5:787–792. doi: 10.1038/1828. [DOI] [PubMed] [Google Scholar]
- Hatch V, Zhi G, Smith L, Stull JT, Craig R, Lehman W. Myosin light chain kinase binding to a unique site on F-actin revealed by three-dimensional image reconstruction. J Cell Biol. 2001;154:611–617. doi: 10.1083/jcb.200105079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AM, McParland BE, Bienkowska A, Tait R, Paré PD, Seow CY. 'Sarcomeres' of smooth muscle: functional characteristics and ultrastructural evidence. J Cell Sci. 2005;118:2381–2392. doi: 10.1242/jcs.02368. [DOI] [PubMed] [Google Scholar]
- Hodgkinson JL, el Mezgueldi M, Craig R, Vibert P, Marston SB, Lehman W. 3-D image reconstruction of reconstituted smooth muscle thin filaments containing calponin: visualization of interactions between F-actin and calponin. J Mol Biol. 1997a;273:150–159. doi: 10.1006/jmbi.1997.1307. [DOI] [PubMed] [Google Scholar]
- Hodgkinson JL, Marston SB, Craig R, Vibert P, Lehman W. Three-dimensional image reconstruction of reconstituted smooth muscle thin filaments: effects of caldesmon. Biophys J. 1997b;72:2398–2404. doi: 10.1016/S0006-3495(97)78885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Hudecki MS, Rosenberg PA, Pollina CM, Kunkel LM. Cell and fiber-type distribution of dystrophin. Neuron. 1988;1:411–420. doi: 10.1016/0896-6273(88)90191-2. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Lehman W. Gestalt-binding of tropomyosin to actin filaments. J Muscle Res Cell Motility. 2008;29:213–219. doi: 10.1007/s10974-008-9157-6. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Yamashiro S, Matsumura F. Differential modulation of actin-severing activity of gelsolin by multiple isoforms of cultured rat cell tropomyosin. Potentiation of protective ability of tropomyosins by 83-kDa nonmuscle caldesmon. J Biol Chem. 1989;264:7490–7497. [PubMed] [Google Scholar]
- Ishikawa R, Yamashiro S, Kohama K, Matsumura F. Regulation of actin binding and actin bundling activities of fascin by caldesmon coupled with tropomyosin. J Biol Chem. 1998;273:26991–26997. doi: 10.1074/jbc.273.41.26991. [DOI] [PubMed] [Google Scholar]
- Jancsó A, Graceffa P. Smooth muscle tropomyosin coiled-coil dimers. Subunit composition, assembly, and end-to-end interaction. J Biol Chem. 1991;266:5891–5897. [PubMed] [Google Scholar]
- Jones KA, Perkins WJ, Lorenz RR, Prakash YS, Sieck GC, Warner DO. F-actin stabilization increases tension cost during contraction of permeabilized airway smooth muscles in dog. J Physiol. 1999;519:527–538. doi: 10.1111/j.1469-7793.1999.0527m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin GJ, Cooke PH, Abramson SB, Fay FS. Periodic organization of the contractile apparatus in smooth muscle revealed by the motion of dense bodies in single cells. J Cell Biol. 1989;108:1465–1475. doi: 10.1083/jcb.108.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil RA, Morgan KG. Enzyme translocations during smooth muscle activation. In: Bárány M, editor. Biochemistry of smooth muscle contraction. San Diego: Acad. Press; 1996. pp. 307–318. [Google Scholar]
- Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signaling pathways in health and disease. J Cell Mol Med. 2008a;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform-dependent and stimulus-dependent. Am J Physiol. 2008b;295:C768–C778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Graceffa P, Ferron F, Gallant C, Boczkowska M, Dominguez R, et al. Actin polymerization in differentiated vascular smooth muscle cells requires vasodilator-stimulated phosphoprotein. Am J Physiol. 2010b;298:C559–C571. doi: 10.1152/ajpcell.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Leavis PC, Graceffa P, Gallant C, Morgan KG. A new method for direct detection of the sites of actin polymerization in intact cells and its application to differentiated vascular smooth muscle. Am J Physiol. 2010a;299:C988–C993. doi: 10.1152/ajpcell.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;2011:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W. Calponin and the composition of smooth muscle thin filaments. J Muscle Res Cell Motil. 1991;12:221–224. doi: 10.1007/BF01745110. [DOI] [PubMed] [Google Scholar]
- Lehman W, Galiñska-Rakoczy A, Hatch V, Tobacman LS, Craig R. Structural basis for the activation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2009;388:673–681. doi: 10.1016/j.jmb.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W, Craig R, Bárány M. Actin and the structure of smooth muscle thin filaments. In: Bárány M, editor. Biochemistry of smooth muscle contraction. San Diego: Acad. Press; 1996. pp. 47–60. [Google Scholar]
- Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, et al. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- Lehman W, Sheldon A, Madonia W. Diversity in smooth muscle thin filament composition. Biochim Biophys Acta. 1987;914:35–39. doi: 10.1016/0167-4838(87)90158-0. [DOI] [PubMed] [Google Scholar]
- Lehman W, Vibert P, Craig R. Visualization of caldesmon on smooth muscle thin filaments. J Mol Biol. 1997;274:310–317. doi: 10.1006/jmbi.1997.1422. [DOI] [PubMed] [Google Scholar]
- Li XE, Holmes KC, Lehman W, Jung H-S, Fischer S. The shape and flexibility of tropomyosin coiled-coils: Implications for actin filament assembly and regulation. J Mol Biol. 2010;395:327–399. doi: 10.1016/j.jmb.2009.10.060. [DOI] [PubMed] [Google Scholar]
- Li Y, Gallant C, Malek S, Morgan KG. Focal adhesion signaling is required for myometrial ERK activation and contractile phenotype switch before labor. J Cell Biochem. 2007;100:129–140. doi: 10.1002/jcb.21033. [DOI] [PubMed] [Google Scholar]
- Li Y, Reznichenko M, Tribe RM, Hess PE, Taggart M, Kim H, DeGnore JP, Gangopadhyay S, Morgan KG. Stretch activates human myometrium via ERK, caldesmon and focal adhesion signaling. PLoS One. 2009;4:e7489. doi: 10.1371/journal.pone.0007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- Mabuchi K, Li Y, Tao T, Wang CL. Immunocytochemical localization of caldesmon and calponin in chicken gizzard smooth muscle. J Muscle Res Cell Motil. 1996;17:243–260. doi: 10.1007/BF00124246. [DOI] [PubMed] [Google Scholar]
- Makuch R, Birukov K, Shirinsky V, Dabrowska R. Functional interrelationship between calponin and caldesmon. Biochem J. 1991;280:33–38. doi: 10.1042/bj2800033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston SB, Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985;231:517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A, Way M, DeRosier D. Determination of the alpha-actinin-binding site on actin filaments by cryoelectron microscopy and image analysis. J Cell Biol. 1994;126:433–443. doi: 10.1083/jcb.126.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough AM, Staiger CJ, Min JK, Simonetti KD. The gelsolin family of actin regulatory proteins: modular structures, versatile functions. FEBS Lett. 2003;552:75–81. doi: 10.1016/s0014-5793(03)00932-3. [DOI] [PubMed] [Google Scholar]
- Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol. 1999;519:820–840. doi: 10.1111/j.1469-7793.1999.0829n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ED, Etter EF, Philipson KD, Carrington WA, Fogarty KE, Lifshitz LM, Fay FS. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993;365:657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Moore ED, Voigt T, Kobayashi YM, Isenberg G, Fay FS, Gallitelli MF, Franzini-Armstrong C. Organization of Ca2+ release units in excitable smooth muscle of the guinea-pig urinary bladder. Biophys J. 2004;87:1836–1847. doi: 10.1529/biophysj.104.044123. Erratum in: Biophys J 2004 Oct;87(4): 2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North AJ, Galazkiewicz B, Byers TJ, Glenney JR, Jr, Small JV. Complementary distributions of vinculin and dystrophin define two distinct sarcolemma domains in smooth muscle. J Cell Biol. 1993;120:1159–1167. doi: 10.1083/jcb.120.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North AJ, Gimona M, Cross RA, Small JV. Calponin is localised in both the contractile apparatus and the cytoskeleton of smooth muscle cells. J Cell Sci. 1994a;107:437–444. doi: 10.1242/jcs.107.3.437. [DOI] [PubMed] [Google Scholar]
- North AJ, Gimona M, Lando Z, Small JV. Actin isoform compartments in chicken gizzard smooth muscle cells. J Cell Sci. 1994b;107:445–455. doi: 10.1242/jcs.107.3.445. [DOI] [PubMed] [Google Scholar]
- Oda T, Iwasa M, Aihara T, Maéda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457:441–445. doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- Parker CA, Takahashi K, Tang JX, Tao T, Morgan KG. Cytoskeletal targeting of calponin in differentiated, contractile smooth muscle cells of the ferret. J Physiol. 1998;508:187–198. doi: 10.1111/j.1469-7793.1998.187br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CA, Takahashi K, Tao T, Morgan KG. Agonist-induced redistribution of calponin in contractile vascular smooth muscle cells. Am J Physiol. 1994;267:C1262–C1270. doi: 10.1152/ajpcell.1994.267.5.C1262. [DOI] [PubMed] [Google Scholar]
- Poole KJ, Lorenz M, Evans G, Rosenbaum G, Pirani A, Tobacman LS, Lehman W, Holmes KC. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J Struct Biol. 2006;155:273–284. doi: 10.1016/j.jsb.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Skau CT, Kovar DR. Fimbrin and tropomyosin competition regulates endocytosis and cytokinesis kinetics in fission yeast. Curr Biol. 2011;20:1415–1422. doi: 10.1016/j.cub.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ Sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G Proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Sousa D, Cammarato A, Jang K, Graceffa P, Tobacman LS, Li XE, Lehman W. Electron microscopy and persistence length analysis of semi-rigid smooth muscle tropomyosin strands. Biophys. J. 2010;99:1–7. doi: 10.1016/j.bpj.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub FB. Actin. Studies Inst Med Chem Univ Szeged. 1942;2:3–16. [Google Scholar]
- Straub V, Ettinger AJ, Durbeej M, Venzke DP, Cutshall S, Sanes JR, Campbell KP. Epsilon-sarcoglycan replaces alpha-sarcoglycan in smooth muscle to form a unique dystrophin-glycoprotein complex. J Biol Chem. 1999;274:27989–27996. doi: 10.1074/jbc.274.39.27989. [DOI] [PubMed] [Google Scholar]
- Stull JT, Gallagher PJ, Herring BP, Kamm KE. Vascular smooth muscle contractile elements. Cellular regulation. Hypertension. 1991;17:723–732. doi: 10.1161/01.hyp.17.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland-Smith AJ, Moores CA, Norwood FL, Hatch V, Craig R, Kendrick-Jones J, et al. An atomic model for actin binding by the CH domains and spectrin-repeat modules of utrophin and dystrophin. J Mol Biol. 2003;329:15–33. doi: 10.1016/s0022-2836(03)00422-4. [DOI] [PubMed] [Google Scholar]
- Tang DD, Mehta D, Gunst SJ. Mechano-sensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Amer J Physiol. 1999;276:C250–C258. doi: 10.1152/ajpcell.1999.276.1.C250. [DOI] [PubMed] [Google Scholar]
- Tang DD, Wu M, Opazo-Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol. 2002;542:501–513. doi: 10.1113/jphysiol.2002.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P, Craig R, Lehman W. Three-dimensional reconstruction of caldesmon-containing smooth muscle thin filaments. J Cell Biol. 1993;123:313–321. doi: 10.1083/jcb.123.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmaier EP, Raff H, Strang KT. Vander's human physiology: The mechanism of body function. 12th edn. New York: McGraw Hill; 2011. [Google Scholar]
- Yuen SL, Ogut O, Brozovich FV. Nonmuscle myosin is regulated during smooth muscle contraction. Am J Physiol Heart Circ Physiol. 2009;297:H191–H199. doi: 10.1152/ajpheart.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol. 2005;288:C1145–C1160. doi: 10.1152/ajpcell.00387.2004. [DOI] [PubMed] [Google Scholar]