Abstract
Introduction
The aim of the study was to evaluate alterations in Th1 and Th2 cytokines during experimental abdominal aortic aneurysm (AAA) formation.
Methods
AAAs were induced in apolipoprotein E null mice by infusing angiotensin II (Ang II, 1000 ng/kg/min). Aortic homogenates were assessed at 0, 7, 14, and 28 days (N=11/time point) for select Th1 and Th2 cytokines by ELISA. Additional mice had co-administration of anti-IgG (N=20) or anti-IL-5 (N=20) and were assessed at 28 days for AAA. Aortic homogenates were assessed forMMP-2 and MMP-9 expression. Mouse aortic SMC (MASMC) and peritoneal-derived macrophages were treated with IL-5 (0–40 ng/ml), and cell extracts and media (0–48 hours) were assessed for MMP-2 and MMP-9 expression.
Results
Ang II infusion was associated with a 3.4-fold (P<.01) and 3.6-fold (P<.01) increase in IL-5 and IL-10 (respectively), and a 0.6-fold reduction in IL-6, by 7 days. Anti-IL-5, but not anti-IgG, ameliorated Ang II-induced AAA formation. Upregulation of MMP-2 and MMP-9 was observed in aneurysmal aortas, but not in the aortas obtained from mice treated with anti-IL-5. IL-5 stimulation of MASMC increased MMP-2 and MMP-9 mRNA (2.1-fold and 2.7-fold, respectively, P<.01) and protein (1.6-fold and 1.9-fold, respectively, P<.01) by 24 hours. IL-5 stimulation of macrophages did not alter MMP expression.
Conclusions
Ang II induces increased Th2 cytokines IL-5 and IL-10 early in the course of experimental AAA formation, and inhibition of IL-5 prevents AAA formation suggesting an important role.. While IL-5 is capable of upregulating MMP-2 and MMP-9 expression in MASMC, investigations into alternate roles in AAA formation is warranted.
INTRODUCTION
The development of abdominal aortic aneurysms (AAA) involves an alteration in the integrity of the extracellular matrix (ECM) and subsequent aortic wall expansion. Key histologic characteristics of AAAs include chronic medial and adventitial inflammatory infiltrates, elastin fragmentation, medial degeneration, aortic smooth muscle cell apoptosis, and micro-vessel accumulation. This is a dynamic process that involves a stage of initiation of aneurysm development, followed by subsequent continued aortic wall destruction and aneurysmal degeneration. Little is known about the initiating event, but multiple mechanisms are involved in the aortic degeneration. Interdependent processes involved in aneurysm formation include genetic abnormalities, biomechanical wall stress, inflammation, and proteolytic degradation of aortic wall connective tissue1. Understanding the mechanisms that coordinate these multiple pathologies, resulting in aneurysm formation, is important in developing methods to limit this process.
The cytokine milieu temporally related to the initiation and subsequent dilation of AAAs is thought to be a putative regulator of disease progression. Multiple cytokines have been implicated in the process2–4, and it is hypothesized that these cytokines promote inflammation through activation of lymphocytes and macrophages which stimulate matrix metalloproteinase (MMP) production4–6. The upregulated lymphocytes include both helper T cells (Th) and cytotoxic T cells (Tc). Within the helper subset, Th1 cells secrete a characteristic set of cytokines including interleukin (IL-1), IL-2, IL-6, tumor necrosis factor (TNF)-α and interferon (IFN)- γ. Th2 cells tend to secrete another, non-overlapping, set of cytokines including IL-4, IL-5, IL-9, 1L-10, and IL-13. Th1 lymphocytes are involved in cell-mediated inflammatory reactions; they are known to activate cytotoxic and inflammatory functions of macrophages, natural killer cells and neutrophils. Th2 cytokines encourage antibody production, particularly IgA and IgE, and also enhance mast cell and eosinophil proliferation and function. The cytokines produced by Th1 or Th2 cells tend to sustain their own development and antagonize each other7, 8.
The literature pertaining to the role of Th1/Th2 cytokines in AAA pathogenesis, however, is contradictory with alterations in both Th1 and Th2 cytokines having been demonstrated9. Some of this difference is due to the variety of methods used to analyze cytokine alterations. Both circulating and systemic levels have been assessed, as well as organ culture and aneurysm-derived cell cultures. Elevated circulating levels of IL-6, TNF-α, and IFN-γ have been demonstrated in patients with AAA3, and organ cultures of aneurysm tissue demonstrate the similar Th1 profile10. T- and B-cells isolated from aortic aneurysmal tissue produced Th1-type cytokines (TNF-α and IFN-γ)11. It has been further demonstrated that IFN-γ producing Th1 CD4+ T cells are required for AAA development in an animal model12. Others, however, have demonstrated that aortic aneurysm formation is predominantly a Th2-disorder based on the detection of IL-4, IL-5, and IL-10 in the lysates from AAA tissue13. To further support the important interplay of these cytokines, it has been demonstrated that blockade of IFN-γ production and response results in IL-4-driven inflammation leading to aneurysm formation in an aortic transplant model14. In support of this, IFN-γ, CXCL10 (the IFN-γ inducible T-cell chemokine), and STAT1 (a downstream modulator of the IFN-γ receptor) deficient mice developed larger aneurysms with higher rates of rupture suggesting a protective role of this Th1 cytokine15, 16.
This data would suggest a dichotomy in the roles of Th1 and Th2 subsets of cytokines in aneurysm formation. These differences, however, may simply reflect the cytokine profile present at a particular stage of aneurysm degeneration (early versus late). We submit as an overall hypothesis that aneurysm degeneration is marked by a shift in the cytokine profile that occurs temporally during the course of aortic wall destruction and attempts at repair. The aim of the current study is to evaluate the changes in aortic wall cytokine levels during the course of aneurysm formation, and to assess the significance of the altered cytokine milieu.
METHODS
All experiments and procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee (ARC08708). All reagents, unless otherwise stated, were obtained from Sigma (St. Louis, Mo).
Animal Model
Aneurysms were induced in male ApoE null mice, 3–6 months of age, 25–30 g, on normal chow diets, by infusion of angiotensin II (AngII, 1000ng/kg/min) for up to 28 days through subcutaneous osmotic mini-pumps (Model 2004, ALZA Scientific Products, Mountain View, CA) as previously described16–18. For cytokine assessment, mice were sacrificed at 0, 7, 14, and 28 days (n=11/time point) and aortic samples snap frozen in liquid nitrogen and stored at −80°C or placed in 10% buffered formalin for use. Before sacrifice, maximum aortic diameter was measured as previously described16. In a separate set of experiments, ApoE null mice received a monoclonal antibody to IL-5 (TRFK-5, Sigma, 1 mg/kg, N=20, “Ang II+TRFK5”), anti-IgG (Sigma, 1 mg/kg, N=20, “Ang II + anti-IgG”), or normal saline (0.5 ml, N=20, “Ang II + saline”) intra-peritoneally at the time of pump implantation and initiation of Ang II infusion19. Results from these mice were compared to a group of mice (N=10) that were infused with saline alone. Administration of TRFK at this dose has been demonstrated to have a half life of 2.4 weeks and an ED50 of 12 weeks20. Control mice (N=10/group) received similar treatments, but were infused with NS instead of Ang II. After 28 days of Ang II or NS infusion, mice were sacrificed and the aortas processed as described above.
Cell culture
Mouse aortic smooth muscle cells (MASMC) (B-Bridge International, Inc., Cupertino, CA) were maintained in DMEM+10% FBS. Peritoneal-derived mouse macrophages (PDM) were maintained in RPMI+10% FBS. PDM were harvested from Apo E null mice by 3% thioglycollate induction and plated at 5×105 cell/cm2. All cultures were supported at 37°C in a humidified, 5% CO2 atmosphere, and all experiments with MASMC were performed within five passages from primary cells. Experimental conditions were carried out in serum-free medium. Confluent monolayers of cells were stimulated with mouse recombinant IL-5 at varying doses (0–40, N=6/dose) for various durations (0–48 hours, N=6/time point). Each experimental condition was performed in triplicate with a new seeding of either MASMC.
Quantitative (real-time) reverse transcriptase-polymerase chain reaction (RT-PCR)
Expression of mRNA levels of ribosomal 18S-rRNA, MMP-2, and MMP-9 were assayed using quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) using Taq-man Gene Expression Assays (Applied Biosystems, Foster City, CA) as previously described16. Briefly, tissue and cellular mRNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). mRNA was reverse transcribed and the resultant cDNA was amplified by Taq Polymerase (Promega, Madison, WI). Gene specific assays were performed and all data were normalized and analyzed using Applied Biosystems 7500 operating software. Final data are presented as expression of indicated molecules relative to expression of internal control probe sets.
Protein Immunoassay
Aortic protein was extracted using Cell Lytic-M lysis buffer, andtotal protein levels determined with the BCA assay (Pierce, Rockford, Ill). Aortic extracts were assessed for specific Th1 (IFNγ, TNFα, IL-2, IL-6)and Th2 (IL-4, IL-5, IL-10, IL-13) cytokines by enzyme linked immunoassay (ELISA) (R&D Systems, Minneapolis, Mn), and results were normalized to total aortic protein and expressed in arbitrary units (AU). Total MMP-2 and MMP-9 protein levels were assessed in aortic and cellular extracts and cell culture media using ELISA (RayBiotech Inc., Norcross, GA). Results for MMP-2 and MMP-9 were verified using an immunoblot assay as previously described21. Briefly, media and cell lysates (10 ug protein/lane) were subjected to electrophoresis, transferred to a nitrocellulose membrane, blocked in 5% milk protein and incubated with antibodies for MMP-2 (1:1000) or MMP-9 (1:1000) (Cell Signaling Technology, Beverly, MA), followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5000). MMP was detected using an ECL Western blotting detection kit (Amersham Bioscience, Piscataway, NJ). For cell lysate loading control, membranes were stripped and reprobed with an antibody to beta-actin (Cell Signaling Technology). Films were scanned using an Epson Perfection 3200 Scanner (Epson America, Long Beach, CA) and band density was analyzed using Gel Analysis Pro software (Bethesda, MD).
Substrate gelatin zymography
Semi-quantitative MMP activity and distribution from aortic extracts and cell culture media were determined by gelatin zymography as previously described17. The relative molecular weight of each band was determined by comparison of the bands against MMP-2 (72 kDa) and MMP-9 (92 kDa) standards (Oncogene Research Products, Boston, MA). MMP activity measured by standard densitometry techniques as described above.
Histologic and Immunohistochemical analysis
Aortic samples were processed for immunohistochemistry as previously described16. Immunostaining was performed with either rabbit monoclonal anti-IL-5 (1:50) or rabbit monoclonal anti-MAC3 (1:20) (Abcam Inc, Cambridge, MA) followed by anti-rabbit biotinylated secondary antibody (1:4000) (Vector Laboratories, Burlingame, CA), Vectastain ABC Reagent (Vector Laboratories) and DAB Slides were counterstained with hematoxylin.
Statistical analysis
Data were analyzed using non-paired T-test for discrete comparisons. Specifically, the T-test was performed to assess changes in aortic size and aortic MMP expression between mice receiving an experimental treatment (“Ang II + saline”, “Ang II + TRFK-5”, or “Ang II + anti-IgG”) and saline controls. One-way analysis of variance (ANOVA) was used to assess for changes in groups over time. Specifically, ANOVA was used to assess for significant changes in the various aortic interleukin levels following Ang II infusion, and to assess the changes in time-dependent, and dose-dependent, MMP expression in MASMC and PDM in response to IL-5 stimulation. If ANOVA was significant, post-hoc analysis was performed using the Turkey procedure. Significance was set at P<.05. Analysis was performed using JMP 6.0.3 software (SAS Institute Inc, Cary, NC). Results are expressed as mean ± standard error of the mean (SEM).
RESULTS
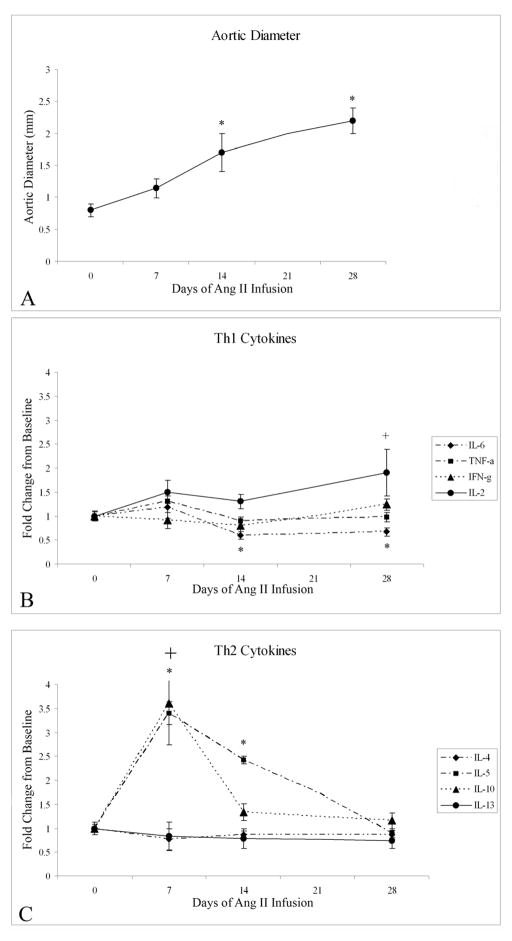
Ang II infusion induced a significant increase in the suprarenal aortic diameter (Fig. 1). Changes in specific cytokines over time were assessed using ANOVA, and when significant discrete data points were compared to baseline using the Turkey procedure. A significant elevation in specific Th2 cytokines IL-5 (ANOVA, P<0.01) and IL-10 (ANOVA, P<0.01) was observed temporally associated with aortic expansion (Fig. 1). After 7 days of Ang II infusion, aortic IL-5 rose from .0577 ±.013 mg/mg total protein (AU) to .196 ±.053 AU (P<.01). IL-5 remained elevated at 14 days of Ang II infusion compared to baseline (.140 ±.036 AU, P<.01), but returned to baseline by 28 days (.051±.012 AU). Similarly, IL-10 rose from a baseline level of .067±.006 AU to .243±.05 AU (P<.001) after 7 days of Ang II infusion, but returned to baseline by 14 days (.090±.012 AU). At seven days this represents a 3.4-fold and 3.6-fold increase in IL-5 and IL-10, respectively (Fig. 1). No significant changes were noted in IL-4 or IL-13. Of the Th1 cytokines, significant alterations were observed over time in IL-6 (ANOVA, P<.05) and IL-2 (ANOVA, P<.05). IL-6 levels diminished from .136 ± .01 AU at baseline to .082 ± .01 AU (P=.02) after 14 days of Ang II infusion (Fig. 1). In addition, IL-2 demonstrated an increase from .046 ± .004 AU at baseline to .089 ± .02 AU (P=.02) by 28 days of Ang II infusion. No significant alterations were noted in TNF-α or IFN-γ during the course of Ang II infusion.
Figure 1.
(A) Graphic representation of significant changes in juxtarenal aortic diameter measured at various time points of Ang II infusion (ANOVA, P<.01). By 14 days there is a significant increase in aortic diameter compared to baseline (* P<.01), and this continues at 28 days of Ang II infusion (*, P<.01). (B) Graphic representation of changes in specific aortic wall Th1 cytokines during Ang II infusion (ANOVA P<.01; *P<.02 compared to baseline). (C) Graphic representation of changes in specific aortic wall Th2 cytokines during Ang II infusion (ANOVA P<.01, *P<.01 compared to baseline).
Immunohistochemistry demonstrated IL-5-positive staining in inflammatory cells adjacent to the aortic wall but within the false lumen of the dissection. These cells similarly stained positive for MAC3suggesting they are of macrophage origin. To assess whether Ang II could be the direct stimulus for IL-5 production, MASMC and PDM were stimulated with varying doses of Ang II (0–100 umol) and media was assessed for IL-5 after 24 hours. Alterations in secreted IL-5 in response to Ang II could not be detected from either cell source suggesting that Ang II alone is insufficient to stimulate IL-5 production in SMC or macrophages.
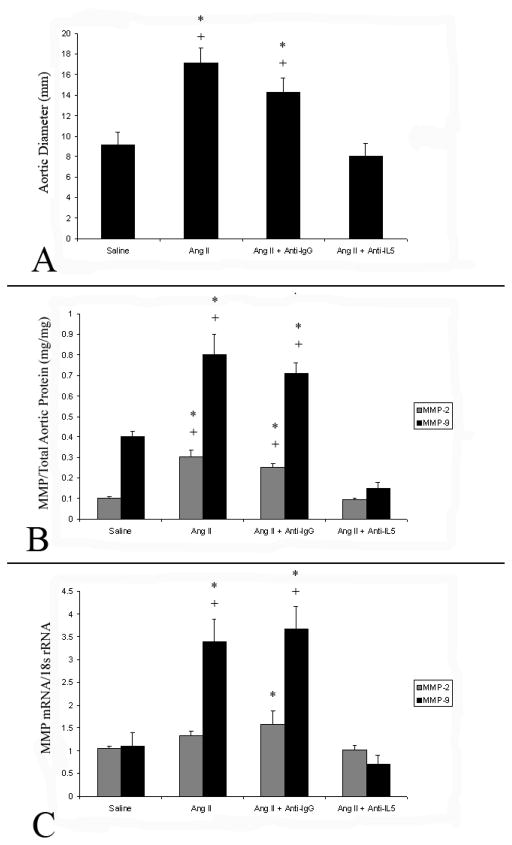
To assess the potential importance of IL-5 in AAA formation, IL-5 was neutralized with the administration of TRFK-5 at the time of Ang II-induced aneurysm formation Saline infusion for 28 days in mice (control series) resulted in no aneurysm formation. In contrast, 90% “Ang II + saline” mice and 80% of the “Ang II + anti-IgG” mice developed AAA. TRFK-5 administration with Ang II infusion (“Ang II + TRFK5), however, reduced aneurysm formation to 20%. Aneurysm rupture occurred in 20% of the “Ang II + saline” group and in 20% of the “Ang II + anti-IgG” group. No aneurysm ruptures were observed in the “Ang II +TRFK5” group. Aortas from “Ang II + saline” mice (17.1 ± 1.5 mm, P<.01) and “Ang II +anti-IgG” mice (14.3 ± 1.2 mm, P<.01) were significantly larger than from mice infused with saline alone (9.1 ± 1.0 mm). Aortic diameter in mice infused with Ang II and treated with TRFK-5 (7.7 ± 1.3 mm) did not differ from saline-infused control mice (Fig. 3)..
Figure 3.
(A) Graphic representation of aortic diameters from mice infused with either saline alone or Ang II with the intraperitoneal co-administration of saline (Ang II), Anti-IgG (AngII+Anti-IgG), or TRFK-5 (Ang II+Anti-IL5). ) for 28 days. Aortic diameters from Ang II and Ang II+Anti-IgG were larger than in saline infused mice (*, P<.01 (T-test)), while Ang II+Anti-IL5 aortic diameters did not differ. (B) Graphic representation of aortic total MMP-2 and MMP-9 protein levels from mice undergoing infusion of saline or Ang II for 28 days, with and without the treatment of anti-IgG or anti-IL5. After 28 days of Ang II infusion alone, or in combination with anti-IgG, aortic total MMP-2 and MMP-9 levels were higher than in those mice infused with saline (*, P<.01), while those mice treated with Ang II+anti-IL5 did not differ from saline infused controls.. (C) Graphic representation of MMP-2 and MMP-9 mRNA levels normalized to baseline (saline infused) levels. Ang II infusion (alone, or in combination with anti-IgG) for 28 days was associated with a significant increase in MMP-9 mRNA compared to those infused with saline (*, P<.01). while similar changes in MMP-9 in those infused with Ang II+anti-IL5 were not observed.. MMP-2 mRNA levels were only elevated in mice infused with Ang II and treated with anti-IgG compared to mice infused with saline (*, P<.05).
Associated with the increase in aortic size was a significant increase in aortic total MMP-2 and total MMP-9 protein levels (normalized to total aortic protein) (Fig. 3). Elevations in total MMP-2 and total MMP-9 protein levels were not observed in mice infused with saline alone, or in “Ang II + TRFK5” mice. Alterations in MMP-2 and MMP-9 mRNA levels are demonstrated in Figure 3.
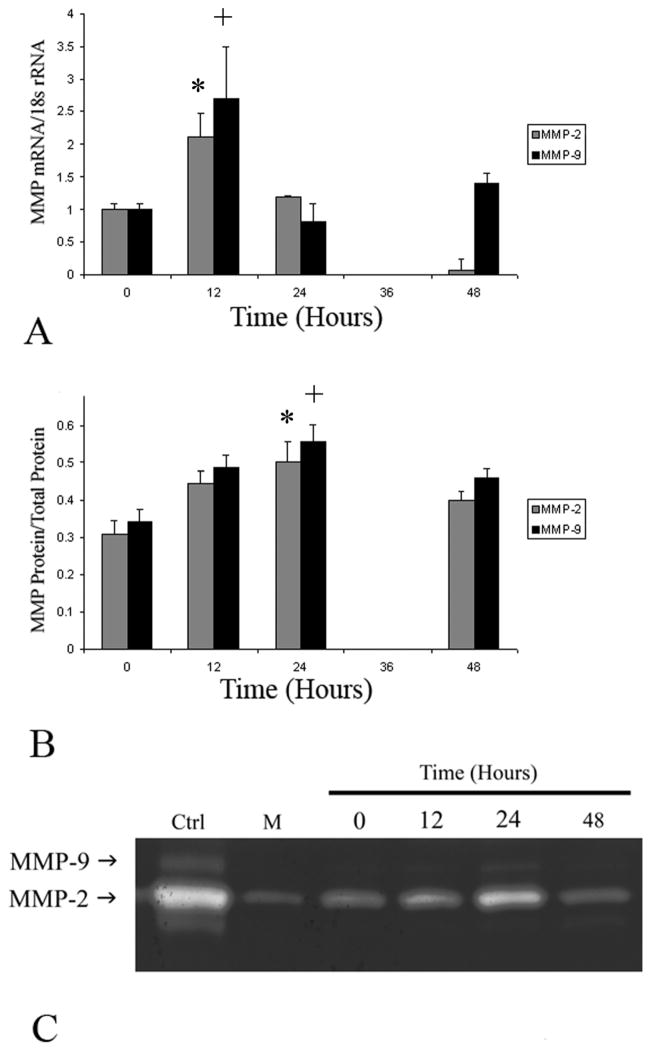
IL-5 is known to be a mediator of the immune response, but its potential role as a regulator of matrix homeostasis has not previously been demonstrated. Given the lack of MMP upregulation in mice treated with the combination of Ang II andTRFK-5, we sought to evaluate whether IL-5 could upregulate MMP expression in vitro. MASCM treated with IL-5 led to a dose (data not shown) and time-dependent increase in both MMP-2 and MMP-9 expression. Gelatin zymography demonstrates increased gelatinolytic activity over time, peaking at 24 hours after IL-5 treatment (Fig. 4). Only pro-forms of MMP-2 and MMP-9 were identified, which was verified by Western blot analysis (data not shown). To further quantify alterations in IL-5-induced-MMP-2 and -MMP-9, secreted levels were assessed by ELISA. IL-5 induced a 1.6-fold increase in total MMP-2 protein (P<.01) and a 1.5-fold increase in total MMP-9 protein (Fig. 4) after 24 hours, compared to baseline. In addition, analysis of MMP-2 and MMP-9 mRNA levels demonstrate a temporal correlation with the increases in secreted protein levels (Fig. 4). Treatment of PDM with IL-5 did not demonstrate any discernable alteration in MMP-2 or MMP-9 expression.
Figure 4.
(A) Graphic representation of the temporal changes in MMP-2 (gray bars) and MMP-9 (black bars) in response to IL-5 stimulation in mouse aortic smooth muscle cells (normalized to 18s rRNA). Stimluation with IL-5 led to a 2.1-fold (*, P<.01) increase in MMP-2 and a 2.7-fold (+, P<.01) increase in MMP-9 mRNA over baseline after 12 hours. (B) Graphic representation of alterations in MMP-2 and MMP-9 total protein levels in response to IL-5 stimulation in MASMC (*, P<.01 and +, P<.01) after 24 hours.. (C) Representative zymogram demonstrating temporal alterations in secreted MMP-2 and MMP-9 after IL-5 treatment of mouse aortic smooth muscle cells. The first lane (CTRL) represents exogenous mouse pro-MMP-2 and pro-MMP-9 to serve as controls, while lane M represents media alone. All of the MMP-2 and MMP-9 appear to be in the pro-form, with no enzyme present in the smaller, active form.
DISCUSSION
Chronic inflammation is a well known characteristic of AAA development. Associated with this inflammation are alterations in various cytokines. The role of cytokines from specific cytokine families, such as Th1 and Th2 cytokines, is not clear in the current literature. It has been suggested that the Th2 cytokines, specifically, are associated with aneurysmal degeneration14. In the Ang II infusion model of AAA formation, increased aortic Th2 cytokines, specifically IL-5 and IL-10, are present early in the course of aneurysm formation. These changes temporally correspond with a significant diminution of the Th1 cytokine IL-6. Inhibition of IL-5 prevented subsequent aneurysm formation in this model. These data lend support to the hypothesis that aneurysm degeneration is mediated by Th2 cytokines15, 16. These data, however, are quite dissimilar from other studies that have demonstrated that members of the Th1 family of cytokines are upregulated in human AAA3, 22. This raises the question of the applicability of the current model to degenerative aneurysms in humans. The main criticism of the Ang II-infusion model of aneurysm formation is that Ang II initiates aneurysm formation by first induceing an aortic dissection that ultimately degenerates into an AAA. No data on aortic cytokine levels are available, however, for aneurysms that have developed from dissections in humans. In addition, the differences may be further exacerbated by differences in the timing of tissue sampling. Human tissue is sampled during end stage disease when surgical intervention is warranted. In the current study, elevations in IL-5 and IL-10 were early in the phase of aneurysm degeneration and shortly after the phase of acute aortic dissection, clearly representing an important difference. While the current data did not evaluate aortas beyond 28 days, an elevation in the Th1 cytokine IL-2 is observed at 28 days. It may be that as the aorta transitions from a phase of acute matrix degradation to one of fibrosis and repair (at later time points), further elevations in Th1 cytokines may be observed.
While elevations in both IL-5 and IL-10 were observed, we sought to evaluate the significance of IL-5 elevation. Evaluations of IL-10 are underway and will be presented in future reports. IL-5 neutralization with TRFK-5 ameliorated significant aneurysm formation and abolished aneurysm rupture. TRFK-5 is a biologic agent used in blocking IL-5-induced eosinophilopoesis, and there is evidence that attenuation of IL-5 mediated immune mechanisms and inflammation may be important in decreasing pathological extracellular matrix remodeling23, 24. In light of these findings, we speculated that a similar role for IL-5 may affect remodeling in the vasculature. It is important to note, however, that the previous putative mechanisms for remodeling were thought to be eosinophil-mediated. Eosinophilia, however, is not hypothesized to play a significant role in either aneurysm formation or dissection.IL-5 neutralization, and the lack of subsequent aneurysm formation, is associated with failure to upregulate MMP-2 and MMP-9 in the aortic wall in response to Ang II infusion. What is not clear is whether upregulation of MMP-2 and MMP-9 in the aortic wall in those mice that developed aneurysms is directly related to IL-5. IL-5 is capable of upregulating the expression, but not activation, of MMP-2 and MMP-9 in smooth muscle cells in vitro, albeit in a relatively minor fashion. If this were the primary effect of IL-5 contributing to AAA formation, we would have anticipated a more dramatic response than that observed. This relatively minor effect, however, may be important in the early stages of aneurysm formation. Minor local injury due to matrix degradation secondary to moderate MMP upregulation could potentially trigger an intense inflammatory reaction leading to subsequent aortic pathology. Support of this hypothesis, however, cannot be discerned from the current data. More likely, IL-5 is contributing to the development of aortic pathology through alternate mechanisms that may ultimately regulate matrix homeostasis. These mechanisms include regulation of the innate and acquired immune response and B-cell differentiation. These immune functions are important in aneurysm formation, and are known to be regulated by IL-5 25, 26.
In summary, in the Ang II murine model of aneurysm formation, Th2 cytokines IL-5 and IL-10 appear to be upregulated early in the process of aneurysmal degeneration. Neutralization of IL-5 limits aneurysm formation, suggesting an important role in this process. While IL-5 appears to have some ability to regulate matrix degradation, the results were not dramatic and suggest this is not its main regulatory mechanism. Alternate potential mechanisms that are worth further investigation include the ability of IL-5 to regulate both the innate and acquired immune response. Further evaluation of these mechanisms using genetically altered mice will help to better delineate the importance of IL-5 in aneurysm formation, as well as to define the mechanisms by which it exerts its influence.
Figure 2.
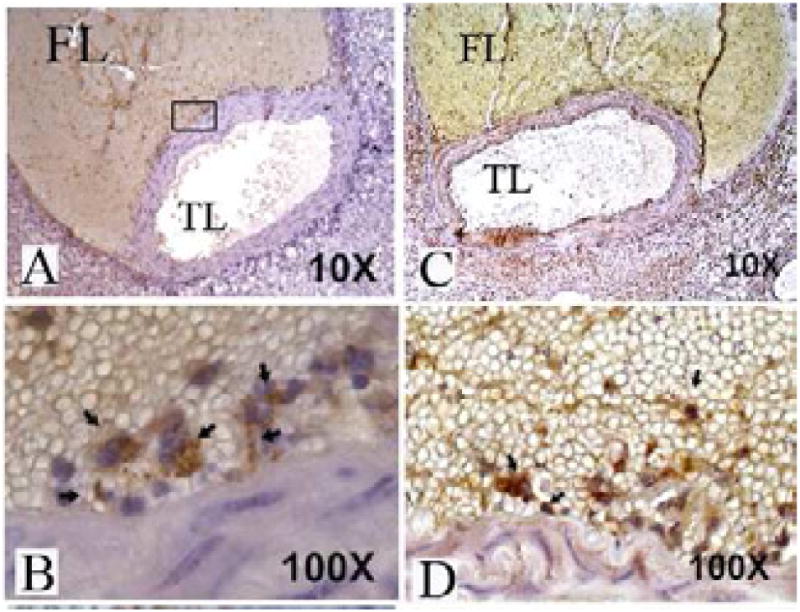
Immunohistochemistry slides from a representative murine aorta excised 7 days post AngII infusion. At lower magnification, (A and C) the aortic dissection is visualized with blood in false lumen (FL). The inset shows representative area of higher magnification in subsequent images. (B) Anti-IL-5 antibodies localize to inflammatory cells within the false lumen adjacent to the aortic wall. (D) Staining with Anti-MAC3 antibodies localizes to cells in a similar location.
Acknowledgments
This research is funded by: National Institutes of Health K08HL087798, the Society for Vascular Surgery Foundation, the American College of Surgeons, and the Foundation for Accelerated Vascular Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wassef M, Baxter BT, Chisholm RL, et al. Pathogenesis of abdominal aortic aneurysms: a multidisciplinary research program supported by the National Heart, Lung, and Blood Institute. J Vasc Surg. 2001 Oct;34(4):730. doi: 10.1067/mva.2001.116966. [DOI] [PubMed] [Google Scholar]
- 2.Pearce W, Sweis I, Yao J, et al. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg. 1992;16:784. [PubMed] [Google Scholar]
- 3.Szekanecz Z, Shah M, Pearce W, Koch A. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994;42:159. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 4.Newman K, Jean-Claude J, Li H, et al. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:224. [PubMed] [Google Scholar]
- 5.Lacraz S, Isler P, Vey E, et al. Direct contact between T lymphocytes and monocytes is a major pathway for induction of metalloproteinase expression. J Biol Chem. 1994;269:22027. [PubMed] [Google Scholar]
- 6.Malik N, Greenfield B, Wahl A, Kiener P. Activation of human monocytes through CD40 induces matrix metalloproteinases. J Immunol. 1996;156:3952–60. [PubMed] [Google Scholar]
- 7.Abbas A, Murphy K, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann T, Li L, Hengartnner H, et al. Differentiation and functions of T cell subsets. Ciba Found Symp. 1997;204:148. doi: 10.1002/9780470515280.ch10. [DOI] [PubMed] [Google Scholar]
- 9.Jagadesham VP, Scott DJ, Carding SR. Abdominal aortic aneurysms: an autoimmune disease? Trends Mol Med. 2008 Dec;14:522. doi: 10.1016/j.molmed.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994;42:159. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 11.Forester ND, Cruickshank SM, Scott DJ, Carding SR. Functional characterization of T cells in abdominal aortic aneurysms. Immunology. 2005;115:262. doi: 10.1111/j.1365-2567.2005.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong W, Zhao Y, Prall A, et al. Key roles of CD4+ T cells and interferon gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2009;2004:2607. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 13.Schonbeck U, Sukhova G, Gerdes N, Libby P. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu K, Shichiri M, Libby P, et al. Th2-predominant inflammation and blockade of IFN gamma signaling induce aneurysm in allografted aortas. J Clin Invest. 2004;114:168. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King V, Yin A, Kristo F, et al. Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation. 2009;119:426. doi: 10.1161/CIRCULATIONAHA.108.785949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eagleton M, Xu J, Liao M, et al. Loss of STAT1 is associated with increased aortic rupture in an experimental model of aortic dissection and aneurysm formation. J Vasc Surg. 2010;51:951. doi: 10.1016/j.jvs.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagleton M, Ballard N, Lynch E, et al. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during angiotensin II induced aneurysm formation. J Surg Res. 2006;135:345. doi: 10.1016/j.jss.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Daugherty A, Manning M, Cassis L. Angiotensin II promoted atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kung T, Stelts D, Zurcher J, et al. Involvement of Il-5 in a murine model of allergic pulmonary inflammation: prophylactic and therapeutic effect of an anti-IL-5 antibody. Am J Respir Cell Mol Biol. 1995;13:360. doi: 10.1165/ajrcmb.13.3.7654390. [DOI] [PubMed] [Google Scholar]
- 20.Garlisi C, Kung T, Wang P, et al. Effects of chronic anti-interleukin-5 monoclonal antibody treatment in a murine model of pulmonary inflammation. Am J Respir Cell Mol Biol. 1999;20:248. doi: 10.1165/ajrcmb.20.2.3327. [DOI] [PubMed] [Google Scholar]
- 21.Eagleton M, Peterson D, Sullivan V, et al. Nitric oxide inhibition increases aortic wall matrix metalloproteinase-9 expression. J Surg Res. 2002;104:15. doi: 10.1006/jsre.2002.6396. [DOI] [PubMed] [Google Scholar]
- 22.Juvonen J, Surcel H-M, Satta J, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1997;17:2843. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Komai M, Nagao K, et al. Role of interleukin-5 and eosinophils in allegen-induced airway remodeling in mice. Am J Respir Cell Mol Biol. 2004;31:62. doi: 10.1165/rcmb.2003-0305OC. [DOI] [PubMed] [Google Scholar]
- 24.Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binder C, Harvigsen K, Chang M-K, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the ling between the innate and acquired immune response. Adv Immunol. 2009;101:191. doi: 10.1016/S0065-2776(08)01006-7. [DOI] [PubMed] [Google Scholar]