Abstract
It is known that estrogen receptors can function as nuclear receptors and transcription factors in the nucleus and as signaling molecules in the plasma membrane. In addition, the localization of the receptors in mitochondria suggests that they may play important roles in mitochondria. In order to identify novel proteins that are involved in ERα-mediated actions of estrogens, we used a proteomic method that integrated affinity purification, two-dimensional gel electrophoresis, and mass spectrometry to isolate and identify cellular proteins that interact with ERα. One of the proteins identified was trifunctional protein β-subunit (HADHB), a mitochondrial protein that is required for β-oxidation of fatty acids in mitochondria. We have verified the interaction between ERα and HADHB by coimmunoprecipitation and established that ERα directly binds to HADHB by performing an in vitro binding assay. In addition, we have shown that ERα colocalizes with HADHB in the mitochondria by confocal microscopy, and the two proteins interact with each other within mitochondria by performing coimmunoprecipitation using purified mitochondria as starting materials. We have demonstrated that the expression of ERα affects HADHB activity, and a combination of 17β-estrodiol and tamoxifen affects the activity of HADHB prepared from human breast cancer cells that express ERα but not from the cells that are ERα deficient. Furthermore, we have demonstrated that 17β-estrodiol plus tamoxifen affects the association of ERα with HADHB in human cell extract. Our results suggest that HADHB is a functional molecular target of ERα in the mitochondria, and the interaction may play an important role in the estrogen-mediated lipid metabolism in animals and humans.
The biological activities of steroid hormone estrogens are mediated by two estrogen receptors (ERs),1 ERα and ERβ, which are widely distributed in different tissues (1). Traditionally, ERs are considered nuclear receptors and classical transcription factors (2). Upon binding to estrogen, ERs undergo a conformational change, translocate to the nucleus, and regulate the expression of estrogen responsive genes through binding to estrogen response elements residing in those genes (3). Since its cloning in the 1980s (4), this classical mechanism has been studied extensively, and a large group of nuclear proteins called co-activators and co-repressors, which interact with ERα, has been identified (5). Much less is known about the relatively newly cloned ERβ (6). Like the majority of other nuclear receptors, ERα and ERβ contain two activation domains, AF1 near the N terminus and AF2 in the ligand binding domain (7). The interactions between ERα/ERβ and co-activators/co-repressors are normally mediated by the binding of the AF2 domain of ERα/ERβ to one or more conserved pentapeptide LXXLL motifs (where X is any amino acid) in co-activators/co-repressors (5).
In addition to the nucleus, ERα and ERβ are also found to be localized in the plasma membrane (8, 9) and the mitochondria (10–12). Plasma membrane localized ERs appear to play important roles in rapid signal transductions (8, 9). While the localization of ERs in mitochondria is well documented (10–12), the biological functions of ERs in the mitochondria are not clear.
In order to identify novel proteins that are involved in ERα-mediated actions of estrogens, we used a proteomic method that integrated affinity purification, two-dimensional gel electrophoresis (2-DE) and MS to isolate and identify cellular proteins that interact with ERα. Multiple proteins were identified to interact with ERα. One of the identified proteins was HADHB, a mitochondrial protein required for fatty acid β-oxidation in the mitochondria. We chose this protein for further characterization because very few mitochondrial targets of ERs have been reported. We found that ERα physically interacts with HADHB and affects HADHB biological activity in fatty acid β-oxidation in the mitochondria.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and Stable Cell Lines
The coding sequence of human ERα was in-frame cloned into the BamHI and XhoI sites of the plasmid pcDNA3.1with an affinity tag (protein G and the streptavidin-binding peptide) (13) at the N terminus. Human 293T cells were routinely maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. Human breast cancer MCF7 cells (ERα positive), MDA-MB-231 cells (ERα negative), and stable cells derived from MDA-MB-231 cells were maintained in α-MEM with 5% FBS and 1% penicillin and streptomycin. For transient transfection of 293T cells for affinity purification, cells in each 150 mm plate were transfected with 25 μg of plasmid DNA using the calcium-phosphate method. Stable cell lines were generated by transfecting MDA-MB-231 cells using Lipofectamine 2000 and selected with 1 mg/ml G418 (Invitrogen, Carlsbad, CA). For 17β-estradiol (E2) and tamoxifen (TAM) treatment, cells were first cultured in phenol-red-free α-MEM (Invitrogen) with 5% charcoal-treated FBS (Hyclone, Logan, UT) for 48 h. Then the cells were treated with indicated concentrations of E2 (Sigma, St. Louis, MO), TAM (MP Biochemicals, Irvine, CA), or a combination of both.
Affinity Purification
Human 293T cells transiently transfected with plasmids expressing tag alone or tagged ERα (∼3.6 × 108 for each) were harvested and washed twice with cold phosphate-buffered saline (PBS). Cells were then lysed in five packed cell pellet volumes of lysis buffer1 (20 mm Tris-HCl, pH 7.5, 125 mm NaCl, 0.5% Nonidet P-40, 10% glycerol, 1 mm EDTA, and 10 nm E2) supplemented with protease inhibitor mixture (Roche, Indianapolis, IN) and phosphatase inhibitors (1 mm Na3VO4, 10 mm NaF, and 10 mm β-glycerophosphate) by incubating the cells on ice for 30 min followed by douncing 50 times. After centrifugation at 20,000 × g for 15 min at 4 °C, the pellets were further extracted twice with 2 ml lysis buffer1 and sonication. The combined and cleared supernatant was incubated with 150 μl pre-washed IgG agarose beads (Sigma) for 2 h at 4 °C with end-to-end rotation. The beads were then washed 4 times (1 ml each time) with lysis buffer1 supplemented with protease inhibitors and phosphatase inhibitors. The bound proteins were eluted three times with a buffer containing 100 mm glycine, pH 2.5 and 150 mm NaCl and then concentrated by TCA precipitation.
Two-Dimensional Electrophoresis (2-DE)
Proteins eluted from each purification were dissolved in a rehydration buffer (8 m urea, 2% w/v 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate, 50 mm dithiothreitol, 0.2% w/v Bio-Lyte and 0.001% w/v bromphenol blue) and loaded onto a 17 cm ReadyStrip IPG Strip (pH 3–10) which was in turn kept at room temperature overnight. Isoelectric focusing was carried out with a Protean IEF Cell using the following conditions: 250 V for 20 min with a linear ramp, 10,000 V for 1 h with a linear ramp, and 10,000 V for a total of 50,000 V/h with a rapid ramp. Other procedures were performed according to the manufacturer's instructions (BioRad, Hercules, CA).
MS Analysis and Database Search
In-gel digestion was performed as described previously (14, 15), and liquid chromatography-tandem MS (LC-MS/MS) analysis was carried out using a LTQ-XL mass spectrometer (Thermo, San Jose, CA) at the Proteomic Facility at the University of Arkansas for Medical Sciences (Little Rock, AR). Briefly, proteins in gel spots were digested with trypsin (Promega, Madison, WI) overnight at 37 °C and the resulting peptides were dissolved in 20 μl 0.1% formic acid for LC-MS/MS analysis. The raw data from LTQ-XL analysis were processed using Mascot Daemon (version 2.3.0) to generate peak lists in mgf format. Mascot (version 2.2; Matrix Science, Boston, MA) was used to search against the International Protein Index (IPI) human protein database (version 3.68; 87061 sequences) using the mgf files as described (14, 15). The parameters for database searching were as follows: (1) 2.0-Da mass error tolerance for MS and 0.65 Da for MS/MS, (2) tryptic enzyme specificity with a maximum of one missed cleavage, and (3) the following variable modifications: acetylation at peptide N terminus, phosphorylation on tyrosine/serine/threonine and oxidation on methionine. Peptide matches with significant homology (p < 0.05) were considered as identified peptides. Search results were further processed by Scaffold software (version 3_00_03; Proteome Software, Portland, OR) for viewing protein and peptide identification information. In the Scaffold analysis, protein identification probability with at least two peptides was set to 99% and the peptide identification probability was set to 95%.
Coimmunoprecipitation and Purification of Mitochondria
MCF7, MDA-MB-231 as well as stable clones derived from MDA-MB-231 (∼1 × 108 cells/each) were lysed in five packed cell pellet volumes of lysis buffer2 (10 mm Hepes-KOH, pH 7.9, 0.5% Nonidet P-40, 140 mm NaCl, 10 mm KCl, 1.5 mm MgCl2 and protease inhibitors) by douncing on ice. The lysate was centrifuged at 16,000 × g for 15 min at 4 °C, and the supernatant was used for immunoprecipitation. After preincubation of each supernatant with 40 μl Protein A (Sigma) (or protein G; Calbiochem, San Diego, CA) beads at 4 °C for 1 h, the precleared supernatant was incubated with 5 μg anti-ERα (or anti-HADHB) antibody and 30 μl protein A (or protein G) beads at 4 °C for 5 h with end-to-end rotation. After washing 4 times with lysis buffer2, the bound proteins were eluted from the beads by boiling in SDS sample buffer. The eluted proteins were analyzed by Western blotting. Mitochondria were purified using differential centrifugation and nonlinear sucrose ultracentrifugation as described by Antonsson et al. (16) and Rezaul et al. (17) (supplemental Fig. S1).
Confocal Microscopy
MCF7 cells grown on a coverslip were stained with 250 nm MitoTracker RedCMXRos (Lonza, Walkersviller, MD) in culture medium for 1 h at 37 °C, washed with culture medium and fixed with 3.7% formaldehydes for 15 min. The cells were then washed with PBS and permeabilized with 0.1% Triton X-100 for 15 min. After blocking with 5% normal goat serum for 30 min, the cells were incubated with ERα antibody (1:100 dilution) overnight at 4 °C, followed by washing with PBS and incubation with goat anti-mouse secondary antibody conjugated with FITC (Santa Cruz Biotechnology, Santa Cruz, CA) (1:100 dilution) for 1 h at room temperature. The cells were then blocked with donkey serum and incubated with goat anti-HADHB antibody (1:100) and donkey anti-goat secondary antibody conjugated with cyanine 5.5 (Cy5.5; Santa Cruz Biotechnology) as described above. Finally, the cells were washed with PBS and stained with 0.1 mg/ml 4′-6-diamidino-2-phenylindole (DAPI; Biotium, Hayward, CA) for 15 min at room temperature. For negative control, MCF7 cells were processed in the same procedures as described above except that the primary antibodies were omitted. Samples were analyzed with a NIKON Eclipse 90i confocal fluorescence microscope (Nikon, Tokyo, Japan).
Plasmids, Protein Expression in E. coli and In vitro Binding Assay
Amino acid substitutions of the last two residues of the LXXLL motif in the HADHB protein sequence (from LXXLL to LXXAA) were carried out using PCR-based site-directed mutagenesis (18). The mutations were confirmed by DNA sequencing. Flag-tagged human ERα and His-tagged human HADHB (wild type or mutant) were in-frame cloned into plasmids pET-21a and pET-28a, respectively. For control purposes, plasmids expressing Flag-tag and His-tag alone respectively were also created. Flag-ERα was expressed in Rosetta DE3 pLysS (EMD Chemicals, Gibbstown, NJ) cells at 28 °C, and the expressed proteins were purified as previously described (15). Expression and purification of His-HADHB was performed as described by Tang et al. (19). The purity of purified proteins was checked with a SDS-PAGE gel. With the same procedures, cell lysates prepared from the cells transformed with plasmids expressing tag alone (Flag- or His-tag) were also purified for control purpose. Equal molar amounts of purified Flag-ERα and His-HADHB were mixed and then incubated with 40 μl anti-Flag beads (Sigma) in the binding buffer (10 mm Tris-HCl, pH 7.5, 50 mm NaCl, 1 mm EDTA, 1.5 mm MgCl2, 0.1% Triton X-100 and protease inhibitors with or without E2 and/or TAM) at 4 °C overnight with end-to-end rotation. The affinity beads were then washed with wash buffer (10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1.5 mm MgCl2, 0.1% Triton X-100, and protease inhibitors) and then eluted with elution buffer (10 mm Tris-HCl, pH 7.5, 350 mm NaCl, 1 mm EDTA, 250 mm 3X Flag peptides, and protease inhibitors). The eluted proteins were examined by 10% SDS-PAGE and Western blotting.
Western Blotting
The eluted proteins from immunoprecipitation, in vitro binding assay, or the total cell lysates extracted in a modified RIPA buffer (50 mm Hepes, pH 7.5, 150 mm NaCl, 1.5 mm MgCl2, 1 mm EGTA, 10% glycerol, 1% Triton X-100, 1% SDS, and protease inhibitors) were dissolved in SDS sample buffer, separated on a SDS-PAGE (20 μg protein/lane) and transferred to a nitrocellulose membrane (Millipore, Billerica, MA), which in turn was incubated with indicated primary antibodies. The blots were then incubated with respective horseradish peroxidase-labeled secondary antibodies. Proteins were detected using enhanced chemiluminescence. Antibodies recognizing ERα, HADHB and actin were from Santa Cruz Biotechnology. Anti-His antibodies were from Roche (Indianapolis, IN). Anti-Flag antibodies were from Sigma.
HADHB Activity Assay
HADHB activity was determined through monitoring thiolytic cleavage of acetoacetyl-CoA. The rate of the thiolytic cleavage was measured spectrophotometrically at 303 nm as described (20). In brief, cells were lysed by sonication in a buffer containing 100 mm Tris-HCl, pH 8.3, 200 mm NaCl, 0.1% hexamethylphosphoric triamide, 2 mm β-mercaptoethanol, 0.5 mm EDTA and 0.5% Tween-20. The enzymatic activity was measured in 1 ml 100 mm Tris-HCl, pH 8.3, 25 mm MgCl2, 100 μm CoA, 40 μm acetoacetyl-CoA and 100 μg cell extract at 30 °C for 5 min. For each treatment, the enzymatic activity was measured 3 times with 3 separate enzyme preparations. One unit of activity was defined as the amount of enzyme that converts 1 μmol acetoacetyl-CoA per min. Calculations were made assuming a molar extinction coefficient of 21,400 M−1 cm−1 at 303 nm.
Statistical Analysis
p values were calculated using a One-way ANOVA (PSI-PLOT, Pearl River, NY). Data were presented as the mean ± S.E. Statistical significance was established when p < 0.05 (denoted as *), p < 0.01 (denoted as **), and p < 0.001 (denoted as ***).
RESULTS
Strategy for Identifying ERα-Associated Proteins
We used a 2-DE based proteomic method to identify the proteins that are associated with ERα. Two populations of 293T cells were transiently transfected with plasmids that express affinity tag alone (13) and tagged ERα, respectively. After affinity purification of total cell lysates from the two populations of cells, the bound proteins eluted from the affinity beads were fractionated with 2-DE. The protein spots uniquely appearing in the “ERα gel” (the gel that resolved the ERα and its associated proteins) were excised, in-gel digested, and the resulting peptides analyzed by MS. A database search revealed the identity of the protein in each spot.
Identifications of Potential ERα Interacting Proteins
The affinity tag we used was a tandem affinity tag containing a protein G and a streptavidin-binding peptide, which was shown to be efficient in affinity purification of protein complexes from mammalian cells (13). We have tested both tags (protein G and streptavidin-binding peptide) separately for purification of ERα protein complexes and found that the protein G tag resulted in higher yield of ERα proteins than the streptavidin-binding peptide, although the latter usually had better specificity. When both tags were used to do two-step affinity purification (i.e. tandem affinity purification), the yield of purified proteins decreased substantially (data not shown). Because the 2-DE system has high resolving power with the capability to resolve up to a thousand protein spots in a single gel (21), we decided to use protein G as an affinity tag to isolate the cellular proteins that are associated with ERα. After affinity purification (supplemental Fig. S2) and 2-DE fractionation, several protein spots uniquely appeared in the “ERα gel” (Fig. 1). LC-MS/MS analysis of the proteins in those unique spots revealed that one of the proteins was HADHB (spot #1; Fig. 1), a mitochondrial protein required for β-oxidation of fatty acids in mitochondria. HADHB was identified with high confidence by LC-MS/MS with 4 unique peptides to the proteins and an 8% sequence coverage (Table I; supplemental Table S1; supplemental Fig. S3). Several other proteins were also identified to potentially interact with ERα (Table I; supplemental Table S1; supplemental Figs. S4–S7). Calmodulin (spot # 2; supplemental Fig. S4) is a calcium-binding protein and has been shown to physically and functionally interact with ERα (22, 23). Tropomyosins are a family of highly conserved actin-binding proteins and regulate actin mechanics. In addition to regulating muscle contraction in muscle cells, tropomyosins also play other important roles in nonmuscle cells, such as the suppression of the transformed phenotype of breast cancer cells (24). It has been reported that tropomyosin 3 is expressed in MCF7 cells (25), and its expression in the cells is affected by E2 and progesterone (26). Two tropomyosin members (isoform 2 of tropomyosin alpha-3 chain and isoform 1 of tropomyosin alpha-4 chain) were identified from the same protein spot in the 2-DE gel (spot # 4; supplemental Table S1; supplemental Figs. S5, S6) because of the close similarity in molecular weight and isoelectric point of the two members (Table I). As calmodulin, EF-hand domain-containing protein D1 (EFHD1) (spot # 3; supplemental Table S1; supplemental Fig. S7) is a calcium-binding protein. EFHD1 is localized in the inner membrane of mitochondria (27), and it has been speculated to be involved in protecting cells from oxidative stress (28). There is no report on the biological relationship between EFHD1 and estrogen.
Fig. 1.
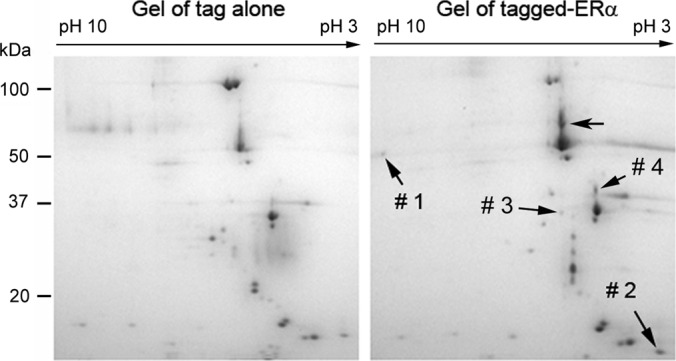
Two-dimensional gel fractionation of the affinity-purified proteins. The tagged ERα and its associated proteins were purified with IgG beads and fractionated with 2-DE. The fractionations of the proteins eluted from control (left panel) and tagged ERα samples (right panel) are shown. LC-MS/MS analysis revealed that spot #1: HADHB; spot #2: calmodulin; spot #3: EFHD1; spot #4: isoform 2 of tropomyosin alpha-3 chain and isoform 1 of tropomyosin alpha-4 chain (Table I).
Table I. List of proteins identified by affinity purification, 2-DE and LC-MS/MS analysis.
| Spot #a | Protein name | Category | IPI acc. no. | UniProt acc. no. | MW (kDa)/pI | Mascot scoreb | No. unique peptide | Sequence coverage (%)c |
|---|---|---|---|---|---|---|---|---|
| 1 | HADHB | Fatty acid β oxidation | IPI00022793 | P55084 | 51.4/9.5 | 87 | 4 | 8.0 |
| 2 | Calmodulin | Calcium-binding protein | IPI00075248 | P62158 | 16.8/4.1 | 395 | 5 | 26.0 |
| 3 | EFHD1 | IPI00031091 | Q9BUP0 | 26.9/5.3 | 62 | 2 | 8.0 | |
| 4 | Isoform 2 of tropomyosin alpha-3 chain | Structure protein | IPI00642042 | Q5HYB6 | 29.0/4.8 | 462 | 9 | 37.0 |
| 4 | Isoform 1 of tropomyosin alpha-4 chain | IPI00010779 | P67936 | 28.5/4.7 | 119 | 3 | 16.0 |
a Spot numbers correspond to those on Fig. 1.
b Mascot protein score. Mascot ion scores, identity threshold scores, and homology threshold scores assigned to individual peptides are listed in supplemental Table S1.
c Coverage of all peptide sequences matched to the identified protein sequence (%).
We excised all protein spots that appeared in the “ERα gel” but not in the control gel and analyzed the proteins in each spot with LC-MS/MS. Some proteins were identified in unexpected locations on the 2-DE gel, presumably because of post-translational modifications (29). For example, the full length of isoform DPI of desmoplakin protein has a theoretical molecular weight of 331 kDa, but the protein was identified on the gel at a location between 50 and 100 kDa markers (Fig. 1; arrow without a spot number). We manually inspected all the proteins identified by LC-MS/MS, and those proteins that were identified at unexpected locations on the gel (i.e. inconsistent with theoretical isoelectric point, molecular weight, or both) were not listed in Table I to reduce the uncertainty of the identified proteins. We were not able to identify the bait protein ERα in the present study. Un-tagged ERα has a molecular weight of 66 kDa and can be resolved by 2-DE (30). Consistent with this, we also found that Flag (a small tag) tagged ERα could be resolved by 2-DE (data not shown). In the present study, we used a relatively large affinity tag (∼20 kDa), and the tagged ERα had an apparent molecular weight of ∼90 kDa (supplemental Fig. S2). Based on the fact that the tagged ERα could be captured by the affinity beads in the affinity purification (supplemental Fig. S2), it is likely that the large affinity tag we used in this study prevented tagged ERα from being resolved by 2-DE.
Validation of the Interaction between ERα and HADHB by Coimmunoprecipitation
Based on the fact that ERs play very important roles in lipid metabolism (31, 32) and that very few mitochondrial targets of ERs have been reported, we decided to further characterize the biological functions of the ERα-HADHB interaction. We first used coimmunoprecipitation to determine if endogenous ERα interacts with endogenous HADHB. As shown in Fig. 2A, the anti-ERα antibody immunoprecipitated the endogenous HADHB from the ERα-positive breast cancer MCF7 cells but failed to do so from the ERα-negative breast cancer MDA-MB-231 cells, suggesting that endogenous ERα is associated with endogenous HADHB. We checked the expression of ERα in the two cell lines we used in this assay (i.e. MCF7 and MDA-MB-231) and confirmed the ERα expression status in the two cell lines (Fig. 2B). We also examined the expression levels of endogenous HADHB in MCF7 and MDA-MB-231 cells, and no significant difference was found (Fig. 2B). In a reciprocal immunoprecipitation with MCF7 cells, anti-HADHB antibody precipitated substantially more ERα proteins (Fig. 2C; right lane) compared with nonimmune goat IgG (Fig. 2C; left lane), suggesting HADHB specifically interacts with ERα. These data demonstrate that endogenous ERα specifically interacts with endogenous HADHB.
Fig. 2.
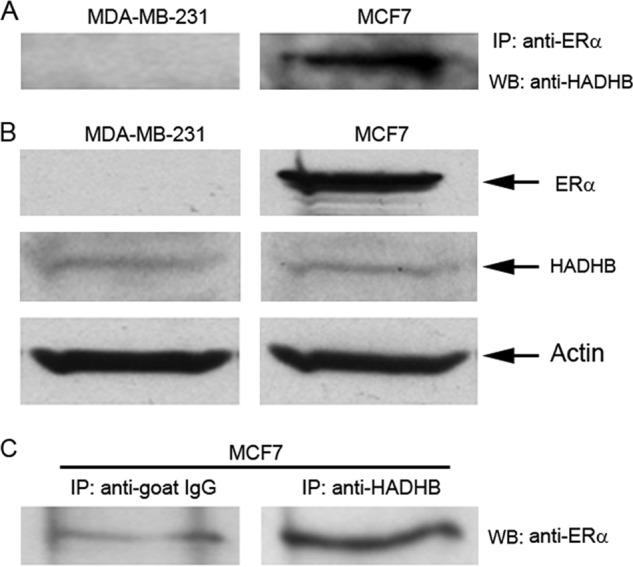
Verification of the interaction between ERα and HADHB by coimmunoprecipitation. A, HADHB was coimmunoprecipitated with ERα. Whole cell lysates from ERα-positive MCF7 cells and ERα-negative MDA-MB-231 cells (1 × 108/each) were immunoprecipitated with anti-ERα antibody. The immunoprecipitated proteins were probed with anti-HADHB antibody in Western blotting. As shown, HADHB was immunoprecipitated by anti-ERα antibody in the ERα-positive MCF7 cells but not in the ERα-negative MDA-MB-231 cells. B, Western blot examination of the expression of ERα and HADHB proteins in MCF7 and MDA-MB-231 cells. As shown, MCF7 cells are ERα-positive and MDA-MB-231 cells are ERα-negative; HADHB expression levels are similar between MCF7 and MDA-MB-231 cells. β-Actin was used as a loading control. C, ERα was coimmunoprecipitated with HADHB by anti-HADHB antibody. Whole cell lysate from MCF7 cells (1 × 108/each) was immunoprecipitated with nonimmune goat IgG (negative control) and anti-HADHB antibody. The immunoprecipitated proteins were probed by anti-ERα antibody in Western blotting. As shown, compared with control IgG, anti-HADHB antibody immunoprecipitated substantially more ERα.
ERα Colocalizes and Interacts with HADHB within Mitochondria
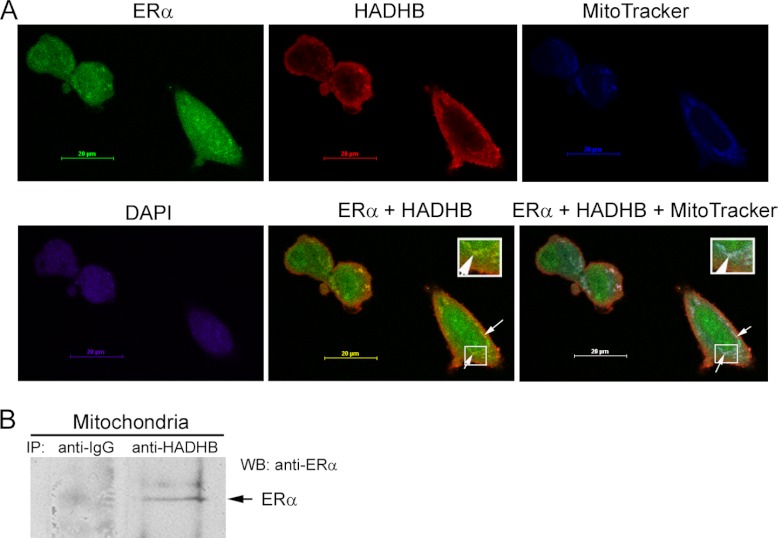
In order to test whether the association between ERα and HADHB takes place in the mitochondria, we performed confocal microscopy analysis using MCF7 cells and a mitochondrial marker MitoTracker, and antibodies against ERα and HADHB (Fig. 3A). The results indicated that ERα colocalized with HADHB (Fig. 3A; panel “ERα + HADHB”) and the colocalization overlapped with MitoTracker (Fig. 3A; panel “ERα + HADHB + MitoTracker”), suggesting that ERα is associated with HADHB in vivo within mitochondria. To further strength this conclusion, we performed immunoprecipitation with purified mitochondria (supplemental Fig. S1) as starting materials. As shown in Fig. 3B, ERα was immunoprecipitated by anti-HADHB antibodies but not by nonimmune goat IgG (negative control), suggesting that ERα interacts with HADHB in the mitochondria.
Fig. 3.
Colocalization and interaction of ERα and HADHB in the mitochondria. A, Colocalization of ERα and HADHB in the mitochondria. MCF7 cells were stained with mitochondrial marker MitoTracker (in blue), 4′-6-diamidino-2-phenylindole (in purple), and antibodies directed against ERα (in green) and HADHB (in red). Images were acquired by using a confocal laser-scanning microscope. Arrows in the merged images (panels “ERα + HADHB” and “ERα + HADHB + MitoTracker”) denote the colocolization of ERα and HADHB (in yellow), and the colocolization of ERα, HADHB and mitochondria (in white). The insets in the two merged images show the zoom-in images of the boxed regions. B, coimmunoprecipitation of ERα with HADHB using purified mitochondria as starting materials. Mitochondria were purified from MDA-MB-231 cells stably expressing ERα. Equal amounts of proteins extracted from the purified mitochondria were immunoprecipitated with non-immune goat IgG (negative control) and anti-HADHB antibody, respectively. The immunoprecipitated proteins were probed with anti-ERα antibody in Western blotting. As shown, ERα was immunoprecipitated by the anti-HADHB antibody, but not by the non-immune goat IgG.
In vitro Binding Assay Reveals Direct Interaction between ERα and HADHB
To determine whether ERα physically interacts with HADHB, we expressed Flag-tagged ERα (Flag-ERα) and His-tagged wild-type HADHB (His-HADHB) proteins in E. coli and performed in vitro binding assay with purified proteins. The binding assay using purified Flag alone (expressed and purified using the same procedures as for Flag-ERα) and purified His-HADHB was also performed as a control. Fig. 4A shows a portion of the Coomassie-blue-stained image of a SDS-PAGE gel by which the precipitated proteins were resolved. As shown in Fig. 4A, compared with the control lane (left lane), incubation of purified Flag-ERα with His-HADHB resulted in two extra protein bands, one was at around 58 kDa and another 68 kDa (Fig. 4A, right lane). Western blot analysis showed that the 58 kDa protein band corresponded to His-HADHB and the 68 kDa protein band corresponded to Flag-ERα protein (Figs. 4B and 4C). These results demonstrate that ERα physically interacts with HADHB under in vitro conditions.
Fig. 4.
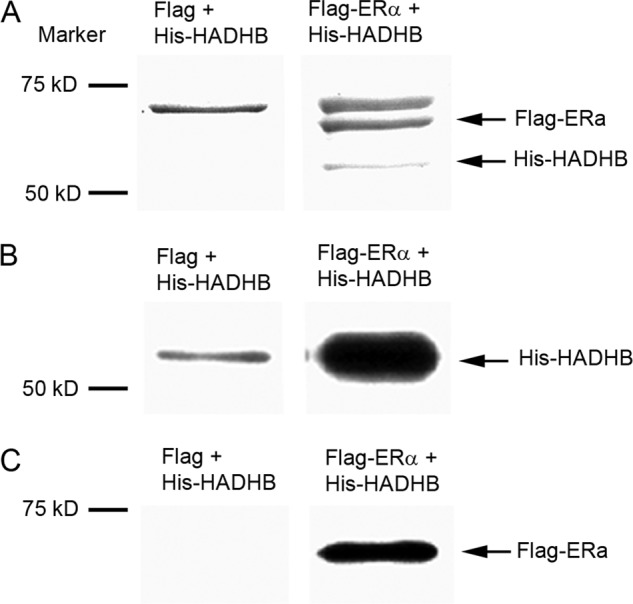
In vitro binding assay reveals direct interaction between ERα and HADHB. A, SDS-PAGE fractionation of the immunoprecipitated ERα-HADHB complex formed in the incubation of purified Flag-ERα with purified His-HADHB. Flag-ERα and His-HADHB were expressed in E. coli, and affinity purified with anti-Flag beads and Ni-NTA agarose beads, respectively. The purified Flag-ERα was mixed with purified His-HADHB, and the mixture was then incubated with anti-Flag beads. The bound proteins were eluted, fractionated by a10% SDS-PAGE gel, and visualized by Coomassie blue staining. The control was performed using the same experimental procedures except that a Flag tag was used to replace Flag-ERα. The binding of purified His-HADHB with purified Flag-ERα is shown. B, Western blot analysis of the ERα-HADHB protein complex. The protein complex was formed and affinity purified in the same way as described in A, but detected by anti-His antibody in Western blotting. C, same as B, except that proteins were probed by an anti-ERα antibody.
The ERα-HADHB Interaction is not Predominately Mediated by the LXXLL Motif in HADHB and is not E2-Dependent
The interaction between ERs and co-activators/co-repressors are normally mediated by the binding of the AF2 domain of ERs to one or more conserved pentapeptide LXXLL motifs in co-activators/co-repressors (5). Protein sequence analysis revealed that HADHB protein contains one LXXLL motif (residues 84–88) (33). In order to examine whether the ERα-HADHB interaction is mediated by the LXXLL motif in HADHB, we generated a His-tagged mutant HADHB, in which the last two amino acids in the LXXLL motif were substituted by alanine (from LXXLL to LXXAA). We then expressed and purified proteins and performed an in vitro binding assay with the mutant HADHB in the same way as for the wild-type HADHB as described in the previous section. As shown in Fig. 5A, anti-Flag affinity beads pulled down similar amount of mutant His-HADHB to that of the wild-type counterpart (compare left with right lane). We checked the amount of Flag-ERα that was associated with beads and confirmed they were similar in each treatment (Fig. 5A; low panel). These results demonstrate that the LXXLL motif in HADHB does not play a predominant role in determining the ERα-HADHB interaction. Binding between LXXLL motifs in co-activators/co-repressors and the AF2 domain of ERα/ERβ is known to be E2-dependent (34). We then performed the in vitro binding assay in the presence or absence of E2 to test whether E2 affects the binding of HADHB to ERα. As shown in Fig. 5B, anti-Flag antibody pulled down comparable amounts of His-HADHB in the presence or absence of E2, suggesting that the ERα-HADHB interaction is not E2-dependent. These results suggest that, different from the interaction between ERα and nuclear co-activators/co-repressors in the nucleus, the LXXLL motif in HADHB does not play a predominant role in determining the interaction between ERα and HADHB in the mitochondria.
Fig. 5.
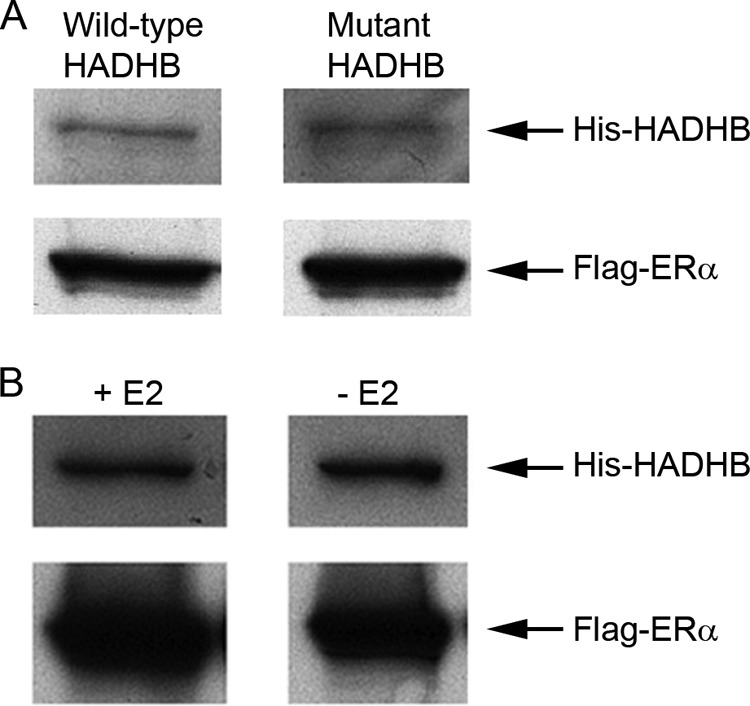
Binding of purified HADHB to ERα is not predominately mediated by the LXXLL motif in HADHB and is not E2-dependent. A, Western blot analysis of the binding of purified ERα to purified wild-type (with LXXLL motif) or mutant (LXXLL was mutated to LXXAA) His-HADHB. Flag-ERα and His-HADHB (wild-type and mutant) were expressed, affinity purified, and the in vitro binding assay was performed (upper panel) as described in Fig. 4. The amounts of Flag-ERα bound to beads were also checked (lower panel). The similar binding profiles of purified wild-type and mutant His-HADHB to Flag-ERα are shown. B, the ERα-HADHB interaction is not E2-dependent. The in vitro binding assay was performed in the absence (right lane) or presence (left lane) of 100 nm E2 in the binding buffer using the same experimental procedures as described in A. Similar amounts of His-HADHB pulled down with Flag-ERα in the presence or absence of E2 are shown. As in A, the amounts of Flag-ERα bound to beads were also checked (lower panel).
Expression of ERα in Breast Cancer Cells Affects HADHB Activity
To determine whether ERα is functionally linked to HADHB in cells, we first generated a cell line that stably expresses ERα in the ERα-negative breast cancer MDA-MB-231 cells, and the resulting stable cell line was designated MDA-MB-231-ERα. A control cell line was also generated by transfecting MDA-MB-231 cells with empty vector, and the resulting stable cell line was designated MDA-MB-231-vector. As shown in Fig. 6B, compared with MDA-MB-231 and MDA-MB-231-vector cells, which are ERα-negative (Fig. 6A), ectopic expression of ERα in cells resulted in significant (p < 0.001) increases of 3-ketoacyl-CoA thiolase activity of HADHB in the MDA-MB-231-ERα cells (a 53 and 46% increase compared with MDA-MB-231 and MDA-MB-231-vector cells, respectively). These data suggest that ERα affects HADHB activity and thus is functionally linked to HADHB in vivo.
Fig. 6.
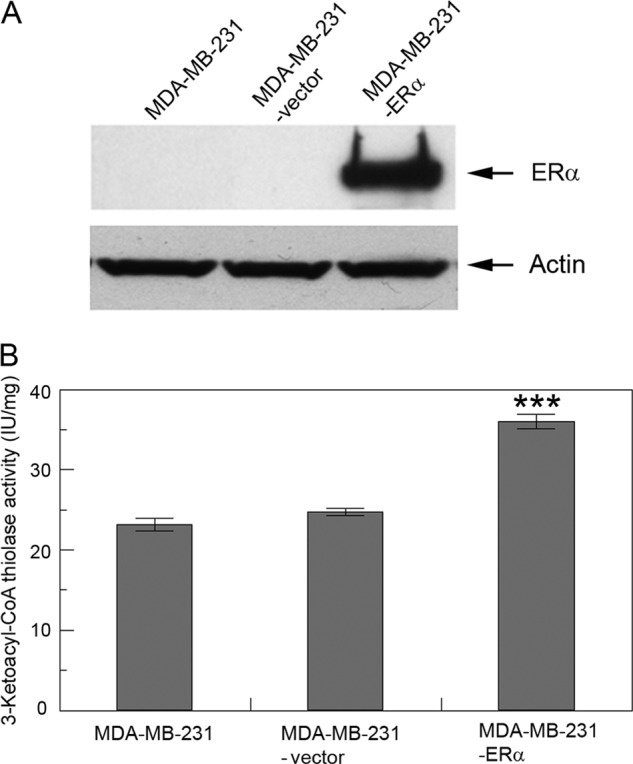
ERα affects HADHB activity. A, Western blot analysis of the expression of ERα in MDA-MB-231, MDA-MB-231-vector and MDA-MB-231-ERα cells. β-Actin was used as a loading control. B, the 3-Ketoacyle-CoA thiolase activity of HADHB in MDA-MB-231, MDA-MB-231-vector, and MDA-MB-231-ERα cells. Dependence of HADHB activity on the status of ERα in cells is shown. Values are the means ± S.E. of three separate enzyme preparations. *** denotes statistical significance of p < 0.001.
E2 and TAM Affect HADHB Activity in MDA-MB-231-ERα Cells
Because the expression of ERα in cells affects HADHB activity, we reasoned that ERα's cognate ligand E2 as well as E2's potent antagonist TAM must also influence HADHB activity in the ERα-positive cells. To test this hypothesis, we first cultured MDA-MB-231-ERα cells as well as MDA-MB-231-vector cells in the phenol-red-free α-MEM supplemented with 5% charcoal-treated fetal bovine serum for 48 h. Then, the E2-starved cells were treated with indicated concentrations of E2, TAM, or a combination of both. Protein extracts from the E2/TAM treated cells were then used for measuring HADHB activity. As shown in Fig. 7A, 100 nm E2, 100 nm TAM, and a combination of both had no significant effects on the activity of HADHB in the MDA-MB-231-vector cells. In contrast, 100 nm TAM and a combination of 100 nm TAM and 100 nm E2 decreased the activity of HADHB in the MDA-HB-231-ERα cells by 11 and 24%, which were both statistically significant (p < 0.05 and 0.01, respectively; Fig. 7B). In order to confirm the effect of E2 and TAM on HADHB activity, we treated cells with increasing concentrations of E2 mixed with a constant concentration of 100 nm TAM (Fig. 7C). As shown, when cells were treated with increasing concentrations of E2 mixed with 100 nm TAM, HADHB activity showed a trend of decreasing. The effect of 100 nm E2 + 100 nm TAM on HADHB activity was reproducibly observed in this assay. These data demonstrate that TAM and a combination of E2 and TAM affect HADHB activity in the cells that ectopically express ERα.
Fig. 7.
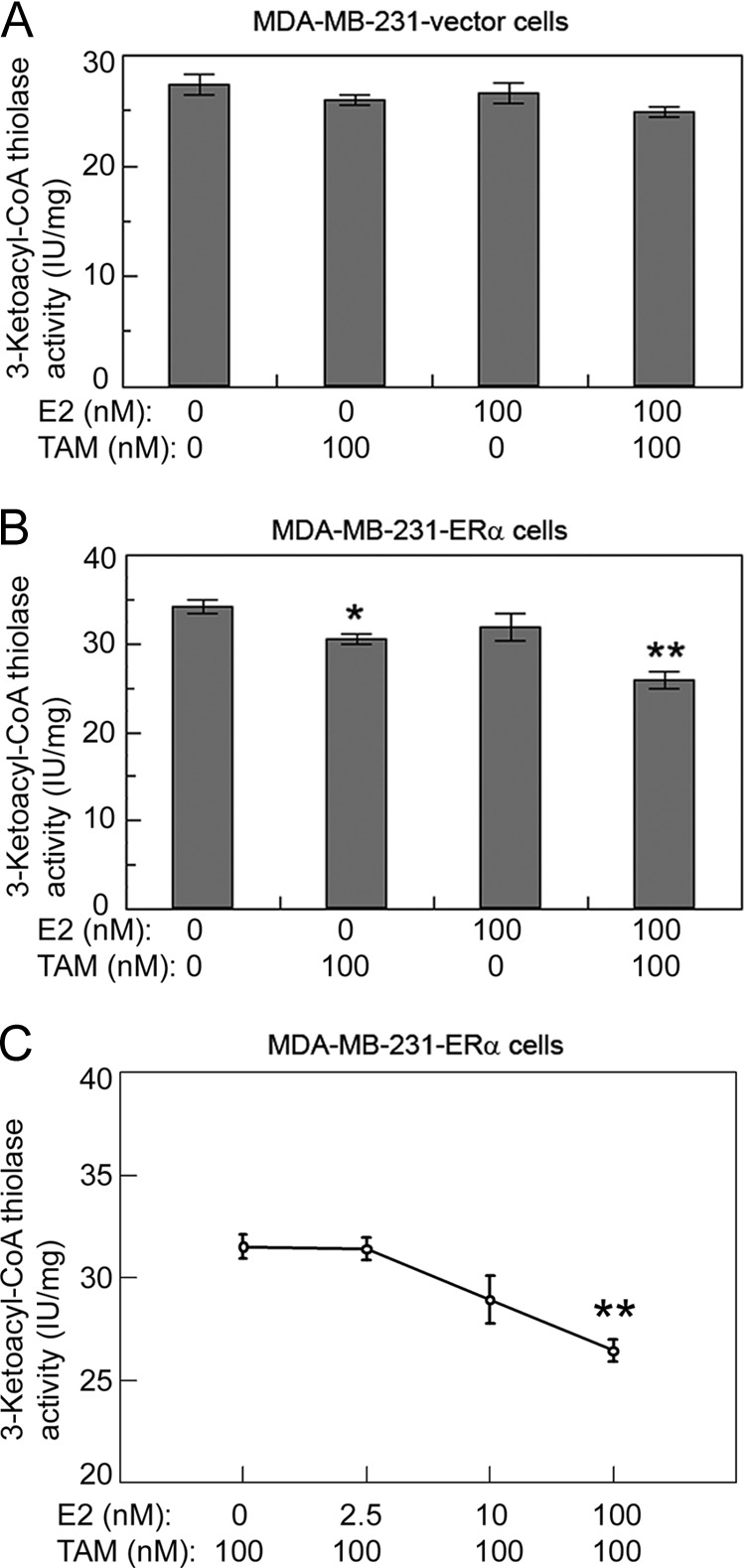
TAM and TAM plus E2 affect HADHB activity in the ERα-positive cells but not in the ERα-negative cells. A, E2 and TAM do not affect HADHB activity in MDA-MB-231-vector cells, which are ERα-negative. B, TAM and a combination of TAM and E2 significantly decrease HADHB activity in MDA-MB-231-ERα cells, which are ERα-positive. C, HADHB activity in MDA-MB-231-ERα cells as a function of increasing concentrations of E2 + a constant concentration of 100 nm TAM. Cells were grown in phenol-red-free α-MEM supplemented with 5% of charcoal-treated FBS for 48 h, and then treated with indicated concentration of TAM, E2, or a combination of both. Values are the means ± S.E. of three separate enzyme preparations. * and ** denote statistical significance of p < 0.05, p < 0.01 respectively, in relation to the data point with lowest E2 or TAM concentrations.
E2 and TAM Affect HADHB Activity in Human Breast Cancer MCF7 Cells
We then sought to examine whether E2 and TAM affect HADHB activity in the cells that express an endogenous ERα. For this purpose, we treated ERα-positive breast cancer MCF7 cells with E2 and TAM and measured HADHB activity in the same way as for MDA-MD-231-ERα cells. Compared with the results of MDA-MB-231-ERα cells, while 100 nm TAM did not cause significant changes in HADHB activity, a combination 100 nm E2 and 100 nm TAM significantly (p < 0.01) decreased HADHB activity. This result was consistent with the results obtained using MDA-MB-231-ERα cells (compare Figs. 8A with 7B). When the MCF7 cells were treated with increasing concentrations of E2 mixed with 100 nm TAM, a similar result was obtained (compare Figs. 8B with 7C). In order to test whether cellular HADHB activity would be affected by E2 at concentrations close to its physiological levels (nm levels) (35–37) and TAM in its pharmacological concentration range (μm levels) (38, 39), we treated MCF7 cells with increasing concentrations (0–10 μm) of TAM mixed with a constant concentration of 10 nm E2. As shown in Fig. 8C, HADHB activity decreased significantly (p < 0.001) when TAM concentrations were higher than 5 μm. These results suggest that a combination of E2 and TAM significantly affects the activity of HADHB in the cells that express an endogenous ERα.
Fig. 8.
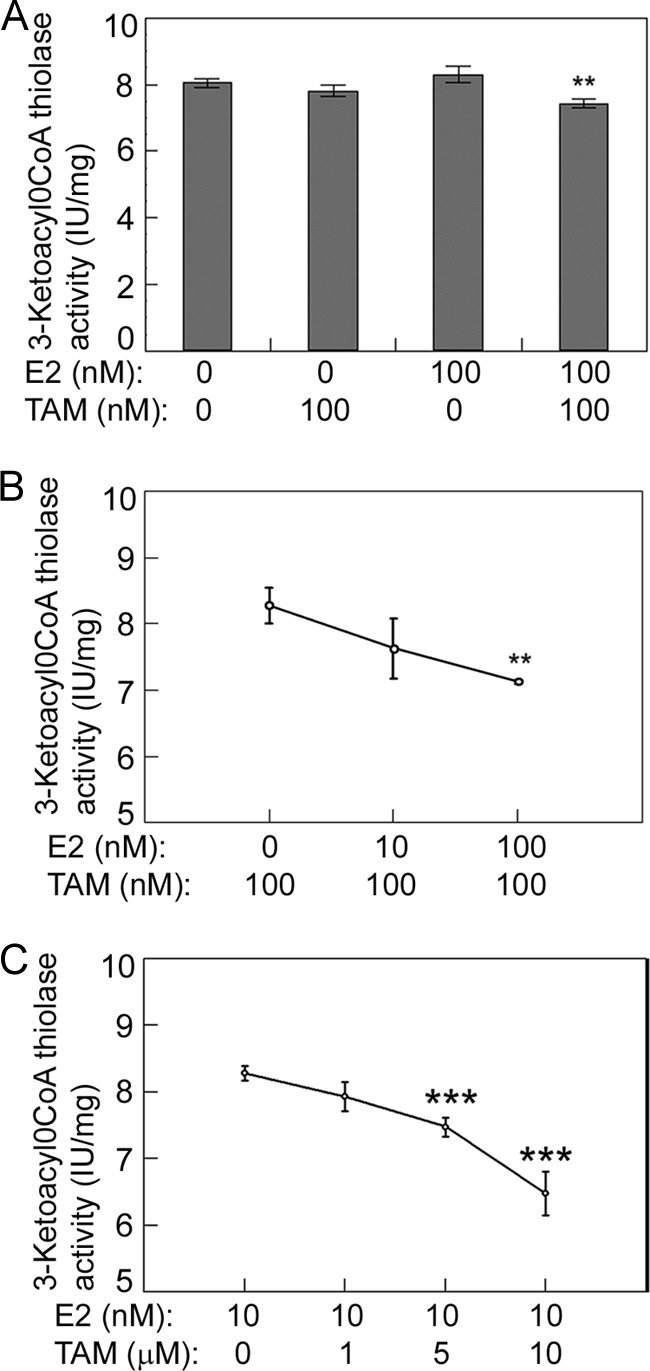
A combination of E2 and TAM affects HADHB activity in the breast cancer MCF7 cells that express an endogenous ERα. A, a combination of TAM and E2 significantly decreases HADHB activity in MCF7 cells. B, HADHB activity in MCF7 cells as a function of increasing concentrations of E2 + a constant concentration of 100 nm TAM. C, HADHB activity in MCF7 cells as a function of increasing concentrations of TAM + a constant concentration of 10 nm E2. Cells were grown and treated as described in Fig. 7. Values are the means ± S.E. of three separate enzyme preparations. ** and *** denote statistical significance of p < 0.01 and p < 0.001 respectively, in relation to the data point with lowest E2 or TAM concentrations.
A Combination of E2 and TAM Does not Affect Binding of Purified HADHB to Purified ERα, but Alters the ERα-HADHB Interaction in Human Cell Extract
To determine whether E2 and TAM alter the binding of ERα to HADHB, we expressed Flag-tagged ERα and His-tagged HADHB in E. coli and affinity-purified them as described in the previous sections. After the purified Flag-ERα was incubated with purified His-HADHB in the presence or absence of E2 and TAM, the ERα-HADHB protein complexes were affinity purified with anti-Flag antibody. As shown in Fig. 9A, there was no difference in the amounts of HADHB precipitated between in the absence and presence of E2 and TAM (even when the E2 and TAM concentrations were raised to 1 μm), suggesting that a combination of E2 and TAM does not alter binding of purified HADHB to ERα. We confirmed that the amounts of Flag-ERα bound to the beads in each treatment were similar (Fig. 9A; lower panel). We next sought to examine whether E2 and TAM alter the interaction of ERα with HADHB in human cells. Total cell extracts prepared from human breast cancer MDA-MB-231-ERα cells that express a tagged [a protein G and a streptavidin-binding peptide (13)] ERα were incubated in the presence or absence of E2 and TAM, and the ERα-HADHB complex was isolated using anti-IgG affinity beads (Fig. 9B). As shown, 100 nm E2 + 100 nm TAM significantly reduced the amount of HADHB pulled down with ERα (upper panel; compare middle lane with left lane). When the cell extract was incubated with 1 μm E2 and 1 μm TAM, HADHB precipitated with ERα became undetectable (right lane). We examined the amount of ERα bound to the beads and observed no difference among treatments (lower panel). In order to test whether E2 and TAM at their physiologically and pharmacologically relevant concentrations (nm and μm, respectively) (35–39) would affect the ERα-HADHB interaction in cells, we repeated the experiment shown in Fig. 9B with a modification of the concentrations of E2 and TAM used in treating the cell extract (Fig. 9C). As shown, 10 nm E2 plus 1 or 10 μm TAM significantly reduced the amount of HADHB precipitated with ERα (upper panel). We examined the amounts of input materials for ERα and HADHB, and the amount ERα bound to the beads, and no differences were observed among treatments (lower panels).
Fig. 9.
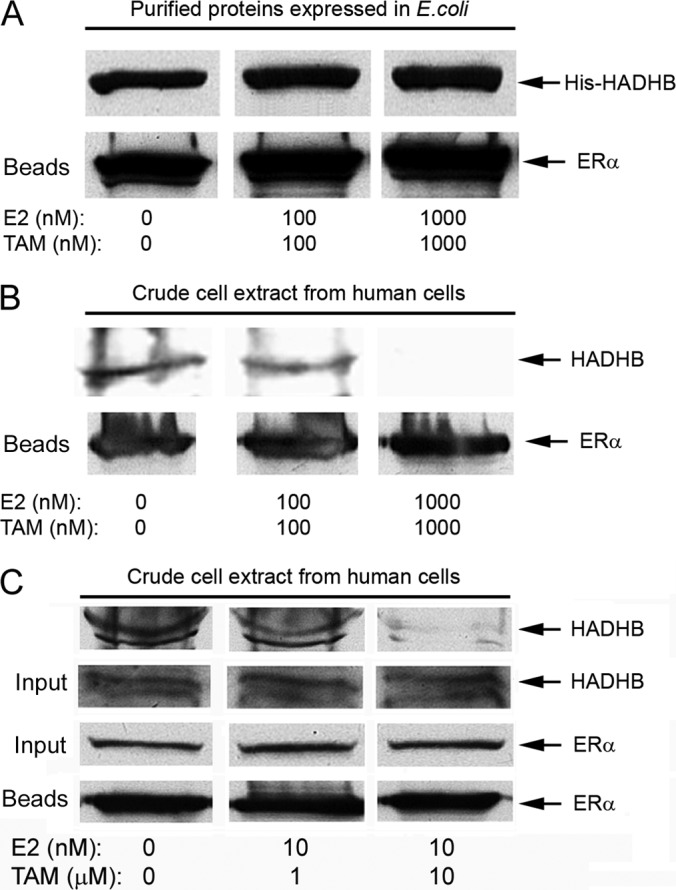
A combination of E2 and TAM does not affect binding of purified HADHB to purified ERα but alters the association of ERα with HADHB in human cell extract. A, binding of purified Flag-ERα to purified His-HADHB is not altered by E2 and TAM. Protein expression, purification, and in vitro binding were performed as described in Fig. 5B except that the in vitro binding was performed in the absence (left lane), or presence of 100 nm E2 + 100 nm TAM (middle lane) or 1000 nm E2 + 1000 nm TAM (right lane) in the binding buffer. The amounts of Flag-ERα bound to beads were also checked (lower panel). The similar amounts of His-HADHB pulled down with Flag-ERα in the presence or absence of E2 + TAM are shown. B, crude extracts of human breast cancer MDA-MB-231-ERα cells that express a protein G tagged-ERα were incubated with the indicated concentration of E2 and TAM, and ERα and its associated proteins were affinity-isolated by anti-IgG affinity beads. The amounts of Flag-ERα bound to beads were also checked (lower panel). C, same as B except that nm concentrations of E2 and μm levels of TAM were used to treat the cell extract. In addition, the amounts of input of ERα and HADHB (∼1% of starting materials/lane) were also checked to make sure similar amounts of starting materials were used in each treatment. In both B and C, the disruptive effects of E2 + TAM on the binding of HADHB to ERα in crude human cell extract are shown.
DISCUSSION
By integration of affinity purification, 2-DE fractionation and MS analysis, we have identified a novel mitochondrial molecular target of ERα (and thus estrogens)
HADHB (Fig. 1), a mitochondrial protein required for β-oxidation of fatty acids in mitochondria. We have verified the interaction between ERα and HADHB by co-immunoprecipitation (Fig. 2) and established that the two proteins physically interact with each other by performing in vitro binding assays (Fig. 4). In addition, we have shown that ERα colocalizes with HADHB within mitochondria by confocal microscopy (Fig. 3). Although HADHB contains an LXXLL motif, the interaction apparently is not predominately mediated by the motif and not E2-dependent (Fig. 5). We have also demonstrated that ERα and its ligands affect the activity of HADHB in human cells (Figs. 6–8). Finally, we have demonstrated that E2 and TAM affect the interaction of ERα-HADHB in human cells (Fig. 9).
In addition to functioning as transcription factors in the nucleus and as signaling molecules in the plasma membrane (5, 8, 9, 40, 41), several lines of evidence suggest that ERs play important roles in the mitochondria. First, a portion of cellular ERα and ERβ was found to be localized in mitochondria, and the relative distribution of ERα and ERβ into the mitochondrial pool is regulated by estrogens (10–12, 42). Second, mitochondrial DNA was found to contain estrogen-response elements (43), suggesting that genes coded by mitochondrial DNA are subject to regulation by estrogens. Third, mitochondrial structure and some important functions are influenced by estrogens and TAM (11, 12, 31, 44, 45). However, the exact molecular mechanism by which ERs function in mitochondria is not clear. Recently, a mitochondrial protein was reported to interact with ERα, but no biological function was characterized (46). In this study, we have found that HADHB is a molecular target of ERα in mitochondria. We have demonstrated that ERα and its ligands E2 and TAM affect HADHB activity. Interestingly, E2 itself does not alter HADHB activity, but combinations of E2 and TAM significantly affect the activity (Figs. 7 and 8). The collaborative effects of E2 and TAM have been reported in other studies (39, 47). The physical and functional interaction between ERα and HADHB in mitochondria may play important roles in regulating lipid metabolism in cells.
Mitochondria are the organelles that power the whole cell, and mitochondrial β-oxidation is an essential metabolic step in energy supply in a cell. β-oxidation is catalyzed by a trifunctional protein complex that is composed of four α-subunits (HADHA) containing the long-chain 2-enoyl-CoA hydratase and the long-chain l-3-hydroxyacyl-CoA dehydrogenase activities and four β-subunits (HADHB) containing the long-chain 3-ketocayl-CoA thiolase activity. Mutations in either HADHA or HADHB result in β-oxidation deficiency, which causes multiple clinical syndromes (48). It has been established that estrogens and ERs play very important roles in lipid metabolism (31, 49). In ERα knock-out mice, increased white adipose tissue and body fat have been observed (50, 51). When aromatase, an enzyme that is responsible for conversion of androgen to estrogen, was knocked out, the mice became obese (52, 53). The inhibitory effect of estrogen on body weight gain and fat accumulation in tissues in animals has been well established (32, 54, 55). All these data suggest that estrogens and ERs are profoundly involved in the lipid metabolism. However, direct, physical evidence of the involvements of ERs in lipid metabolism has not been reported. The physical and functional interaction between ERα and HADHB observed in this study provides the direct evidence that ER is involved in lipid metabolism.
As a selective estrogen receptor modulator, TAM has been used for treatment of postmenopausal women with breast cancer for decades. TAM usually is administered at a daily dose of 20 mg/day, which leads to TAM serum concentrations of 50 and 300 ng/ml (∼0.15–0.85 μm) (38, 56). However, steady state TAM concentrations in various tissues are 10- to 60-fold higher than in serum, reaching the range of tens of μm levels (38). Because of the local production and/or uptake from circulation, estrogen levels in a variety of tissues are 10- to 20-fold higher than the plasma estrogen levels, reaching nm range (35–37). One of the standards of care for breast cancer in the clinics is a 5 year TAM administration (57), which generates a large population of patients whose physiologies are influenced by both endogenous E2 and the administrated TAM. In this study, we found that HADHB activity in human breast cancer cells was significantly affected by combinations of E2 and TAM at their physiologically and pharmacological relevant concentrations (Fig. 8C). These results suggest that E2 and TAM may have significant impacts on the lipid metabolisms for the breast cancer patients with TAM administration. Concomitant with the effect of E2 and TAM on HADHB activity, we found that combinations of E2 and TAM affected the association of ERα with HADHB in human breast cancer cells (Fig. 9B). Significantly, this effect was still effective when E2 was in the physiologically relevant level and TAM in its pharmacological concentration ranges (Fig. 9C). Thus, it is highly likely that the effect of E2 and TAM on HADHB activity was realized via affecting the interaction between ERα and HADHB in cells.
Interestingly, E2 and TAM did not affect the binding of purified ERα to purified HADHB (Fig. 9A), but altered the association of ERα with HADHB in human cell extract (Figs. 9B and 9C). The results strongly suggest that other cellular factor(s) is (are) involved in the action of E2 and TAM on the interaction of ERα-HADHB in cells. It is well known that TAM normally functions as an antagonist of estrogen. However, TAM can also function as an agonist of estrogen under certain circumstances and in certain tissues (58). Cell-type (or tissue-) specific effector(s) has (have) been proposed to play an important role in determining the action of TAM and hormones in different types of cells or tissues (59, 60). Lipid catabolism is carried out primarily in liver and heart cells in animals and humans, and it will be interesting to examine the ER-HADHB interactions and the impact of E2 and TAM on lipid catabolism in those tissues. Furthermore, different from ERα, ERβ has been speculated to be a mitochondrial protein rather than a nuclear receptor and may play an important role in mediating estrogen's effects in mitochondria (12). It will also be interesting to examine whether ERβ also interacts with HADHB, and how ERα and ERβ may coordinate and regulate HADHB activity. Detailed mechanistic studies in those regards may provide clues of how estrogens and ERs influence lipid metabolism in animals and humans.
HADHA (α) and HADHB (β) form α4β4 complexes in the inner membrane of mitochondria (61, 62). If HADHB was identified to interact with ERα via affinity purification, one would expect that HADHA should also be identified. However, in the present study HADHA was not on the list of the identified proteins (Table I). A potential cause for this was that a portion of the identified HADHB was in the monomeric form (i.e. the monomers that are not yet assembled with HADHA). The HADHB protein spot in the 2-DE gel was weak (Fig. 1). If only a portion of the HADHB in the protein spot was in complex, then the HADHA level in the protein spot might not be in the range of MS detection. On the other hand, HADHA-independent function of HADHB has been reported (63). It is also possible that HADHB may function in a novel way when it complexes with ERα. However, considering the fact that HADHB and ERα colocalize in the mitochondria (Fig. 3) and that the ERα-HADHB interaction affects the thiolytic cleavage activity in β-oxidation (Figs. 6–8), it seems that the first scenario makes more sense.
Acknowledgments
We thank Dr. Cornelius Joel Funk for helpful comments.
Footnotes
* This work was supported in part by the Arkansas Biosciences Institute (ABI) and an NIH grant NCRR COBRE 5P30RR031154. Zhenqi Zhou is a recipient of Research Assistantship from the Program in Cell and Molecular Biology at the University of Arkansas.
 This article contains supplemental Figs S1 to S7 and Table S1.
This article contains supplemental Figs S1 to S7 and Table S1.
1 The abbreviations used are:
- ER
- estrogen receptor
- 2-DE
- two-dimensional gel electrophoresis
- E2
- 17β-estradiol
- EFHD1
- EF-hand domain containing protein D1
- HADHA
- hydroxyacyl-CoA dehydrogenase/trifunctional protein, alpha subunit
- HADHB
- hydroxyacyl-CoA dehydrogenase/trifunctional protein, beta subunit
- TAM
- tamoxifen.
REFERENCES
- 1. Gustafsson J. A. (1999) Estrogen receptor beta–a new dimension in estrogen mechanism of action. J. Endocrinol. 163, 379–383 [DOI] [PubMed] [Google Scholar]
- 2. Jensen E. V., Jacobson H. I., Walf A. A., Frye C. A. (2010) Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol. Behav. 99, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKenna N. J., Lanz R. B., O'Malley B. W. (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20, 321–344 [DOI] [PubMed] [Google Scholar]
- 4. Walter P., Green S., Greene G., Krust A., Bornert J. M., Jeltsch J. M., Staub A., Jensen E., Scrace G., Waterfield M., et al. (1985) Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. U.S.A. 82, 7889–7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klinge C. M. (2000) Estrogen receptor interaction with co-activators and co-repressors. Steroids 65, 227–251 [DOI] [PubMed] [Google Scholar]
- 6. Mosselman S., Polman J., Dijkema R. (1996) ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 392, 49–53 [DOI] [PubMed] [Google Scholar]
- 7. Danielian P. S., White R., Lees J. A., Parker M. G. (1992) Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 11, 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin E. R. (2009) Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 20, 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lösel R., Wehling M. (2003) Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 4, 46–56 [DOI] [PubMed] [Google Scholar]
- 10. Chen J. Q., Delannoy M., Cooke C., Yager J. D. (2004) Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. Am. J. Physiol. Endocrinol. Metab. 286, E1011–1022 [DOI] [PubMed] [Google Scholar]
- 11. Pedram A., Razandi M., Wallace D. C., Levin E. R. (2006) Functional estrogen receptors in the mitochondria of breast cancer cells. Mol. Biol. Cell 17, 2125–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang S. H., Liu R., Perez E. J., Wen Y., Stevens S. M., Jr., Valencia T., Brun-Zinkernagel A. M., Prokai L., Will Y., Dykens J., Koulen P., Simpkins J. W. (2004) Mitochondrial localization of estrogen receptor beta. Proc. Natl. Acad. Sci. U.S.A. 101, 4130–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bürckstummer T., Bennett K. L., Preradovic A., Schütze G., Hantschel O., Superti-Furga G., Bauch A. (2006) An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat. Methods 3, 1013–1019 [DOI] [PubMed] [Google Scholar]
- 14. Du Y., Zhou J., Fan J., Shen Z., Chen X. (2009) Streamline proteomic approach for characterizing protein-protein interaction network in a RAD52 protein complex. J. Proteome Res. 8, 2211–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du Y. C., Gu S., Zhou J., Wang T., Cai H., Macinnes M. A., Bradbury E. M., Chen X. (2006) The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca(2+)/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol. Cell. Proteomics 5, 1033–1044 [DOI] [PubMed] [Google Scholar]
- 16. Antonsson B., Montessuit S., Sanchez B., Martinou J. C. (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276, 11615–11623 [DOI] [PubMed] [Google Scholar]
- 17. Rezaul K., Wu L., Mayya V., Hwang S. I., Han D. (2005) A systematic characterization of mitochondrial proteome from human T leukemia cells. Mol. Cell. Proteomics 4, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 19. Tang H., Severinov K., Goldfarb A., Ebright R. H. (1995) Rapid RNA polymerase genetics: one-day, no-column preparation of reconstituted recombinant Escherichia coli RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 92, 4902–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamijo T., Wanders R. J., Saudubray J. M., Aoyama T., Komiyama A., Hashimoto T. (1994) Mitochondrial trifunctional protein deficiency. Catalytic heterogeneity of the mutant enzyme in two patients. J. Clin. Invest. 93, 1740–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Görg A., Weiss W., Dunn M. J. (2004) Current two-dimensional electrophoresis technology for proteomics. Proteomics 4, 3665–3685 [DOI] [PubMed] [Google Scholar]
- 22. Li L., Sacks D. B. (2007) Functional interactions between calmodulin and estrogen receptor-alpha. Cell Signal 19, 439–443 [DOI] [PubMed] [Google Scholar]
- 23. Li Z., Joyal J. L., Sacks D. B. (2001) Calmodulin enhances the stability of the estrogen receptor. J. Biol. Chem. 276, 17354–17360 [DOI] [PubMed] [Google Scholar]
- 24. Mahadev K., Raval G., Bharadwaj S., Willingham M. C., Lange E. M., Vonderhaar B., Salomon D., Prasad G. L. (2002) Suppression of the transformed phenotype of breast cancer by tropomyosin-1. Exp. Cell Res. 279, 40–51 [DOI] [PubMed] [Google Scholar]
- 25. O'Neill G. M., Stehn J., Gunning P. W. (2008) Tropomyosins as interpreters of the signalling environment to regulate the local cytoskeleton. Semin. Cancer Biol. 18, 35–44 [DOI] [PubMed] [Google Scholar]
- 26. Sukocheva O. A., Yang Y., Gierthy J. F. (2009) Estrogen and progesterone interactive effects in postconfluent MCF-7 cell culture. Steroids 74, 410–418 [DOI] [PubMed] [Google Scholar]
- 27. Tominaga M., Kurihara H., Honda S., Amakawa G., Sakai T., Tomooka Y. (2006) Molecular characterization of mitocalcin, a novel mitochondrial Ca2+-binding protein with EF-hand and coiled-coil domains. J. Neurochem. 96, 292–304 [DOI] [PubMed] [Google Scholar]
- 28. Dütting S., Brachs S., Mielenz D. (2011) Fraternal twins: Swiprosin-1/EFhd2 and Swiprosin-2/EFhd1, two homologous EF-hand containing calcium binding adaptor proteins with distinct functions. Cell Commun. Signal 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Person M. D., Shen J., Traner A., Hensley S. C., Lo H. H., Abbruzzese J. L., Li D. (2006) Protein fragment domains identified using 2D gel electrophoresis/MALDI-TOF. J. Biomol. Tech. 17, 145–156 [PMC free article] [PubMed] [Google Scholar]
- 30. Nalvarte I., Schwend T., Gustafsson J. A. (2010) Proteomics analysis of the estrogen receptor alpha receptosome. Mol. Cell. Proteomics 9, 1411–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen J. Q., Brown T. R., Russo J. (2009) Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim. Biophys. Acta 1793, 1128–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Djouadi F., Weinheimer C. J., Saffitz J. E., Pitchford C., Bastin J., Gonzalez F. J., Kelly D. P. (1998) A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J. Clin. Invest. 102, 1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamijo T., Aoyama T., Komiyama A., Hashimoto T. (1994) Structural analysis of cDNAs for subunits of human mitochondrial fatty acid beta-oxidation trifunctional protein. Biochem. Biophys. Res. Commun. 199, 818–825 [DOI] [PubMed] [Google Scholar]
- 34. Needham M., Raines S., McPheat J., Stacey C., Ellston J., Hoare S., Parker M. (2000) Differential interaction of steroid hormone receptors with LXXLL motifs in SRC-1a depends on residues flanking the motif. J. Steroid Biochem. Mol. Biol. 72, 35–46 [DOI] [PubMed] [Google Scholar]
- 35. Geisler J. (2003) Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J. Steroid Biochem. Mol. Biol. 86, 245–253 [DOI] [PubMed] [Google Scholar]
- 36. Pasqualini J. R., Chetrite G., Blacker C., Feinstein M. C., Delalonde L., Talbi M., Maloche C. (1996) Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 81, 1460–1464 [DOI] [PubMed] [Google Scholar]
- 37. Vermeulen A., Deslypere J. P., Paridaens R., Leclercq G., Roy F., Heuson J. C. (1986) Aromatase, 17 beta-hydroxysteroid dehydrogenase and intratissular sex hormone concentrations in cancerous and normal glandular breast tissue in postmenopausal women. Eur. J. Cancer Clin. Oncol. 22, 515–525 [DOI] [PubMed] [Google Scholar]
- 38. Lien E. A., Solheim E., Ueland P. M. (1991) Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 51, 4837–4844 [PubMed] [Google Scholar]
- 39. Moreira P. I., Custódio J., Moreno A., Oliveira C. R., Santos M. S. (2006) Tamoxifen and estradiol interact with the flavin mononucleotide site of complex I leading to mitochondrial failure. J. Biol. Chem. 281, 10143–10152 [DOI] [PubMed] [Google Scholar]
- 40. Mo R., Rao S. M., Zhu Y. J. (2006) Identification of the MLL2 complex as a coactivator for estrogen receptor alpha. J. Biol. Chem. 281, 15714–15720 [DOI] [PubMed] [Google Scholar]
- 41. Schultz-Norton J. R., Ziegler Y. S., Likhite V. S., Yates J. R., Nardulli A. M. (2008) Isolation of novel coregulatory protein networks associated with DNA-bound estrogen receptor alpha. BMC Mol. Biol. 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yager J. D., Chen J. Q. (2007) Mitochondrial estrogen receptors–new insights into specific functions. Trends Endocrinol. Metab. 18, 89–91 [DOI] [PubMed] [Google Scholar]
- 43. Demonacos C. V., Karayanni N., Hatzoglou E., Tsiriyiotis C., Spandidos D. A., Sekeris C. E. (1996) Mitochondrial genes as sites of primary action of steroid hormones. Steroids 61, 226–232 [DOI] [PubMed] [Google Scholar]
- 44. Chen J. Q., Yager J. D., Russo J. (2005) Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim. Biophys. Acta 1746, 1–17 [DOI] [PubMed] [Google Scholar]
- 45. Moreira P. I., Custódio J. B., Oliveira C. R., Santos M. S. (2005) Brain mitochondrial injury induced by oxidative stress-related events is prevented by tamoxifen. Neuropharmacology 48, 435–447 [DOI] [PubMed] [Google Scholar]
- 46. Jazbutyte V., Kehl F., Neyses L., Pelzer T. (2009) Estrogen receptor alpha interacts with 17beta-hydroxysteroid dehydrogenase type 10 in mitochondria. Biochem. Biophys. Res. Commun. 384, 450–454 [DOI] [PubMed] [Google Scholar]
- 47. Hashimoto M., Inoue S., Muramatsu M., Masliah E. (1997) Estrogens stimulate tamoxifen-induced neuronal cell apoptosis in vitro: a possible nongenomic action. Biochem. Biophys. Res. Commun. 240, 464–470 [DOI] [PubMed] [Google Scholar]
- 48. Spiekerkoetter U., Sun B., Khuchua Z., Bennett M. J., Strauss A. W. (2003) Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum. Mutat. 21, 598–607 [DOI] [PubMed] [Google Scholar]
- 49. Nemoto Y., Toda K., Ono M., Fujikawa-Adachi K., Saibara T., Onishi S., Enzan H., Okada T., Shizuta Y. (2000) Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J. Clin. Invest. 105, 1819–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heine P. A., Taylor J. A., Iwamoto G. A., Lubahn D. B., Cooke P. S. (2000) Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U.S.A. 97, 12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ohlsson C., Hellberg N., Parini P., Vidal O., Bohlooly-Y M., Bohlooly M., Rudling M., Lindberg M. K., Warner M., Angelin B., Gustafsson J. A. (2000) Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem. Biophys. Res. Commun. 278, 640–645 [DOI] [PubMed] [Google Scholar]
- 52. Jones M. E., Thorburn A. W., Britt K. L., Hewitt K. N., Misso M. L., Wreford N. G., Proietto J., Oz O. K., Leury B. J., Robertson K. M., Yao S., Simpson E. R. (2001) Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J. Steroid Biochem. Mol. Biol. 79, 3–9 [DOI] [PubMed] [Google Scholar]
- 53. Jones M. E., Thorburn A. W., Britt K. L., Hewitt K. N., Wreford N. G., Proietto J., Oz O. K., Leury B. J., Robertson K. M., Yao S., Simpson E. R. (2000) Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. U.S.A. 97, 12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao Q., Mezei G., Nie Y., Rao Y., Choi C. S., Bechmann I., Leranth C., Toran-Allerand D., Priest C. A., Roberts J. L., Gao X. B., Mobbs C., Shulman G. I., Diano S., Horvath T. L. (2007) Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat. Med. 13, 89–94 [DOI] [PubMed] [Google Scholar]
- 55. Roesch D. M. (2006) Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol. Behav. 87, 39–44 [DOI] [PubMed] [Google Scholar]
- 56. Jordan V. C. (1990) Long-term adjuvant tamoxifen therapy for breast cancer. Breast Cancer Res. Treat. 15, 125–136 [DOI] [PubMed] [Google Scholar]
- 57. Early Breast Cancer Trialists' Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351, 1451–1467 [PubMed] [Google Scholar]
- 58. Lin S. X., Chen J., Mazumdar M., Poirier D., Wang C., Azzi A., Zhou M. (2010) Molecular therapy of breast cancer: progress and future directions. Nat. Rev. Endocrinol. 6, 485–493 [DOI] [PubMed] [Google Scholar]
- 59. Katzenellenbogen J. A., O'Malley B. W., Katzenellenbogen B. S. (1996) Tripartite steroid hormone receptor pharmacology: interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol. Endocrinol. 10, 119–131 [DOI] [PubMed] [Google Scholar]
- 60. Sato M., Rippy M. K., Bryant H. U. (1996) Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. FASEB J. 10, 905–912 [DOI] [PubMed] [Google Scholar]
- 61. Carpenter K., Pollitt R. J., Middleton B. (1992) Human liver long-chain 3-hydroxyacyl-coenzyme A dehydrogenase is a multifunctional membrane-bound beta-oxidation enzyme of mitochondria. Biochem. Biophys. Res. Commun. 183, 443–448 [DOI] [PubMed] [Google Scholar]
- 62. Uchida Y., Izai K., Orii T., Hashimoto T. (1992) Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J. Biol. Chem. 267, 1034–1041 [PubMed] [Google Scholar]
- 63. Adams D. J., Beveridge D. J., van der Weyden L., Mangs H., Leedman P. J., Morris B. J. (2003) HADHB, HuR, and CP1 bind to the distal 3′-untranslated region of human renin mRNA and differentially modulate renin expression. J. Biol. Chem. 278, 44894–44903 [DOI] [PubMed] [Google Scholar]