Abstract
Survivin is responsible for cancer progression and drug resistance in many types of cancer. YM155 selectively suppresses the expression of survivin and induces apoptosis in cancer cells in vitro and in vivo. However, the mechanism underlying these effects of YM155 is unknown. Here, we show that a transcription factor, interleukin enhancer-binding factor 3 (ILF3)/NF110, is a direct binding target of YM155. The enhanced survivin promoter activity by overexpression of ILF3/NF110 was attenuated by YM155 in a concentration-dependent manner, suggesting that ILF3/NF110 is the physiological target through which YM155 mediates survivin suppression. The results also show that the unique C-terminal region of ILF3/NF110 is important for promoting survivin expression and for high affinity binding to YM155.
Survivin is a member of the inhibitor of apoptosis protein family and intersects the multiple pathways involved in tumor proliferation and inhibition of apoptosis, as a nodal molecule (1–3). Although survivin is highly expressed in all primary tumors (4, 5), it is rarely expressed in normal adult tissues except the thymus, placenta, and CD34+ stem cells (6). Because of this significant differential expression between cancer and corresponding normal tissues, the dependence of tumor cell proliferation and progression on survivin, and its global effects on multiple signaling pathways in tumor cells (7–9), survivin is thought to be a potential therapeutic target in cancers. However, targeting survivin for therapeutic purposes appears to be difficult because survivin has few potential sites for drug binding on its surface (10), and little is known about the regulation mechanism of the survivin gene. Despite these limitations, some novel therapeutic strategies have been developed to target survivin function and expression, and clinical trials are currently ongoing (7, 11, 12).
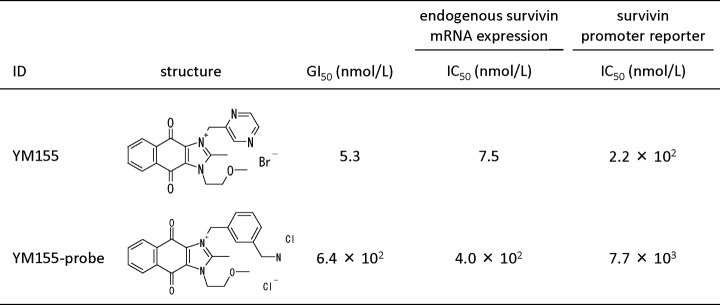
YM155, a novel small molecule suppressant of survivin, was identified in a survivin gene promoter assay by high throughput screening of in-house chemical libraries (11). It selectively suppresses the expression of survivin and induces apoptosis in p53-deficient cancer cells in vitro and in vivo (11). YM155 alone or in combination with chemotherapy or radiotherapy shows potent antitumor activities in human carcinoma xenograft models and induces in vivo antitumor activity without causing severe systemic toxicity in mice (13). Although YM155 has a unique mechanism of action, and clinical studies as a first-in-class compound are ongoing (14–16), the target molecules of YM155 have remained unclear.
A wide range of techniques can be used to identify unknown molecular targets of small molecule compounds. The most powerful method for this purpose is chemical proteomics. This involves the binding of active compound analogs to solid supports, which are then incubated with a cell or tissue lysate. Proteins that bind specifically to the compound are then identified (17–22). To understand the mechanism by which YM155 suppresses survivin expression, we sought to identify the molecules that bind to YM155 and to elucidate their function in the regulation of survivin expression.
EXPERIMENTAL PROCEDURES
Compounds and Antibodies
YM155 and the YM155 amino derivative (YM155 probe) for the affinity probe were synthesized by Astellas Pharma Inc. The antibodies used in these experiments are listed in supplemental Table 1.
Cells
Human hormone-refractory prostate cancer (PC-3), non-small cell lung cancer (Calu-6), uterine cervix (HeLa), and HEK-293 cell lines were purchased from American Type Culture Collection. The cells were maintained in RPMI 1640 (Invitrogen) or Dulbecco's modified Eagle's medium (Invitrogen), supplemented with 10% heat-inactivated fetal calf serum (JRH Biosciences).
Affinity Purification Using Chemical Probes
For affinity purification, we used a polysaccharide-coated polyacrylic resin, JSR LNS1001, with carboxyl-functional surface chemistry obtained from JSR Corporation (Japan). To prepare the YM155 probe-coupled beads (YM155 beads), 3 mg of the beads were first incubated with 400 mm 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (Peptide Institute) and 100 mm N-hydroxysuccinimide (Fluka) at room temperature for 30 min. The activated beads were centrifuged to remove 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxysuccinimide and then incubated with 1 ml of 100 μm YM155 probe in DMSO (Sigma-Aldrich) at room temperature for 3 h. Residual reactive groups on the beads were blocked with 1 m ethanolamine (Sigma-Aldrich).
Extracts of PC-3, Calu-6, and HeLa cells were prepared from 1 × 108 cells in lysis buffer (1% digitonin (Wako Pure Chemical), 10 mm HEPES, pH 7.4, 150 mm NaCl with EDTA-free protease inhibitor mixture (Roche) and benzonase (Merck)) or ProteoExtract subcellular extraction kit (Merck). The YM155 beads were washed with HBS-EP buffer (GE Healthcare) and incubated with the extracts at 4 °C for 90 min with agitation. After washing five times with HBS-EP buffer, the bound proteins were eluted with 100 μl of 0.5% SDS at 100 °C for 5–10 min. The eluted samples were concentrated by evaporation (Genevac) and subjected to SDS-PAGE and silver staining (Wako Pure Chemicals). Protein spots of interest were excised from the gel and then subjected to in-gel trypsin digestion (23).
Mass Spectrometry Analysis
The digested peptide mixture was concentrated on an on-line trap column (PepMap C18) and then separated with a reversed phase microcapillary column (nano-HPLC capillary column) using a CapLC system (Waters). The mobile phases consisted of Solvent A (2% acetonitrile, 0.1% formic acid) and Solvent B (90% acetonitrile, 0.1% formic acid). The column was initially equilibrated at 10% B at a flow rate of 200 nl/min. The separation was performed by a linear gradient of 10% B to 50% B over 35 min, 90% B over 10 min, and 10% B over 15 min at a flow rate of 200 nl/min at room temperature. The LC effluent was directly interfaced with the nanoelectrospray ion source on a Q-TOF Ultima API mass spectrometer (Waters). An MS survey scan was obtained for the m/z range of 400–1500, and MS/MS spectra were acquired for the two most intense ions from the survey scan with precursor charge limitation (2 or greater). Dynamic exclusion of 2-min duration was used to acquire MS/MS spectra from low intensity ions. All the MS/MS spectra were converted into text files (.pkl) using Protein Lynx software (Waters; purchased in February 2003) combining sequential scans with the same precursor, smoothing the spectrum with Savitzky-Golay smoothing, and measuring the peak top with a centroid top of 80%.
Protein identification was performed using MASCOT software (version 2.1.0; Matrix Science Inc., Boston, MA). An in-house customized database (built in June 2007; 231,942 sequences) based on the NCBI nonredundant protein sequence database with species limitation (only human, mouse, rat, and bovine proteins can pass) and with locus redundancy removal by NCBI EntrezGene was used. The MASCOT search parameters were as follows. Peptide tolerance was 0.45 Da, and MS/MS tolerance was 0.15 Da (monoisotopic mass). Fixed modification of carbamidomethylation (Cys) and variable modifications of oxidation (Met), acetylation (N terminus), and pyro-Glu (Glu and Gln) were selected, and up to four missed trypsin cleavages were allowed. A protein score of 40 (p < 0.05) and a peptide score of 25 were the minimum identification criteria, and manual examination was conducted for all proteins identified with fewer than 80 points on the protein score or fewer than four unique peptides. The criteria used for manual validation included (a) peptide fragment ions being clearly above base-line noise, (b) intense y or b ions corresponding to the N-terminal cleavage of proline or glycine and absence of C-terminal cleavage of proline or glycine (except where proline follows), (c) major five fragment ions being interpretable, and (d) not matching a common contaminant (such as silicone or polyethylene glycol) or trypsin-derived signals. In addition, each MS/MS spectrum of proteins identified with a small number (one or two) of peptides was compared with the same spectrum of the same proteins (if it exists) identified using a large set (three or more) of peptides for further manual confirmation. If peptides matched to multiple database entries, the one RefSeq accession number belonging to the species Homo sapiens was assigned for the identification protein.
Western Blotting
The eluted samples from the affinity purification and pulldown assay were resolved by SDS-PAGE and transferred to PVDF membranes (Bio-Rad). The membranes were blocked and incubated sequentially with the primary then secondary antibodies. The blots were developed using an enhanced chemiluminescence system (GE Healthcare) in accordance with the manufacturer's instructions. The primary antibodies used for this study are listed in supplemental Table 1.
Construction of Expression Vectors
To amplify the ILF31 isoforms (ILF3–1a, -1b, -2a, -2b, -3a, and -3b) and deletion mutants (ILF3-ΔN and ILF3-ΔC), PCR was performed using human testis Marathon-Ready cDNA (Clontech) with the primers listed in supplemental Table 2. The resulting fragments were ligated into the pcDNA3.1 (+) expression vector (Invitrogen) digested with EcoRI and NotI and sequenced to confirm that the inserts were correct. A FLAG peptide was inserted at the C terminus of all recombinant proteins as a detection marker for Western blotting analysis.
Pulldown Assay
The pulldown assays were performed using lysates of PC-3 cells overexpressing ILF3 isoforms or deletion mutants, all of which contained a C-terminal FLAG epitope tag. Transfection of each vector was performed using Lipofectamine LTX reagent (Invitrogen) in accordance with the manufacturer's instructions. The samples captured with the YM155 beads were subjected to Western blotting analysis.
Promoter-Luciferase Reporter Assay
For the luciferase reporter assay, we used a pSUR-luc reporter gene vector containing the promoter fragment (−2,751/+16) of the human survivin gene (11), and vectors containing truncated promoter fragments of the survivin promoter region (D1, −328/+16; D2, −152/+16; and D3, −109/+16). The truncated fragments of survivin promoter prepared from pSUR-luc by the PCR-based method were ligated into the XhoI/HindIII cleavage site of a pGL3-Basic vector (Promega).
HEK-293 cells were transiently co-transfected with 200 ng of ILF3 expression plasmid (ILF3–1a, ILF3–2a, ILF3–3a, or empty pcDNA3.1 (+) vector), 100 ng of reporter gene plasmid (pSUR-luc, D1, D2, or D3) and 1 ng of pGL4.75 (hRluc/CMV) Renilla plasmid (Promega) by using Lipofectamine LTX. Forty-eight hours after transfection of plasmids, dual luciferase reporter assays (Promega) were performed according to the manufacturer's instructions. Firefly luciferase activity was normalized to Renilla activity.
siRNA Experiments
The siRNA for survivin was purchased from Invitrogen. The siRNAs for ILF3 and negative control were designed using siDirect software (RNAi Co.) and were preconfirmed to reduce the mRNA expression of the target gene by over 80%. Sequences are listed in supplemental Table 3. The siRNAs were transfected at a final concentration of 10 nm into PC-3 cells (2.0 × 104 cells/well) using RNAiMax (Invitrogen) in accordance with the manufacturer's instructions. The cells were used for gene expression and Western blotting 72 h after transfection.
Real Time RT-PCR
Total RNA was prepared from siRNA-transfected PC-3 using an RNeasy kit (Qiagen), and cDNA was synthesized using the ThermoScript RT-PCR system (Invitrogen) in accordance with the manufacturer's instructions. Primer sequences were designed using Primer Express 1.0 software (Applied Biosystems) and are listed in supplemental Table 4. Quantitative PCR was performed using an ABI PRISM 7900HT sequence detection system (Applied Biosystems) with Power SYBR Green PCR master mix (Applied Biosystems).
RESULTS
ILF3/NF110 Is a Binding Target of YM155
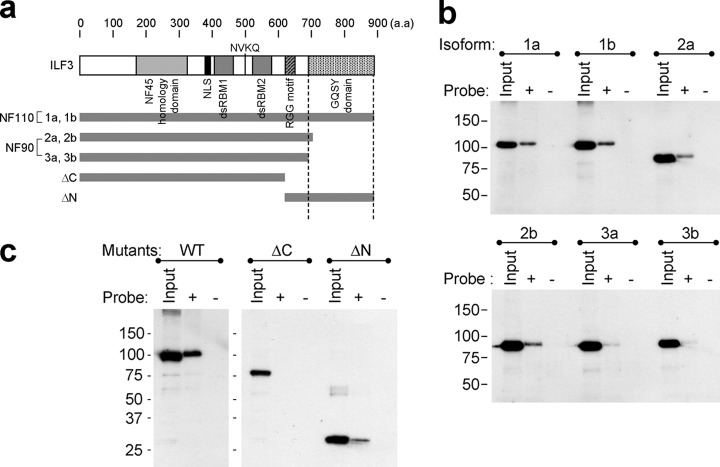
To identify the proteins that bind directly to YM155, we performed chemical proteomics studies. Affinity purification using a YM155 probe (Fig. 1) showed highly purified protein patterns on the SDS-PAGE gels in all experiments using different cell lysates (Fig. 2a). The molecular masses of the major and minor bands were ∼100 and 45 kDa, respectively. In MS analysis, interleukin enhancer binding factor 3 isoform 1 (ILF3/NF110, NP_036350) was identified with ILF3/NF110-specific peptides in the 100-kDa major band (supplemental Fig. 1, a–c) and interleukin enhancer binding factor 2 (ILF2/NF45, NP_004506), a heterodimer partner of ILF3, was detected in the 45-kDa minor band (supplemental Fig. 1d). The identification of ILF3 and ILF2 was further confirmed by Western blotting (Fig. 2b). ILF3 isoforms are classified by molecular mass, with 110- and 90-kDa isoforms referred to as ILF3/NF110 and ILF3/NF90, respectively (24, 25). It has been reported that ILF3/NF100, ILF3/NF90, and ILF2/NF45 are principally localized in the nucleus (25). Although the existence of the three molecules in the nucleus was confirmed in an input sample used for our affinity purification, only ILF3/NF110 and ILF2/NF45 were purified from the nuclear extract by YM155 beads (Fig. 2b). It is known that ILF2 forms complexes with both ILF3/NF110 and ILF3/NF90 (24), so if ILF2 bound directly to YM155, both ILF3/NF90 and ILF3/NF110 would have been detected via this indirect association. Given that this was not the case, we considered that it was instead ILF3/NF110 that bound directly to YM155 and that ILF2/NF45 was co-purified with the YM155 probe via ILF3/NF110.
Fig. 1.
YM155 probe used for affinity purification. The figure shows the chemical structure and activity of YM155 and YM155 probe. In vitro activities are shown as growth inhibitory concentrations (GI50) of PC-3 cells treated with YM155 or YM155 probe for 24 h and inhibitory concentrations (IC50) determined in endogenous survivin mRNA expression and survivin promoter reporter assays using HEK-293 cells treated with YM155 or YM155 probe for 24 h.
Fig. 2.
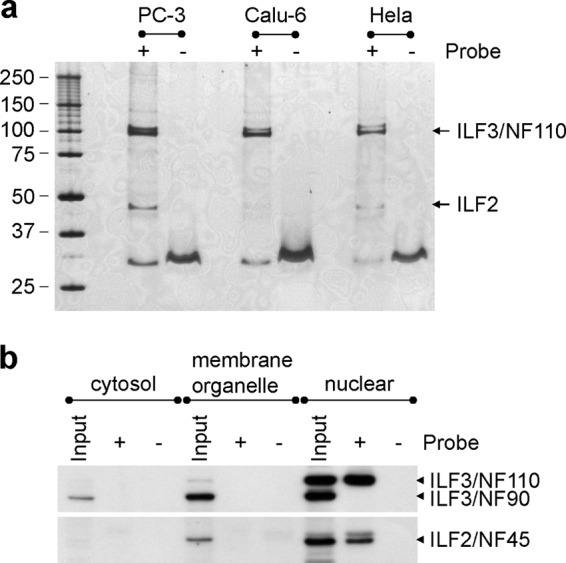
Identification of proteins binding to the YM155 probe. a, elution profile of proteins labeled with the YM155 probe. Cell lysates of human hormone-refractory prostate cancer (PC-3), non-small cell lung cancer (Calu-6), and uterine cervix (HeLa) cell lines were used for affinity purification. The SDS-PAGE profile was visualized by silver staining. The proteins (∼30 kDa) purified using beads with or without the YM155 probe were identified as fragments of keratin 1 (NP_006112) and keratin 10 (NP_000412). b, Western blotting analysis of the proteins purified from the subcellular extracts of PC-3 cells. ILF3 was detected as two bands in the input lane of the nuclear extract. The upper and lower bands correspond to ILF3/NF110 and ILF3/NF90, respectively. The validity of the subcellular extracts is demonstrated in supplemental Fig. 2.
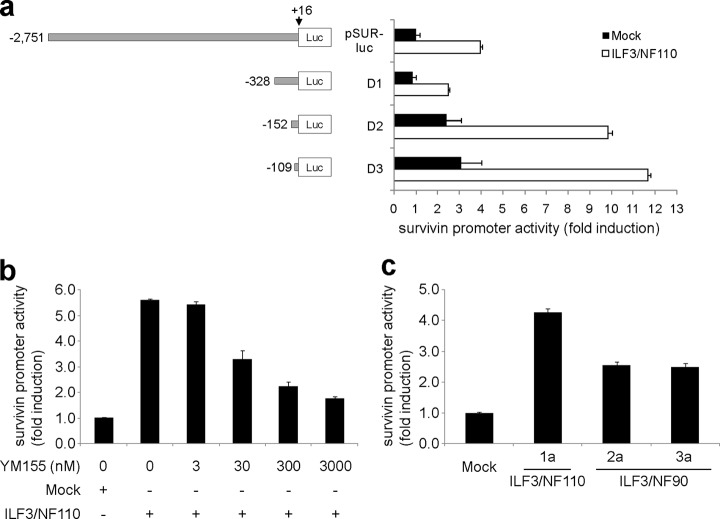
To find the YM155-binding site on ILF3, a pulldown assay was performed using ILF3 isoforms (ILF3–1a, -1b, -2a, -2b, -3a, and -3b) and two deletion mutants encoding the Met1–Val608 (ILF3-ΔC) and Arg609–Arg894 (ILF3-ΔN) fragments of ILF3–1a, constructed from human testis cDNA (Fig. 3a). Analyses performed using all of the ILF3 isoforms showed that although all of the isoforms were able to bind to YM155, shorter C-terminal length was correlated with reduced binding (Fig. 3b). As for deletion mutants, ILF3-ΔN was captured by YM155 beads, unlike ILF3-ΔC (Fig. 3c). These results indicate that the C-terminal region containing the GQSY domain is important for the stronger binding of ILF3/NF110 to YM155 than that of ILF3/NF90 to YM155.
Fig. 3.
Pulldown assay using ILF3 isoforms and deletion mutants. a, the structure of ILF3 showing the predicted binding site of YM155. The ΔC fragment contains an ILF2 homology domain and two double-stranded RNA binding motifs (dsRBM). The ΔN fragment has an RGG motif and a GQSY-rich domain. b, pulldown assay using ILF3 isoforms. The amino acid sequences of the isoforms are shown in supplemental Fig. 3. The isolated proteins from YM155 beads were detected by Western blotting using an antibody against the FLAG tag. c, pulldown assay using ILF3 deletion mutants. Wild-type (WT), ΔC, and ΔN consist of Met1–Arg894, Met1–Val608, and Arg609–Arg894 residues of ILF3/NF110 (NP_036350), respectively.
Overexpression and Knockdown of ILF3/NF110 Influence Survivin Expression
To determine whether ILF3/NF110 regulates survivin expression, promoter activities were investigated using various lengths of the survivin gene promoter region in HEK-293 cells (Fig. 4a). Overexpression of ILF3/NF110 enhanced survivin promoter activity. The region of −109 to +16 base pairs relative to the transcription start site was critical for survivin expression via ILF3/NF110. The enhanced promoter activity induced by overexpression of ILF3/NF110 was attenuated by YM155 in a concentration-dependent manner (Fig. 4b), indicating that YM155 regulates survivin expression via ILF3/NF110 and that ILF3/NF110 is the physiological target of YM155 for survivin suppression. Overexpression of ILF3/NF110 was more effective than that of ILF3/NF90 (Fig. 4c) and ILF3-ΔC (supplemental Fig. 5) at enhancing survivin promoter activity, indicating the importance of the C-terminal region of ILF3/NF110 for survivin expression.
Fig. 4.
Enhancement of survivin expression by ILF3/NF110 overexpression. a, promoter activities were examined using various lengths of the survivin gene promoter region in HEK-293 cells. Luciferase activity was measured in the presence of ILF3–1a (white) or control plasmid (black). The firefly luciferase enzyme activity was normalized to Renilla luciferase enzyme activity. The values are the means ± S.D. from three independent experiments. b, promoter activities of D3 containing −109 to +16 bp relative to the transcription start site were examined at different concentrations of YM155 in the presence of ILF3–1a or control plasmid in HEK-293 cells. c, the promoter activities of D3 (−109 to +16 bp) were examined in the presence of ILF3–1a, -2a, or -3a or control plasmid in HEK-293 cells. Comparable protein expression levels following overexpression were confirmed by Western blotting (supplemental Fig. 4).
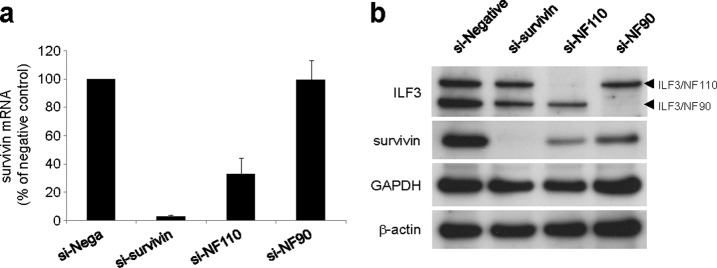
To confirm the positive regulation of endogenous survivin expression by ILF3/NF110, we measured survivin expression after siRNA knockdown of ILF3 in PC-3 cells. Knockdown of ILF3/NF110 suppressed endogenous survivin expression at both mRNA and protein levels, whereas knockdown of ILF3/NF90 did not (Fig. 5). These results indicate that ILF3/NF110 positively regulates survivin expression and that the C-terminal region of ILF3/NF110 is a key factor in this activity.
Fig. 5.
Inhibition of survivin expression by ILF3/NF110 knockdown. a, PC-3 cells were treated with the indicated siRNAs. The mRNA levels of survivin were measured by real time RT-PCR (n = 3). The values are the means ± S.D. The expression levels of survivin mRNA are normalized to those of GAPDH and expressed relative to those cells receiving negative siRNA treatment. b, protein expression levels following siRNA treatment were confirmed by Western blotting.
DISCUSSION
Using affinity purification with a YM155 probe, we identified ILF3/NF110 and ILF2/NF45, which are known to form a complex (24). Our results suggest that the main binding target of YM155 is ILF3/NF110 and that the C-terminal region of ILF3/NF110 is important for high affinity binding to YM155.
ILF3 is known to be a transcription factor (26–28) and is overexpressed in doxorubicin- and cyclophosphamide-resistant tumors and in lung cancer development and progression (29, 30), implying an important link to cancer. ILF3/NF110 is reported to stimulate the transcription of other genes and, consistent with our survivin results, does so more strongly than ILF3/NF90 (27, 28). ILF3/NF110 contains a characteristic C-terminal GQSY domain (24–26), which is known to form a protein-protein interaction with other proteins such as FUS and PRMT1 (28, 31). Recently, it was reported that FUS and PRMT1 synergistically activate survivin expression (32). Our results showed that survivin expression was regulated by ILF3/NF110 through the −109 to +16 bp region of the survivin promoter, which is consistent with the region reported to be targeted by FUS and PRMT1 (32). Therefore, we hypothesize that survivin expression could be regulated by ILF3/NF110 binding to FUS and PRMT1 on its C-terminal region. Studies to test this hypothesis are currently underway. Regarding ILF3/NF90, it is also a positive regulator of survivin expression (Figs. 4c and 5b), whereas survivin mRNA levels were significantly reduced by depletion of ILF3/NF110, not ILF3/NF90 (Fig. 5a). This contrast indicates that ILF3/NF110 and ILF3/NF90 might regulate survivin expression through different mechanisms.
Our proteomics approach using a chemical probe resulted in identification of ILF3/NF110 as the target molecule of YM155. To our knowledge, this is the first report showing that ILF3/NF110 regulates survivin expression and can be a drug target molecule for the treatment of cancer. The exact mechanism by which YM155 suppresses survivin expression via ILF3/NF110 remains to be elucidated, but better understanding of these mechanisms may contribute to the creation of a new generation of survivin suppressants.
Acknowledgments
We are grateful to A. Ohtsu, M. Mito, I. Sato, N. Kamata, K. Yodoya, and M. Masumoto for technical assistance and to other members of the staff at Astellas Pharma for help. We thank T. Ogawa, M. Takahashi, and other members of the staff at JSR Corporation for providing the affinity beads. All authors are employees of Astellas Pharma, Inc.
Footnotes
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- ILF
- interleukin enhancer-binding factor
- siRNA
- small interfering RNA
- NF
- nuclear factor.
REFERENCES
- 1. Tamm I., Wang Y., Sausville E., Scudiero D. A., Vigna N., Oltersdorf T., Reed J. C. (1998) IAP-family protein Survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 58, 5315–5320 [PubMed] [Google Scholar]
- 2. Altieri D. C. (2006) The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr. Opin. Cell Biol. 18, 609–615 [DOI] [PubMed] [Google Scholar]
- 3. Lens S. M., Vader G., Medema R. H. (2006) The case for Survivin as mitotic regulator. Curr. Opin. Cell Biol. 18, 616–622 [DOI] [PubMed] [Google Scholar]
- 4. Ambrosini G., Adida C., Altieri D. C. (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3, 917–921 [DOI] [PubMed] [Google Scholar]
- 5. O'Connor D. S., Grossman D., Plescia J., Li F., Zhang H., Villa A., Tognin S., Marchisio P. C., Altieri D. C. (2000) Regulation of apoptosis at cell division by p34(cdc2) phosphorylation of survivin. Proc. Natl. Acad. Sci. U.S.A. 97, 13103–13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukuda S., Pelus L. M. (2001) Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34+ cells by hematopoietic growth factors: Implication of survivin expression in normal hematopoiesis. Blood 98, 2091–2100 [DOI] [PubMed] [Google Scholar]
- 7. Altieri D. C. (2008) Opinion: Survivin, cancer networks and pathway-directed drug discovery. Nat. Rev. Cancer 8, 61–70 [DOI] [PubMed] [Google Scholar]
- 8. Mita A. C., Mita M. M., Nawrocki S. T., Giles F. J. (2008) Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 14, 5000–5005 [DOI] [PubMed] [Google Scholar]
- 9. Ryan B. M., O'Donovan N., Duffy M. J. (2009) Survivin: A new target for anti-cancer therapy. Cancer Treat. Rev. 35, 553–562 [DOI] [PubMed] [Google Scholar]
- 10. Chantalat L., Skoufias D. A., Kleman J. P., Jung B., Dideberg O., Margolis R. L. (2000) Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual α-helical extensions. Mol. Cell 6, 183–189 [PubMed] [Google Scholar]
- 11. Nakahara T., Takeuchi M., Kinoyama I., Minematsu T., Shirasuna K., Matsuhisa A., Kita A., Tominaga F., Yamanaka K., Kudoh M., Sasamata M. (2007) YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 67, 8014–8021 [DOI] [PubMed] [Google Scholar]
- 12. Li F., Ackermann E. J., Bennett C. F., Rothermel A. L., Plescia J., Tognin S., Villa A., Marchisio P. C., Altieri D. C. (1999) Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat. Cell Biol. 1, 461–466 [DOI] [PubMed] [Google Scholar]
- 13. Iwasa T., Okamoto I., Suzuki M., Nakahara T., Yamanaka K., Hatashita E., Yamada Y., Fukuoka M., Ono K., Nakagawa K. (2008) Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin. Cancer Res. 14, 6496–6504 [DOI] [PubMed] [Google Scholar]
- 14. Satoh T., Okamoto I., Miyazaki M., Morinaga R., Tsuya A., Hasegawa Y., Terashima M., Ueda S., Fukuoka M., Ariyoshi Y., Saito T., Masuda N., Watanabe H., Taguchi T., Kakihara T., Aoyama Y., Hashimoto Y., Nakagawa K. (2009) Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin. Cancer Res. 15, 3872–3880 [DOI] [PubMed] [Google Scholar]
- 15. Tolcher A. W., Mita A., Lewis L. D., Garrett C. R., Till E., Daud A. I., Patnaik A., Papadopoulos K., Takimoto C., Bartels P., Keating A., Antonia S. (2008) Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of Survivin. J. Clin. Oncol. 26, 5198–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giaccone G., Zatloukal P., Roubec J., Floor K., Musil J., Kuta M., van Klaveren R. J., Chaudhary S., Gunther A., Shamsili S. (2009) Multicenter phase II trial of YM155, a small-molecule suppressor of Survivin, in patients with advanced, refractory, non-small-cell lung cancer. J. Clin. Oncol. 27, 4481–4486 [DOI] [PubMed] [Google Scholar]
- 17. Shimizu N., Sugimoto K., Tang J., Nishi T., Sato I., Hiramoto M., Aizawa S., Hatakeyama M., Ohba R., Hatori H., Yoshikawa T., Suzuki F., Oomori A., Tanaka H., Kawaguchi H., Watanabe H., Handa H. (2000) High-performance affinity beads for identifying drug receptors. Nat. Biotechnol. 18, 877–881 [DOI] [PubMed] [Google Scholar]
- 18. Murray C. M., Hutchinson R., Bantick J. R., Belfield G. P., Benjamin A. D., Brazma D., Bundick R. V., Cook I. D., Craggs R. I., Edwards S., Evans L. R., Harrison R., Holness E., Jackson A. P., Jackson C. G., Kingston L. P., Perry M. W., Ross A. R., Rugman P. A., Sidhu S. S., Sullivan M., Taylor-Fishwick D. A., Walker P. C., Whitehead Y. M., Wilkinson D. J., Wright A., Donald D. K. (2005) Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 1, 371–376 [DOI] [PubMed] [Google Scholar]
- 19. Kotake Y., Sagane K., Owa T., Mimori-Kiyosue Y., Shimizu H., Uesugi M., Ishihama Y., Iwata M., Mizui Y. (2007) Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 3, 570–575 [DOI] [PubMed] [Google Scholar]
- 20. Kaida D., Motoyoshi H., Tashiro E., Nojima T., Hagiwara M., Ishigami K., Watanabe H., Kitahara T., Yoshida T., Nakajima H., Tani T., Horinouchi S., Yoshida M. (2007) Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat. Chem. Biol. 3, 576–583 [DOI] [PubMed] [Google Scholar]
- 21. Leslie B. J., Hergenrother P. J. (2008) Identification of the cellular targets of bioactive small organic molecules using affinity reagents. Chem. Soc. Rev. 37, 1347–1360 [DOI] [PubMed] [Google Scholar]
- 22. Huang S. M., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C. J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M. W., Lengauer C., Finan P. M., Tallarico J. A., Bouwmeester T., Porter J. A., Bauer A., Cong F. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 [DOI] [PubMed] [Google Scholar]
- 23. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 24. Guan D., Altan-Bonnet N., Parrott A. M., Arrigo C. J., Li Q., Khaleduzzaman M., Li H., Lee C. G., Pe'ery T., Mathews M. B. (2008) Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control. Mol. Cell. Biol. 28, 4629–4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parrott A. M., Walsh M. R., Reichman T. W., Mathews M. B. (2005) RNA binding and phosphorylation determine the intracellular distribution of nuclear factors 90 and 110. J. Mol. Biol. 348, 281–293 [DOI] [PubMed] [Google Scholar]
- 26. Reichman T. W., Mathews M. B. (2003) RNA binding and intramolecular interactions modulate the regulation of gene expression by nuclear factor 110. RNA 9, 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reichman T. W., Muñiz L. C., Mathews M. B. (2002) The RNA binding protein nuclear factor 90 functions as both a positive and negative regulator of gene expression in mammalian cells. Mol. Cell. Biol. 22, 343–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohno M., Komakine J., Suzuki E., Nishizuka M., Osada S., Imagawa M. (2011) Interleukin enhancer-binding factor 3 functions as a liver receptor homologue-1 co-activator in synergy with the nuclear receptor co-activators PRMT1 and PGC-1α. Biochem. J. 437, 531–540 [DOI] [PubMed] [Google Scholar]
- 29. Cleator S., Tsimelzon A., Ashworth A., Dowsett M., Dexter T., Powles T., Hilsenbeck S., Wong H., Osborne C. K., O'Connell P., Chang J. C. (2006) Gene expression patterns for doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) response and resistance. Breast Cancer Res. Treat. 95, 229–233 [DOI] [PubMed] [Google Scholar]
- 30. Guo N. L., Wan Y. W., Tosun K., Lin H., Msiska Z., Flynn D. C., Remick S. C., Vallyathan V., Dowlati A., Shi X., Castranova V., Beer D. G., Qian Y. (2008) Confirmation of gene expression-based prediction of Survival in non-small cell lung cancer. Clin. Cancer Res. 14, 8213–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang J., Kao P. N., Herschman H. R. (2000) Protein-arginine methyltransferase I, the predominant protein-arginine methyltransferase in cells, interacts with and is regulated by interleukin enhancer-binding factor 3. J. Biol. Chem. 275, 19866–19876 [DOI] [PubMed] [Google Scholar]
- 32. Du K., Arai S., Kawamura T., Matsushita A., Kurokawa R. (2011) TLS and PRMT1 synergistically coactivate transcription at the survivin promoter through TLS arginine methylation. Biochem. Biophys. Res. Commun. 404, 991–996 [DOI] [PubMed] [Google Scholar]