Abstract
Purpose
The purpose of this study was to determine whether increasing the Ti6Al4V surface oxide negative charge through heat (600℃) or radiofrequency plasma glow discharge (RFGD) pretreatment, with or without a subsequent coating with fibronectin, stimulated osteoblast gene marker expression in the MC3T3 osteoprogenitor cell line.
Methods
Quantitative real-time polymerase chain reaction was used to measure changes over time in the mRNA levels for osteoblast gene markers, including alkaline phosphatase, bone sialoprotein, collagen type I (α1), osteocalcin, osteopontin and parathyroid hormone-related peptide (PTH-rP), and the osteoblast precursor genes Runx2 and osterix.
Results
Osteoprogenitors began to differentiate earlier on disks that were pretreated with heat or RFGD. The pretreatments increased gene marker expression in the absence of a fibronectin coating. However, pretreatments increased osteoblast gene expression for fibronectin-coated disks more than uncoated disks, suggesting a surface oxide-mediated specific enhancement of fibronectin's bioactivity. Heat pretreatment had greater effects on the mRNA expression of genes for PTH-rP, alkaline phosphatase and osteocalcin while RFGD pretreatment had greater effects on osteopontin and bone sialoprotein gene expression.
Conclusions
The results suggest that heat and RFGD pretreatments of the Ti6Al4V surface oxide stimulated osteoblast differentiation through an enhancement of (a) coated fibronectin's bioactivity and (b) the bioactivities of other serum or matrix proteins. The quantitative differences in the effects of the two pretreatments on osteoblast gene marker expression may have arisen from the unique physico-chemical characteristics of each resultant oxide surface. Therefore, engineering the Ti6Al4V surface oxide to become more negatively charged can be used to accelerate osteoblast differentiation through fibronectin-dependent and independent mechanisms.
Keywords: Cell differentiation, Dental implants, Fibronectins, Integrin alpha5beta1, Osteoblasts
INTRODUCTION
Although the outcomes of dental and orthopedic implant procedures are usually successful, in many instances the long-term stability and functionality of the implant cannot be achieved. Despite the reported long-term predictability of dental implants [1,2], failures do occur in 10% of cases within a 5-year period [3]. The survival rates decrease to 71 to 83.5% over a 5 to 6 year period for dental implants placed in previously failed implant sites [4,5]. The quality and quantity of bone that forms at the implant-skeletal interface is generally believed to be one of the major determinants of implant success. Therefore, improving fixation by enhancing the attachment and function of osteoblastic cells at the implant surface is likely to substantially decrease the likelihood of failure, especially for implants placed in previously failed implant sites.
Commercially pure titanium (cpTi) and titanium alloys are widely employed in orthopedic and dental implants due to their biocompatibility [6,7]. Titanium-aluminum-vanadium alloy is used in the fabrication of prosthetic joint replacements. The Ti6Al4V alloy has also been successfully utilized in the dental field [8]. Both titanium and its alloys form an active oxide layer that readily interacts with extracellular matrix proteins produced by the cells and cell surface proteins. It is this superficial oxide found on both metals, titanium dioxide (TiO2) being the most abundant, that provides an interface that is biocompatible with peri-implant tissues [9]. The surface oxide of Ti6Al4V is similar to that of pure titanium except that it is enriched with aluminum-oxide when present in air [10]. Altering the surface of titanium implant materials has been shown to affect protein adsorption, cell-substrate interactions, and tissue development [11]. However, the mechanisms by which surface oxide properties modulate the bioactivity of bound osteogenic proteins to influence osteoblast behavior and osseointegration are poorly understood.
A variety of methods have been explored to prevent implant failure including the use of bioactive adhesive peptides or extracellular matrix proteins such as fibronectin (FN) to facilitate the attachment of osteogenic cells to the implant surface [12-16]. Our laboratory and others have studied extracellular matrix proteins such as FN or human bone sialoprotein (hBSP) or hBSP peptides following their non-covalent adsorption [12-16] to the implant surface oxide to increase the adhesion of osteoblasts. FN is also thought to play an important role in skeletal development by regulating osteoblast differentiation and mineralization [17]. Most importantly, since we have shown that FN binds rapidly and irreversibly to TiO2 [15], the protein can be efficiently adsorbed to titanium materials without the use of intervening chemical coupling agents. However, the precise influence of the metal surface oxide on the capacity of FN to modulate osteoblast attachment and differentiation has yet to be fully elucidated.
When added to implant materials, exogenous FN is likely to manifest a capacity to enhance osteoblast attachment/function or integration that is not obscured or overcome by the effects of serum FN for a number of reasons. Histological analyses of immediately loaded titanium dental implants demonstrated a direct contact between titanium and bone over the whole surface area of the implant immediately after insertion. No substantial blood clotting or inflammatory repair process was observed in the implant-tissue interface preceding bone cell adhesion [18]. Other studies have shown that soluble FN in serum, which appears more inert than the insoluble fibrillar form [19], does not bind to tooth surfaces [20], and a number of serum and matrix proteins can compete with FN for binding to bone [21,22]. Therefore, due to immediate direct implant-bone contact after insertion, the low intrinsic binding activity of serum FN, and competition from other proteins for binding to the implant surface, it is unlikely that endogenous FN is able to bind to implant materials in vivo (or in vitro). These findings suggest that the effects of exogenous FN on bone cells and integration, when it is adsorbed to an implant surface, will supercede those of endogenous FN, which are likely to be minimal or negligible.
Our earlier studies examined the effects of modifying the surface oxide properties, such as oxide chemistry or topography, of Ti6Al4V on adsorbed FN's bioactivity toward osteoblasts [10,23-25]. These studies demonstrated that pretreating Ti6Al4V surface oxide with heat or radiofrequency plasma glow discharge (RFGD) increased the oxide's negative net charge and dramatically increased the number of osteoblastic cells that attached to surface adsorbed FN [23,24]. In a later investigation of the mechanism for the effects of pretreatments on FN bioactivity, we showed that modified Ti6Al4V disks increased the exposure of FN's integrin binding domain to enhance its binding to α5β1 integrin receptors in osteoblasts [25]. These results suggested that heat or RFGD pretreatments of Ti6Al4V amplified FN's intrinsic biological activity toward osteoblast integrins by inducing conformational changes in the matrix protein upon its adsorption. More recently, our laboratory has shown that when FN is precoated on Ti6Al4V disks prior to the addition of osteoprogenitors, the peak expression of all of the osteoblast gene markers analyzed was increased modestly in comparison to the uncoated disks [26]. These latter results suggest that the coated FN has the capacity to stimulate osteoblast differentiation.
In view of the findings that heat and RFGD pretreatments increased FN's integrin binding activity [24,25] and cell spreading [24], the purpose of this study was to determine if these pretreatments also augment FN-promoted osteoblast differentiation as well as baseline differentiation. In this study, we have tested the hypothesis that surface pretreatments of Ti6Al4V, which make the surface oxide more negatively charged [23], increase FN-promoted osteoblast differentiation. We have also tested the sub-hypothesis that engineering the surface oxide to become more negatively charged exerts a general stimulatory effect on the bioactivities of other serum and matrix proteins to increase osteoblastogenesis without a FN coating. These hypotheses were tested by using quantitative real-time polymerase chain reaction (qRT-PCR) to measure changes in the mRNA levels for several osteoblast genes over time.
MATERIALS AND METHODS
Disk preparation
Cylindrical implant disks were initially prepared from Ti6Al4V sheets obtained from TIMET (Wentzville, MO, USA). Metal sheets were cut into strips, polished by machining, and later punched into disks (Industrial Tool & Die Co., Troy, NY, USA) as previously described [23]. The disks were washed successively in isopropanol, acetone, xylene, acetone, and 1 M ammonium hydroxide, and then rinsed with deionized water to remove organic and inorganic contaminants. The disks were then passivated in 40% nitric acid to form a stable surface oxide layer and rinsed three times with deionized water. Next, the disks were dried, transferred into acid-washed scintillation vials in a HEPA-filtered isolation hood (USA Scientific Inc., Ocala, FL, USA), and stored closed in an auto-desiccator cabinet (Sanplatec Co., Osaka, Japan). All of the disks were sterilized using a rapid dry heat oven (Alpha Medical, Hempstead, NY, USA) for 5 minutes. Some of the cleaned and passivated disks were further treated with RFGD or heat (600℃) as previously described [23]. For heat pretreatment, the disks were heated to a temperature of 600℃ in air for 1 hour [23]. RFGD pretreatment of disks was performed using a modified Harrick RF unit (PDC-002, Harrick Scientific Co., Ossining, NY, USA) with a quartz chamber to subject samples to an oxygen plasma pretreatment [23]. The passivated Ti6Al4V disks were placed on a clean quartz tray. The tray was inserted into the RF unit, and the unit was placed under dry vacuum (EcoDry-M oil-less vacuum pump; Leybold Vakuum, Köln, Germany). When the vacuum was low enough (1,600 mTorr) to remove all water vapor, oxygen was gradually bled into the system via a needle valve. The gas flow rate was monitored using an Omega shielded flow meter (Omega Technologies Co., Stamford, CT, USA) at a rate of 150 mL/min. All oxygen gas was prefiltered prior to its entry into the chamber (Advantec MFS Inc., Pleasanton, CA, USA). Samples of titanium alloy were treated with a 13.56 MHz RF power-generated oxygen plasma for 5 minutes at 29.6 W [23]. Following heat or RFGD pretreatment, disks were sterilized and stored as previously described for untreated specimens [10]. The treated disks were produced in parallel to those analyzed previously [23]. Continuous quality control was maintained for disk preparation/pretreatment. A regular analysis of disk topography and charge was performed to insure that the treated disks employed in the current investigation possessed the same physico-chemical properties as those analyzed previously [23].
Cell culture
MC3T3-E1 cells (subclone 4; American Type Culture Collection; Manassas, VA, USA), which exhibit high levels of osteoblast differentiation [27], were cultured in minimum essential medium (MEM)-α with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA).
Cell culture conditions for measuring the effects of surface pretreatments on osteoblast gene marker expression
Ti6Al4V disks were placed into 24-well plates (Laboratory Disposable Products Inc., North Haledon, NJ, USA) and incubated with 1× phosphate buffered saline (PBS) or a 1 nM FN (Sigma-Aldrich Co., St. Louis, MO, USA)/1× PBS solution overnight at room temperature under a cell culture hood. At this concentration of FN solution, a surface concentration of 120 to 170 ng adsorbed FN/cm2 was obtained for the untreated, heat-treated and RFGD-treated disks. No significant differences in FN adsorption were found among the three groups [24]. We have shown that this concentration of FN increased the attachment of MC3T3 cells to the titanium alloy 6- to 8-fold compared to uncoated disks [24]. The PBS and FN/PBS solutions were removed and each disk was plated with 500,000 cells in MEM-α with 10% FBS. A confluent cell monolayer was obtained three days following cell plating. Since this study focused on the effects of the alloy surface and FN coating on osteoblast differentiation, not proliferation, three days following the initial cell plating was designated as day 0 of the experimental period. RNA was then extracted at days 3, 7, 10, 14, 21, and 28 of the experimental period.
qRT-PCR
Total RNA was isolated using RNeasy Mini Kits (Qiagen, Valencia, CA, USA) following the direct lysis protocol. The quality and yield of the recovered RNA were evaluated by absorption at 260 and 280 nm. The total RNA was reverse transcribed into cDNA by using First Strand cDNA Synthesis Kits (Fermentas Inc., Glen Burnie, MD, USA). Equal amounts of RNA from each sample were reverse transcribed and the resultant cDNA was amplified by polymerase chain reaction using iQ SYBR Green Supermix (Bio-Rad Laboratories Inc., Hercules, CA, USA). The primer pairs are shown in Table 1. qRT-PCR was performed using the My iQ Single Color Real Time PCR Detection System (Bio-Rad Laboratories Inc.). The specificity of each primer pair for the corresponding mouse cDNA (shown by the gene accession number in Table 1) was confirmed using the National Center for Biotechnology Information Basic Local Alignment Search Tool program to search its nucleotide database for homologous sequences. The PCR program used the following parameters in sequential order: one cycle of 3 minutes at 95℃; 40 cycles of 10 seconds at 95℃ followed by 45 seconds at 60℃; one cycle of 1 minute at 95℃; one cycle of 1 minute at 55℃; followed by a final extension cycle of 800 seconds at 55℃. Beta-actin was used as an internal control for sample normalization. PCR products were analyzed within the linear range of amplification for the various genes examined. Changes in the relative levels of expression of specific genes over the time of the cell culture (days 0, 3, 7, 10, 14, 21, and 28) were monitored. The control samples received no FN pre-coating. The expression of each specific mRNA was measured at the selected points in time (relative to day 0 in the control group). For each experimental condition (untreated, heat-treated, or RFGD-treated), experiments measuring the relative mRNA expression at all of the points in time were repeated at least three times (each experiment was performed with a separate independent cell culture). The data presented for each point in time was averaged from multiple independent cultures so that artifacts arising from atypical single cultures (associated with passage number, differences between lots of serum, or subtle alterations in subcultivation protocol) could be minimized.
Table 1.
Primers used for quantitative real time polymerase chain reaction.
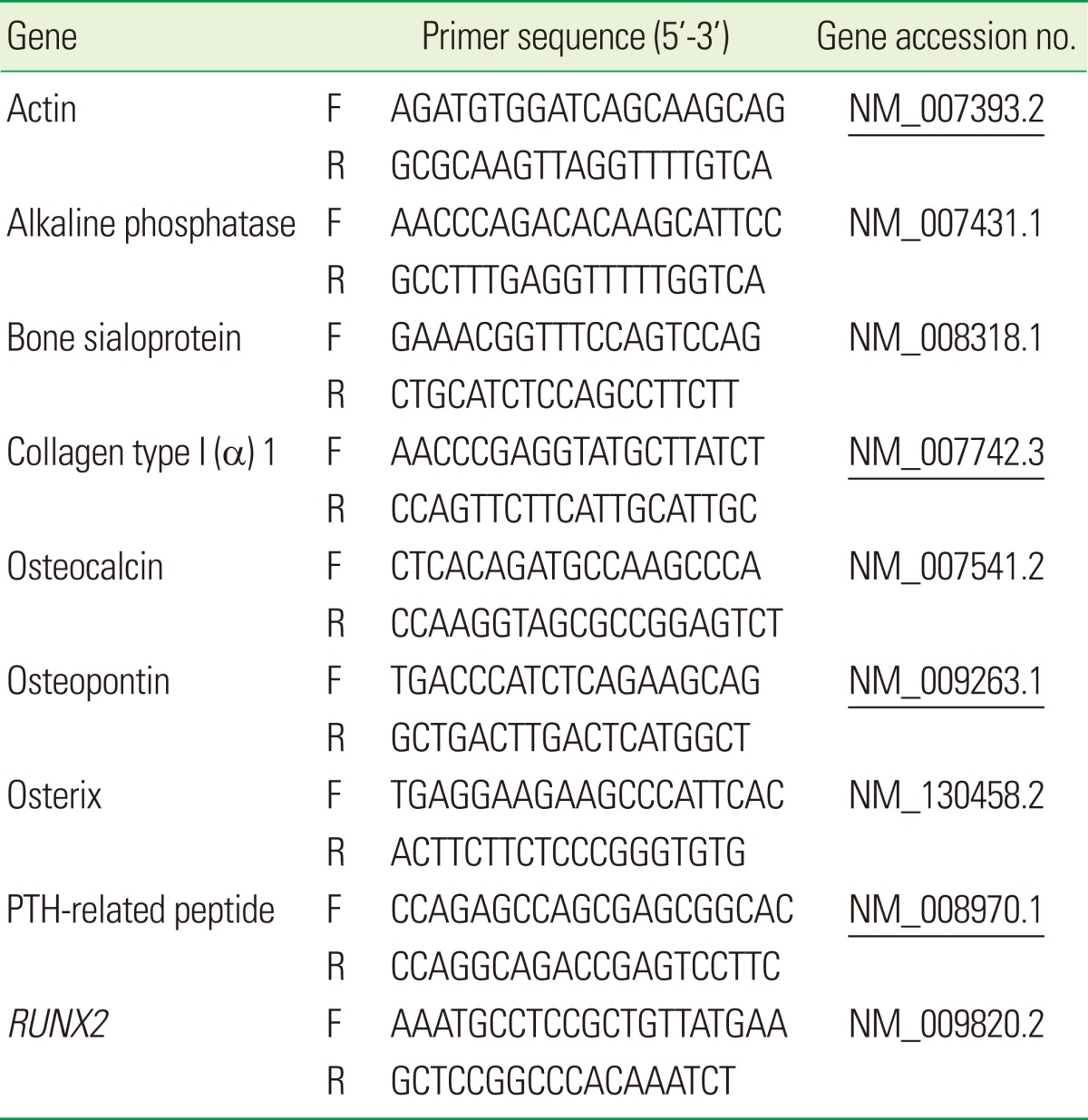
F: forward, R: reverse.
Statistical analysis
Data are presented as mean±standard error of the mean (N, total number of independent cell cultures) for each experimental condition. Statistical comparisons were performed using analysis of variance (with the alpha level set at 0.05) comparing all of the experimental conditions (untreated, heat-pretreated, and RFGD-pretreated) at a given point in time.
RESULTS
The effects of heat and RFGD pretreatments on Runx2 and osterix mRNA expression
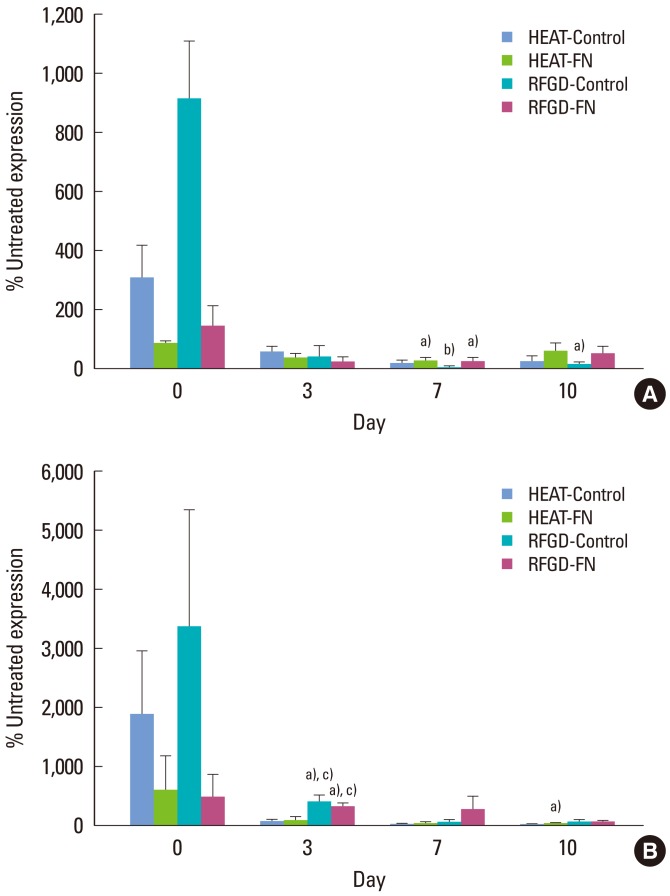
Fig. 1A shows the levels of mRNA expression for Runx2 and osterix genes in MC3T3 cells attached to pretreated Ti6Al4V disks presented as a percentage of the corresponding levels of mRNA expression measured for untreated disks. In general, both heat and RFGD pretreatments produced an early stimulation in Runx2 mRNA expression on day 0 for both control disks and disks precoated with 1 nM FN, although mRNA levels were lower in the latter group. After day 0, both pretreatments promoted a decrease in Runx2 mRNA expression compared to the untreated disks (Fig. 1A).
Figure 1.
Effects of heat and radiofrequency plasma glow discharge (RFGD) pretreatments on (A) Runx2 and (B) osterix mRNA expression in MC3T3 osteoprogenitor cells cultured on Ti6Al4V disks. Both untreated and pretreated disks were either uncoated (control) or precoated (fibronectin, FN) with 1 nM FN overnight and plated with MC3T3 cells. The levels of Runx2 and osterix mRNA expression measured by quantitative real time polymerase chain reaction for treated disks at the indicated points in time are presented as a percentage of the corresponding levels of mRNA expression measured for untreated disks. The data represent means±standard error for 3 to 7 independent cultures at each point in time. a)Significantly greater (P<0.05) than untreated disks. b)Significantly greater (P<0.01) than untreated disks. c)Significantly greater (P<0.05) than heat-pretreated disks based on analysis of variance.
In general, the pretreatments produced an early stimulation in osterix mRNA expression on day 0, although transcript levels were lower for coated compared to uncoated disks (Fig. 1B). The RFGD pretreatment also increased the osterix gene expression on day 3. In general, the stimulatory effects of pretreatments on osterix mRNA expression in MC3T3 cells decreased with time in culture. Heat pretreatment promoted a decrease in osterix mRNA expression compared to that of the untreated disks on day 10 (Fig. 1B).
The effects of heat and RFGD pretreatments on type I collagen (α1), parathyroid hormon-related peptide (PTH-rP), and alkaline phosphatase mRNA expression
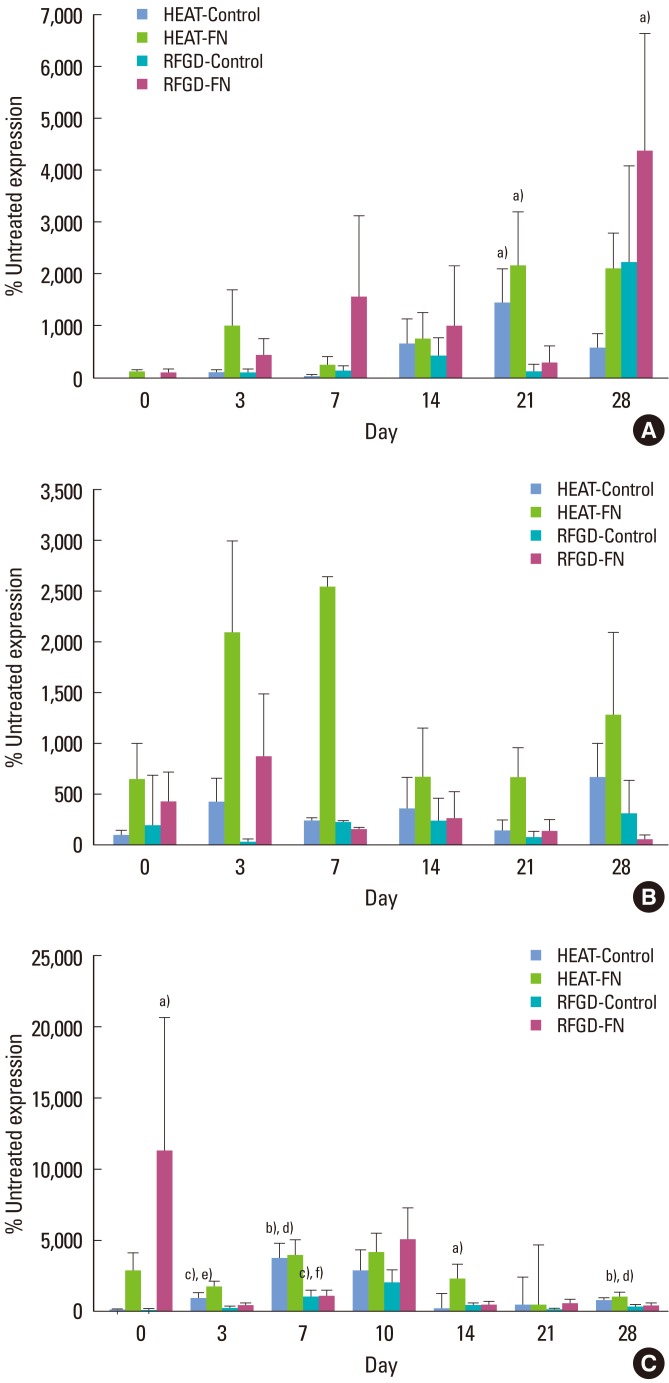
Fig. 2A shows that heat and RFGD pretreatments generally increased type I collagen (α1) mRNA expression compared to untreated disks at points in time from 3 to 28 days. Both pretreatments appeared to promote a pattern in which type I collagen (α1) mRNA expression was increased for FN-coated disks more than control disks at almost every point in time. The percent stimulation of gene expression produced by both pretreatments generally increased over time (Fig. 2A).
Figure 2.
Effects of heat and radiofrequency plasma glow discharge (RFGD) pretreatments on (A) type I collagen (α1), (B) parathyroid hormon-related peptide (PTH-rP), and (C) alkaline phosphatase mRNA expression in MC3T3 osteoprogenitor cells cultured on Ti6Al4V disks. The levels of type I collagen (α1), PTH-rP, and alkaline phosphatase mRNA expression measured by quantitative real time polymerase chain reaction for treated disks at the indicated points in time are presented as a percentage of the levels of mRNA expression measured for untreated disks. Data represent means±standard error for 3 to 8 independent cultures at each point in time. FN: fibronectin. a-c)Significantly greater (P<0.05, 0.01, 0.001) than untreated disks. d-f)Significantly greater (P<0.05, 0.01, 0.001) than RFGD-pretreated disks based on analysis of variance.
Both heat and RFGD pretreatments promoted a general pattern of increased PTH-rP mRNA expression on days 0 and 3 (Fig. 2B). At these points in time, the pretreatments appeared to increase PTH-rP mRNA expression in the FN group more than the control group. Heat pretreatment appeared to increase PTH-rP mRNA expression from day 7 to day 28, showing generally greater effects for FN-coated disks compared to control disks. In general, heat pretreatment produced a pattern of greater stimulation in PTH-rP gene expression compared to the RFGD pretreatment (Fig. 2B).
Pretreatments caused an early increase in alkaline phosphatase mRNA expression on day 0 for FN-coated disks, although only RFGD produced a statistically significant effect (Fig. 2C). At points in time later than day 0, both RFGD and heat pretreatments appeared to increase alkaline phosphatase mRNA expression. However, heat pretreatment exhibited greater effects than RFGD pretreatment at every point in time after day 0 and statistically significant increases in gene expression were observed only for the heat-treated disks. The heat pretreatment generally promoted greater percentage increases in alkaline phosphatase gene expression for FN-coated disks compared to control (uncoated) disks (Fig. 2C).
The effects of heat and RFGD pretreatments on osteopontin, bone sialoprotein, and osteocalcin mRNA expression
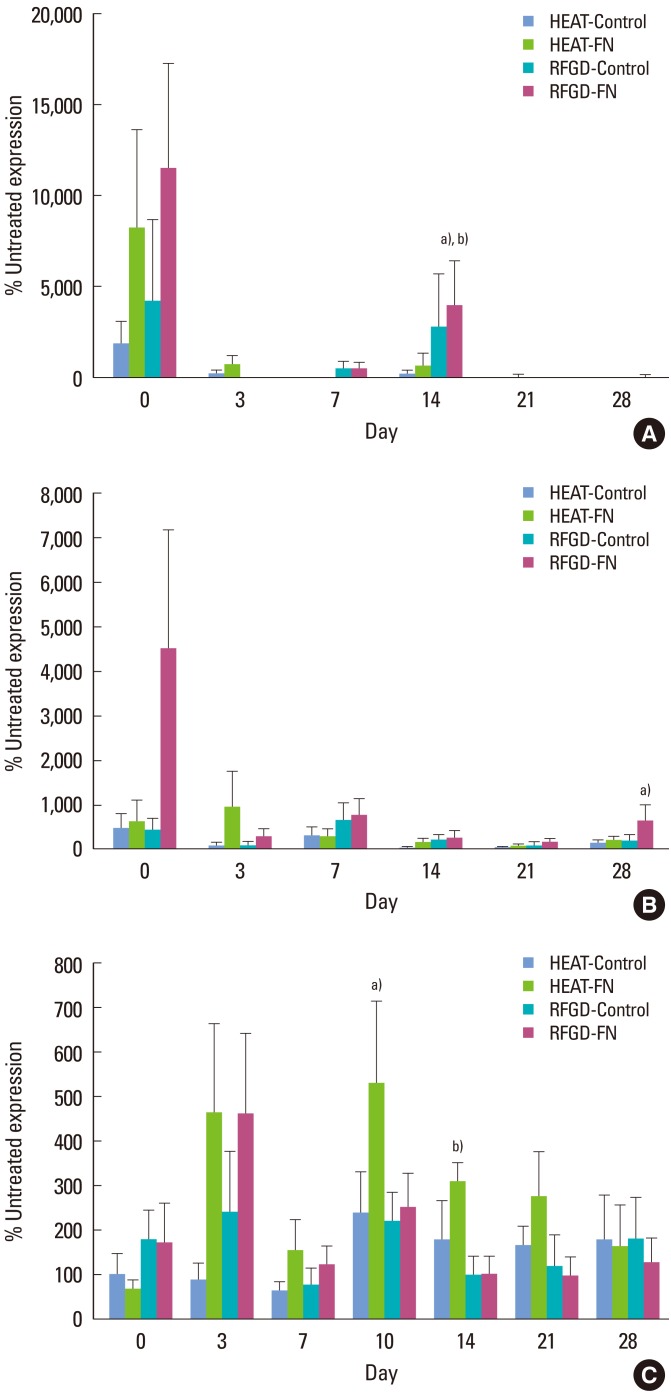
On day 0, heat and RFGD pretreatments also appeared to promote early increases in osteopontin mRNA expression that were greater in the FN group compared to the control group, although these effects diminished at later points in time (Fig. 3A). The RFGD pretreatment produced a 40-fold increase in osteopontin mRNA expression at day 14 for FN-coated disks that was statistically significant. In general, RFGD pretreatment produced a pattern of greater stimulation of osteopontin gene expression compared to heat pretreatment (Fig. 3A). Both pretreatments generally showed weaker effects on osteopontin mRNA expression at points in time later than day 0 compared to the other gene markers analyzed (Fig. 3A).
Figure 3.
Effects of heat and radiofrequency plasma glow discharge (RFGD) pretreatments on (A) osteopontin, (B) bone sialoprotein, and (C) osteocalcin mRNA expression in MC3T3 osteoprogenitor cells cultured on Ti6Al4V disks. The levels of osteopontin, bone sialoprotein, and osteocalcin mRNA expression measured by quantitative real time polymerase chain reaction for treated disks at the indicated points in time are presented as a percentage of the levels of mRNA expression measured for untreated disks. Data represent means±standard error for 3 to 9 independent cultures at each point in time. FN: fibronectin. a)Significantly greater (P<0.05) than untreated disks. b)Significantly greater (P<0.05) than heat-pretreated disks based on analysis of variance.
The RFGD pretreatment appeared to increase bone sialoprotein mRNA expression for FN-coated disks more than control disks on day 0 (Fig. 3B). The effects of both pretreatments began to gradually decline after day 0. After day 3, the heat pretreatment produced a general pattern of weaker stimulation of bone sialoprotein gene expression compared to the RFGD pretreatment (Fig. 3B). The RFGD pretreatment produced a statistically significant 6-fold increase in bone sialoprotein mRNA expression in the FN group on day 28. RFGD pretreatment had generally greater effects on bone sialoprotein gene expression for FN-coated disks compared to control (uncoated) disks (Fig. 3B).
Stimulatory effects for both heat and RFGD pretreatments on osteocalcin mRNA expression did not become apparent until day 3 (Fig. 3C). The heat pretreatment produced statistically significant increases in osteocalcin mRNA expression for FN-coated disks on days 10 and 14. The levels of osteocalcin mRNA expression appeared to diminish for both heat and RFGD-pretreated disks after day 10. In general, the effects of the heat pretreatment on osteocalcin gene expression were greater for FN-coated disks than control (uncoated) disks (Fig. 3C). After day 3, heat pretreatment generally increased osteocalcin mRNA expression more than the RFGD pretreatment did (Fig. 3C).
DISCUSSION
A key finding in this study is that, beginning on day 0, heat and RFGD pretreatments increased osteoblast gene marker expression for FN-coated disks more than uncoated disks, suggesting a specific enhancement of FN's bioactivity. Similarly, by day 3, pretreatments also increased the expression of all of the osteoblast gene markers in the absence of adsorbed FN (control). These results suggest that osteoblastic cells began to differentiate earlier on pretreated disks whether they were FN-coated or uncoated. This conclusion is also supported by our findings that the pretreatments down-regulated the mRNA expression of Runx2 and osterix, markers for osteoprogenitors [28] and preosteoblasts [28], respectively, for both coated and uncoated disks. Findings that Runx2 and osterix transcript levels were lower for coated than uncoated disks on day 0 further suggest that both of these genes became down-regulated earlier on disks that were pretreated and coated with FN. All of these findings collectively suggest that our surface oxide pretreatments by RFGD or heat accelerated the maturation of osteogenic precursor cells into osteoblasts.
The heat or RFGD pretreatment of FN-coated or uncoated disks also increased gene marker transcript levels during late osteoblastogenesis (14 to 28 days), suggesting that more osteoprogenitors differentiated and attained peak levels of gene marker expression earlier on modified disks. The overall greatest effects were observed for pretreated samples coated with FN compared to pretreated and uncoated controls, supporting the specific stimulation of adsorbed FN's bioactivity. Our laboratory has reported that FN coated on untreated Ti6Al4V disks modestly increased the levels of osteoblast gene marker expression during the later stage (28 days) of development compared to uncoated disks [26]. In the current study, surface pretreatments combined with a FN coating increased osteoblast gene expression during the later phase of development (days 14 to 28) to levels that were far greater than those measured in the presence of FN alone (with no pretreatment). Therefore, these results suggest that our pretreated implant surfaces stimulate osteoblast differentiation by activating precoated FN and perhaps other adsorbed osteogenic serum and matrix proteins (even in the absence of a FN coating).
Our laboratory has investigated the influence of Ti6Al4V oxide physicochemical properties on the bioactivity of adsorbed FN [10,23,24]. Using atomic force microscopy, we previously showed that the preheated (600℃) surface oxide exhibited root mean square roughness values (12.8±1.7 nm) that were three-fold higher than those of untreated (4.1±1.1 nm) or RFGD-treated (3.6±0.9 nm) disks [23]. Heat pretreatment altered oxide topography by creating a pattern of oxide elevations that were approximately 50 to 100 nm in diameter [23]. In contrast, the RFGD pretreatment of Ti6Al4V disks did not alter the topography of the alloy surface (Fig. 1) since the RFGD-pretreated surface appeared to be at least as smooth as the control polished surface [23].
Both heat and RFGD pretreatments altered the Ti6Al4V oxide's net surface charge [23]. In these latter experiments, the electrostatic forces between the oxide surface and a negatively charged silicon nitride probe were used to estimate the net charge of the oxide [23]. Atomic force spectroscopy measurements demonstrated that both the RFGD and heat pretreatments made the surface oxide of Ti6Al4V more negatively charged at physiological pH [23]. The local metal oxide charge can be influenced by the acid-base balance of metal-hydroxo complexes [29]. The effects of the pretreatments on the net negative charge of the oxide at physiological pH are likely to have arisen from an elevation in the surface concentration of uncompensated negative charges from [M-O]- groups. In addition, we have demonstrated that heat and RFGD pretreatment induced increases in adsorbed FN's cell attachment activity [24], the conformational exposure of its integrin-binding domain [25], and binding to α5β1 integrins [25]. Moreover, all of these parameters were more highly correlated with changes in oxide charge rather than roughness or topography [24,25]. Taken together, these findings suggest that charged functional groups in the Ti6Al4V surface oxide can modulate the conformation of adsorbed FN, thereby increasing its capacity to bind to α5β1 integrins. In the current study, we showed that heat and RFGD pretreatments produced qualitatively similar effects on FN's capacity to induce a panel of osteoblast gene markers that were more highly correlated with increases in oxide charge than with oxide topography or roughness. These findings indicate that increases in oxide charge promoted by either pretreatment may enhance FN's capacity to promote osteoblast differentiation.
A number of studies have investigated the effects of non-metallic surface charge on FN structure and bioactivity. One study compared the effects of varying surface functionalities in self-assembled monolayers (SAMs) of alkanethiol on adsorbed FN's bioactivity toward MC3T3 cells [30]. The study found that FN displayed the greatest exposure of its integrin binding domain and binding to α5β1 integrins on a surface functionalized with hydroxyl groups (OH) with a negative charge compared to surfaces containing positively charged or hydrophobic functionalities [30]. It has been observed that a specific increase in FN binding to α5β1 integrins is associated with a switch in the cellular program from proliferation to differentiation [31]. Keselowsky et al. [32] showed that a OH-functionalized SAM surface also induced the highest levels of FN-stimulated focal adhesion kinase (FAK) signaling, which is mediated via integrin receptor activation. FAK can regulate a number of cell functions including differentiation [33]. In addition, OH-functionalized surfaces coated with FN have been shown to up regulate osteoblast-specific gene expression to a greater degree than other substrates [34]. In the latter study, the HFN7.1 monoclonal antibody, which blocks the binding of α5β1 integrins to the central integrin binding domain of FN, completely inhibited MC3T3 osteoblast differentiation and mineralization on FN-coated OH-functionalized surfaces [34]. We have also shown that the HFN7.1 antibody completely inhibited MC3T3 cell attachment to FN-coated heat or RFGD-treated disks [25], thereby complicating any measurements of osteoblast differentiation or mineralization. These studies suggest that [M-O]- groups in the Ti6Al4V oxide modulate FN-mediated osteoblast differentiation by increasing the protein's binding to α5β1 integrins and activation of integrin-dependent cell signaling pathways. Whether or not negatively charged oxides also modulate the conformational bioactivities of other serum or matrix proteins awaits further investigation.
We have previously shown that MC3T3 osteoprogenitor cells cultured on a rigorously cleaned Ti6Al4V alloy were found to demonstrate a normal pattern of osteoblast differentiation [26] similar to that observed on a polystyrene substrate. Nevertheless, this and another study that employed surface cleaning methods to increase hydrophilicity [35] provide only a limited understanding of how surface oxide chemistry influences osteoblast gene expression. In comparison, the current study showed that RFGD pretreatment, which increased oxide charge without altering topography [23], was able to stimulate osteoblast differentiation even after surface contamination was removed by our rigorous cleaning protocol. By using RFGD treatment to selectively perturb oxide chemistry without affecting topography, our study has provided insight into the mechanism by which negatively charged Ti6Al4V oxide groups influence protein bioactivity and osteoblast differentiation. Our findings show that by engineering a surface oxide to become more negatively charged, an implant surface can be created that promotes osteoblast differentiation by enhancing the biological activities of adsorbed osteogenic proteins.
The quantitative differences between our two pretreatments (heat and RFGD) and their effects on the expression of osteoblast gene markers may have arisen from the unique physico-chemical characteristics of each resultant oxide surface. Heat pretreatment, for example, had greater effects on the mRNA expression of genes for PTH-rP, alkaline phosphatase, and osteocalcin, while RFGD pretreatment had greater effects on osteopontin and bone sialoprotein gene expression. These findings suggest that structural oxide properties such as roughness or topography that are altered by heat pretreatment may induce some osteoblast gene markers while decreasing the expression of others.
A number of studies have examined the influence of titanium surface topography on the capacity of osteoblastic cells to differentiate in vitro. TiO2 surfaces containing nanofibers [36] or nanotubes [37] facilitated a higher cellular differentiation capacity than surfaces devoid of nanostructures. Zhao et al. [38] found that the microroughening of Ti6Al4V surfaces by grit-blasting with hydroxyapatite/beta tricalcium phosphate particles markedly increased the expression of osteocalcin but decreased alkaline phosphatase activity. In contrast, another study reported that microstructured titanium surfaces created by sandblasting/acid etching increased both alkaline phosphatase and osteocalcin expression in osteogenic cells compared to smoother machined surfaces [35]. The expression of alkaline phosphatase and osteocalcin was even further enhanced when the microstructured surfaces were made more hydrophilic by reducing surface contamination [35]. These findings suggest that changes in surface oxide chemistry, topography, and micro and/or nanoroughness interact in a complex manner to cooperatively alter the expression of individual osteoblast gene markers. Notably, variations in the expression of specific extracellular matrix genes such as those for type I collagen (α1), osteopontin, and bone sialoprotein, alter the protein composition of the matrix and the biochemical cues it provides for osteoblast gene marker expression and osteoblast function. Interactive effects of oxide chemistry and structure on protein conformational bioactivity, matrix protein synthesis/bioactivity, and osteoblast behavior may underlie the differences in gene marker expression observed in the current study between RFGD and heat-pretreated Ti6Al4V.
In summary, our results suggest that heat and RFGD pretreatments of the FN-coated Ti6Al4V surface oxide stimulated osteoblast differentiation through an enhancement of (a) FN's bioactivity and (b) the bioactivities of other serum and matrix proteins. These findings also suggest that negatively charged oxides can increase the capacity of coated FN, and perhaps other adsorbed osteogenic proteins, to promote osteoblast differentiation. Heat and RFGD pretreatments had different quantitative effects on osteoblast gene expression that may be related to possible effects of heat pretreatment-induced increases in oxide roughness. Our studies suggest that by combining FN coatings of Ti6Al4V with pretreatments that either alter surface oxide charge (RFGD) or charge and roughness (heat), an implantable surface can be developed that will more effectively support osteoblast differentiation. The investigation of how multiple surface oxide properties interact with selected matrix proteins or peptides to affect bone cell behavior may reveal novel strategies to improve implant fixation during the critical early healing phase.
ACKNOWLEDGEMENTS
The project described was supported by Grant Number NIH RO1 DE017695 (Awarded to DEM). This material is also the result of work supported with resources and the use of facilities at the James J. Peters VA Medical Center, Bronx, New York. This investigation was also conducted at the HSS research facility constructed with support of Grant C06-RR12538-01 from the National Center for Research Resources, NIH. We would like to acknowledge Dr. Xiaoyu Hu for technical advice with the qRT-PCR analyses. Special thanks goes to Paul Lee, Carrie Guan, and Jani Jae Eun Lee for the preparation and treatment of titanium alloy disks.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Adell R, Eriksson B, Lekholm U, Branemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347–359. [PubMed] [Google Scholar]
- 2.Adell R, Lekholm U, Rockler B, Branemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 3.Hardt CR, Grondahl K, Lekholm U, Wennstrom JL. Outcome of implant therapy in relation to experienced loss of periodontal bone support: a retrospective 5-year study. Clin Oral Implants Res. 2002;13:488–494. doi: 10.1034/j.1600-0501.2002.130507.x. [DOI] [PubMed] [Google Scholar]
- 4.Grossmann Y, Levin L. Success and survival of single dental implants placed in sites of previously failed implants. J Periodontol. 2007;78:1670–1674. doi: 10.1902/jop.2007.060516. [DOI] [PubMed] [Google Scholar]
- 5.Machtei EE, Mahler D, Oettinger-Barak O, Zuabi O, Horwitz J. Dental implants placed in previously failed sites: survival rate and factors affecting the outcome. Clin Oral Implants Res. 2008;19:259–264. doi: 10.1111/j.1600-0501.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 6.Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, Kasemo B, et al. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996;17:605–616. doi: 10.1016/0142-9612(96)88711-4. [DOI] [PubMed] [Google Scholar]
- 7.Tengvall P, Lundstrom I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992;9:115–134. doi: 10.1016/0267-6605(92)90056-y. [DOI] [PubMed] [Google Scholar]
- 8.Morris HF, Winkler S, Ochi S. A 48-month multicentric clinical investigation: implant design and survival. J Oral Implantol. 2001;27:180–186. doi: 10.1563/1548-1336(2001)027<0180:AMCIID>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Imam MA, Fraker AC. Titanium Alloys as Implant Materials. In: Brown SA, Lemons JE, editors. Medical applications of titanium and its alloys. Philadelphia: American Society for Testing and Materials; 1996. pp. 3–16. [Google Scholar]
- 10.MacDonald DE, Rapuano BE, Deo N, Stranick M, Somasundaran P, Boskey AL. Thermal and chemical modification of titanium-aluminum-vanadium implant materials: effects on surface properties, glycoprotein adsorption, and MG63 cell attachment. Biomaterials. 2004;25:3135–3146. doi: 10.1016/j.biomaterials.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Sousa SR, Lamghari M, Sampaio P, Moradas-Ferreira P, Barbosa MA. Osteoblast adhesion and morphology on TiO2 depends on the competitive preadsorption of albumin and fibronectin. J Biomed Mater Res A. 2008;84:281–290. doi: 10.1002/jbm.a.31201. [DOI] [PubMed] [Google Scholar]
- 12.Sieving A, Wu B, Mayton L, Nasser S, Wooley PH. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J Biomed Mater Res A. 2003;64:457–464. doi: 10.1002/jbm.a.10368. [DOI] [PubMed] [Google Scholar]
- 13.Rapuano BE, Wu C, MacDonald DE. Osteoblast-like cell adhesion to bone sialoprotein peptides. J Orthop Res. 2004;22:353–361. doi: 10.1016/S0736-0266(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 14.Sauberlich S, Klee D, Richter EJ, Hocker H, Spiekermann H. Cell culture tests for assessing the tolerance of soft tissue to variously modified titanium surfaces. Clin Oral Implants Res. 1999;10:379–393. doi: 10.1034/j.1600-0501.1999.100505.x. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials. 2002;23:1269–1279. doi: 10.1016/s0142-9612(01)00317-9. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald DE, Markovic B, Allen M, Somasundaran P, Boskey AL. Surface analysis of human plasma fibronectin adsorbed to commercially pure titanium materials. J Biomed Mater Res. 1998;41:120–130. doi: 10.1002/(sici)1097-4636(199807)41:1<120::aid-jbm15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Moursi AM, Damsky CH, Lull J, Zimmerman D, Doty SB, Aota S, et al. Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci. 1996;109(Pt 6):1369–1380. doi: 10.1242/jcs.109.6.1369. [DOI] [PubMed] [Google Scholar]
- 18.Meyer U, Joos U, Mythili J, Stamm T, Hohoff A, Fillies T, et al. Ultrastructural characterization of the implant/bone interface of immediately loaded dental implants. Biomaterials. 2004;25:1959–1967. doi: 10.1016/j.biomaterials.2003.08.070. [DOI] [PubMed] [Google Scholar]
- 19.Hormann H. Fibronectin--mediator between cells and connective tissue. Klin Wochenschr. 1982;60:1265–1277. doi: 10.1007/BF01727483. [DOI] [PubMed] [Google Scholar]
- 20.Wagle JE, Virji AS, Williams KB, Rapley JW, MacNeill SR, Cobb CM. Can application of exogenous fibronectin enhance periodontal regeneration? J Clin Periodontol. 2002;29:440–447. doi: 10.1034/j.1600-051x.2002.290509.x. [DOI] [PubMed] [Google Scholar]
- 21.Pearson BS, Klebe RJ, Boyan BD, Moskowicz D. Comments on the clinical application of fibronectin in dentistry. J Dent Res. 1988;67:515–517. doi: 10.1177/00220345880670021701. [DOI] [PubMed] [Google Scholar]
- 22.Bentley KL, Klebe RJ. Fibronectin binding properties of bacteriologic petri plates and tissue culture dishes. J Biomed Mater Res. 1985;19:757–769. doi: 10.1002/jbm.820190704. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald DE, Rapuano BE, Schniepp HC. Surface oxide net charge of a titanium alloy: comparison between effects of treatment with heat or radiofrequency plasma glow discharge. Colloids Surf B Biointerfaces. 2011;82:173–181. doi: 10.1016/j.colsurfb.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapuano BE, MacDonald DE. Surface oxide net charge of a titanium alloy: modulation of fibronectin-activated attachment and spreading of osteogenic cells. Colloids Surf B Biointerfaces. 2011;82:95–103. doi: 10.1016/j.colsurfb.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapuano BE, Lee JJ, MacDonald DE. Titanium alloy surface oxide modulates the conformation of adsorbed fibronectin to enhance its binding to α(5) β(1) integrins in osteoblasts. Eur J Oral Sci. 2012;120:185–194. doi: 10.1111/j.1600-0722.2012.954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapuano BE, Hackshaw KM, Schniepp HC, MacDonald DE. Surface coating of a titanium alloy with fibronectin augments expression of osteoblast gene markers in the MC3T3 osteoprogenitor cell line. J Oral Maxillofac Implants. 2012 Forthcoming. [PMC free article] [PubMed] [Google Scholar]
- 27.Erli HJ, Ruger M, Ragoss C, Jahnen-Dechent W, Hollander DA, Paar O, et al. The effect of surface modification of a porous TiO2/perlite composite on the ingrowth of bone tissue in vivo. Biomaterials. 2006;27:1270–1276. doi: 10.1016/j.biomaterials.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Glass DA, 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148:2630–2634. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- 29.Vargo TG, Bekos EJ, Kim YS, Ranieri JP, Bellamkonda R, Aebischer P, et al. Synthesis and characterization of fluoropolymeric substrata with immobilized minimal peptide sequences for cell adhesion studies. I. J Biomed Mater Res. 1995;29:767–778. doi: 10.1002/jbm.820290613. [DOI] [PubMed] [Google Scholar]
- 30.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 31.García AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 33.Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728–2735. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z, Daniels RH, Enzerink RJ, Hardev V, Sahi V, Goodman SB. Effect of nanofiber-coated surfaces on the proliferation and differentiation of osteoprogenitors in vitro. Tissue Eng Part A. 2008;14:1853–1859. doi: 10.1089/ten.tea.2007.0399. [DOI] [PubMed] [Google Scholar]
- 37.Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28:2821–2829. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]