Abstract
The architecture of secondary lymphoid organs (SLOs) is supported by several non-hematopoietic stromal cells. Currently it is established that two distinct stromal subsets, follicular dendritic cells and fibroblastic reticular cells, play crucial roles in the formation of tissue compartments within SLOs, i.e., the follicle and T zone, respectively. Although stromal cells in the anlagen are essential for SLO development, the relationship between these primordial cells and the subsets in adulthood remains poorly understood. In addition, the roles of stromal cells in the entry of antigens into the compartments through some tissue structures peculiar to SLOs remain unclear. A recently identified stromal subset, marginal reticular cells (MRCs), covers the margin of SLOs that are primarily located in the outer edge of follicles and construct a unique reticulum. MRCs are closely associated with specialized endothelial or epithelial structures for antigen transport. The similarities in marker expression profiles and successive localization during development suggest that MRCs directly descend from organizer stromal cells in the anlagen. Therefore, MRCs are thought to be a crucial stromal component for the organization and function of SLOs.
Keywords: CXCL13, fibroblastic reticular cell, follicular dendritic cell, lymph node, marginal reticular cell, organizer, secondary lymphoid organ, stromal cell
INTRODUCTION
Secondary lymphoid organs/tissues (SLOs) are essential for the efficient induction of adaptive immune responses. Several types of SLOs, including the lymph nodes (LNs), spleen, and mucosal-associated lymphoid tissues (MALTs) such as Peyer’s patches (PPs), are strategically positioned throughout various places within the body. SLOs are an elaborate filter that samples antigens and is equipped with highly sensitive immune sensors. In order to collect and filtrate foreign antigens, SLOs contain specialized tissue structures that are associated with the endothelium or epithelium. Immune cells such as lymphocytes, dendritic cells (DCs), and macrophages accumulate to high densities and form compartments. A remarkable feature common to all SLOs is the segregated localization of B cells and T cells. The architecture of SLOs is supported by several types of non-hematopoietic stromal cells of mesenchymal origin, which construct networks and define compartments (Mueller and Germain, 2009; Roozendaal and Mebius, 2011). Stromal networks provide not only a functional foothold but also a space for immune cell activities, as well as a physical framework for the tissue. Moreover, growing evidence indicates that stromal cells also play critical roles in immune cell function and homeostasis (Link et al., 2007; Fletcher et al., 2010; Suzuki et al., 2010; Lukacs-Kornek et al., 2011). SLOs are programmed to develop from the anlagen that occur at certain places and during restricted periods in the fetus and infant. In addition, stromal cells are known to be important for SLO development and maintenance (Mebius, 2003). Here, I focus on a recently identified stromal subset, marginal reticular cells (MRCs), and summarize their characteristics. I also discuss the relationship between MRCs and tissue structure, other stromal subsets, and immunological functions.
T AND B CELL COMPARTMENTS AND TWO CONVENTIONAL STROMAL CELL SUBSETS
As described above, B cells and T cells localize to distinct regions within SLOs. B cells accumulate to form follicles (B zone) in the outer cortex beneath the capsule in LNs or in the outer periarteriolar lymphoid sheath (PALS) in the spleen. A cluster of large follicles is the core tissue of PPs. During immune responses, activated B cells in the follicles form germinal centers, where they differentiate into high-affinity antibody producers (Cyster et al., 2000). In contrast, T cells localize to a separate area (T zone) adjacent to the follicles, i.e., the paracortex in LNs, inner PALS in the spleen, or interfollicular region (IFR) in PPs. DCs also accumulate in the T zone to present antigen and prime T cells (Steinman et al., 1997).
There are two different types of mesenchymal stromal subsets in the B and T zones (Figure 1A). Follicular dendritic cells (FDCs) form a dense network in the center of the follicles and have received considerable attention because of their importance in antibody production by B cells (Tew et al., 1997; Cyster et al., 2000; Victoratos et al., 2006; Suzuki et al., 2010). FDCs express CR1/CD35, CD23, and occasionally MAdCAM-1 (Szabo et al., 1997; Cyster et al., 2000). By contrast, an elaborate network of fibroblastic reticular cells (FRCs) comprises the scaffold of the T zone, which produces podoplanin/gp38 and various extracellular matrix (ECM) components (Gretz et al., 1997; Luther et al., 2000; Kaldjian et al., 2001; Katakai et al., 2004a,b). In general, immune cell migration and localization are regulated by a variety of chemokines (Mackay, 2001). Resting lymphocytes and mature DCs are highly responsive to “homeostatic chemokines” (Cyster, 1999; Müller et al 2003). Consistent with this, there are clear correlations between chemokines produced by stromal cell subsets and the localization of immune cells expressing the corresponding receptors; CXCL13 produced by FDCs is an attractant of B cells expressing CXCR5, while CCL19 and CCL21 from T zone FRCs attract T cells and mature DCs through the common receptor CCR7 (Cyster, 1999; Cyster et al., 2000; Luther et al., 2000). Moreover, lymphocytes that migrate robustly in the tissue parenchyma to scan antigens are thought to use stromal networks as a foothold (Bajénoff et al 2006). A variety of factors produced by stromal subsets are also required for the activation and survival of immune cells (Cyster et al., 2000; Huber et al., 2005; Link et al., 2007; Suzuki et al., 2010; Lukacs-Kornek et al., 2011; Malhotra et al., 2012). Therefore, the structure and function of the stromal network in each compartment is likely optimized for the activity of immune cell subsets.
FIGURE 1.
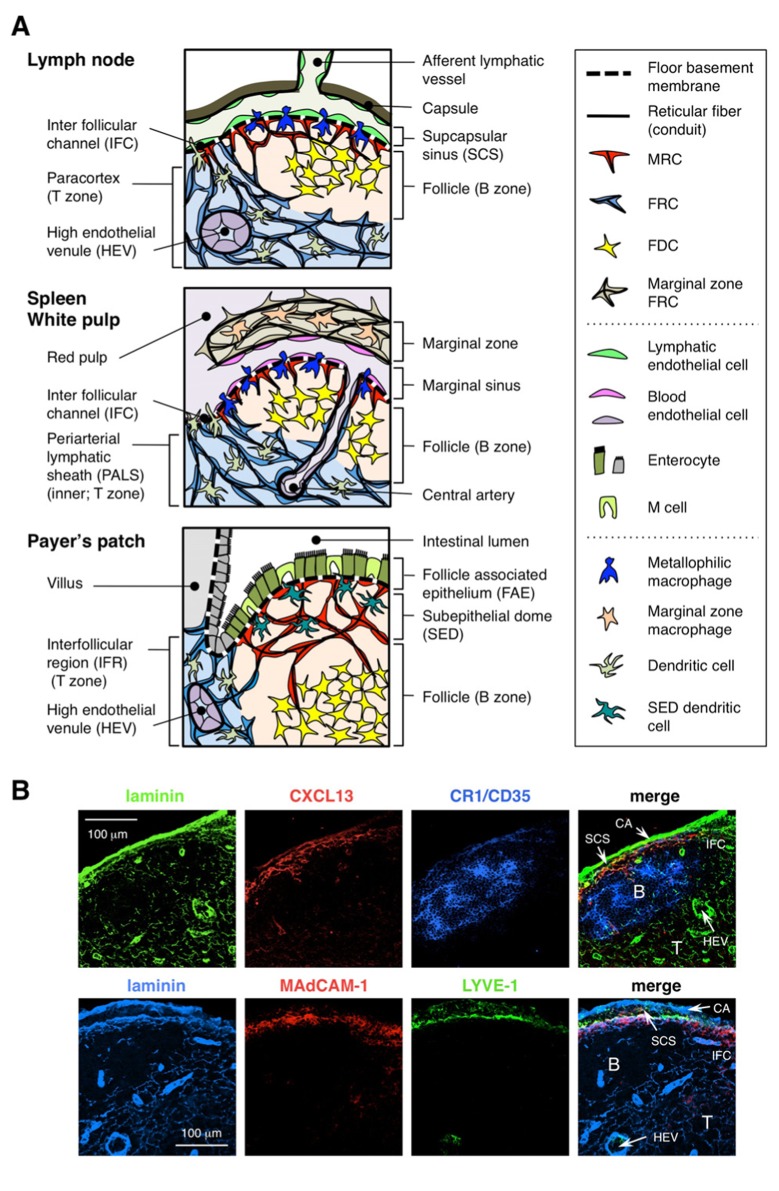
Stromal cell subsets and tissue structures of SLOs. (A) Schematic representation of tissue structures in mouse SLOs, with an emphasis on stromal cells and the antigen-transporting apparatus. Stromal elements, including mesenchymal, endothelial, and epithelial cells as well as myeloid cells such as macrophages and dendritic cells (listed in the right panel), are drawn for emphasis. (B) Upper: The tissue framework constructed by three different types of stromal cells in the outer cortical region of LN. Fluorescent immunostaining of a mouse LN section. In the upper micrograph, there is a small resting follicle (B) without a germinal center, which is supported by FDCs expressing CR1/CD35 but undetectable levels of CXCL13. Instead, MRCs form a laminin+CXCL13+ reticular network in the outer edge of the follicle underneath the capsule (CA) and SCS. The network constructed by FRCs in the paracortex (T) is laminin+CXCL13-CR1/CD35-. Lower: MAdCAM-1+ MRC layer is closely associated with LYVE-1+ lymphatic endothelial layer in the SCS. Note that MRC layer is extended to the IFC area.
The stromal network in the T zone is composed of FRCs and an ECM bundle known as a reticular fiber, which forms a “conduit” that facilitates the passage of low molecular weight substances (Gretz et al., 1997, 2000; Nolte et al., 2003). This conduit transports various factors and soluble antigens deep within the tissue, while particles and large molecules are predominantly excluded from the lymphocyte compartments. Indeed, lymph-borne chemokines from peripheral tissues are rapidly transported to the high endothelial venule through the conduit to control the mobilization of circulating cells into the LNs (Palframan et al., 2001). In addition, some resident DCs directly contact the conduit and capture lymph-borne antigens (Sixt et al., 2005).
A NEWLY IDENTIFIED STROMAL SUBSET, MARGINAL RETICULAR CELLS
The outer margin of the LN cortex, just beneath capsule, is surrounded by the subcapsular sinus (SCS; Figure 1A). The luminal surface of the SCS is covered with lymphatic endothelial cells and their cortical side is backed by the basement membrane, called the “floor” (Szakal et al., 1983). Particularly within the IFRs, reticular fibers spread out from the floor into the paracortex (Gretz et al., 1997). Importantly, a thin layer of reticular structure is also observed in the outermost region of the follicles. FRC-like stromal cells in the follicular reticulum express CXCL13 and MAdCAM-1 but not CCL21, which indicates that these cells are distinct from T zone FRCs, while the FDC marker CR1/CD35 was undetected or only weakly expressed in these cells (Katakai et al., 2008; Figures 1B and 2A). FDCs do not generate reticular fibers, and accordingly, the reticular marker ER-TR7 is virtually absent in the center of the follicles (Katakai et al., 2004a,b). In addition, reticular cells in the subcapsular region specifically express RANKL/TRANCE, which is a TNF family cytokine that is essential for LN development (Dougall et al., 1999; Kong et al., 1999). Therefore, these stromal cells were thought to be a new stromal subset and were designated marginal reticular cells (MRCs; Katakai et al., 2008). Of note, a substantial amount of CXCL13 is constitutively expressed in MRCs in resting small follicles even if FDCs express undetectable levels of CXCL13 (Figure 1B). It is well established that the outer edge of PALS in the spleen, especially that which lies over the follicles, is bordered by a MAdCAM-1+ stromal layer. The cells that constitute the lining of basement membrane beneath the marginal sinus (MS) also express CXCL13 and RANKL, indicating that these cells are a type of MRCs (Katakai et al., 2008). In MALTs, reticular cells similar to MRCs form a network in the subepithelial dome (SED) region just under the basement membrane of the follicle-associated epithelium (FAE; Katakai et al., 2008; Knoop et al., 2009). Taken together, MRCs are thought to be a stromal subset common to SLOs but distinct from FDCs and FRCs.
FIGURE 2.
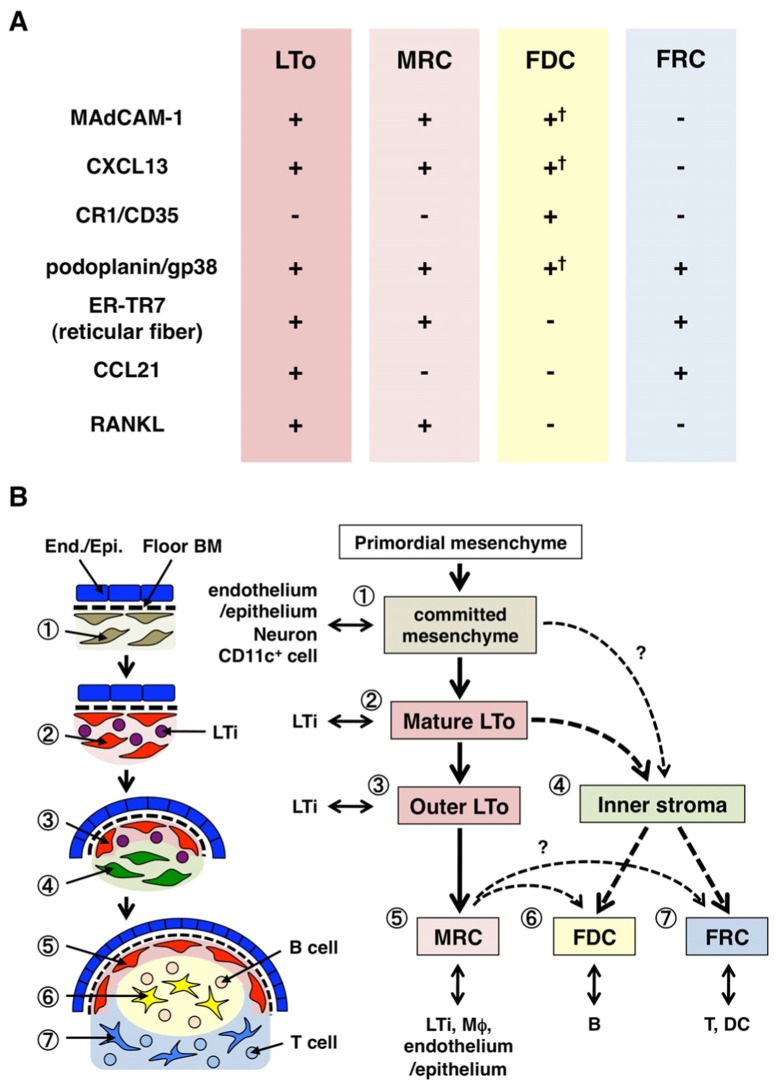
Relationship between MRCs and other stromal cell subsets in SLOs. (A) Pattern of marker expression in stromal cell subsets. Expression levels of MAdCAM-1, CXCL13, and podoplanin/gp38 in FDCs are often weak or undetectable by immunohistochemistry (†). (B) Schematic of a generalized model of SLO development with the locations of stromal cell subsets (left) and a putative genealogy of stromal cell subsets (right). Numbers indicating cell elements in the left drawing represent stromal cell subsets shown in the right scheme. BM, basement membrane; End./Epi., endothelial or epithelial layer.
MRCs AND THE ANTIGEN ENTRY ROUTE IN SLOs
Secondary lymphoid organs are characterized by peculiar structures that filtrate and transport antigens into the lymphocyte compartments (Figure 1A). LNs are connected with lymphatic vessels to survey lymph-borne antigens. Because the afferent lymphatics are open to the SCS, the sinus lymphatic endothelium and floor basement membrane are, as it were, the front surface of the LN filter. The spleen filters blood, in which branches of the central artery open to the MS and marginal zone (MZ), where immune cells survey the blood contents. In these specialized sinus structures, the border barriers are the lymphatic endothelium in the SCS of the LN and the blood endothelium in the MS of the spleen, both of which are supported by the MRC reticulum. Interestingly, CD169+ metallophilic macrophages are selectively distributed near the sinus lining over the follicles, some of which settle across the endothelial barrier and convey particulate antigens from the sinus lumen into the lymphocyte compartment (Szakal et al., 1983; Taylor et al., 2005; Carrasco and Batista, 2007; Phan et al., 2009). DCs that carry antigens from the tissues via the lymphatic vessels arrive at the SCS and subsequently move into the paracortex across the floor of the interfollicular channel (IFC; Steinman et al., 1997; Katakai et al., 2004b; Braun et al., 2011). In MALTs, the FAE functions as the barrier in which a specialized epithelial cell called M cells transfers bacteria or particles from the gut lumen to underlying DCs or macrophages (Kraehenbuhl and Neutra, 2000). Therefore, MRCs support the frontline antigen-transporting apparatus in each SLO. The basic design of SLOs is that the follicles are primarily arranged toward the site of antigen entry and are accompanied by T zones. Thus, from a stromal viewpoint, the whole tissue architecture appears to be organized toward the MRC layer.
Although the immunological functions of MRCs remain elusive, it was previously shown that the conduit network constructed by MRCs in the outer follicle transports small soluble antigens to follicular B cells and FDCs (Bajénoff and Germain, 2009; Roozendaal et al., 2009). Most CD169+ macrophages are positioned at the cortical side of the SCS floor and protrude an extension into the sinus lumen to capture particles (Phan et al., 2009). Thus, MRCs might be involved in the localization, morphology, and function of these macrophages. M cell development in PPs requires RANKL-RANK signaling, which is likely controlled by a RANKL-expressing MRC network just beneath the FAE (Knoop et al., 2009). Inhibiting the LT pathway, which abolishes MRC signatures in the splenic white pulp, disturbs the MS structure (Koike et al., 1996; Balogh et al., 2007; Katakai et al., 2008; Zindl et al., 2009). Moreover, elevated ICAM-1, VCAM-1, and CXCL13 expression implies that MRCs are involved in the dynamic interstitial migration of follicular B cells as a functional scaffold. CD169+ macrophages have been shown to directly deliver particulate antigens to antigen-specific B cells in this area (Carrasco and Batista, 2007; Phan et al., 2009), suggesting that MRC network-mediated control of B cell migration may impact this process. Since MRCs are also present in the IFC region, they possibly regulate the transmigration of DCs from the SCS toward the T zone.
MRCs AND ORGANIZER STROMAL CELLS IN SLO DEVELOPMENT
Secondary lymphoid organs develop from the anlagen, which are aggregates of mesenchymal and hematopoietic cells associated with vessels or epithelium, at a defined site and period in the fetus or infant (Mebius, 2003). A critical event in the development of the SLO anlagen is the accumulation of CD45+CD4+CD3- hematopoietic cells, which are also known as lymphoid tissue inducer (LTi) cells that interact with mesenchymal stromal cells called lymphoid tissue organizer (LTo) cells (Mebius et al., 1997). A TNF family cytokine, lymphotoxin (LT)-α1β2 that is expressed by LTi cells transmits signals to LTo cells via the LT-β receptor. LTo cells subsequently induce the expression of adhesion molecules, including ICAM-1, VCAM-1, and MAdCAM-1, and chemokines CXCL13, CCL19, and CCL21 (Honda et al., 2001; Cupedo et al., 2004a; Bénézech et al 2010). In particular, CXCL13 is especially important in attracting LTi cells to the anlagen via its receptor CXCR5 (Finke et al., 2002; Luther et al., 2003; Ohl et al., 2003). It is assumed that a positive feedback loop, i.e. newly immigrating LTi cells that produce LT further activate LTo stromal cells, promoting the organization of the anlagen. As lymphocytes began to accumulate after birth, the tissue expands and the compartments supported by different stromal cell subsets are induced (Cupedo et al., 2004b; Bajénoff and Germain 2009).
Marginal reticular cells and LTo cells express a very similar pattern of various markers (Figure 2A), suggesting that there is some relationship between these two stromal cells. LTo cells seem to be more concentrated in the marginal region of the LN anlagen adjacent to the lymphatic sinus of the presumptive SCS (Finke et al., 2002; Cupedo et al., 2004a; Eberl et al., 2004; Katakai et al., 2008). This LTo cell layer appears to expand outwardly with the growth of the anlagen, which ultimately appears to become the MRC layer (Katakai et al., 2008). Likewise, as lymphocytes accumulate around the central artery in the postnatal spleen, LTo cells expressing MAdCAM-1 and RANKL expand with the layer and become MRCs in the white pulp. These findings strongly suggest that MRCs are a direct descendant of the LTo stroma, which preserve the characteristics of LTo cells at specialized sites within SLOs. Even RAG-deficient mice exhibit a subcapsular MRC layer in atrophic LNs and shrunken periarterial MRC sheathes in the spleen, indicating that MRC development occurs independently of B and T cells and is programmed before their colonization (Katakai et al., 2008). Since SLOs efficiently sample antigens to trigger immune responses, the antigen-collecting structures that are constructed during development need to be maintained thereafter. Accordingly, it is reasonable that these tissue structures are maintained by organizer-like stromal cells throughout adulthood.
RELATIONSHIP BETWEEN MRCs AND OTHER STROMAL SUBSETS
Figure 2B shows the possible genealogy of stromal subsets in SLOs. In the embryo, the primordial mesenchyme that is in close proximity to the vasculature or epithelium is committed to form the anlage core and attracts LTi cells. Nerve cells or other less characterized cells are also involved in the initial process (Veiga-Fernandes et al., 2007; van de Pavert et al., 2009). The accumulation of LTi cells facilitates the maturation of mesenchymal cells into LTo cells, which ultimately facilitates the construction of the basic architecture. Typically, LTo cells tend to concentrate at the periphery of the anlage and expand outward with tissue growth, while stromal cells exhibiting weak or no LTo signatures conversely increase in the inner portion of the anlage. Postnatal colonization of B and T cells leads to compartments with the induction of conventional stromal subsets.
If this scheme is correct, it follows that all the stromal subsets in adult SLOs are originally derived from LTo cells. Thus, do MRCs that preserve the characteristics of LTo cells have the ability to differentiate into FDCs or FRCs? MRCs share many signatures with other subsets (Figure 2A), suggesting that this is possible. One speculation is that MRCs function as stromal stem cells that continuously supply all of the stromal subsets throughout adulthood. Although this idea is intriguing, it will require cautious consideration and further validation in the future. Mesenchymal cells are generally highly flexible in nature depending on the surrounding environment, and thus specific features can be easily changed. Extended culturing of stromal cells isolated from LNs results in lost expression for many genes, particularly homeostatic chemokines (Katakai et al., 2004a; Tomei et al., 2009). This suggests that the in vivo phenotypes of stromal cell subsets are optimally maintained by the tissue circumstances, which are reversible and not due to terminal differentiation. In addition, inflammatory stimuli induce the robust proliferation of stromal cells and the dramatic remodeling of SLOs (Katakai et al., 2004a; Chyou et al., 2011). Therefore, although MRCs could be converted to other subsets, conventional stromal subsets might also self-renew and be interchangeable in adult SLOs.
REMODELING OF SLOs AND TERTIARY LYMPHOID STRUCTURES
During immune responses, stromal structures within SLOs are dramatically remodeled (Gretz et al., 1997; Katakai et al., 2004a). Some infections cause a severe disruption of tissue structures within the LNs and splenic white pulp within a few days, which is restored as the pathogens are eradicated (Mueller et al., 2007a,b; Scandella et al., 2008; St John and Abraham, 2009). This restoration process likely recapitulates a self-organizing process via a feedback reaction similar to SLO development. Importantly, LTi-like cells are also present in adult tissues and regulate infection-associated remodeling of SLOs (Kim et al., 2003; Scandella et al., 2008). Although the role of MRCs in such processes is unknown, they possibly have an organization role in collaboration with LTi-like cells and determine the outer frame of SLOs during reconstruction.
In chronic inflammation associated with various organ pathologies, large numbers of infiltrating lymphocytes often lead to organizations that are similar to SLOs, known as tertiary lymphoid tissues (TLTs; Drayton et al., 2006). B and T cells are segregated and corresponding networks of stromal cell subsets are induced. It would be interesting to determine whether MRCs are present in these ectopic lymphoid structures; however, MRC-like cells and related tissue structures are not observed in TLTs that developed during mouse autoimmune gastritis (Katakai et al., 2006, 2008). It should be emphasized that TLTs are fundamentally not programmed lymphoid organizations and naturally do not associate with the antigen-transporting structures. Therefore, MRCs are likely absent in TLTs. Even though stromal cells that are phenotypically similar to MRCs might be induced in some lesions, they would not be identified as MRCs unless they closely associate with the specific endothelial or epithelial structure and form a layered reticulum at the outer follicles.
CONCLUSION
Marginal reticular cells are a unique stromal cell subset common to SLOs. MRCs are clearly different from conventional subsets that are induced or maturated through interactions with lymphocytes after birth, and are directly derived from LTo stromal cells in the anlagen and independent of lymphocytes. The network of MRCs is closely associated with the antigen-transporting apparatus of SLOs and is thought to directly or indirectly control antigen delivery to lymphocyte compartments as well as the localization and migration of immune cells. MRCs likely play pivotal roles in maintaining SLO structures as the outer framework and may be converted to other stromal subsets at steady state or during tissue remodeling. It will be important to collectively consider all the stromal subsets, tissue structures, and immunological microenvironments in order to comprehensively understand the SLO system.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by Grant-in-Aid for Young Scientist and for Scientific Research on Innovative Areas from The Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations
- DC
dendritic cell
- FAE
follicle-associated epithelium
- FDC
follicular dendritic cell
- FRC
fibroblastic reticular cell
- HEV
high endothelial venue
- IFC
interfollicular channel
- IFR
interfollicular region
- LN
lymph node
- LT
lymphotoxin
- LTi
lymphoid tissue inducer
- LTo
lymphoid tissue organizer
- MALT
mucosal associated lymphoid tissue
- MRC
marginal reticular cell
- MS
marginal sinus
- MZ
marginal zone
- PALS
periarteriolar lymphoid sheath
- PP
Peyer’s patch
- SED
subepithelial dome
- SLO
secondary lymphoid organ
- SCS
subcapsular sinus
- TLT
tertiary lymphoid tissue
REFERENCES
- Bajénoff M., Egen J. G., Koo L. Y., Laugier J. P., Brau F., Glaichenhaus N., Germain R. N. (2006). Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajénoff M., Germain R. N. (2009). B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood 114 4989–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh P., Balázs M., Czömpöly T., Weih D. S., Arnold H. H., Weih F. (2007). Distinct roles of lymphotoxin-beta signaling and the homeodomain transcription factor Nkx2.3 in the ontogeny of endothelial compartments in spleen. Cell Tissue Res. 328 473–486 [DOI] [PubMed] [Google Scholar]
- Bénézech C., White A., Mader E., Serre K., Parnell S., Pfeffer K., Ware C. F., Anderson G., Caamaño, J. H. (2010). Ontogeny of stromal organizer cells during lymph node development. J. Immunol. 184 4521–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Worbs T., Moschovakis G. L., Halle S., Hoffmann K., Bölter J., Münk A, Förster R. (2011). Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 12 879–887 [DOI] [PubMed] [Google Scholar]
- Carrasco Y. R., Batista F. D. (2007). B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27 160–171 [DOI] [PubMed] [Google Scholar]
- Chyou S., Benahmed F., Chen J., Kumar V., Tian S., Lipp M., Lu T. T. (2011). Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J. Immunol. 187 5558–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T., Vondenhoff M. F., Heeregrave E. J., De Weerd A. E., Jansen W., Jackson D. G., Kraal G., Mebius R. E. (2004a). Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J. Immunol. 173 2968–2975 [DOI] [PubMed] [Google Scholar]
- Cupedo T., Lund F. E., Ngo V. N., Randall T. D., Jansen W., Greuter M. J., de Waal-Malefyt R., Kraal G., Cyster J. G., Mebius R. E. (2004b). Initiation of cellular organization in lymph nodes is regulated by non-B cell-derived signals and is not dependent on CXC chemokine ligand 13. J. Immunol. 173 4889–4896 [DOI] [PubMed] [Google Scholar]
- Cyster J. G. (1999). Chemokines and cell migration in secondary lymphoid organs. Science 286 2098–2102 [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Ansel K. M., Reif K., Ekland E. H., Hyman P. L., Tang H. L., Luther S. A., Ngo V. N. (2000). Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176: 181–193 [DOI] [PubMed] [Google Scholar]
- Dougall W. C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., Daro E., Smith J., Tometsko M. E., Maliszewski C. R., Armstrong A., Shen V., Bain S., Cosman D., Anderson D., Morrissey P. J., Peschon J. J., Schuh J. (1999). RANK is essential for osteoclast and lymph node development. Genes Dev. 13 2412–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton D. L., Liao S., Mounzer R. H., Ruddle N. H. (2006). Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 7 344–353 [DOI] [PubMed] [Google Scholar]
- Eberl G., Marmon S., Sunshine M. J., Rennert P. D., Choi Y., Littman D. R. (2004). An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5 64–73 [DOI] [PubMed] [Google Scholar]
- Finke D., Acha-Orbea H., Mattis A., Lipp M., Kraehenbuhl J. (2002). CD4+CD3- cells induce Peyer’s patch development: role of α4β1 integrin activation by CXCR5. Immunity 17 363–373 [DOI] [PubMed] [Google Scholar]
- Fletcher A. L., Lukacs-Kornek V., Reynoso E. D., Pinner S. E., Bellemare-Pelletier A., Curry M. S., Collier A. R., Boyd R. L., Turley S. J. (2010). Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J. Exp. Med. 207 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretz J. E., Anderson A. O., Shaw S. (1997). Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156 11–24 [DOI] [PubMed] [Google Scholar]
- Gretz J. E., Norbury C. C., Anderson A. O., Proudfoot A. E., Shaw S. (2000). Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Nakano H., Yoshida H., Nishikawa S., Rennert P., Ikuta K., Tamechika M., Yamaguchi K., Fukumoto T., Chiba T., Nishikawa S.-I. (2001). Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J. Exp. Med. 193 621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C., Thielen C., Seeger H., Schwarz P., Montrasio F., Wilson M. R., Heinen E., Fu Y. X., Miele G., Aguzzi A. (2005). Lymphotoxin-β receptor-dependent genes in lymph node and follicular dendritic cell transcriptomes. J. Immunol. 174 5526–5536 [DOI] [PubMed] [Google Scholar]
- Kaldjian E. P., Gretz J. E., Anderson A. O., Shi Y., Shaw S. (2001). Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int. Immunol. 13 1243–1253 [DOI] [PubMed] [Google Scholar]
- Katakai T., Hara T., Sugai M., Gonda H., Shimizu A. (2004a). Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200 783–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakai T., Hara T., Lee J. H., Gonda H., Sugai M., Shimizu A. (2004b). A novel reticular stromal structure in lymph node cortex: an immuno-platform for interactions among dendritic cells, T cells and B cells. Int. Immunol. 16 1133–1142 [DOI] [PubMed] [Google Scholar]
- Katakai T., Nomura T., Gonda H., Sugai M., Agata Y., Nishio A., Masuda T., Sakaguchi S., Shimizu A. (2006). Spontaneous large-scale lymphoid neogenesis and balanced autoimmunity versus tolerance in the stomach of H+/K+-ATPase-reactive TCR transgenic mouse. J. Immunol. 177 7858–7867 [DOI] [PubMed] [Google Scholar]
- Katakai T., Suto H., Sugai M., Gonda H., Togawa A., Suematsu S., Ebisuno Y., Katagiri K., Kinashi T., Shimizu A. (2008). Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J. Immunol. 181 6189–6200 [DOI] [PubMed] [Google Scholar]
- Kim M. Y., Gaspal F. M., Wiggett H. E., McConnell F. M., Gulbranson-Judge A., Raykundalia C., Walker L. S., Goodall M. D., Lane P. J. (2003). CD4+ CD3- accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity 18 643–654 [DOI] [PubMed] [Google Scholar]
- Knoop K. A., Kumar N., Butler B. R., Sakthivel S. K., Taylor R. T., Nochi T., Akiba H., Yagita H., Kiyono H., Williams I. R. (2009). RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J. Immunol. 183 5738–5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike R., Nishimura T., Yasumizu R., Tanaka H., Hataba Y., Hataba Y., Watanabe T., Miyawaki S., Miyasaka M. (1996). The splenic marginal zone is absent in alymphoplastic aly mutant mice. Eur. J. Immunol. 26 669–675 [DOI] [PubMed] [Google Scholar]
- Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999). OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397 315–323 [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. (2000). Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol. 16 301–332 [DOI] [PubMed] [Google Scholar]
- Link A., Vogt T. K., Favre S., Britschgi M. R., Acha-Orbea H., Hinz B., Cyster J. G., Luther S. A. (2007). Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8 1255–1265 [DOI] [PubMed] [Google Scholar]
- Lukacs-Kornek V., Malhotra D., Fletcher A. L., Acton S. E., Elpek K. G., Tayalia P., Collier A. R., Turley S. J. (2011). Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 12 1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther S. A., Tang H. L., Hyman P. L., Farr A. G., Cyster J. G. (2000). Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. U.S.A. 97 12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther S. A., Ansel K. M., Cyster J. G. (2003). Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J. Exp. Med. 197 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R. (2001). Chemokines: immunology’s high impact factors. Nat. Immunol. 2 95–101 [DOI] [PubMed] [Google Scholar]
- Malhotra D., Fletcher A. L., Astarita J., Lukacs-Kornek V., Tayalia P., Gonzalez S. F., Elpek K. G., Chang S. K., Knoblich K., Hemler M. E., Brenner M. B., Carroll M. C., Mooney D. J., Turley S. J. The Immunological Genome Project Consortium. (2012). Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 13 499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius R. E., Rennert P., Weissman I. L. (1997). Developing lymph nodes collect CD4+ CD3- LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7 493–504 [DOI] [PubMed] [Google Scholar]
- Mebius R. E. (2003). Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3 292–303 [DOI] [PubMed] [Google Scholar]
- Mueller S. N., Hosiawa-Meagher K. A., Konieczny B. T., Sullivan B. M., Bachmann M. F., Locksley R. M., Ahmed R., Matloubian M. (2007a). Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317 670–674 [DOI] [PubMed] [Google Scholar]
- Mueller S. N., Matloubian M., Clemens D. M., Sharpe A. H., Freeman G. J., Gangappa S., Larsen C. P., Ahmed R. (2007b). Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc. Natl. Acad. Sci. U.S.A. 104 15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. N., Germain R. N. (2009). Stromal cell contributes to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9 618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Höpken U. E., Lipp M. (2003). The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 195 117–135 [DOI] [PubMed] [Google Scholar]
- Nolte M. A., Beliën, J. A., Schadee-Eestermans I., Jansen W., Unger W. W., van Rooijen N., Kraal G., Mebius R. E. (2003). A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med. 198 505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L., Henning G., Krautwald S., Lipp M., Hardtke S., Bernhardt G., Pabst O., Förster R. (2003). Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J. Exp. Med. 197 1199–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palframan R. T., Jung S., Cheng G., Weninger W., Luo Y., Dorf M., Littman D. R., Rollins B. J., Zweerink H., Rot A., von Andrian U. H. (2001). Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T. G., Green J. A., Gray E. E., Xu Y., Cyster J. G. (2009). Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 10 786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R., Mempel T. R., Pitcher L. A., Gonzalez S. F., Verschoor A., Mebius R. E., von Andrian U. H., Carroll M. C. (2009). Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R., Mebius R. E. (2011). Stromal cell-immune cell interactions. Annu. Rev. Immunol. 29 23–43 [DOI] [PubMed] [Google Scholar]
- Scandella E., Bolinger B., Lattmann E., Miller S., Favre S., Littman D. R., Finke D., Luther S. A., Junt T., Ludewig B. (2008). Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat. Immunol. 9 667–675 [DOI] [PubMed] [Google Scholar]
- Sixt M., Kanazawa N., Selg M., Samson T., Roos G., Reinhardt D. P., Pabst R., Lutz M. B., Sorokin L. (2005). The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22 19–29 [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Pack M., Inaba K. (1997). Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156 25–37 [DOI] [PubMed] [Google Scholar]
- St John A. L., Abraham S. N. (2009). Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines. Nat. Med. 15 1259–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Maruya M., Kawamoto S., Sitnik K., Kitamura H., Agace W. W., Fagarasan S. (2010). The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 33 71–83 [DOI] [PubMed] [Google Scholar]
- Szabo M. C., Butcher E. C., McEvoy L. M. (1997). Specialization of mucosal follicular dendritic cells revealed by mucosal addressin-cell adhesion molecule-1 display. J. Immunol. 158 5584–5588 [PubMed] [Google Scholar]
- Szakal A. K., Holmes K. L., Tew J. G. (1983). Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J. Immunol. 131 1714–1727 [PubMed] [Google Scholar]
- Taylor P. R., Martinez-Pomares L., Stacey M., Lin H. H., Brown G. D., Gordon S. (2005). Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23 901–944 [DOI] [PubMed] [Google Scholar]
- Tew J. G., Wu J., Qin D., Helm S., Burton G. F., Szakal A. K. (1997). Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol. Rev. 156 39–52 [DOI] [PubMed] [Google Scholar]
- Tomei A. A., Siegert S., Britschgi M. R., Luther S. A., Swartz M. A. (2009). Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 183 4273–4283 [DOI] [PubMed] [Google Scholar]
- van de Pavert S. A., Olivier B. J., Goverse G., Vondenhoff M. F., Greuter M., Beke P., Kusser K., Höpken U. E., Lipp M., Niederreither K., Blomhoff R., Sitnik K., Agace W. W., Randall T. D., de Jonge W. J., Mebius R. E. (2009). Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat. Immunol. 10 1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Fernandes H., Coles M. C., Foster K. E., Patel A., Williams A., Natarajan D., Barlow A., Pachnis V., Kioussis D. (2007). Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature 446 547–551 [DOI] [PubMed] [Google Scholar]
- Victoratos P., Lagnel J., Tzima S., Alimzhanov M. B., Rajewsky K., Pasparakis M., Kollias G. (2006). FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity 24 65–77 [DOI] [PubMed] [Google Scholar]
- Zindl C. L., Kim T. H., Zeng M., Archambault A. S., Grayson M. H., Choi K., Schreiber R. D., Chaplin D. D. (2009). The lymphotoxin LTα1β2 controls postnatal and adult spleen marginal sinus vascular structure and function. Immunity 30 408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]