Abstract
Identification and reversal of treatment resistance mechanisms of clinically refractory tumor cells is critical for successful cancer therapy. Here we show that ATP-binding cassette member B5 (ABCB5) identifies therapy-refractory tumor cells in colorectal cancer patients following fluorouracil (5-FU)-based chemoradiation therapy and provide evidence for a functional role of ABCB5 in colorectal cancer 5-FU resistance. Examination of human colon and colorectal cancer specimens revealed ABCB5 to be expressed only on rare cells within healthy intestinal tissue, whereas clinical colorectal cancers exhibited substantially increased levels of ABCB5 expression. Analysis of successive, patient-matched biopsy specimens obtained prior to and following neoadjuvant 5-FU-based chemoradiation therapy in a series of colorectal cancer patients revealed markedly enhanced abundance of ABCB5-positive tumor cells when residual disease was detected. Consistent with this finding, the ABCB5-expressing tumor cell population was also treatment-refractory and exhibited resistance to 5-FU-induced apoptosis in a colorectal cancer xenograft model of 5-FU monotherapy. Mechanistically, shRNA-mediated ABCB5 knockdown significantly inhibited tumorigenic xenograft growth and sensitized colorectal cancer cells to 5-FU-induced cell killing. Our results identify ABCB5 as a novel molecular marker of therapy-refractory tumor cells in colorectal cancer patients and point to a need for consistent eradication of ABCB5-positive resistant tumor cell populations for more effective colorectal cancer therapy.
Keywords: ABCB5, 5-FU, fluorouracil, colorectal cancer, CD133
Introduction
Colorectal cancer is a leading cause of cancer-related mortality. It arises from the accumulation of genetic mutations in normal epithelium, leading to the development of adenomas with eventual neoplastic transformation into invasive, metastatic carcinomas (1). Despite significant progress regarding early detection and treatment of colorectal cancer, a high proportion of patients with surgically resectable tumors eventually succumb to metastatic disease originating from residual microscopic malignancy not evident at the time of surgery (1, 2). Adjuvant therapeutic modalities such as radiotherapy and chemotherapy are designed to target residual tumor cells; however, their success is currently limited by the existence of therapy-resistant cancer cell populations (2-4).
Fluorouracil (5-FU) is one of the main chemotherapeutic agents for colorectal cancer. In patients with advanced colorectal cancer, treatment with 5-FU reduces tumor size by approximately 50 percent and prolongs median survival by 5 months (5). Identification and targeting of 5-FU-resistant populations might therefore be expected to lead to further improvements in advanced colorectal cancer survival.
Recent studies have revealed heterogeneity of human colorectal cancers with regard to molecular markers that identify highly tumorigenic and potentially therapy-resistant cancer subpopulations, including CD133, ESAhighCD44+, CD166, ALDH1 and WNT (6-10). For example, in vitro treatment of human HT-29 colorectal cancer cells with high doses of 5-FU resulted in significant enrichment of CD133+ colorectal cancer subpopulations (11) and CD133+ colorectal cancer cells can be sensitized to 5-FU- or oxaliplatin-mediated cell killing using an anti-IL-4 neutralizing antibody (12) or specific gene silencing of the Aurora-A kinase (13). In addition, CD133-overexpressing tumors were found to be more resistant to 5-FU-based chemotherapy and CD133 expression was associated with poor prognosis in a recent study of 501 cases of human colorectal cancer (14). While additional studies are required to further delineate whether all heterogeneously-expressed colorectal cancer markers (6-10, 15) identify hierarchical tumor organization as posited by the cancer stem cell model (16), and while more work is necessary to define their specific relationships, the data to date suggest preferential survival of CD133+ colorectal cancer cells following chemotherapy, and underline the importance of identifying and ultimately targeting all possible resistance mechanisms of these aggressive tumor subpopulations (2, 3).
We have recently cloned and characterized ABCB5 (ATP-binding cassette, sub-family B (MDR/TAP), member 5) (17-22), a chemoresistance gene in human melanomas (18, 23, 24) and hepatocellular carcinomas (25) preferentially expressed on CD133+ tumor cells (18, 25), which correlates with clinical tumor progression in these malignancies according to results from several laboratories (19, 25-27) and serves as a major independent biomarker of tumor recurrence and poor survival in human hepatocellular carcinoma patients (25). Our previous analysis of mRNA expression across diverse physiological and malignant human tissues demonstrated that ABCB5 is also expressed in human colorectal cancer (18). Therefore, we hypothesized that ABCB5 might identify therapy-refractory tumor populations in patients with colorectal cancer and that ABCB5, similar to its role in melanoma (23), might contribute to 5-FU resistance in this malignancy. Our results identify ABCB5 overexpression in clinical colorectal cancers compared to healthy controls and show that ABCB5 marks therapy-refractory tumor subpopulations following neoadjuvant 5-FU-based chemoradiation treatment in colorectal cancer patients. Mechanistically, utilization of a colorectal cancer xenotransplantation model reveals resistance of ABCB5+ tumor subpopulations to 5-FU-induced apoptosis. Moreover, stable shRNA-mediated ABCB5 knockdown in human colorectal cancer cells enhances 5-FU-mediated cell killing.
Materials and Methods
Clinical colorectal cancer specimens
Clinical colorectal tumor specimens were obtained from patients according to human subjects research protocols approved by institutional IRBs at the VA Boston Healthcare System and the University of Würzburg Medical School. Baseline ABCB5 expression was examined in tumors of diverse stages resected from fifteen patients not subjected to pre-operative treatment (Supplementary Table 1). In addition, patient-matched biopsy specimens derived from seven rectal cancer patients prior to and following neoadjuvant treatment with chemoradiation (bolus 5-FU treatment and long-course 40-52 Gy total dose radiotherapy) and subsequent curative surgical resection were included in the study (Supplementary Table 2), for analysis of ABCB5 expression in pre-treatment tumor biopsies and post-treatment surgically resected tumors.
Colorectal cancer cells and culture methods
Authenticated human colorectal cancer cell lines (HT-29 and SW480) were obtained from American Type Culture Collection (ATCC; Manassas, VA) and were cultured and passaged for fewer than six months in RPMI 1640 medium (Lonza Bio-Whittaker, Walkersville, MD) supplemented with 10% (v/v) fetal bovine serum (Invitrogen GIBCO, Carlsbad, CA) and 1% (v/v) penicillin/streptomycin (Lonza Bio-Whittaker). COLO205, HCT-116, HCT-15, HT-29, HCC-2998, KM12, and SW620 mRNA specimens were provided by the NCI/NIH Developmental Therapeutics Program.
Antibodies
The anti-ABCB5 monoclonal antibody (mAb) 3C2-1D12 (17-19), commercially available from AbD Serotec (Raleigh, NC), was used for flow-cytometric and immunohistochemical analyses. Unconjugated MOPC-31C mouse isotype control mAb was purchased from BD Pharmingen (San Diego, CA) and APC-conjugated secondary Ab from eBioscience (San Diego, CA). For Western blots the following polyclonal Abs (pAbs) were used: rabbit anti-human ABCB5 (Abgent, San Diego, CA), rabbit anti-human α-Tubulin (Abcam, Cambridge, MA), and HRP-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA). The following Abs were used for immunohistochemistry or immunofluorescence staining: Rabbit anti-CD133 (Abcam, Cambridge, MA), Alkaline phosphatase horse anti-mouse or goat anti-rabbit IgG, peroxidase goat anti-rabbit IgG, Alexa Fluor 594 donkey anti-rabbit IgG and Alexa Fluor 488 donkey anti-mouse IgG (Invitrogen, Carisbad, CA).
Histopathology and immunohistochemistry
For single-label immunohistochemistry, 5 μm sections were deparaffinized in xylene 2×10 minutes, followed with 2×100% ethanol, 95% and 75% ethanol, 3xdH2O, 2 minutes for each. Subsequently, sections were placed in 1x target retrieval solution (Dako, Carpenteria, CA) and boiled in a Pascal pressure chamber (Dako) at 125°C for 30 seconds, 90°C for 10 seconds, and were then allowed to cool to room temperature. Immunohistochemistry was performed as described previously (19, 22). Briefly, the sections were incubated with primary Ab at 4°C overnight. After washing out unbound primary Ab with Tris buffered saline-0.05% tween 20 (TBST), the tissue sections were incubated with peroxidase-conjugated or alkaline phosphatase (AP)-conjugated secondary Ab at room temperature for 30 minutes, then washed with TBST 3×5 minutes. Immunoreactivity was detected using NovaRed peroxidase substrate or AP red substrate (Vector Laboratories, Burlingame, CA, USA). For double-label immunohistochemistry, sections were deparaffinized and heat-induced antigen retrieval with Target Retrieval Solution (Dako) was used. Sections were incubated with ABCB5 mAb and CD133 mAb at 4°C overnight and then with peroxidase-conjugated horse anti-rabbit Ab (Vector) and AP-conjugated horse anti-mouse Ab at room temperature for 30 minutes. CD133 staining was detected with NovaRed peroxidase substrate and ABCB5 staining was detected with AP red substrate (Vector Laboratories). For double-label immunofluorescence, sections were deparaffinized and heat-induced antigen retrieval with Target Retrieval Solution (Dako) was used. Slides were blocked for 30 minutes with serum, incubated with ABCB5 mAb and CD133 mAb at 4°C overnight and then incubated with the appropriate secondary Abs in the dark for 1 hour. After washing out secondary Ab, slides were then coverslipped with ProLong Gold Anti-Fade with DAPI (Invitrogen). Sections were analyzed with a BX51/BX52 microscope (Olympus America Inc, Melville, NY, USA). Images were captured using the CytoVision 3.6 software (Applied Imaging, San Jose, CA, USA). For quantitative analysis of ABCB5 expression, ImageJ software (http:/rsbweb.nih.gov/ij/), which calculates percentage pixel area, coupled with the Color Deconvolution plug-in (http://www.dentistry.bham.ac.uk/landinig/software/cdeconv/cdeconv.html), was used to quantify the percentage of tissue that showed immunoreactivity for ABCB5 in microscopically acquired JPEG images of normal colon, clinical colorectal cancers, and 5-FU-treated cancer xenografts. Measurements of ABCB5 expression in normal colon were performed in clinically and histologically uninvolved portions of surgical specimens of the same group of colorectal cancer patients (Supplemental Table 1). ABCB5 staining intensity in full thickness colonic wall specimens was hereby evaluated in histologically selected areas representing only colonic epithelium in order to avoid inclusion of nonepithelial stromal, submucosa and muscular elements. In each case, ImageJ analysis software was used to quantify the pixel intensity of the selected areas as described previously (28).
TUNEL/ABCB5 co-staining
The ApopTag TdT Enzyme Kit (Chemicon International, Billerica, MA) was used for TUNEL staining. After deparaffinisation and antigen retrieval, the colorectal cancer sections were incubated with TdT enzyme mixture which 77 ml ApopTag reaction buffer + 33 ml ApopTag TdT Enzyme (33 ml dH2O instead of TdT enzyme was used as a negative control) at 37°C for 1 hour and then washed with dH2O and PBS once for each assay. The sections were incubated with ABCB5 mAb at 4°C overnight and then with peroxidase-conjugated sheep anti-DIG Ab (Roche, Branchburg, NJ) and AP-conjugated horse anti-mouse Ab at room temperature for 30 minutes. TUNEL staining was detected with NovaRed peroxidase substrate (Vector) and ABCB5 staining was detected with AP NBT/BCIP.
Flow cytometry
Cells were harvested using Versene (Invitrogen) as described (17, 18) and dissociated HT-29 and SW480 cell cultures were passed through a 40 μm nylon mesh to exclude cell aggregates followed by trypan blue examination for cell viability, as described previously (29, 30). Single cell suspensions used for flow cytometry consistently showed >99% cell viability (trypan blue exclusion) and absence of cell detritus or cell clusters. Analysis of ABCB5 expression in HT-29 and SW480 colon cancer cells was performed by single-color flow cytometry as described previously (18, 19). Briefly, 1 × 106 cells were incubated for 30 minutes at 4°C with APC-conjugated anti-ABCB5 mAb or APC-conjugated isotype control mAb (10 μg/mL), and single-color flow cytometry was subsequently performed on ungated cells with acquisition of fluorescence emission at the FL4 (APC) spectrum on a Becton Dickinson FACScan (Becton Dickinson, San Jose, CA). The positive gates were set at a detection threshold of 0.1% of cells in control mAb-stained samples.
Real-time quantitative reverse transcription-PCR
Total RNA was prepared from all 7 of the NCI-60 colorectal cancer cell cultures maintained at the National Cancer Institute and real-time quantitative reverse transcription-PCR was performed as described previously (18). The primers for ABCB5 (Homo sapiens ATP-binding cassette, sub-family B (MDR/TAP), member 5 (ABCB5), transcript variant 2, mRNA NCBI Reference Sequence: NM_178559.5) detection were 5’-CACAAAAGGCCATTCAGGCT-3’ (forward) and 5’-GCTGAGGAATCCACCCAATCT-3’ (reverse) and the primers for ß-actin were 5’-CCTGGCACCCAGCACAAT-3’ and 5’-GCCGATCCACACGGAGTACT-3’ (18). Relative gene expression was measured with the GeneAmp 7000 Sequence Detection System (Applied Biosystems) and ABCB5 expression was assessed by the ratio of the expression level in the sample against mean expression in all samples as described previously (18).
Full ABCB5 mRNA open reading frame (ORF) identification in HT-29 and SW480 colorectal cancer cells
RNA was prepared using RNeasy Mini kit (Qiagen, Germantown, MD) and reverse-transcribed using the Advantage RT-for-PCR kit (Clontech, Mountain View, CA) according to the manufacturers’ instructions. cDNA was then subjected to PCR amplification of the full ABCB5 ORF (transcript variant 2, mRNA NCBI Reference Sequence: NM_178559.5) as previously described for human melanocytes (17). For sequencing reactions, the PCR product was then used as a template for nested PCR of 3 overlapping fragments encompassing the ORF.
N-terminal: Forward (ATGGTGGATGAGAATGACATCAGAGCTTT), Reverse (GAATTAAATAGGCTCCAAATCGAAACCCT);
Middle: Forward (AATGACTGGATTTGCCAACAAAGATAAGC), Reverse (TTCTCAGGGAGACCTTCAATAAAAGAATG);
C-terminal: Forward (AAATAG CAATCGTTCCTCAAGAGCCTGTG), Reverse (TCACTGCACTGACTGTGCATTCACTAACT).
The full ORF sequences of ABCB5 (transcript variant 2, mRNA NCBI Reference Sequence: NM_178559.5) expressed by the human HT-29 and SW480 colorectal cancer cell lines were submitted to the Genbank database under the following accession numbers: GU437216 for HT-29, and GU437217 for SW480.
Animals
NOD/SCID and NOD/SCID IL-2Rγ-/- mice were purchased from the Jackson Laboratory (Bar Habor, ME). The human colorectal cancer to NOD/SCID mouse xenotransplantation model was used to dissect colorectal cancer drug resistance, because it represents an established model system (6, 7). NOD/SCID IL-2Rγ-/- (NSG) mice, which have a higher tumor take for some cancers due to more profound host immune suppression (22), were selected as the model of choice to assess intrinsic molecular function of ABCB5, independent of potential tumor microenvironmental interactions (22). Mice were maintained in accordance with the institutional guidelines of Children’s Hospital Boston and Harvard Medical School and experiments were performed according to approved experimental protocols.
Human colorectal cancer xenotransplantation and 5-FU treatment
HT-29 or SW480 human colorectal cancer cells (1×106 each) were injected s.c. into the right flanks of recipient NOD/SCID mice. Tumor formation was assayed weekly and tumor volumes were calculated as described previously (19). At 5 weeks (HT-29) or 9 weeks (SW480) post tumor cell inoculation mice were randomized into 5-FU (Teva, Irvine, CA) or vehicle control treatment groups with similar tumor volumes. 5-FU was administered by daily i.p. injection for 5 consecutive days, as described (31, 32), at 50mg/kg body weight, and control animals were given PBS at equal volumes. Tumor xenografts were harvested 1 day following administration of the final treatment dose, and frozen or paraffin-embedded colon cancer sections were prepared for subsequent immunohistochemical analysis. In additional experiments, HT-29 or SW480 human colorectal cancer cells, or their corresponding stable ABCB5 shRNA knockdown or vector control variants (1×106 cells/mouse, respectively) were injected s.c. into the right flanks of recipient NOD/SCID IL-2Rγ-/- mice. Tumorigenic growth of untreated HT-29 or SW480 human colorectal cancer cells or their corresponding stable ABCB5 shRNA knockdown or vector control variants were assayed weekly as a time course, at least up to the endpoint of 7 weeks, unless excessive tumor size or disease state required protocol-stipulated euthanasia earlier, by determination of tumor volume as described previously (19).
Generation of stable ABCB5 knockdown colorectal cancer cell variants
Generation of stable HT-29-pSUPER-Retro-Puro-ABCB5, SW480-pSUPER-Retro-Puro-ABCB5 or their respective empty vector cell variants was accomplished by transfection of plasmids with Fugene6 transfection reagent followed by puromycin selection (1μg/ml and 2μg/ml, respectively). The ABCB5 shRNA target sequence GCTGGAAAGATAGCAACTGAA was as previously described (24). Knockdown was confirmed by RT-PCR amplification using ABCB5-specific primers and 35 cycles of amplification and comparison relative to GAPDH RT-PCR amplification from the corresponding samples, with 28 cycles of amplification. The primers were as follows: ABCB5: Forward (GCGAGCAAAGGTCGGACTACAATCGTGG), Reverse (CCCAGAACCACAAAAGGCCATTCAGGC); GAPDH: Forward (ACCACAGTCCATGCCATCAC), Reverse (TCCACCACCCTGTTGCTGTA). Confirmation of ABCB5 knockdown at the protein level was performed utilizing an IP-Western blot using a rabbit anti-ABCB5 pAb. Briefly, 1mg (HT-29) or 4mg (SW480) total cell lysate and their respective knockdown lysates were sonicated and precleared before incubation with 2μg ABCB5 Ab with protein G agarose for 3hr at 4°C. IPs were then washed 4x before SDS-PAGE and Western blot. The buffer used for lysis and IP was 50mM Tris pH7.5, 1%NP-40, 0.1% SDS, 150mM NaCl, 0.5mM EDTA, 1mM DTT, 1xcomplete protease inhibitor cocktail (Roche, Indianapolis, IN). A tubulin Western blot was used as a control for lysate concentrations.
MTT cell growth and cytotoxicity assays
In vitro growth kinetics of HT-29 or SW480 human colorectal cancer cells or their corresponding stable ABCB5 shRNA knockdown or vector control variants were assayed using the MTT cell proliferation assay as described previously (18). To determine the effect of ABCB5 gene knockdown on 5-FU-induced cell killing, cells were seeded in 96-well dishes and exposed to a range of 5-FU concentrations (0.5-100nM) and the dose response of cell viability was measured after 7 days of drug treatment using a TACS MTT Cell Proliferation Assay kit (Trevigen, Gaithersburg, MD) according to the manufacturer’s protocol, as previously described for doxorubicin in human melanoma cells (18).
Statistical Analysis
Statistical differences between expression levels of markers, between surviving cell fractions or between tumor volumes were determined using the nonparametric Mann-Whitney test, with two-sided P values of P<0.05 considered significant. Pearson correlation coefficients were calculated for assessment of ABCB5 expression-drug potency relationships. The dual criteria of P<0.05 and r>0.3 were used to identify significant negative correlations as described previously (18).
Results
ABCB5 is expressed only on rare cells in healthy colonic mucosa but markedly increased in human colorectal cancer
In healthy human colon, we found ABCB5 to be expressed only on rare, distinct cells residing preferentially at or near the base of colonic crypts (Fig.1A), which also CD133-positive cells (Fig.1A). Furthermore, ABCB5-positive crypt cells co-expressed CD133, a candidate marker of intestinal stem cells (33, 34) and this cell population represented a subset of the CD133-expressing component (Fig.1B). Interestingly, ABCB5 and CD133 were expressed on opposite poles of individual cells, in keeping with the phenomenon of apico-basal polarity attributed to normal columnar epithelial cells (35). In clinical human colorectal cancer specimens, ABCB5 could also be detected to co-express CD133, a marker of 5-FU-resistant tumor populations in colorectal cancer (14, 36) and a candidate marker of more aggressive colorectal cancer cells according to some reports (7, 8), in the same tumor regions and on the same cancer cells (Fig.1C). Intriguingly, ABCB5 was expressed at higher levels in lesser differentiated areas of human colorectal cancer specimens compared to better differentiated areas (Fig.1D, left panels), based on conventional histopathologic evaluation of the correlative architecture and cytology of individual patient samples (37), similar to previous observations in malignant melanoma where ABCB5 expression was found to correlate with more advanced stages of disease (19, 26). Aggregate quantitative analysis of all tissue specimens examined (Supplementary Table 1) demonstrated marked ABCB5 overexpression in colorectal cancer specimens compared to normal colonic mucosa (ABCB5 positivity 15.39±4.06% vs. 0.66±0.24% of cells, respectively; mean±SEM, P=0.0016) (Fig.1D, right panel). These results provide initial evidence that the chemoresistance mediator ABCB5 is overexpressed in human colorectal cancer.
Figure 1. ABCB5 expression in physiological colon and colorectal cancer.
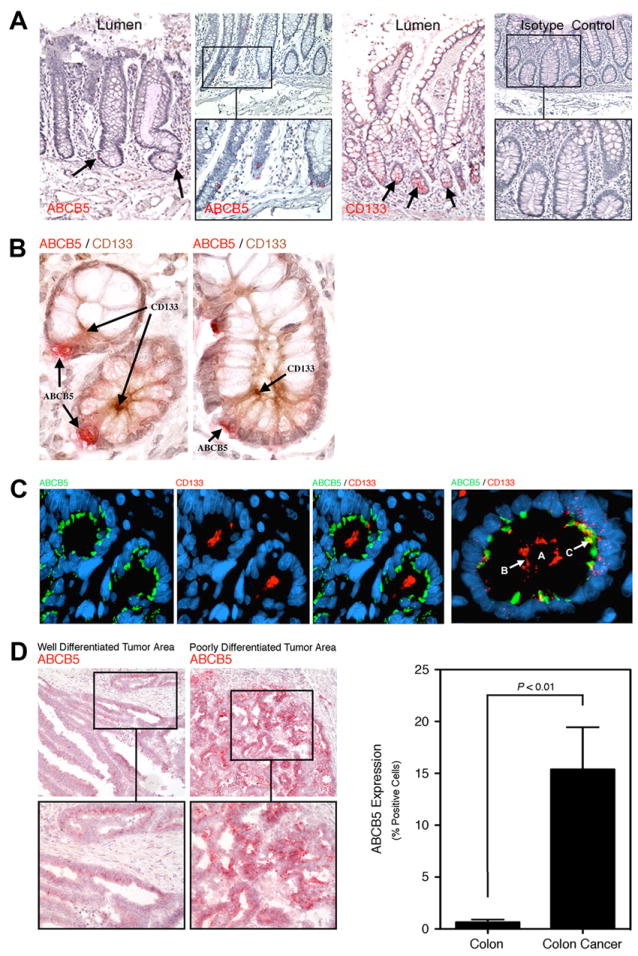
A, Representative immunohistochemical analysis of ABCB5 protein expression (AP, red, left two panels) or CD133 expression (third panel) in physiological human colon tissue compared to isotype control staining (right panel). Arrows demarcate cells expressing ABCB5 and CD133 in sections of full-length crypts from the lumenal surface of the colon to the crypt bases bordered by underlying connective tissue. B, Representative ABCB5/CD133 coexpression in human colon crypt cells showing basilar staining for ABCB5 (AP, red) and apical staining for CD133 (HRP, brown) in both a cross and lateral section. C, Representative immunofluorescence analysis of ABCB5 (green) and CD133 (red) co-expression in a clinical human colon cancer specimen. The central lumen in the far-right panel (labeled A) is defined at its periphery by apical membrane reactivity for CD133 (labeled B), with associated underlying foci of ABCB5 reactivity (labeled C). D, Left panels: Immunohistochemical analysis of ABCB5 (AP, red) protein expression in well-differentiated versus poorly-differentiated tumor areas of a representative clinical colon cancer. Right panel: ABCB5 protein expression (% positive cells, mean±SEM) in physiological colon (n=9) versus clinical colon cancer specimens (n=29), as determined by quantitative image analysis of ABCB5 immunohistochemistry.
ABCB5+ colorectal cancer cells exhibit increased resistance to 5-FU therapy
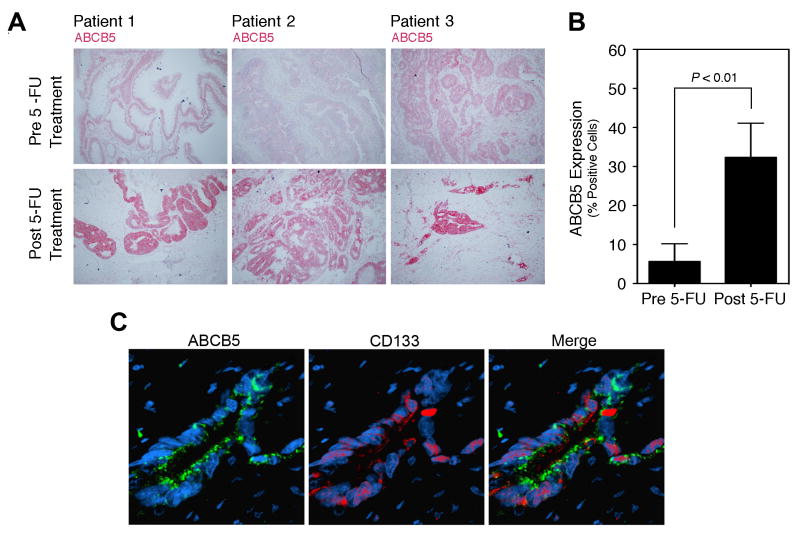
Based on previously demonstrated functions of ABCB5 as a chemoresistance mediator in human melanoma (18, 23, 24) and hepatocellular carcinoma (25), and on the here-identified preferential co-expression of ABCB5 with CD133, a marker of 5-FU-resistant tumor populations in colorectal cancer (14, 36), we investigated ABCB5 expression prior to and following standard 5-FU-based neoadjuvant chemoradiation therapy in a series of human rectal cancer patients (Supplementary Table 2). Analysis of matched diagnostic rectal cancer biopsy specimens in a series of patients who underwent neoadjuvant treatment prior to curative surgical tumor resection revealed ABCB5 positivity in only 5.7±4.6% of rectal cancer cells (mean±SEM) in pre-treatment specimens (Fig.2A, B), whereas post-treatment specimens showed markedly increased levels of ABCB5 expression (P<0.01), with 32.3±8.8% of cancer cells staining positively for ABCB5 (Fig.2A, B). Post-treatment rectal cancer specimens also revealed preferential co-expression of ABCB5 with CD133, in the same tumor regions and on the same cancer cells (Fig.2C). These findings show that ABCB5+ rectal cancer cells are more resistant to neoadjuvant chemoradiation therapy involving 5-FU.
Figure 2. Response of ABCB5 expression to 5-FU treatment in human patients.
A, ABCB5 expression in matched, patient-identical rectal cancer specimens before and after 5-FU treatment. ABCB5 immunohistochemistry (AP, red) pre and post 5-FU treatment is shown for three representative patients. B, Quantitative ABCB5 protein expression analysis (% positive cells, mean±SEM) in patient-matched rectal cancer specimens obtained before and after 5-FU chemotherapy (n=7, respectively). C, Representative immunofluorescence analysis of ABCB5 (green) and CD133 (red) co-expression in a clinical rectal cancer specimen after 5-FU chemotherapy.
To further dissect a potential role of ABCB5 in colorectal cancer 5-FU resistance, we examined ABCB5 expression and 5-FU treatment response of ABCB5+ cancer cell populations in an established in vivo model system utilizing NOD/SCID murine recipients of human colorectal cancer xenografts (6, 7). First, we found that ABCB5 expression levels correlated significantly with colorectal cancer resistance to 5-FU (Pearson correlation r=0.531, P=0.019) (Fig.3A) when ABCB5 mRNA expression was determined by quantitative real-time PCR analyses in seven colorectal cancer lines from the NCI-60 panel used by the National Cancer Institute for drug screening and compared to the respective 5-FU GI50 values available from the NCI Developmental Therapeutics Program (18). SW480 and HT-29 cells were selected for further study based on their well-characterized in vivo tumorigenic potential, and found to express the principal ABCB5 mRNA isoform first identified in human malignant melanoma (17) (NCBI Reference Sequence: NP_848654.3), as determined by RT-PCR amplification, cloning and sequencing of the corresponding complete 2784-bp cDNA ORF (deposited to the GenBank database under accession numbers GU437217 and GU437216, respectively) (Fig. 3B, left panel). ABCB5 protein expression by SW480 and HT-29 cells was demonstrated by flow cytometry (Fig.3B, right panels) and IP and Western blotting (Fig.4A), with 20.1% of SW480 and 4.9% of HT-29 colorectal cancer cells found to express ABCB5 (Figure 3B, right panels). In vivo, quantitative immunohistochemical expression analyses of HT-29 or SW480 tumor xenograft specimens resected from 5-FU-treated or vehicle control-treated NOD/SCID recipient mice demonstrated that 38.8±5.8% of cancer cells in tumors of 5-FU-treated xenograft recipients expressed ABCB5, a significantly increased proportion compared to 18.4±3.6% ABCB5 positivity in tumors derived from vehicle control-treated xenograft recipients (mean±SEM, P=0.011) (Fig. 3C, D). ABCB5/TUNEL double staining of tumor specimens derived from 5-FU-treated xenograft recipients revealed enhanced resistance to 5-FU-induced apoptotic cell killing of ABCB5+ cells (predominantly TUNEL-negative) compared to ABCB5- cells (predominantly TUNEL-positive) following systemic 5-FU therapy (Fig. 3C). In aggregate, these results reveal increased therapeutic resistance of ABCB5+ colorectal cancer cells, compared to ABCB5- tumor populations, to 5-FU treatment.
Figure 3. Response of ABCB5 expression to 5-FU treatment in human colorectal cancer xenografts.

A, Correlation between ABCB5 expression and 5-FU resistance in human NCI-60 colon cancer cell lines: (1) COLO205, (2) HCT-116, (3) HCT-15, (4) HT-29, (5) HCC-2998, (6) KM12, and (7) SW620. B, Left panel: RT-PCR expression analysis of full-length ABCB5 mRNA (NM_178559) in human SW480 and HT-29 colon cancer cell lines. The human melanoma cell line G3361 was used as a positive control. Right panels: Flow cytometric determination of ABCB5 protein expression in SW480 and HT-29 cells. Bottom panels depict isotype control-stained cells. C, ABCB5 expression in human colorectal cancer xenografts in response to 5-FU treatment. Panels depict (from left to right) representative ABCB5 staining (HRP, brown; nuclei are counterstained with DAPI, blue) or ABCB5 (AP, blue) / TUNEL (HRP, brown) co-staining of SW480 (top rows) and HT-29 (bottom rows) colon cancer xenografts dissected from vehicle control- versus 5-FU treated animals. D, Quantitative ABCB5 protein expression analysis in colon cancer xenografts (% positive cells, mean±SEM) dissected from vehicle-control (n=13) versus 5-FU-treated specimens (n=18).
Figure 4. Inhibition of tumorigenic growth and 5-FU resistance reversal of human colorectal cancer cells by ABCB5 knockdown (KD).

A, Stable HT-29 (top) or SW480 (bottom) ABCB5-KD cells or vector controls were generated using shRNA gene silencing. Confirmation of ABCB5-KD at mRNA (bottom rows, determined by exon-exon RT-PCR), and protein levels (top rows, determined by Western blotting), using GAPDH and αTubulin as controls, respectively. B, Analysis of in vitro growth kinetics of stable HT29ABCB5 KD (red line) vs. HT-29control (blue line) cells (top), or SW480ABCB5 KD (red line) vs. SW480control (blue line) cells bottom). C, In vivo tumor growth kinetics of stable HT29ABCB5 KD (red line) vs. HT-29control (blue line) xenografts (top), or SW480ABCB5 KD (red line) vs. SW480control (blue line) xenografts (bottom). D, 5-FU-dependent cell killing for HT-29control (blue line) vs. HT29ABCB5 KD cells (red line) (top) and SW480control (blue line) vs. SW480ABCB5 KD (red line) cells (bottom) as determined using the MTT assay. Illustrated are surviving cell fractions as a function of 5-FU concentration (nM) for n=6 replicate samples, respectively (*: P<0.05; ***: P<0.0001).
ABCB5 mediates 5-FU resistance in human colorectal cancer cells
The findings of increased resistance of ABCB5+ colorectal cancer cells to 5-FU therapy suggested that ABCB5 might functionally contribute to 5-FU resistance in this malignancy, similar to the role of a related ABCB5 isoform (NCBI Reference Sequence: NM_001163942.1) in human melanoma (23). To explore the potential role of ABCB5 as a 5-FU resistance mediator in colorectal cancer, we generated stable ABCB5 shRNA knockdown (ABCB5-KD) or pSUPER vector control variants for both SW480 and HT-29 colorectal cancer cells. Significant ABCB5 knockdown was demonstrated for both HT-29 and SW480 cells (Fig. 4A), at both the mRNA level using RT-PCR and real-time PCR (HT-29: 64% knockdown and SW480: 65% knockdown), and at the protein level using IP-western blot analysis (Fig. 4A). First, in vitro and in vivo growth characterization of ABCB5 knockdown variants vs. controls revealed that in vitro proliferation was not significantly different for HT-29ABCB5-KD vs. HT-29control or SW480ABCB5-KD vs. SW480control cells (Fig. 4B), even upon long-term, 3-week in vitro culture (culture doubling time HT-29ABCB5-KD vs. HT-29control: 18.7±1.8 h vs. 17.9±1.4 h, mean±SD, NS; culture doubling time SW480ABCB5-KD vs. SW480control: 17.9±0.8 h vs. 18.0±0.2 h, mean±SD, NS). In contrast, in vivo tumorigenic growth resulting from xenotransplantation of HT-29ABCB5-KD or SW480ABCB5-KD cells was significantly inhibited compared to that resulting from xenotransplantation of HT-29control or SW480control cells, respectively (HT-29ABCB5-KD vs. HT-29control at 7 weeks: 336±52 mm3 vs. 1150±269 mm3, 71% inhibition, P=0.0163; SW480ABCB5-KD vs. SW480control at 5 weeks: 429±23 mm3 vs. 983±108 mm3, 66% inhibition, P=0.001) (Fig. 4C), pointing to a functional role of ABCB5 in tumorigenic growth. Next, based on identical growth characteristics of untreated control and ABCB5-KD colorectal cancer cells in vitro, we subjected ABCB5-KD or control cells to 5-FU-induced cell killing as a dose-response to increasing 5-FU concentrations in 1-week drug exposure assays, followed by cell viability measurements to assess relative cell death, using an MTT assay (18). ABCB5 knockdown significantly reversed the 5-FU resistance of both HT-29 and SW480 colorectal cancer cells (Fig. 4D), resulting in significant enhancement of cell killing at 5-FU concentrations as low as 0.5 nM in HT-29 cells, and at all 5-FU concentrations >5 nM in both HT-29 and SW480 cells, with significant reductions of the LD50 for both HT-29ABCB5-KD vs. HT-29control cells (LD50 2.6 nM vs. 12 nM, respectively, 4.6-fold sensitization, P < 0.0001) and SW480ABCB5-KD vs. SW480control cells (LD50 2.6 nM vs. 22 nM, respectively, 8.5-fold sensitization, P < 0.0001). These results show that ABCB5 mediates 5-FU resistance in human colorectal cancer cells.
Discussion
In this study, based on our previous identification of ABCB5 mRNA expression in human colorectal cancer (18), we performed an in-depth analysis of ABCB5 expression and function involving healthy human colon specimens from nine distinct individuals, colorectal cancer specimens from twenty-three patients, and eight established colorectal cancer cell lines.
First, we found ABCB5 expression to be restricted to rare cells within the healthy human colon, but detected marked overexpression in human colorectal cancer. These results identify ABCB5 expression in clinical human colorectal cancer and parallel findings in melanoma and hepatocellular carcinoma of absent or rare ABCB5 expression in the respective non-malignant tissues, but overexpression in the corresponding malignancies (19, 25-27). Intriguingly, we found ABCB5 to be co-expressed with CD133 on the same individual cells in healthy colon and in human colorectal cancer, also resembling previous findings of co-expression of these molecular markers in human skin and malignant melanoma (17-19) and hepatocellular carcinoma (25). Since CD133 represents a candidate intestinal stem cell marker (34) and potential marker of colorectal cancer stem cells (7, 8), and because colorectal cancers can originate from physiological intestinal stem cells (34, 38) including CD133+ colonic crypt populations (34), ABCB5 might provide a novel molecular link between the physiological colonic stem cell niche and the cell-of-origin for colon cancer.
Second, our results reveal that ABCB5+ malignant subpopulations are resistant to 5-FU-based chemoradiation therapy in rectal cancer patients, thereby establishing ABCB5 as a novel molecular marker of clinically therapy-refractory colorectal cancer cells. The preferential co-expression of CD133 by 5-FU-resistant ABCB5+ cancer cells is consistent with previous findings that documented increased CD133 expression among 5-FU-resistant colorectal cancer cell subsets in vitro (11), and clinical findings of increased resistance of CD133+ colorectal tumors to 5-FU based chemotherapy in human patients (14). In addition, our results show that ABCB5+ malignant subpopulations are also resistant to clinical radiation therapy. Importantly, while a functional role in cancer therapeutic resistance has not been attributed to date to CD133, ABCB5 isoforms have been shown to confer resistance to multiple chemotherapeutic agents in malignant melanoma and hepatocellular carcinoma (18, 23-25). Our findings therefore establish a novel link between biomarkers of therapeutic resistance and a molecular drug resistance mechanism in clinical colorectal cancer.
Finally, the observed expression of the drug resistance mediator ABCB5 by 5-FU-refractory colorectal cancer cell populations suggested a potential functional role of ABCB5 as a mediator of 5-FU resistance in colorectal cancer. Emergence of 5-FU resistance is a major impediment to successful colorectal cancer therapy, but the various potential mechanisms of 5-FU resistance in this malignancy, particularly of more aggressive and resistant CD133+ cancer subpopulations that correlate with recurrence and poor prognosis (14, 36, 39), have not been fully explored. Our results provide initial evidence for a functional contribution of ABCB5 to 5-FU resistance in human colorectal cancer, because stable shRNA-mediated ABCB5 knockdown significantly sensitized human colorectal cancer cells to 5-FU-induced cell killing. Thus, therapy-refractory colorectal cancer cells that might cause tumor recurrence possess a protective mechanism, ABCB5, potentially intrinsic to CD133+ populations from which the tumor arises (7, 8, 34). This function parallels the roles of additional ABC transporters in cancer drug resistance, including of ABCB1 and ABCG2, which afford protection to normal stem cells and cancer cell subsets in the corresponding malignancies in other tissues (40). Our results therefore suggest a novel molecular link between clinical drug resistance and potential tumor-initiating cells in human colorectal cancer.
In aggregate, our results identify ABCB5 as a novel molecular marker of therapy-refractory tumor cell population in colorectal cancer patients and point to a need for consistent eradication of ABCB5-positive resistant tumor cell populations for more effective colorectal cancer therapy. ABCB5-targeted resistance reversal or ABCB5-positive cancer cell ablation might therefore represent novel, clinically relevant strategies to ultimately enhance tumor eradication in colorectal cancer patients, in order to produce more durable clinical responses than those obtained by therapeutic strategies directed predominantly at the bulk population of tumor cells.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by funds provided by the NIH/NCI (grants 1RO1CA113796 and 1R01CA138231 to M.H.F.) and the U.S. Department of Veterans Affairs (BLR&D VA CDA-2 Award to J.S.G. and BLR&D VA Merit Award 10688354 to N.Y.F.).
References
- 1.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson BJ, Schatton T, Frank MH, Frank NY. Colorectal Cancer Stem Cells: Biology and Therapeutic Implications. Curr Colorectal Cancer Rep. 2011;7:128–35. doi: 10.1007/s11888-011-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 5.Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766–75. doi: 10.1200/JCO.2004.03.104. [DOI] [PubMed] [Google Scholar]
- 6.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 8.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–15. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 9.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–89. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 11.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, 2nd, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–57. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–02. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Cammareri P, Scopelliti A, Todaro M, Eterno V, Francescangeli F, Moyer MP, et al. Aurora-a is essential for the tumorigenic capacity and chemoresistance of colorectal cancer stem cells. Cancer Res. 2010;70:4655–65. doi: 10.1158/0008-5472.CAN-09-3953. [DOI] [PubMed] [Google Scholar]
- 14.Ong CW, Kim LG, Kong HH, Low LY, Iacopetta B, Soong R, et al. CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Mod Pathol. 2010;23:450–57. doi: 10.1038/modpathol.2009.181. [DOI] [PubMed] [Google Scholar]
- 15.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–20. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 17.Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–65. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- 18.Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–33. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- 19.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–49. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–08. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Lin JY, Alloo A, Wilson BJ, Schatton T, Zhan Q, et al. Isolation of tumorigenic circulating melanoma cells. Biochem Biophys Res Commun. 2010;402:711–17. doi: 10.1016/j.bbrc.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, et al. VEGFR-1 expressed by malignant melanoma initiating cells is required for tumor growth. Cancer Res. 2011;71:1474–85. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, et al. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004;64:4294–01. doi: 10.1158/0008-5472.CAN-03-3884. [DOI] [PubMed] [Google Scholar]
- 24.Elliott AM, Al-Hajj MA. ABCB8 mediates doxorubicin resistance in melanoma cells by protecting the mitochondrial genome. Mol Cancer Res. 2009;7:79–87. doi: 10.1158/1541-7786.MCR-08-0235. [DOI] [PubMed] [Google Scholar]
- 25.Cheung ST, Cheung PF, Cheng CK, Wong NC, Fan ST. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344–55. doi: 10.1053/j.gastro.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Sharma BK, Manglik V, Elias EG. Immuno-expression of human melanoma stem cell markers in tissues at different stages of the disease. The Journal of surgical research. 2010;163:e11–15. doi: 10.1016/j.jss.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Gazzaniga P, Cigna E, Panasiti V, Devirgiliis V, Bottoni U, Vincenzi B, et al. CD133 and ABCB5 as stem cell markers on sentinel lymph node from melanoma patients. Eur J Surg Oncol. 2010;36:1211–14. doi: 10.1016/j.ejso.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Frank NY, Kho AT, Schatton T, Murphy GF, Molloy MJ, Zhan Q, et al. Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin. J Cell Biol. 2006;175:99–10. doi: 10.1083/jcb.200511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, et al. Human CD271-Positive Melanoma Stem Cells Associated with Metastasis Establish Tumor Heterogeneity and Long-term Growth. Cancer Res. 2011;71:3098–109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 30.Frank MH, Pomer S. Interferon alpha2b differentially affects proliferation of two human renal cell carcinoma cell lines differing in the P-glycoprotein-associated multidrug-resistant phenotype. J Cancer Res Clin Oncol. 1999;125:117–20. doi: 10.1007/s004320050252. [DOI] [PubMed] [Google Scholar]
- 31.Ciccolini J, Peillard L, Evrard A, Cuq P, Aubert C, Pelegrin A, et al. Enhanced antitumor activity of 5-fluorouracil in combination with 2’-deoxyinosine in human colorectal cell lines and human colon tumor xenografts. Clin Cancer Res. 2000;6:1529–35. [PubMed] [Google Scholar]
- 32.Jorgensen TJ, Tian H, Joseph IB, Menon K, Frost D. Chemosensitization and radiosensitization of human lung and colon cancers by antimitotic agent, ABT-751, in athymic murine xenograft models of subcutaneous tumor growth. Cancer Chemother Pharmacol. 2007;59:725–32. doi: 10.1007/s00280-006-0326-2. [DOI] [PubMed] [Google Scholar]
- 33.Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2187–94z. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–07. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–74. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–98. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 37.Kumar V, Abbas A, Fausto N, Aster J. Robbins and Cotran Pathologic Basis of Disease, Professional Edition. 8. Elsevier; 2010. The Gastrointestinal Tract. [Google Scholar]
- 38.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 39.Artells R, Moreno I, Diaz T, Martinez F, Gel B, Navarro A, et al. Tumour CD133 mRNA expression and clinical outcome in surgically resected colorectal cancer patients. Eur J Cancer. 2010;46:642–49. doi: 10.1016/j.ejca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Moitra K, Lou H, Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin Pharmacol Ther. 2011;89:491–502. doi: 10.1038/clpt.2011.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.