Abstract
Outcomes following lung transplant remain suboptimal. This is attributable to variable post-transplant recovery of lung function, and inconsistent degrees of lung function loss after peak function is reached. Granzyme B is elevated in the blood and bronchoalveolar lavage (BAL) in acute rejection. We hypothesized that persistent exposure to T cells high in granzymeB would negatively correlate with lung function. We investigated cumulative exposure measured as the area-under-the-curve (AUC) of CD8+ T cell granzyme Bhi cells in the first year post transplant in both BAL and blood in 24 transplant recipients. We assessed the correlation between cumulative 1-year exposure and FEV1 slope. There was a negative correlation between 1-year exposure and FEV1 slope within the first year (r−.63, p.001). This relationship persisted even when adjusted for transplant type, gender, age, rejection, and indication for transplantation. In contrast, no relationship was seen with the 1-year AUC and lung function after 1 year post transplant. In contrast to the BAL granzyme B hi levels, granzyme B hi levels from the blood showed no relationship with lung function. These findings suggest that CD8+ T cell driven factors are responsible for early improvements in lung function after transplantation.
Keywords: Lung Transplantation, rejection, granzyme B, FEV1
Introduction
Despite advances in surgical technique and improvements in early outcomes, only 50% of lung transplant patients survive longer than five years(1). The major limitation to long-term survival is a high rate of chronic allograft rejection termed bronchiolitis obliterans (BO), which manifests clinically as bronchiolitis obliterans syndrome (BOS)(2). Many factors are proposed as triggers to OB/BOS. These include ischemia-reperfusion injury, acute cellular rejection (ACR), infections, and gastroesophageal reflux disease (GERD) leading to alloimmune-and non-alloimmune-dependent lung injury(3–6). T cells stimulated during alloimmume response to lung injury respond by secreting granzyme B, a serine protease that induces apoptosis of target epithelial cells via the members of the caspase family(7, 8). Several clinical studies in solid organ transplantation including series in kidney, liver and lung transplantation have shown that granzyme B mRNA expression serves as a biomarker for ACR(9, 10). Additional studies in human lung transplant recipients have shown an increased level of T cell granzyme B in the blood and bronchoalveolar lavage of these patients in ACR and BOS(11).
Patients are labeled as having BOS once their forced expiratory volume in one second (FEV1) measured by spirometry drops below 80% of their best post transplant value. Because BOS is a ratio between current and best FEV1, BOS may underestimate chronic lung dysfunction because patients with early lung injury may never manifest high post-transplant “best” FEV1 values. It is likely though that morbidity and mortality are affected not simply by the decline in lung function after a peak is achieved, but also by the height of the peak of best lung function after transplant.
Transbronchial biopsy remains the standard for diagnosing ACR in lung transplantation(12). However this procedure can cause morbidity, is confounded by inter-rater variability and is subject to sampling error.(13–15). Thus, detection of makers of injury in the cellular compartment of bronchoalveolar lavage alone or in combination with transbronchial biopsy and physiologic measures of lung function could improve the utility of bronchoscopies. We, and many others, have hypothesized that cumulative exposure to injurious cells and soluble constituents of the immune system subsequently leads to pulmonary dysfunction. While perhaps self-evident, this idea has been difficult to prove because of the practical difficulty of estimating cumulative exposure. Here we took advantage of our program’s clinical management protocol in lung transplantation, which calls for multiple surveillance bronchoscopy procedures in the first year. We analyzed residual BALF by cellular analysis to determine the extent to which cumulative exposure to CD8+ granzyme B cells in the lung predicted early and late lung function after transplant.
Methods
Patients
The study was approved by the institutional review board and informed consent was obtained from all patients. In our center, patients undergo surveillance bronchoscopy at 2 weeks, and 1, 2, 3, 6, 9 and 12 months post transplantation. Bronchoscopy was perfomed in a standardized manner in which a total of 150mL of saline was instilled into the right middle lobe or lingula followed by aspiration of BALF. A total of 52 subjects were screened during the study period of June 2006 to July 2009. Of these, 24 patients had at least 4 surveillance bronchoscopies performed where samples were obtained for assessment of T cell granzyme B, a complete set of PFT data post-transplantation, and at least one year of post transplant survival. These 24 patients with a complete set of data were included for analysis. There were 16 (67%) males and the median age at transplant was 59 (IQR 51–63) years. The primary indication for transplantation was emphysema (13/24; 54%) and idiopathic pulmonary fibrosis (IPF) (8/24; 33%). All but one subject received bilateral lung transplant. 16/24 had at least one episode of acute cellular rejection (≥A1), 8/24 had stable allograft (no episodes of rejection) and one developed OB during the first year after transplant. None of the 24 patients studied had significant lymphocytic bronchiolitis (>B1R).
Flow cytometry
The cellular fraction of the BAL was obtained by brief centrifugation followed by red blood cell lysis. Mononuclear cells were then stained with various combinations of antibodies to define different subpopulations of cells. All cell preparations were prepared and labeled fresh on the day of bronchoscopy. Granzyme B frequency was obtained in the CD8+ population using a gating strategy that first demonstrated the lymphocyte population by forward and side scatter qualities. CD8 cells were positive for CD8 and CD3 and negative for CD4. In this way, CD4/8 double positives, which also express Granzyme B, were excluded from analysis. We defined the gates for granzyme B by initially analyzing peripheral blood mononuclear cells taken from subjects not assessed in this study. When analyzed this way, CD8+ peripheral blood mononuclear cells (PBMCs) show a strong bimodal distribution of granzyme B+ cells (sample flow gating is shown in figure 1). We applied the gates from these initial experiments to all the study samples. We utilized mid-range calibration beads (BD) to set identical positive and negative peaks between samples run at different time points. We calculated the area-under- the-curve (AUC) of the percentage of CD8+ cells that were granzyme B+ for each subject over a 12-month period post-transplant. In order to estimate absolute numbers of CD8+ granzyme Bhi T cells in each BAL sample, we multiplied the mononuclear cell count of the BAL obtained from our clinical lab by the percentage of BAL cells which were CD3+CD8+CD45+ and in a similar manner calculated the AUC for granzyme B absolute cell counts.
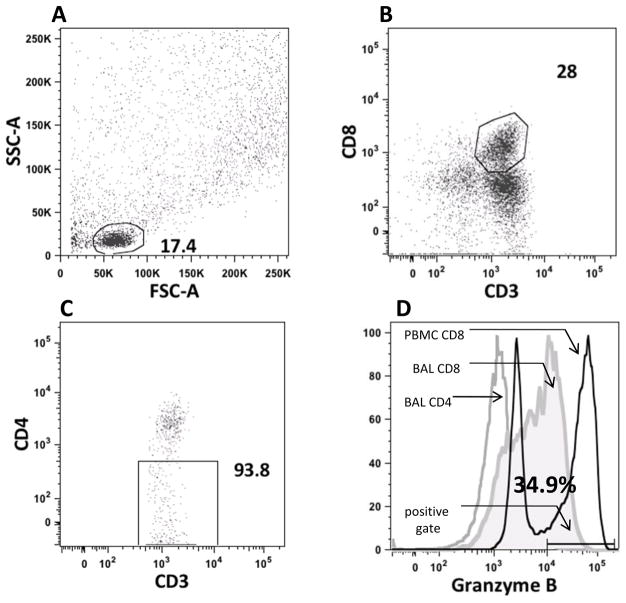
Figure 1.
Sample gating strategy to quantify CD8 granzyme Bhi frequency. Panel A shows light scatter plots for a BAL sample. Small lymphocytes were gated on. Panel B demonstrates the CD8 positive population derived from panel A. Panel C demonstrates the CD4 negative component of the gate derived from panel B. Panel D compares the Granzyme B frequencies of BAL CD8 cells (solid grey area) to BAL CD4 cells (open grey line) to PBMC CD8 cells (open black line). Also shown is the positive gate used for each sample analyzed.
Pulmonary Function Testing
Each subject underwent monthly pulmonary function testing on from month one post transplant onwards: forced expiratory flow rate in one second (FEV1) was measured during each testing. We calculated the slope of FEV1 between month 1 and subsequent time points post-transplant for each subject assessed as a linear term. We then assessed the association between the area under the curve of BAL CD8+ granzyme Bhi T cells and FEV1 slope using Spearman’s rank correlation.
Statistical Analysis
Descriptive analyses were performed to characterize the study population with reporting of median values and standard error of mean (SEM). The cumulative level of BAL granzymeB hi CD8+ T cells was measured by calculating the AUC of the percentage of CD8+ T cells in the BAL that were granzymeB positive over a period of 12 month post transplant. The association between the AUC of BAL CD8+ granzyme Bhi T cells and FEV1 slope was assessed using Spearman’s rank correlation. A 2-tailed P value of .05 was considered statistically significant. In order to estimate the effects of relevant clinical parameters on FEV1 we fit the FEV1 for each patient using a least-squares means model. The a-priori variables chosen to fit the model were indication for transplant (fibrotic vs. obstructive disease), recipient age, recipient sex, laterality of transplant (single vs. bilateral), donor height, donor age, the presence of treated ≥ grade 2 acute rejection in the first year, and the presence of ≥ grade 2 primary graft dysfunction at 48 or 72 hours post transplant. Of these variables only gender and recipient age at enrollment were found to be significant. All statistical analyses were performed using SAS 9.1 (Cary NC).
Results
CD8+ T cell Granzyme B is highly variable between samples in BAL and PBMC
Key to understanding how CD8+ granzyme Bhi T cells influenced lung function was determining the procedure to procedure variability of this biomarker. To estimate within subject variability, we analyzed CD8+ from 52 subjects who had at least 3 longitudinal data points. The samples contained in this analysis included BAL from episodes of quiescence as well as rejection. This initial analysis provided an estimate of the upper bound of CD8+ granzyme Bhi T cells variability. In BAL, the percentage of total variance due to between subject variance was 25.3% whilst 74.7% of the total variance was due to within subject variance. In the PBMC the percentage of total variance due to between subject variance was 39.0% whilst 61.0% of the total variance was due to within subject variance. This shows that both BAL and PBMC have high within subject variability relative to between-subject variability. Based on these findings we analyzed the relationship of CD8+ granzyme Bhi T cells to spirometry utilizing only patients that had an entire year’s worth of BAL and spirometry to limit the effect of within subject variability among patients with on a few discreet data points. This limited our analyses to 24 of the initial 52 patients screened for inclusion into this study.
Cumulative 1-year bronchoalveolar lavage CD8+ granzyme Bhi T cell level negatively correlates with FEV1 slope in the first year following lung transplantation
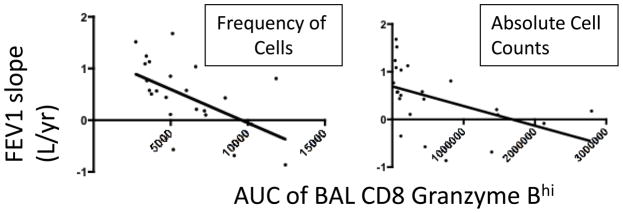
While episodic inflammation is known to cause allograft damage, we hypothesized that cumulative exposure to cytotoxic T cells, though perhaps more indolent, could give rise to substantial lung damage, at times independent of overt clinical inflammatory events. We therefore assessed the cumulative exposure to CD8+granzymeBhi T cells in the BAL as it related to indices of lung allograft function. As shown in figure 2, there was a significant negative correlation between cumulative 1-year BAL CD8+ granzyme Bhi T cell frequency and FEV1 slope for 1 to 6 month, 1 to 9 month and 1 to 12 month post lung transplant. A similar negative correlation was observed when absolute BAL CD8+ granzyme Bhi counts were assessed. Importantly, as the time interval of FEV1 slope was increased, the degree of negative correlation between granzyme B decreased (summarized in table 1). Hence, when the relationships between FEV1 slopes for the time intervals 1 to 18 months or 9 to18 months and cumulative 1-year BAL CD8+ granzyme Bhi T cell level were analyzed, no statistically significant negative correlation was noted. The relationships between early time intervals and the absence of a relationship during late time intervals suggest that the predominant effect of T cell inflammation is established early.
Figure 2.
Inverse relationship between cumulative BAL CD8 granzyme Bhi exposure and FEV1 slope over the first 9 months post lung transplant. r=−.61 for cell frequency and −.68 for absolute cell count, p=.0017 and .0003.
Table 1.
| Variable | Value |
|---|---|
| No of patients | 24 |
| Male | 16 (67%) |
| Female | 8 (33%) |
| Race | |
| Caucasian | 20 (83 %) |
| African American | 4 (17%) |
| Median Age at Transplant (±SEM) | 59 (±2.3) |
| Indication for Transplant | |
| Restrictive Lung Disease (eg. fibrosis, silicosis, sarcoid) | 11 (46%) |
| Obstructive Lung Disease (eg. COPD, cystic fibrosis) | 13 (54%) |
| Type of Transplant | |
| Bilateral | 23 (96%) |
| Single | 1 (4%) |
Baseline patient characteristics for the 24 patients assessed for cumulative 1 year CD8 Granzyme Bhi BAL and PBMC exposure
Peripheral blood CD8+ granzyme Bhi levels do not strongly correlate with either early or late physiology post transplant
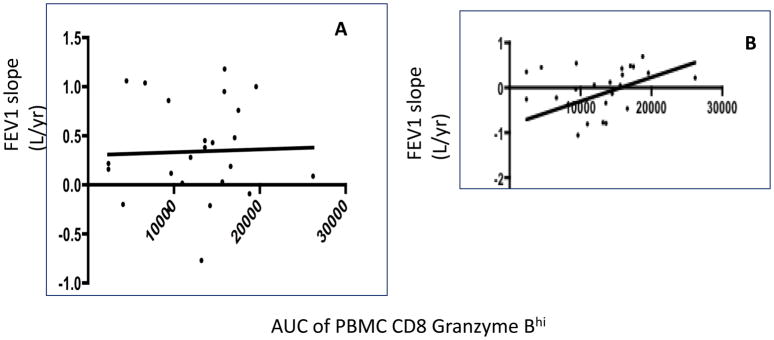
To determine whether the effect of CD8+granzymeBhi T cells was localized to the allograft, or the result of a systemic inflammatory response, we then evaluated the relationship between CD8+ granzyme Bhi T cells in the peripheral blood and pulmonary function. As shown in figure 3, and indicated in the table, we did not observe a statistically meaningful relationship between PBMC CD8 granzyme Bhi T cell frequency over 12 months and FEV1 slope over 12 months. Similarly, no correlation existed when shorter FEV1 slope intervals were assessed or when absolute counts of PBMC CD8 GranzymeB hi cells were assessed (not shown). When longer-term lung function was assessed, we found a positive, yet not statistically significant correlation between blood CD8+ granzyme Bhi T cell exposure and FEV1 slope between 9–18 months (figure 2b).
Figure 3.
No relationship between blood CD8 granzyme Bhi exposure and early or late lung function. Panel A shows relationship of pbmc CD8 granzyme Bhi AUC to FEV 1 slope between 1 and 9 months. Panel B shows relationship of pbmc CD8 granzyme Bhi AUC to FEV1 slope between 9 and 18 months. (r=−.11, p.6 for 1–9 months and r= .38, p=.07 for 9–18 months.
Cumulative CD8+ granzyme Bhi T cell exposure predicts early lung function after multivariable adjustment
In order to adjust for the potential effect of other variables on post transplant FEV1, we performed multivariable analysis of a-priori determined baseline and clinical variables. We fit our FEV1 data into a least squares means models, which had the advantage of not being constrained to whether the FEV1 change observed during the period of observation corresponded to a linear or quadratic relationship. Using this methodology, only age and recipient gender were found to be statistically significantly related to post transplant mean FEV1, although our cohort only contained 24 patients, so it is possible the effects of some variables (e.g. presence of at least one episode of rejection) would have some additional observed effect with a larger sample size. We then assessed the AUC for CD8+ granzyme Bhi T cells adjusted for these variables in deciles. As shown in table 2, for each decile increase there was a corresponding decrease in the FEV1 observed, p<.0038.
Table 2.
| AUC Parameter measured | Time of Observation for FEV1 slope | Correlation (r) | 95% CI | p value |
|---|---|---|---|---|
| BAL CD8 GranzymeB frequency | 1–6 month | −.63 | −.83 to−.29 | 0.001 |
| BAL CD8 GranzymeB frequency | 1–9 month | −.61 | −.82 to−.26 | .001 |
| BAL CD8 GranzymeB frequency | 1–12 month | −.52 | −.77 to−.13 | .01 |
| BAL CD8 Granzyme B Absolute Cell count | 1–9 month | −.68 | −.86 ti−.29 | .0003 |
| BAL CD8 GranzymeB frequency | 1–18 month | −.43 | −.73 to 0 | .05 |
| BAL CD8 GranzymeB frequency | 9–18 month | −.13 | −.53 to .31 | .5 |
| BAL CD8 GranzymeB frequency | 1 month- last data point | −.34 | −.66 to .09 | .1 |
| BAL CD8 GranzymeB frequency | 9 month- last data point | −.11 | −.5 to .32 | .6 |
| PBMC CD8 Granzyme B frequency | 1–12 month | −.11 | −.32 to .50 | .6 |
Correlation of 1 year Granzyme B exposure measured in the BAL or pbmc with lung function trends over different time intervals post transplant.
Discussion
In lung transplantation there has been an emerging concern that repetitive episodes of immunologic-mediated injury predisposes patients to subsequent development of BO. This concept underlies the rationale behind clinical protocols that rely on surveillance transbronchial biopsies to detect rejection when it can be classified by histology prior to being clinically obvious. There are several practical problems related to the use of surveillance transbronchial biopsies. These include the fact that such biopsies may miss rejection when present, they may overestimate rejection and hence lead to over treatment, and they may lead to complications such as pulmonary hemorrhage. Importantly, the threshold for establishing ACR is not set at a level void of all inflammation. As such, indolent inflammation can persist, and evoke cumulative damage, without ever reaching the diagnostic threshold for ACR. We hypothesized a measure of cumulative T cell-mediated immunologic activation in the lung over time could be calculated by quantifying the sum total of a relevant T cell biomarker, granzyme B obtained from BALF obtained during routine bronchoscopy. We further characterized the relationship between granzyme B exposure and early changes in spirometry compared to later changes. While this is the first report to estimate effector CD8 exposure using an AUC analysis, it is noteworthy that other investigators have used a similar approach linking cumulative TGFb exposure to kidney function after renal transplantation(16). Further, within the field of lung transplantation other investigators have attempted to link cumulative exposures such as rejection episodes to subsequent lung function(17). Collectively these reports as well as our report here support the concept of modeling repetitive exposure over time to important clinical outcomes.
Considerable data link granzyme B levels either at the transcript level or protein level to acute adverse outcomes in solid organ transplantation. Our own group has reported elevation of this biomarker in both patients with ACR and who have severe gastroesophageal reflux(18). But acute rejection by itself in the setting of lung transplantation is of unclear clinical concern. For example, ACR usually is managed easily by treating patients with a short course of high-dose corticosteroids. In this work, we show that cumulative measurement of BAL CD8+ granzyme Bhi T cells correlates with an important marker of lung function, FEV1. We found the highest correlation with the FEV1 slope measure from the time of transplant to 9 months post transplant. This corresponds to the post transplant period in which most patients achieve peak lung function following transplantation. The clinical significance of our findings is that patients with high cumulative levels of CD8+ granzyme Bhi T cells experience less exuberant increases in FEV1. The end result is that they ultimately achieved a lower peak lung function. With lower peak lung function, they have less of a physiologic cushion with which to buffer against subsequent insults to the transplanted lungs. We predict that patients with high cumulative 1-year CD8+ granzyme Bhi T cell exposure would be at increased risk of subsequent mortality, which is a focus of ongoing investigation with this cohort.
The premise that early events would predispose to subsequent late-term clinical outcomes has precedence; primary graft dysfunction in the first hours after transplant increases the risk for BOS(19–21). Here we found that CD8+ granzyme Bhi T cell exposure over the first post transplant year did not correlate with subsequent FEV1 slope from one year onward. This is also an interesting finding. While our study could be underpowered to detect a small effect of granzyme B on later lung function, our findings suggest that CD8+ granzyme Bhi T cells are likely not the primary determinant of what drives loss of airflow once peak lung function is achieved. A growing body of work has focused on linking the frequency of certain cell populations in both blood and BAL and longer-term lung function. Among these is the finding that a population of blood CD4+ lymphocytes expressing granzyme B and low in CD28 are highly predictive of patients at risk of developing BOS(22). At the time points studied in this work, we did not detect a significant population of CD4+ lymphocytes that expressed granzyme B, when CD4/8 double positives were excluded from analysis (not shown). Hence it is possible that some cells with phenotypic features of both CD8 and CD4 cells, perhaps belonging to the gamma-delta T cell subset, could also contribute a Granzyme B signal. These cells would have been missed by our conservative gating stategy, and this may explain the differences between our findings and those of other investigators who have described CD4+ Granzyme Bhi cells as biomarkers for adverse outcome in lung transplantation(23). It is also notable that for the early time points assessed, none of the surveillance biopsies contained high-grade lymphocytic bronchiolitis, a condition associated with increased effector CD4 cells(24). Hence it is possible that T cell subets beyond conventional CD8 cells are also responsible for ongoing lung injury, particularly at later time points.
CD8+ T cells are the predominant lymphocyte in early rejection(25). Further, in the early aftermath of lung transplant the pulmonary epithelium expresses allogeneic class I molecules that support CD8 division via direct allorecognition. But as the balance of direct and indirect allorecognition changes with time, it also is plausible that the relative contribution of CD8+ versus CD4+ cells to ongoing lung injury would evolve(26). In this regard, both CD4+ and CD8+ T cells have been shown to participate with differing roles in the murine model of obliterative airway disease(27–29). Finally, it is likely that additional mechanisms unrelated to traditional T cell mediated adaptive immune responses also are responsible for loss of lung function once peak post transplant function has been established, including repetitive epithelial injury from reflux, aspiration, and infection.
In summary, our findings show a relationship between the presence of CD8+ granzyme Bhi T cells in the BAL and achievement of peak lung function in the allograft. It remains to be determined whether these T cells exist as a result of rejection, infection, traumatic or chemical injury, or as is likely the case, a combination of all of these events. They support the concept that measurement of CD8+ granzyme Bhi T cells is a clinically meaningful biomarker with respect to lung injury.
Table 3.
| Variable | Category | Mean FEV1 | 95% CI | P Value |
|---|---|---|---|---|
| Gender | Male | 2.76 | 2.58, 2.94 | < 0.0001 |
| Female | 2.19 | 2.03, 2.35 | ||
| Age at Enrollment | 40 | 2.31 | 2.16, 2.47 | 0.01 |
| 50 | 2.44 | 2.32, 2.57 | ||
| 60 | 2.56 | 2.42, 2.71 | ||
| AUC GranzymeB | 1000 | 2.88 | 2.62, 3.15 | 0.0038 |
| 2000 | 2.80 | 2.58, 3.02 | ||
| 3000 | 2.72 | 2.54, 2.90 | ||
| 4000 | 2.64 | 2.49, 2.78 | ||
| 5000 | 2.55 | 2.43, 2.68 | ||
| 6000 | 2.47 | 2.35, 2.59 | ||
| 7000 | 2.39 | 2.25, 2.53 | ||
| 8000 | 2.31 | 2.13, 2.48 | ||
| 9000 | 2.22 | 2.01, 2.44 | ||
| 10000 | 2.14 | 1.88, 2.40 |
Multivariable analysis of FEV1 (liter/sec) with respect to gender, recipient age and CD8 granzymeBhi frequency assessed by decile
Acknowledgments
David C. Neujahr is supported by a grant from the Roche Organ Transplant Research Foundation and by an NIH Career Development Award (K08AI079166). The investigators wish to thank the Atlanta Clinical and Translational Science Institute and the Emory Transplant Center Biorepository for supporting this research. The authors wish to thank Drs Allan Ramirez and Andres Pelaez for assistance with sample collection.
Abbreviations
- BAL
Bronchoalveolar Lavage
- BO
Bronchiolitis Obliterans
- BOS
Bronchiolitis Obliterans Syndrome
- ACR
Acute Cellular Rejection
- AUC
Area Under the Curve
- FEV1
Forced Expiratory Flow 1 Second
- PBMC
Peripheral Blood Mononuclear Cells
- PFT
Pulmonary Function Test
Footnotes
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
A Mohammed, Email: aminu.mohammed@gmail.com.
O Ulukpo, Email: oulukpo@med.miami.edu.
EC Lawrence, Email: clint.lawrence@emoryhealthcare.org.
F Fernandez, Email: felix.fernandez@emoryhealthcare.org.
A Pickens, Email: allan.pickens@emoryheatlhcare.org.
AA Gal, Email: agal@emory.edu.
SD Force, Email: sforce@emory.edu.
KC Easley, Email: keasle2@emory.edu.
CP Larsen, Email: clarsen@emory.edu.
AD Kirk, Email: adkirk@emory.edu.
DC Neujahr, Email: david.neujahr@emoryhealthcare.org.
References
- 1.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009 Feb;9(2):389–96. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke CM, Theodore J, Dawkins KD, Yousem SA, Blank N, Billingham ME, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest. 1984 Dec;86(6):824–9. doi: 10.1378/chest.86.6.824. [DOI] [PubMed] [Google Scholar]
- 3.Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. Semin Thorac Cardiovasc Surg. 2008 Summer;20(2):173–82. doi: 10.1053/j.semtcvs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2004 Nov 1;170(9):1022–6. doi: 10.1164/rccm.200302-165OC. [DOI] [PubMed] [Google Scholar]
- 5.Bando K, Paradis IL, Similo S, Konishi H, Komatsu K, Zullo TG, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995 Jul;110(1):4–13. doi: 10.1016/S0022-5223(05)80003-0. discussion -4. [DOI] [PubMed] [Google Scholar]
- 6.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999 Mar;159(3):829–33. doi: 10.1164/ajrccm.159.3.9607099. [DOI] [PubMed] [Google Scholar]
- 7.Krupnick AS, Kreisel D, Popma SH, Balsara KR, Szeto WY, Krasinskas AM, et al. Mechanism of T cell-mediated endothelial apoptosis. Transplantation. 2002 Sep 27;74(6):871–6. doi: 10.1097/00007890-200209270-00022. [DOI] [PubMed] [Google Scholar]
- 8.Krams SM, Villanueva JC, Quinn MB, Martinez OM. Expression of the cytotoxic T cell mediator granzyme B during liver allograft rejection. Transpl Immunol. 1995 Jun;3(2):162–6. doi: 10.1016/0966-3274(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 9.Sharma VK, Bologa RM, Li B, Xu GP, Lagman M, Hiscock W, et al. Molecular executors of cell death--differential intrarenal expression of Fas ligand, Fas, granzyme B, and perforin during acute and/or chronic rejection of human renal allografts. Transplantation. 1996 Dec 27;62(12):1860–6. doi: 10.1097/00007890-199612270-00031. [DOI] [PubMed] [Google Scholar]
- 10.Shi R, Yang J, Jaramillo A, Steward NS, Aloush A, Trulock EP, et al. Correlation between interleukin-15 and granzyme B expression and acute lung allograft rejection. Transpl Immunol. 2004 Jan;12(2):103–8. doi: 10.1016/j.trim.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Hodge S, Hodge G, Ahern J, Liew CL, Hopkins P, Chambers DC, et al. Increased levels of T cell granzyme b in bronchiolitis obliterans syndrome are not suppressed adequately by current immunosuppressive regimens. Clin Exp Immunol. 2009 Nov;158(2):230–6. doi: 10.1111/j.1365-2249.2009.04008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007 Dec;26(12):1229–42. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Valentine VG, Gupta MR, Weill D, Lombard GA, LaPlace SG, Seoane L, et al. Single-institution study evaluating the utility of surveillance bronchoscopy after lung transplantation. J Heart Lung Transplant. 2009 Jan;28(1):14–20. doi: 10.1016/j.healun.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Herf SM, Suratt PM, Arora NS. Deaths and complications associated with transbronchial lung biopsy. Am Rev Respir Dis. 1977 Apr;115(4):708–11. doi: 10.1164/arrd.1977.115.4.708. [DOI] [PubMed] [Google Scholar]
- 15.Sibley RK, Berry GJ, Tazelaar HD, Kraemer MR, Theodore J, Marshall SE, et al. The role of transbronchial biopsies in the management of lung transplant recipients. J Heart Lung Transplant. 1993 Mar-Apr;12(2):308–24. [PubMed] [Google Scholar]
- 16.Harris S, Coupes BM, Roberts SA, Roberts IS, Short CD, Brenchley PE. TGF-beta1 in chronic allograft nephropathy following renal transplantation. J Nephrol. 2007 Mar-Apr;20(2):177–85. [PubMed] [Google Scholar]
- 17.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008 May 1;177(9):1033–40. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 18.Neujahr DC, Mohammed A, Ulukpo O, Force SD, Ramirez AM, Pelaez A, et al. Surgical correction of gastroesophageal reflux in lung transplant patients is associated with decreased effector CD8 cells in lung lavages: a case series. Chest. Oct;138(4):937–43. doi: 10.1378/chest.09-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005 Jun 1;171(11):1312–6. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008 Nov;8(11):2454–62. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008 Jul;86(1):189–95. doi: 10.1016/j.athoracsur.2008.03.073. discussion 96–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studer SM, George MP, Zhu X, Song Y, Valentine VG, Stoner MW, et al. CD28 down-regulation on CD4 T cells is a marker for graft dysfunction in lung transplant recipients. Am J Respir Crit Care Med. 2008 Oct 1;178(7):765–73. doi: 10.1164/rccm.200701-013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodge G, Hodge S, Li-Liew C, Chambers D, Hopkins P, Reynolds PN, et al. Time post-lung transplant correlates with increasing peripheral blood T cell granzyme B and proinflammatory cytokines. Clin Exp Immunol. Sep;161(3):584–90. doi: 10.1111/j.1365-2249.2010.04186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodge G, Hodge S, Li-Liew C, Chambers D, Hopkins P, Reynolds PN, et al. Lymphocytic bronchiolitis is associated with inadequate suppression of blood T-cell granzyme B, IFN-gamma, and TNF-alpha. Transplantation. May 27;89(10):1283–9. doi: 10.1097/TP.0b013e3181d75971. [DOI] [PubMed] [Google Scholar]
- 25.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008 Apr 1;180(7):4754–62. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999 Jan 1;162(1):352–8. [PubMed] [Google Scholar]
- 27.West EE, Lavoie TL, Orens JB, Chen ES, Ye SQ, Finkelman FD, et al. Pluripotent allospecific CD8+ effector T cells traffic to lung in murine obliterative airway disease. Am J Respir Cell Mol Biol. 2006 Jan;34(1):108–18. doi: 10.1165/rcmb.2005-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards DM, Dalheimer SL, Ehst BD, Vanasek TL, Jenkins MK, Hertz MI, et al. Indirect minor histocompatibility antigen presentation by allograft recipient cells in the draining lymph node leads to the activation and clonal expansion of CD4+ T cells that cause obliterative airways disease. J Immunol. 2004 Mar 15;172(6):3469–79. doi: 10.4049/jimmunol.172.6.3469. [DOI] [PubMed] [Google Scholar]
- 29.Chalermskulrat W, Neuringer IP, Brickey WJ, Felix NJ, Randell SH, Ting JP, et al. Hierarchical contributions of allorecognition pathways in chronic lung rejection. Am J Respir Crit Care Med. 2003 Apr 1;167(7):999–1007. doi: 10.1164/rccm.200209-1099OC. [DOI] [PubMed] [Google Scholar]