Abstract
During mitosis, kinetochores couple chromosomes to the dynamic tips of spindle microtubules. These attachments convert chemical energy stored in the microtubule lattice into mechanical energy, generating force to move chromosomes. In addition to mediating robust microtubule attachments, kinetochores also integrate and respond to regulatory signals that ensure the accuracy of chromosome segregation during each cell division. Signals for corrective detachment act specifically on kinetochore-microtubule attachments that fail to generate normal levels of tension, although it is unclear how tension is sensed and how the attachments are released. In this review, we discuss the mechanisms by which kinetochore-microtubule attachments generate force during chromosome biorientation, and the pathways of maturation and regulation that lead to the formation of correct attachments.
Keywords: Mitosis, kinetochore, microtubule, chromosome segregation, tension, phosphorylation, Aurora B, Ndc80 complex, Dam1 complex
Force generation by kinetochore-microtubule attachments
Kinetochores couple chromosomes to the dynamic tips of spindle microtubules, forming attachments that exhibit a striking combination of strength and plasticity. These attachments are mobile and robust under tension, but can also rapidly destabilize in response to regulatory signals that selectively cue the release of incorrect attachments. Thirty years ago, Nicklas directly measured the magnitude of forces that can be exerted by a mitotic spindle by pulling on anaphase chromosomes with a glass microneedle [1]. Since his iconic experiment, the field has focused on understanding how kinetochore-microtubule attachments generate tension during chromosome biorientation and segregation. More recently, the identification of functional units in the kinetochore (typically multi-protein complexes) has enabled production of these components in recombinant form for biochemical and biophysical interrogation [2].
Due to the prevailing interest in force generation by kinetochores, the first in vitro biophysical studies were performed on components that directly bind microtubules. The budding yeast Dam1 complex alone can form stable attachments to microtubule ends, even against physiologically relevant tensile loads [3, 4]. The ability to form load-bearing attachments to microtubule tips was found to be a property also of the conserved Ndc80 complex [5]. Although these components appear individually sufficient to reconstitute kinetochore-microtubule coupling, a surprising result arose from the recent purification of kinetochore-derived particles from budding yeast. These particles bind microtubules with much greater strength as compared to the Ndc80 and Dam1 complexes individually or in combination [6, 7]. This finding suggests that structural components of the kinetochore (e.g., the Mis12/Mtw1 and Ctf19 complexes) indirectly enhance the strength of microtubule attachments. Structural complexes could strengthen microtubule interactions by organizing or oligomerizing components at the microtubule attachment interface, and/or by inducing allosteric changes in these components to drive robust microtubule coupling. Determining if and how each kinetochore component contributes to microtubule binding requires systematic reconstitution of the kinetochore in vitro. Through this approach, a minimal particle can be built to match the microtubule-binding capability of whole kinetochores, and provide a means to probe the molecular basis for the remarkable stability of these attachments during mitosis.
Mechanisms of error correction
Kinetochore-microtubule attachments that are too stable can be detrimental. For example, improper connections can be made during initial kinetochore capture by the mitotic spindle, resulting in chromosome misalignment. Thus, the accuracy of chromosome segregation during each cell division depends on a mechanism for the targeted release of aberrant attachments [8–10]. It is widely accepted that this mechanism relies upon the stabilization of kinetochore-microtubule attachments in a tension-dependent manner [11]. Less clear is whether this stabilizing effect is direct, or indirectly mediated by other factors that sense and respond to kinetochore tension.
Direct tension-dependent stabilization is exemplified in the “catch bond” behavior of cell-cell adhesion molecules, for which bond lifetimes become longer with increasing applied tension. In vitro, purified kinetochore-derived particles exhibit a biphasic response in attachment lifetime with increasing applied force, indicating a catch bond-like behavior [6]. This phenomenon can be explained by a two-state binding model, in which applied force biases attachments to the stronger of two binding modes. Consistent with this model, kinetochore-derived particles bind more strongly to assembling microtubule tips as compared to disassembling tips, and applied force strongly biases microtubule tips to the assembly state [6]. The notion that error correction relies upon such a simple and self-contained mechanism is elegant and attractive. However, kinetochores do not readily detach during anaphase, when they are coupled to disassembling microtubules under relatively low levels of tension [12]. Additionally, the rates of microtubule assembly and disassembly are much different in vivo as compared to in vitro, owing to the presence of microtubule-associated proteins that regulate microtubule dynamics in cells [13]. If microtubule dynamic rates determine the relative stability of attachments to assembling and disassembling tips, it is unclear if the two-state binding model will apply under in vivo circumstances. Finally, normal error correction in cells requires phospho-regulation by the Aurora B kinase. Altogether, these observations suggest that direct tension-dependent stabilization is only one part of the mechanism upon which cells rely to correct aberrant kinetochore-microtubule attachments.
The conserved Aurora B kinase localizes to the inner centromere (the chromatin on which the kinetochore assembles) and phosphorylates diverse targets at kinetochores that fail to generate normal levels of tension [14–17]. These targets include core kinetochore components, such as the Ndc80, Dam1, KNL1/Spc105, and Mis12/Mtw1 complexes, as well as microtubule-associated proteins, like the microtubule-depolymerizing motor, MCAK [8]. It is thought that Aurora B phosphorylation destabilizes kinetochore-microtubule linkages, driving their release for another attempt at proper attachment [18–21]. Tension could indirectly affect the stability of kinetochore-microtubule attachments by altering the localization of Aurora B relative to its substrates at the microtubule attachment interface.
One possibility is that Aurora B activity forms a gradient centered at the inner centromere, and envelops kinetochores that generate low tension. When tension across a sister kinetochore pair (“inter-kinetochore tension”) increases, the microtubule-binding components would be displaced from the Aurora B activity zone, allowing their dephosphorylation. Using a FRET-based sensor for Aurora B activity, it was found that inner centromere components show constitutive Aurora B phosphorylation [22]. By contrast, components at the kinetochore-microtubule interface are only phosphorylated when inter-kinetochore tension is low. Retargeting Aurora B to the kinetochore alters the distribution of Aurora B activity, such that kinetochore targets show constitutive phosphorylation. These findings are consistent with the spatial separation model, but how tension might displace Aurora B from its kinetochore targets remains unclear.
Spatial separation is proposed to occur through tension-dependent structural deformations in kinetochores. Inter-kinetochore tension correlates with the distance between sister kinetochores (“inter-kinetochore stretching”) due to elasticity in the centromeric chromatin connecting them. It has been proposed that high inter-kinetochore tension could also result in the elongation of compliant components within each sister kinetochore (“intra-kinetochore stretching”). Therefore, a simple hypothesis is that inter-kinetochore tension causes spatial separation through intra-kinetochore stretching. In Drosophila S2 cells, the distance from the inner centromere to the Ndc80 complex at the microtubule attachment site is ~65 nm for unattached kinetochores; during biorientation, this distance increases to ~100 nm [23]. Importantly, a reporter epitope for Aurora B activity showed an inverse correlation between intra-kinetochore stretch and kinetochore phosphorylation by Aurora B. However, intra-kinetochore stretching is not correlated with inter-kinetochore stretching [23, 24]. Thus, if spatial separation of Aurora B from the microtubule-binding components is required for attachment stabilization, it does not appear to depend on inter-kinetochore tension.
An alternative possibility is that error correction instead relies upon tension-dependent redistribution of Aurora B. In this model, Aurora B could be actively recruited to kinetochores that generate low levels of intra-kinetochore stretch. For example, Aurora B could be recruited to the kinetochore via the microtubule-binding activity of its binding partners in the chromosomal passenger complex, and/or by kinetochore-localized Bub1 [25–27].
Although the tension-sensing mechanism has yet to be elucidated, several lines of evidence suggest that Aurora B phosphorylation drives destabilization of kinetochore-microtubule attachments. In yeast cells, activity of the Aurora B kinase, Ipl1, generates unattached kinetochores in response to defective kinetochore tension [20]. In vitro, phosphorylation of the Dam1 complex by Ipl1 directly weakens the Dam1-microtubule interaction [28]. Phosphorylation of Dam1 complex also blocks its interaction with the Ndc80 complex, through which the Dam1 complex enables processive Ndc80-based coupling to microtubule tips [7]. This two-pronged effect could strongly disrupt the microtubule-binding interface of a target kinetochore to drive corrective detachment. Importantly, no clear homolog of the Dam1 complex has been found outside of fungal organisms, though recent evidence suggests phosphorylation-mediated disruption of interactions between kinetochore components at the microtubule interface is a conserved effect of Aurora B activity [29, 30]. In vitro assays suggest that phosphorylation-dependent weakening of microtubule binding affinity is also conserved, and is targeted primarily to the Ndc80 complex in C. elegans and humans [31, 32]. In marsupial PtK1 cells, mutations that mimic phosphorylation at target sites in Ndc80 result in unattached kinetochores [33].
The regulation of kinetochore-microtubule attachments in higher eukaryotes is further complicated because each kinetochore associates with a bundle of dynamic microtubules, rather than a single microtubule as in budding yeast. First, a kinetochore with multiple microtubule attachment sites can bind microtubules emanating from both spindle poles, forming a “merotelic” attachment (Figure 1B). Importantly, merotelic kinetochore pairs can generate normal levels of tension, and they do not activate the spindle assembly checkpoint [34–36]. Thus, merotelic attachments can cause lagging chromosomes during anaphase, which sometimes result in chromosome missegregation [34, 36]. Correction mechanisms act to minimize the abundance of these errors, as merotelic attachments are prevalent early in mitosis, but are normally shed as cells progress to anaphase [37, 38]. Pharmacological inhibition of Aurora B increases the number of merotelic attachments, suggesting that merotelic resolution depends at least in part on Aurora B activity [35]. Furthermore, Aurora B is enriched at the site of merotelic attachments, where it recruits and regulates MCAK [39]. However, it is unclear how these attachments are subsequently released, without disrupting correct attachments on the same kinetochore. Further experiments are required to determine how MCAK contributes to error correction at merotelic kinetochores, and if other factors are similarly regulated by Aurora B to drive targeted release at these sites.
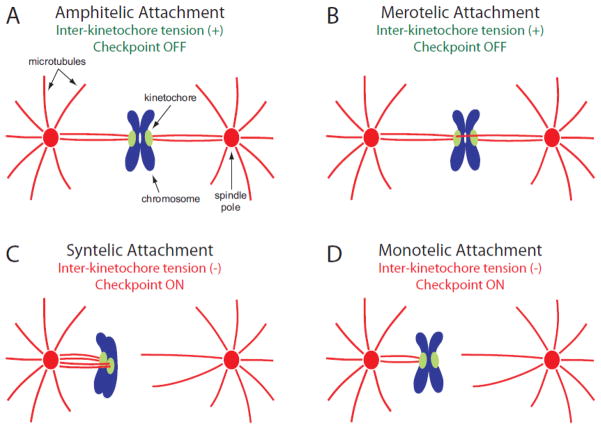
Figure 1.
Types of kinetochore attachment to the mitotic spindle. Chromosomes are shown in blue, with associated kinetochores in green. Spindle poles are shown as red ovals, from which microtubules emanate outwards, shown as red lines. (A) At metaphase, kinetochores normally form amphitelic attachments on the spindle. In this orientation, one kinetochore attaches to microtubules emanating from one spindle pole, and its sister kinetochore attaches exclusively to microtubules from the opposite spindle pole. Amphitelic sister kinetochores are bioriented and under tension, and do not activate the spindle assembly checkpoint. (B) A merotelic attachment occurs when a single kinetochore of a sister pair attaches to microtubules emanating from both spindle poles. Although incorrect, these attachments can generate normal levels of tension and do not activate the spindle checkpoint. (C) Syntelic attachments are a form of erroneous linkage in which both kinetochores of a sister pair attach to microtubules emanating from the same spindle pole. Syntelic kinetochores are not under tension and activate the spindle checkpoint. (D) Monotelic attachment describes the case in which one kinetochore attaches to microtubules emanating from one spindle pole, while its sister kinetochore remains unattached. Monotelic kinetochores are not under tension, and the presence of an unattached kinetochore activates the spindle checkpoint. This type of attachment is typically observed early in mitosis, during initial capture of kinetochores by the mitotic spindle.
An additional complexity in higher eukaryotes is that Aurora B activity also appears to control the mobility of kineotchores on the mitotic spindle, possibly by tuning the ability of kinetochores to modulate the tip dynamics of attached microtubules. When Aurora B phosphorylation of Ndc80 is blocked by alanine mutation, hyperstable kinetochore-microtubule attachments are observed [40, 41]. The metaphase oscillatory movements of phospho-blocked kinetochores are severely attenuated compared to those of wild-type kinetochores. In human cells, activation of Aurora B promotes the movement of syntelic kinetochore pairs (Figure 1C) to the spindle pole, driven by the disassembly of the attached microtubules [42]. At the pole, error correction occurs by a completely uncharacterized mechanism. First, it is unclear if Aurora B activity simply drives poleward movement of these kinetochores, or if it is additionally required to trigger attachment release at the pole. Second, the attachment intermediates that occur during error correction are unknown. One possibility is that these kinetochores become completely detached from the spindle, and must start the initial capture and biorientation process anew (see the following section). Alternatively, release might occur at only one of the sister kinetochores to form a mono-oriented attachment. The relative kinetics of microtubule capture and detachment could dictate which pathway predominates in this correction mechanism. Ultimately, investigation using biophysical assays will be necessary to determine if Aurora B has a key role in controlling microtubule tip dynamics at kinetochores, in addition to regulating microtubule attachment strength. We propose that both effects, which could be tuned independently, combine to facilitate the resolution of erroneous attachments in higher eukaryotes. The evidence for an important correction mechanism that resides in or near the spindle pole also warrants further inquiry in order to compile a complete picture of error correction.
Kinetochore assembly and attachment maturation
While the field has largely focused on understanding the events surrounding metaphase (force generation and error correction, see above), comparatively little is known about the initial stages of kinetochore assembly and microtubule attachment. Kinetochores assemble on centromeric DNA, which in most organisms is epigenetically specified by the presence of nucleosomes containing the histone H3 variant, CENP-A [2]. Together with a variety of other proteins that remain stably associated with the centromere, CENP-A forms the core scaffolding site for hierarchical assembly of the rest of the kinetochore. However, the order and kinetics of events in kinetochore assembly are still unknown. This question could be readily addressed using a recently published method for building kinetochores on reconstituted CENP-A chromatin in cell-free extracts [43]. Combined with targeted synthetic dye labeling techniques [44], this system would allow direct visualization of kinetochore assembly at the single-molecule level with high temporal resolution.
Whole assembled kinetochores are competent for initial capture by the mitotic spindle through a lateral or “side-on” microtubule attachment, and subsequently undergo poleward transport [45–47]. The early and late stages of attachment, in which kinetochores are captured and eventually bioriented, respectively, are widely conserved among eukaryotes. However, the intermediary events, during which kinetochores transition from lateral to end-on microtubule binding modes, are poorly understood. Following poleward transport, kinetochores congress to the spindle equator. Congression could increase the probability that monotelic kinetochores (Figure 1D) will form bioriented attachments. Congression is facilitated by the presence of bundled microtubules that penetrate into the spindle midzone [48]. The unattached kinetochore of a monotelic pair can glide along these microtubules through lateral interactions mediated by the plus-end directed motor, CENP-E. This process might also function as an important means by which kinetochores become bioriented after aberrant attachments are released at or near the spindle pole. Such a mechanism could be particularly expedient if error correction proceeds through a monotelic attachment. However, it is unclear how side-on CENP-E attachments are handed off to form end-on, Ndc80-based attachments during biorientation.
In fact, it is not even known why bioriented kinetochores generally associate with microtubule tips. The kinetochores of budding yeast cells carrying an S221F mutation in Dam1 (also known as Dam1-765) are uncoupled from microtubule ends [49]. The only clear phenotype of DAM1-765 cells is loss of microtubule length regulation in the spindle. Microtubule-associated proteins (Bik1/CLIP-170, Stu2/XMAP215, Cin8/kinesin-5, and Kip3/kinesin-8) remain properly localized on microtubules in these cells, suggesting that normal microtubule length regulation requires kinetochore attachment to microtubule ends [50]. Despite the microtubule length regulation defect, these cells grow at a normal rate and the checkpoint is fully satisfied, indicating that stable end-on attachment is dispensable in yeast. In fact, the DAM1-765 mutation alleviates the lethality and chromosome missegregation conferred at semi-restrictive temperatures by the temperature-sensitive ipl1-321 allele, suggesting that the lateral microtubule attachments made by Dam1-765 kinetochores are less error-prone, or more easily corrected [50]. Dam1-765 kinetochores also recruit more Bub1 than wild-type kinetochores. This result supports the possibility that kinetochore-localized Bub1 promotes Aurora B activity (see above), as any residual activity at semi-restrictive temperature is more efficiently targeted to the kinetochore. Thus, although much is known about the behavior of bioriented kinetochores, additional study is necessary to determine what factors target kinetochores to microtubule ends, and to assess the advantage (if any) afforded by achieving end-on attachment.
Conclusions
Kinetochores play a central role in the life of a cell by providing for timely and accurate segregation of the genetic material during each cell division. Proper chromosome segregation relies upon the ability of kinetochores to form stable attachments to the dynamic tips of microtubules, and on the activity of error correction mechanisms that monitor attachment fidelity. Disrupting either of these functions almost universally causes catastrophic failure that results in cell death. Past decades have shed light on the basic principles of kinetochore-microtubule coupling and force generation. Presently, these activities are being reconstituted in vitro from purified kinetochore components. These studies should elucidate how each component contributes to the ability of the whole kinetochore to form a processive, load-bearing microtubule attachment. Another outstanding question is how these attachments are tuned and modulated to promote error correction. In vitro biophysical assays allow precise manipulation and quantitative assessment of the effects of phosphorylation at target sites that are important for regulation in vivo. These measurements are necessary to tease apart complex effects that act concertedly and inextricably within the context of a living cell. Finally, there are still many mysteries surrounding the steps of kinetochore assembly and maturation, through which bioriented, end-on microtubule attachments are formed. Here, single-molecule techniques can provide invaluable insights into the kinetics and order of events that govern each of these processes, which often occur too quickly for visualization and manipulation in vivo. Thus, the key questions outlined here still stand between us and a clear understanding of the intricacies of the kinetochore, a structure as fundamental and ancient as it is robust and dynamic.
Acknowledgments
We thank C.L. Asbury, J.F. Tien, E.M. Mazanka, and A.D. Franck for helpful suggestions and scientific discussion. This work was supported by NIH grant T32 GM008268 to N.T.U., and NIGMS grant R01 GM40506 to T.N.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicklas RB. Measurements of the force produced by the mitotic spindle in anaphase. J Cell Biol. 1983;97:542–548. doi: 10.1083/jcb.97.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 3.Asbury CL, Gestaut DR, Powers AF, Franck AD, Davis TN. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc Natl Acad Sci U S A. 2006;103:9873–9878. doi: 10.1073/pnas.0602249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franck AD, Powers AF, Gestaut DR, Gonen T, Davis TN, Asbury CL. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat Cell Biol. 2007;9:832–837. doi: 10.1038/ncb1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers AF, Franck AD, Gestaut DR, Cooper J, Gracyzk B, Wei RR, Wordeman L, Davis TN, Asbury CL. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010 doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tien JF, Umbreit NT, Gestaut DR, Franck AD, Cooper J, Wordeman L, Gonen T, Asbury CL, Davis TN. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J Cell Biol. 2010;189:713–723. doi: 10.1083/jcb.200910142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khodjakov A, Pines J. Centromere tension: a divisive issue. Nat Cell Biol. 2010;12:919–923. doi: 10.1038/ncb1010-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly AE, Funabiki H. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr Opin Cell Biol. 2009;21:51–58. doi: 10.1016/j.ceb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicklas RB, Ward SC. Elements of error correction in mitosis: microtubule capture, release, and tension. J Cell Biol. 1994;126:1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicklas RB. Chromosome Velocity during Mitosis as a Function of Chromosome Size and Position. J Cell Biol. 1965;25(SUPPL):119–135. doi: 10.1083/jcb.25.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline-Smith SL, Walczak CE. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol Cell. 2004;15:317–327. doi: 10.1016/j.molcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- 15.Pinsky BA, Tatsutani SY, Collins KA, Biggins S. An Mtw1 complex promotes kinetochore biorientation that is monitored by the Ipl1/Aurora protein kinase. Dev Cell. 2003;5:735–745. doi: 10.1016/s1534-5807(03)00322-8. [DOI] [PubMed] [Google Scholar]
- 16.King EM, Rachidi N, Morrice N, Hardwick KG, Stark MJ. Ipl1p-dependent phosphorylation of Mad3p is required for the spindle checkpoint response to lack of tension at kinetochores. Genes Dev. 2007;21:1163–1168. doi: 10.1101/gad.431507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang C, Hazbun TR, Cheeseman IM, Aranda J, Fields S, Drubin DG, Barnes G. Kinetochore protein interactions and their regulation by the Aurora kinase Ipl1p. Mol Biol Cell. 2003;14:3342–3355. doi: 10.1091/mbc.E02-11-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewar H, Tanaka K, Nasmyth K, Tanaka TU. Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature. 2004;428:93–97. doi: 10.1038/nature02328. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 20.Pinsky BA, Kung C, Shokat KM, Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol. 2006;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- 21.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184:373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandall S, Severin F, McLeod IX, Yates JR, 3rd, Oegema K, Hyman A, Desai A. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell. 2006;127:1179–1191. doi: 10.1016/j.cell.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimogawa MM, Widlund PO, Riffle M, Ess M, Davis TN. Bir1 is required for the tension checkpoint. Mol Biol Cell. 2009;20:915–923. doi: 10.1091/mbc.E08-07-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricke RM, van Deursen JM. Aurora B hyperactivation by Bub1 overexpression promotes chromosome missegregation. Cell Cycle. 2011;10:3645–3651. doi: 10.4161/cc.10.21.18156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gestaut DR, Graczyk B, Cooper J, Widlund PO, Zelter A, Wordeman L, Asbury CL, Davis TN. Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nat Cell Biol. 2008;10:407–414. doi: 10.1038/ncb1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan YW, Jeyaprakash AA, Nigg EA, Santamaria A. Aurora B controls kinetochore-microtubule attachments by inhibiting Ska complex-KMN network interaction. J Cell Biol. 2012;196:563–571. doi: 10.1083/jcb.201109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guimaraes GJ, Dong Y, McEwen BF, Deluca JG. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol. 2008;18:1778–1784. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimini D, Fioravanti D, Salmon ED, Degrassi F. Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J Cell Sci. 2002;115:507–515. doi: 10.1242/jcs.115.3.507. [DOI] [PubMed] [Google Scholar]
- 35.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 38.Gregan J, Polakova S, Zhang L, Tolic-Norrelykke IM, Cimini D. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 2011;21:374–381. doi: 10.1016/j.tcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 40.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 41.DeLuca KF, Lens SM, DeLuca JG. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J Cell Sci. 2011;124:622–634. doi: 10.1242/jcs.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 43.Guse A, Carroll CW, Moree B, Fuller CJ, Straight AF. In vitro centromere and kinetochore assembly on defined chromatin templates. Nature. 2011;477:354–358. doi: 10.1038/nature10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gautier A, Juillerat A, Heinis C, Correa IR, Jr, Kindermann M, Beaufils F, Johnsson K. An engineered protein tag for multiprotein labeling in living cells. Chem Biol. 2008;15:128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–994. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 48.Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, McEwen BF, Khodjakov A. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimogawa MM, Graczyk B, Gardner MK, Francis SE, White EA, Ess M, Molk JN, Ruse C, Niessen S, Yates JR, 3rd, Muller EG, Bloom K, Odde DJ, Davis TN. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr Biol. 2006;16:1489–1501. doi: 10.1016/j.cub.2006.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimogawa MM, Wargacki MM, Muller EG, Davis TN. Laterally attached kinetochores recruit the checkpoint protein Bub1, but satisfy the spindle checkpoint. Cell Cycle. 2010;9:3619–3628. doi: 10.4161/cc.9.17.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]