Abstract
Background
Respiratory syncytial virus (RSV) can cause severe lower respiratory tract infection (LRI) and is a risk factor for the development of bronchiolitis obliterans syndrome (BOS) after lung transplantation (LTx). Currently, the most widely used therapy for RSV is inhaled ribavirin. However, this therapy is costly and cumbersome. We investigated the utility of using oral ribavirin for the treatment of RSV infection after LTx.
Methods
RSV was identified in nasopharyngeal swabs (NPS) or bronchoalveolar lavage (BAL) using direct fluorescent antibody (DFA) in 5 symptomatic LTx patients diagnosed with LRI. Data were collected from December 2005 and August 2007 and included: age; gender; type of LTx; underlying disease; date of RSV; pulmonary function prior to, during and up to 565 days post-RSV infection; need for mechanical ventilation; concurrent infections; and radiographic features. Patients received oral ribavirin for 10 days with solumedrol (10 to 15 mg/kg/day intravenously) for 3 days, until repeat NPS were negative.
Results
Five patients had their RSV–LRI diagnosis made at a median of 300 days post-LTx. Mean forced expiratory volume in 1 second (FEV1) fell 21% (p < 0.012) during infection. After treatment, FEV1 returned to baseline and was maintained at follow-up of 565 days. There were no complications and no deaths with oral therapy. A 10-day course of oral ribavirin cost $700 compared with $14,000 for nebulized ribavirin at 6 g/day.
Conclusions
Treatment of RSV after LTx with oral ribavirin and corticosteroids is well tolerated, effective and less costly than inhaled ribavirin. Further studies are needed to directly compare the long-term efficacy of oral vs nebulized therapy for RSV.
Respiratory syncytial virus (RSV) is a single-stranded, negative-sense RNA virus and a member of the Paramyxoviridae family. RSV is known to lead to respiratory illnesses and has been recognized as a major cause of respiratory compromise in solid-organ transplant recipients.1 In addition to mild acute respiratory complications, RSV can produce severe lower respiratory tract infections, such as bronchiolitis, pneumonia and respiratory failure, in lung transplant (LTx) recipients.2 RSV infections have also been associated with the development of bronchiolitis obliterans syndrome (BOS) in lung allograft recipients.3–5
Despite incremental improvements in the supportive care of all patients with viral respiratory infections, the mortality of RSV infection approaches 10% to 20%. Early therapy with the anti-viral nucleoside analog ribavirin appears to be associated with improved survival in other solid-organ transplant and bone marrow transplant recipients.6 Ribavirin (1-β-d-ribofuranosyl-12-triazole-3-carboximide) is a synthetic nucleoside analog with a broad spectrum and in vivo activity against RNA and DNA viruses. Its mechanism of action in RSV infection appears to be interference with the expression of viral mRNA and viral proteins at the translatory level.
Unfortunately, the available pharmacologic therapy in the USA, nebulized ribavirin with or without corticosteroids, is costly and cumbersome. Nebulized ribavirin must be administered in well-ventilated rooms (at least 6 air changes/hour) for 18 hours/day. In addition, it is mutagenic, tumorigenic and gonadotoxic.7 Side effects associated with nebulized ribavirin are common and include dyspnea, cough and nasal congestion.6 One alternative, intravenous ribavirin, has been shown to be safe and cost-effective in LTx patients, but it is currently unavailable in the USA.8
Among the various organ transplant populations, the highest rates of RSV infection have been reported in LTx recipients, which can impact as much as 21% of these patients.4 Patients with lower respiratory tract involvement, in the form of bronchiolitis or pneumonitis, typically present with fever, dyspnea, cough and wheezing.9 Chest radiographs may be normal or may show only subtle interstitial changes. The combination of respiratory bronchiolitis and an abnormal chest radiograph, in these patients, is associated with a high incidence of ventilatory failure and an overall poor prognosis.10 LTx patients who develop severe pulmonary symptoms may also show a significant decline in their forced expiratory volume in 1 second (FEV1). High-risk patients with symptoms of RSV infection should be tested early for rapid diagnosis,with viral cultures and enzyme-linked immunosorbent assays or immunoflourescence techniques.11
Although early therapy is associated with improved survival in other types of transplant recipients,6 there are limited data regarding the efficacy of treating RSV after LTx. Standard therapy for RSV is nebulized ribavirin with or without corticosteroids, but this treatment is associated with significant cost, difficulty of administration, and an unacceptable failure rate. Hence, we investigated the safety, efficacy and cost of oral ribavirin combined with corticosteroids for the reduction of morbidity and mortality of pulmonary RSV infection after LTx.
METHODS
Patient Population
Three bilateral lung transplant (BLT) recipients, and 2 single-lung transplant (SLT) recipients (Table 1) were diagnosed with RSV between Days 90 and 730 (median 300 days) post-transplant (Table 2). All patients diagnosed with RSV plus lower respiratory tract infection (LRI) were treated with oral ribavirin as per our institution protocol.
Table 1.
Patient Demographics
| Patient | Age/gender | Reason for LTx | Type of LTx |
|---|---|---|---|
| 1 | 44/M | Sarcoidosis | BLT |
| 2 | 45/M | IPF | BLT |
| 3 | 66/M | COPD | SLT |
| 4 | 26/M | CF | BLT |
| 5 | 62/F | IPF | SLT |
LTx, lung transplantation; BLT, bilateral lung transplantation; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease; SLT, singlelung transplantation; CF, cystic fibrosis.
Table 2.
Correlation of Pulmonary Function, Infection and Treatment for the 5 Patients Studied
| Date of transplant | Date of RSV | FEV1 max. measured | FEV1 during RSV | FEV1 at 575 days post-RSV |
|---|---|---|---|---|
| 9/05 | 1/06 | 2.52 | 2.0 | 2.63 |
| 1/04 | 1/06 | 2.98 | 1.6 | 2.59 |
| 9/05 | 12/05 | 1.58 | 1.1 | 1.63 |
| 12/03 | 1/06 | 3.09 | 2.8 | 3.06 |
| 12/04a | 12/05 | 1.91 | 1.3 | 1.2 |
This patient underwent right middle-lobe resection for a lung mass (discovered at the time of RSV infection) that was consistent with angiocentric rejection.
These patients were followed up to 565 days after diagnosis. No alternative therapies, such as inhaled ribavirin or IV ribavirin, were utilized in cases of RSV–LTI. There were 4 men and 1 woman with a mean age of 46 (range 26 to 66) years. Underlying diagnoses included cystic fibrosis (n = 1), emphysema (n = 1), sarcoidosis (n = 1) and idiopathic pulmonary fibrosis (n = 2). Patients were studied during the period between December 2005 and August 2007. Only patients with lower respiratory RSV infection, with or without radiographic changes, were included in the study. All patients had a documented fall in FEV1 of >10%. Patients were tested with NPS and also underwent bronchoscopy with BAL. Patients with positive nasopharyngeal swabs (NPS) for RSV were admitted and treated with oral ribavirin and intravenous corticosteroids.
Anti-viral Therapy With Oral Ribavirin
At our institution, patients diagnosed with RSV–LRI are treated with oral ribavirin (since 2005). All 5 patients received oral ribavirin (15 to 20 mg/kg in 3 divided doses for total of 10 days), until repeat NPS swabs were negative for RSV on direct fluorescent antibody (DFA) for at least 10 days in all patients. In the absence of a prior established therapeutic protocol for patients with RSV at our institution, we do not have an adequately sized control group of patients who have undergone treatment for this condition.
Corticosteroid Therapy
All patients received solumedrol (10 to 15 mg/kg/day intravenously) for 3 days and then resumed the previous prednisone maintenance dose.
Clinical Definitions
Diagnosis of LRI induced by RSV was defined by any combination of hypoxemia, respiratory wheezing and ≥10% drop in FEV1. In the setting of a positive NPS, bronchoscopy with bronchoalveolar lavage (BAL) was performed to rule-out alternative pathogens. BOS was defined by criteria established by the International Society for Heart and Lung Transplantation (ISHLT).7
Microbiologic Analysis
The methods for viral identification were antigen detection by virus antigen-specific monoclonal antibodies (MAb) visualized by direct fluorescent antibody (DFA) in 5 symptomatic patients; in addition, the samples were inoculated into cell culture tubes. All patients evaluated with symptoms of a respiratory viral illness with evaluated with either NPS or BAL. After DFA, respiratory samples were inoculated for culture into R-Mix (Diagnostic Hybrids, Athens, OH). Culture samples were checked at 24 and 48 hours after inoculation with DFA for multiple respiratory viruses including RSV. Specimens with positive pooled DFA staining after culture were then tested with specific DFA staining according to the manufacturer’s protocol.
Transplant Immunosuppression Protocols
All patients received our standard immunosuppression after transplantation. Immediately after surgery, patients received intravenous doses of 125 mg methylprednisolone every 8 hours for 6 doses, followed by an oral prednisone taper to 20 mg/day, cyclosporine (5 mg/kg/day) and mycophenolate mofetil (MMF) 1,000 mg orally twice daily. At our institution, if patients fulfill criteria for RSV–LRI and they do not have co-existent pathogens or they do not have contraindications to steroid therapy, they receive 3 days of intravenous solumedrol, as noted earlier. Otherwise, a patient’s immunosuppression is not changed during the acute episode of RSV infection.
Transplant Infection Prophylaxis Protocols
All patients at risk for cytomegalovirus (CMV) infection (donor or recipient serology positive for CMV) received prophylaxis after transplantation with twice-daily gancyclovir at 5 mg/kg intravenously at least for 2 weeks. The patients were transitioned to oral valgancyclovir at a dose of 900 mg/day orally for up to 6 months. CMV hyperimmune globulin (Cytogam) at 150 mg/kg per dose for a total of 6 doses was added to patients who were donor-positive and recipient-negative for CMV. Patients also received prophylaxis for Pneumocystis jirovecii with sulfamethoxazole and trimethoprim (TMP/SMX) indefinitely. Anti-fungal therapy against Aspergillus was provided with itraconazole or voriconazole for to 3 months after lung transplantation. All patients received a pneumococcal vaccine before LTx and a yearly single-dose trivalent influenza vaccine after LTx.
Statistical Analysis
Descriptive statistics are reported as mean, standard deviation, range and counts. Repeated measures were analyzed with paired Student’s t-test with statistical significance set at p < 0.05.
RESULTS
From December 2005 through August 2007, 5 lung allograft recipients developed lower respiratory infection with RSV and were treated with oral ribavirin and high-dose intravenous solumedrol according to the treatment protocol. FEV1 decreased by a mean of 21% (range 4% to 42%, p < 0.012) at the time of infection. After clearance of RSV infection, there was resolution of FEV1 to baseline after 3 to 10 months, which was maintained at follow-up of 565 days (Figure 1). There was no post-RSV BOS documented up to 1.5 years after ribavirin therapy, although 1 patient was discovered to have angiocentric rejection of a lung mass that was present at the time of RSV infection. The cost for 10-day course of oral ribavirin was $700 per patient. The mean time taken for the NPS to become negative for RSV was 7 to 10 days. Consequently, the duration of oral ribavirin treatment was 10 days.
Figure 1.
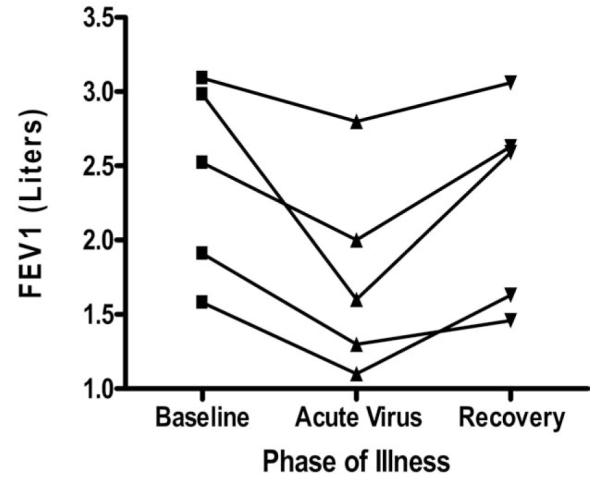
Changes in FEV1 over time for all LTx patients infected with RSV. All patients infected with RSV underwent a decline in FEV1 with subsequent recovery after therapy with oral ribavirin. It is important to note that the final patient (see footnote in (Table 2), representing the same patient shown second from the bottom in this figure, had angiocentric rejection in a lung mass that was discovered at the time of RSV infection that required right middle-lobe resection, and was left with a residual lower FEV1.
Complications
There were no complications with RSV therapy. No patient died or developed respiratory failure requiring mechanical ventilation at the time of RSV infection. One patient developed mild anemia, which did not require therapy.
DISCUSSION
We found that treatment with oral rivabirin for 10 days was well tolerated and helped with viral clearance post-LTx, as reflected by a negative nasopharyngeal DFA after therapy. Despite a >10% drop in FEV1, all patients’ lung function returned to baseline and has remained stable up to 1.5 years after therapy. Furthermore, the medication cost for a 10-day course of oral ribavirin is $700 as compared with $14,000 for nebulized ribavirin at 6 g/day.
Treatment of RSV infection in LTx patients is critical because it can affect short- and long-term survival in these patients. Palmer et al detected an 8% incidence of respiratory viral infection among LTx patients, all of whom had signs or symptoms of acute infection.10 They also found that obliterative bronchiolitis (OB) occurred in 50% of patients who survived the illness, which is higher than expected for patients at >2 years post-LTx. Furthermore, RSV has been associated with acute allo-graft rejection in a small series of LTx recipients.3
Treatment options for respiratory viral infections are mainly supportive. Oral ribavirin is available in the USA, and is currently being used to treat patients with hepatitis C. Reports of the use of oral ribavirin therapy for other clinical indications are limited to small case series and case reports, primarily involving the hematopoietic stem cell transplantation population.6 Sparrelid et al reported that ribavirin in post–bone marrow transplant patients was well tolerated orally and intravenously and may, if instituted before development of hypoxia, reduce morbidity and mortality due to RSV.12 However, 30% of their patients had to discontinue inhalations due to bronchoconstriction and/or respiratory distress. Inhaled ribavirin is also difficult to administer because it requires full face-mask application for 15 to 18 hours/day and is associated with an increased risk of respiratory distress that necessitates discontinuation. In addition, aerosolized ribavirin is teratogenic, so women of child-bearing age should avoid treatment areas, thereby limiting support staff availability for patient care. The difficulty in delivering aerosolized ribavirin, compounded by patient discomfort and the financial burden, makes widespread acceptance of this approach unlikely. Moreover, in the absence of randomized studies, the clinical benefit from such an approach remains unsubstantiated. Systemic administration is also supported by the fact that only 0.2% to 0.1% of inhaled ribavirin is absorbed from the respiratory tract.
Intravenous ribavirin and corticosteroids have recently been reported to be well tolerated and effective in reducing RSV-related complications.8 However, ribavirin is unavailable in the USA and therefore other effective therapies warrant investigation. We have shown that oral ribavirin is generally well tolerated without any adverse effects in LTx recipients, and outcomes after treatment, in our small study, were favorable. In addition, the direct cost of oral therapy is significantly lower than the cost of nebulized ribavirin. It is important to mention that, despite adequate response and tolerability to oral ribavirin in these patients with prior LTx, the superiority of the oral preparation compared with the inhaled or intravenous preparations cannot be concluded from this small study. This combination holds promise as a cost-effective therapeutic strategy in other instances of single-organ or bone marrow transplantation. Moreover, the ease of delivery and enhanced safety of oral therapy, to both staff and patients, represents a major advance over the risks and inconvenience associated with standard nebulized therapy using ribavirin.
Acknowledgments
Supported the McKelvey Center for Lung Transplantation and Pulmonary Vascular Diseases, Emory University.
REFERENCES
- 1.Ison MG. Respiratory viral infections in transplant recipients. Antivir Ther. 2007;12:627–38. [PubMed] [Google Scholar]
- 2.Respiratory syncytial virus activity–United States, July 2006–November 2007. MMWR Morbid Mortal Wkly Rep. 2007;56:1263–5. Brief report. [PubMed] [Google Scholar]
- 3.Kumar D, Erdman D, Keshavjee S, Peret T, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–6. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCurdy LH, Milstone A, Dummer S. Clinical features and outcomes of paramyxoviral infection in lung transplant recipients treated with ribavirin. J Heart Lung Transplant. 2003;22:745–53. doi: 10.1016/s1053-2498(02)00569-7. [DOI] [PubMed] [Google Scholar]
- 5.Estenne M, Maurer J, Boehler A, et al. Bronchiolitis obliterans 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Collingham KE, Holder K, et al. Pre-emptive oral ribavirin therapy of paramyxovirus is after haematopoietic stem cell transplantation: a pilot study. Bone Marrow Transplant. 2001;28:759–63. doi: 10.1038/sj.bmt.1703216. [DOI] [PubMed] [Google Scholar]
- 7.Flynn JD, Akers WS, Jones M, et al. Treatment of respiratory syncytial virus pneumonia in a lung transplant recipient: case report and review of the literature. Pharmacotherapy. 2004;24:932–8. doi: 10.1592/phco.24.9.932.36090. [DOI] [PubMed] [Google Scholar]
- 8.Glanville AR, Scott AI, Morton JM, et al. Intravenous ribavirin is a safe and cost-effective treatment for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant. 2005;24:2114–9. doi: 10.1016/j.healun.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Ko JP, Shepard JA, Sproule MW, et al. CT manifestations of respiratory syncytial virus infection in lung transplant recipients. J Comput Assist Tomogr. 2000;24:235–41. doi: 10.1097/00004728-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Palmer SM, Jr, Henshaw NG, Howell DN, et al. Community respiratory viral infection in adult lung transplant recipients. Chest. 1998;113:944–50. doi: 10.1378/chest.113.4.944. [DOI] [PubMed] [Google Scholar]
- 11.Costa C, Libertucci D, Solidoro P, et al. Rapid shell vial culture for the detection of respiratory viruses from bronchoalveolar lavage in immunocompromised patients. Panminerva Med. 2007;49:1–6. [PubMed] [Google Scholar]
- 12.Sparrelid E, Ljungman P, Ekelof-Andstrom E, et al. Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997;19:905–8. doi: 10.1038/sj.bmt.1700752. [DOI] [PubMed] [Google Scholar]


