Abstract
Outcomes following lung transplant are suboptimal owing to chronic allograft failure termed bronchiolitis obliterans syndrome (BOS). Prior work in both mice and humans has shown that interferon gamma (IFNG)-induced chemokines, including CXCL9 and CXCL10, are elevated in patients with established BOS. We hypothesized that patients who ultimately developed BOS would have elevations in these chemokines before losing lung function. We utilized a high throughput multiplex enzyme-linked immunosorbent assay (ELISA) to measure biomarkers in bronchoalveolar lavage fluid (BALF). We modeled cumulative exposure to seven biomarkers (CXCL9, CXCL10, RANTES, IL1-RA, IL-17, MCP1 and IL-13) by calculating the 1-year area under the curve (AUC) for each biomarker in the BALF of 40 lung transplant patients who had at least four samples obtained in the first year posttransplant. Cumulative elevations in CXCL9 and CXCL10 were associated with a significant risk of subsequent graft failure after transplant (HR 9.37 and 5.52, respectively; p < 0.01 for both). Further these chemokines were also elevated in patients before the onset of BOS. CXCL9 and CXCL10 elevations were seen between 3 and 9 months before graft failure. Our data show that persistent presence of CXCL9 and CXCL10 portents worsening lung allograft function; measuring these IFNG-induced chemokines might prospectively identify patients at risk for BOS.
Keywords: Area-under-curve, bronchiolitis obliterans, bronchoalveolar lavage, chemokines, lung transplantation
Introduction
Lung transplantation is a viable therapy for select patients with end-stage lung disease. In spite of surgical advances over the past two decades, outcomes in lung transplantation have lagged behind those of other solid organs. In particular, the 5-year survival rate following lung transplantation is 50% (1). The largest hurdle to long-term survival and to continued improved quality of life following lung transplantation is bronchiolitis obliterans (BO), a form of chronic allograft rejection characterized by obliteration of the terminal bronchiole by fibrosis. Many putative factors have been shown to increase the risk of developing BO, including ischemia reperfusion injury, infections, episodes of acute rejection, degree of antigenic mismatch between donor and recipient and aspiration of gastric contents (reviewed in Ref. 2). It is the known association with acute cellular rejection (ACR) early after transplant and the subsequent development of BO that has formed the rationale for periodic surveillance lung biopsy protocols, which are performed by many centers around the world. Biopsies, performed during fiberoptic bronchoscopy procedures, provide samples to be scored for rejection so that patients experiencing early rejection can be aggressively treated and subsequent BO averted.
Implicit in protocols that employ surveillance biopsy procedures are two important premises. One, that the small tissue samples obtained at biopsy give an accurate representation of the degree of immunologic injury happening within the lung and second, that the knowledge of such processes is meaningful clinically with respect to future lung function. The practical use of surveillance lung biopsy is hampered by a small but finite risk related to performing multiple lung biopsies over time, a requirement for significant pathologic expertise in interpreting lung biopsies and an unquantifiable risk of false negative biopsy specimens (3).
We have hypothesized that better immune monitoring tools could enhance the ability of lung biopsy to project future risk of BO for patients. An untapped resource from a biomarker standpoint is bronchoalveolar lavage fluid (BALF). This fluid, obtained by injecting saline into an entire region of the lung and then aspirating that saline, contains both a cellular component with multiple potential prognostic biomarkers as well as a supernatant, which contains constituents that may be involved in recruitment of injurious cells. Among these constituents are the inter-feron gamma (IFNG)-dependent chemokines CXCL9 and CXCL10, which have already been shown to be elevated in patients with existing BO as well as experimental lung rejection in animals (4–6). Additional work has shown that interleukin (IL)-13 is elevated in patients with chronic rejection, and that blocking the effects of IL-13 can diminish experimental fibrosis in mice (7). Chemoattractants capable of increasing lung migration of monocyte/macrophage subsets into the lung also have been shown to be elevated in human lung transplant recipients at risk for the development of chronic rejection (8). Given these findings, we embarked on a study to utilize multiplex enzyme-linked immunosorbent assay (ELISA) of human lung transplant BALF assessing multiple candidate biomarkers to determine which would be associated with a risk of graft failure, chronic rejection, acute rejection and lung function posttransplant. We took advantage of an Institutional Review Board–approved tissue acquisition protocol actuating a commitment to store for later analysis any lung fluid obtained during clinical protocol driven bronchoscopy. In this study, we were able to assess these important outcomes in 40 lung transplant patients in whom we had an entire year of BALF to analyze. We chose seven candidate biomarkers (CXCL9, CXCL10, MCP-1, IL1-RA, RANTES, IL-13 and IL-17) and measured their concentrations in the first year posttransplant. These biomarkers were chosen for analysis based on their high degree of detectability in previous pilot experiments (RANTES, IL1-RA and MCP-1) as well as a strong animal and human literature supporting their potential role (CXCL9, CXCL10, IL-13 and IL-17). Importantly, our ability to evaluate these patients longitudinally permitted the estimation both of episodic and cumulative inflammation. Overall, we found that CXCL9 and CXCL10 exposure had the highest association with graft function and survival.
Methods
Patients
The Institutional Review Board at Emory approved this study. Patient outcomes including graft function, rejection and survival were prospectively assessed. BAL samples were prospectively collected and used for biomarker measurement in a batched fashion after patients had accrued more than a year of posttransplant survival. All subjects underwent transplantation at Emory University Hospital between 2006 and 2008. Informed consent was obtained from all patients. Patients accrued to this study were required to have at least 1 year of posttransplant survival, at least four bronchoscopies obtained in the first posttransplant year for which BALF was available for analysis and the 1-month and 12-month BALF was available for study. Patients enrolled agreed to allow us to perform assays on BALF, which was left over from routine protocol or for-cause bronchoscopies. Of the 53 lung transplants performed during this time period, 40 patients met these criteria and were included in this analysis. The characteristics of our study populations are shown in Table 1. Of the 40 patients assessed, there were six single lung transplants and 34 bilateral transplants. All patients were treated with the same initial immunosuppressive therapy, which included induction therapy with basiliximab and maintenance immunosuppression with prednisone, tacrolimus and azathioprine. Following transplantation, each patient had, at least, monthly pulmonary function testing performed, during which the forced expiratory volume in 1 s (FEV1) was calculated.
Table 1.
Baseline characteristics of patients included and excluded for assessment. COPD includes emphysema and alpha 1 antitrypsin deficiency. Other diseases were cystic fibrosis, idiopathic bronchiolitis, silicosis, sarcoidois, hypersensitivity pneumonitis and Langerhans cell histiocytosis
| Type of transplant | Indications for transplant | Gender | Age | Reason for exclusion | |
|---|---|---|---|---|---|
| Patients included (N = 40) | 34 bilateral 6 single |
19 COPD 16 IPF 5 other |
26 Male 14 Female |
54.8 (17–66) | NA |
| Patients excluded (N = 13) | 10 bilateral 3 single |
5COPD 6IPF 2 other |
6Male 7Female |
57.3 (45–65) | 5 insufficient samples 4 deferred procedures 4 deaths <1year |
| p = 0.67 | p = 0.85 | p = 0.32 | p = 0.69 |
Sample collection
Surveillance bronchoscopy was performed at predetermined time-points posttransplant: 2 weeks, 1, 2, 3, 6, 9 and 12 months. Not all patients had bronchoscopy performed at each time point at the clinical discretion of the treating pulmonologist. Further, in the aftermath of histologic rejection some patients received follow-up bronchoscopy outside of the surveillance time points. Finally, not every bronchoscopy yielded sufficient extra fluid for our analysis. In total, from 40 patients analyzed over the first year posttransplant, we performed multiplex ELISA on 290 separate samples. Rejection was graded on transbronchial lung biopsy pieces obtained during bronchoscopy by a dedicated pulmonary pathologist in 270 of the 290 samples analyzed.
BALF was obtained in a standardized fashion during routine bronchoscopy by wedging the bronchoscope into either the right middle lobe or lingular bronchus. A total of five 30 mL aliquots of normal saline were instilled into the lung and lavage fluid was obtained by suctioning. Fluid return was generally 70–100 mL total. BALF was immediately centrifuged at 1300 rpm × 5 min and the supernatant was frozen at –80°C, until analyzed by ELISA.
Luminex assay
Custom multiplex bead kits were obtained from Invitrogen (Carlsbad, CA, USA). Capture beads with unique bead regions suitable for analysis on a multiplex plate reader were made for CXCL9, CXCL10, MCP-1, IL-1 RA, RANTES, IL-13 and IL-17. Twenty microliters of freshly thawed BALF super-natant from each sample was incubated with capture beads. Following incubation, the protocol for luminex multiplex analysis was followed according to the manufacturer (cat#LHC6001). All samples and standards were run in duplicate. Biomarker concentrations were obtained using a Luminex200 instrument (Millipore, Billerica, MA, USA). For all analytes tested, any sample falling below the lowest concentration on the standard curve was assigned a level of 0 ng/mL.
Statistical analysis
We measured relevant clinical outcomes of graft survival, freedom from bronchiolitis obliterans syndrome (BOS), acute rejection and FEV1. The exposure for each patient for each biomarker studied was calculated by measuring the concentration of each analyte over the course of a year. Different transformations of each biomarker were modeled: mean of all measurements, the sum of all measurements, the highest measure, the area under the curve (AUC), assessed by integration of each biomarkers concentration over time and the last measured concentration of each biomarker. Two patients developed BOS at the same time point as the last biomarker assessment (12 months posttransplant). In these cases, the cumulative biomarker AUC did not include this last measure. To employ models using repeated measures, these transformations were calculated for each time where a biomarker was recorded. Thus, each patient would have a new biomarker AUC for each new time their biomarker was measured.
To assess for differences between included and excluded subjects, we used Fisher's Exact test and t tests for categorical and continuous variables. Survival was assessed using a Kaplan–Meier analysis with a log-rank test performed on subjects with AUCs above and below the median (because AUCs for each analyte were not normally distributed).
To assess the impact of chemokines levels as continuous variables, we utilized univariate and multivariate Cox regression models. Lung function (FEV1) was analyzed using univariate and multivariate linear mixed models accounting for the repeated measures nature of the data. Models used an autoregressive correlation structure to model the fact that related measurements were taken over time, and a random intercept effect because subjects had different starting FEV1s. Acute rejection was modeled using general estimating equation (GEE) models with autoregressive correlation structures to model the longitudinal nature of the data as well. For all multivariate models the main interest was whether the relationship between the relevant endpoint and CXCL9 AUC or CXCL10 AUC stayed the same when controlling for other variables. Patient and transplant characteristics were tested as possible confounders of this relationship by checking whether adding these to models, including CXCL9 AUC or CXCL10 AUC, changed the crude parameter estimates by more than 10%. Variables tested included recipient and donor age, sex, indication for transplant, laterality of transplant, recipient and donor height. All analyses were performed using SAS (Cary, NC, USA). A p-value ≤ 0.05 was considered statistically significant.
Results
Increased CXCL9 and CXCL10 exposure is associated with increased graft loss and mortality
For each analyte assessed, there were 20 patients in the high group and 20 patients in the low group. We assessed overall graft survival for each analyte by Kaplan–Meier analysis. A graft was considered failed when the patient died or went for retransplantation. Of the 40 patients studied, there were 10 graft failures (eight deaths and two retrans-plantations).
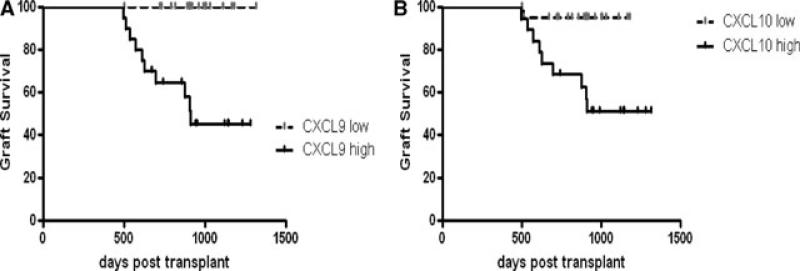
As shown in Table 2 and demonstrated graphically in Figure 1, patients with 1-year CXCL9 AUCs above the median for the group were responsible for all of the graft failures. Similarly, patients with 1-year CXCL10 AUCs above the median accounted for 9 of 10 graft failures. Collectively the hazard ratio (HR) for a high CXCL9 AUC was 9.37 (95% CI 2.69–32.7) and for a high CXCL10 AUC was 5.52 (95% CI 1.59–19.1), p < 0.01 for both biomarkers. As shown in Table 2, there was a trend toward better survival in patients with low MCP-1 AUC (HR 2.82, 95% CI 0.81–9.8), p 0.1, but this did not reach statistical significance. =
Table 2.
Comparison of graft survival in patients with high- or low-cumulative exposures to seven BALF biomarkers in the first-year posttransplant. BALF IL-13 levels were not detectable in our patient samples; survival could not be defined for IL-13 exposure
| Biomarker | Biomarker median pg/mL (25th and 75th percentile) | % Graft survival AUC > Median | % Graft survival AUC < Median | HR for AUC > Median (confidence interval) | p-Value |
|---|---|---|---|---|---|
| CXCL9 | 1255 (53, 8739) | 50% | 100% | 9.37 (2.69-32.7) | 0.0007 |
| CXCL10 | 12 847 (4159, 27 191) | 55% | 95% | 5.52 (1.59-19.1) | 0.007 |
| MCP-1 | 18 588 (8920, 101 081) | 65% | 85% | 2.82 (0.81-9.8) | 0.096 |
| RANTES | 5947 (2500, 9425) | 65% | 85% | 2.56 (0.74-8.94) | 0.14 |
| IL1-RA | 57 869 (16 813, 108 125) | 70% | 80% | 1.46 (0.42-5.06) | 0.56 |
| IL-17 | 4600 (170, 9304) | 80% | 70% | 0.53 (0.15-1.86) | 0.27 |
| IL-13 | Undetected | Undetected | Undetected |
Figure 1. Graft survival of lung transplant patients with high or low levels of BAL CXCL9 (A) or CXCL10 (B).
Analyte area under the curve was assessed for 40 patients over the first year postlung transplant. Patients were divided into high and low levels, if they were above or below the median. Event free survival indicates freedom from death or listing for retransplantation. p < 0.01 by log-rank test. N = 20 patients per group.
Because even modest elevations in biomarkers values could put patients at risk for graft failure, we modeled exposure as a continuous variable. Using univariate Cox regression, we assessed the association of each transformation of the biomarkers of interest with graft failure. As shown in Table 3, for CXCL9 and CXCL10, multiple transformations of these biomarkers demonstrated an increased HR for graft failure. We also found that the last measure of MCP-1 and IL-17 were associated with increased graft failure, but the cumulative exposures of MCP-1 and IL-17 did not correlate with graft failure. After finding that CXCL9 and CXCL10 AUC had a strong association with graft failure we were interested in establishing if patient variables or other biomarker transformations affected the relationship with these two metrics. None of the patient variables changed the effect of CXCL9 or CXCL10 AUC on graft survival. Models adjusting for the last measured CXCL9 and CXCL10 also failed to alter the crude HR for CXCL9 and CXCL10 AUCs respectively by more than 10%.
Table 3.
Unadjusted (A) and adjusted (B) Cox regression for graft survival. Shown are the various transformations of BALF biomarkers assessed. In the adjusted model, no covariates affected the model; covariates were recipient and donor gender, age, height, transplant type, diagnosis (restrictive/obstructive) and number of episodes of acute rejection. The adjusted effect of last measured CXCL9 and CXCL10 are shown here, even though they did not change the HR. The HR shown depicts the increased risk for incremental increase in biomarker measure. For the case of the last assessment of each biomarker, the HR reflects each 10 pg/mL increase. For the case of AUC, the HR reflects each 1000 pt increase in AUC
| Hazard ratio (95% CI) | p-Value | |
|---|---|---|
| (A) Univariate biomarker transformation | ||
| CXCL9 last measure | 1.06 (0.99–1.14) | 0.07 |
| CXCL9 AUC | 1.07 (1.02–1.11) | <0.01 |
| CXCL9 sum | 1.22 (1–1.5) | 0.05 |
| CXCL9 mean | 1.17 (1–1.38) | 0.05 |
| CXCL10 last measure | 1.08 (1.03–1.13) | <0.01 |
| CXCL10 AUC | 1.03 (1.01–1.04) | <0.01 |
| CXCL10 sum | 1.13 (1.03–1.24) | 0.01 |
| CXCL10 AUC | 1.08 (1.01–1.16) | 0.02 |
| IL-17 last measure | 1.26 (1.05–1.52) | 0.01 |
| IL-17 AUC | 0.96 (0.83–1.10) | 0.55 |
| IL1-RA last measure | 1 (0.99–1.01) | 0.81 |
| IL1-RA AUC | 1 (0.99–1.01) | 0.75 |
| MCP-1 last measure | 1.02 (1.01–1.03) | <0.01 |
| MCP-1 AUC | 1 (0.99–1.01) | 0.42 |
| RANTES last measure | 1.07 (0.92–1.24) | 0.41 |
| RANTES AUC | 1.02 (1–1.04) | 0.12 |
| (B) Adjusted biomarker | ||
| CXCL9 AUC (adjusted for last measured CXCL9) | 1.06 (1.02–1.11) | 0.01 |
| CXCL10 AUC (adjusted for last measured CXCL10) | 1.02 (1.01–1.04) | 0.01 |
Increased CXCL9 and CXCL10 exposure is associated with increased risk of early chronic rejection
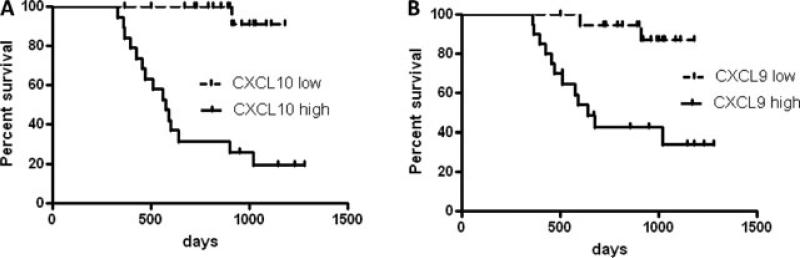
BOS is a surrogate clinical endpoint, characterizing chronic rejection in lung transplantation and is defined by a sustained 20% reduction of FEV1 not explained by other causes. Using this definition, there were 15 cases of BOS in our cohort, with a range of 365–1018 days posttrans-plant. When assessed by Kaplan–Meier analysis, patients with high CXCL9 and CXCL10 AUC had increased risk of chronic rejection (HR 6.4, p < 0.01 and HR 9.6, p < 0.01 respectively; Figure 2). We assessed risk of BOS using Cox regression on all patients in univariate and multivariate models. Similar to the Kaplan–Meier assessment, we found a strong association between multiple transformations of CXCL9 and CXCL10 and risk of BOS. As shown in Table 4, for CXCL9, the last reading, the mean, the AUC and the maximum value obtained, all had an association with BOS; whereas for CXCL10, the last reading, the mean and the AUC, all had an association with BOS, but the maximum value did not. Similar to the graft survival models, we tested whether patient factors or other biomarker transformations could alter the HR of CXCL9 or CXCL10 AUC. As shown in Table 4, several patient factors did alter the HR of CXCL9 or CXCL10 AUC by more than 10% (although the last biomarker measures did not), but did not change the overall finding that the AUC of these biomarkers was associated with BOS.
Figure 2. Graft survival of lung transplant patients with high or low levels of BAL CXCL9 (A) or CXCL10 (B).
Analyte area under the curve was assessed for 40 patients over the first year postlung transplant. Patients were divided into high and low levels if they were above or below the median. Event free survival indicates freedom from diagnosis of BOS or histologic BO. p < 0.01 for CXCL9 and CXCL10 by log-rank test. N = 20 patients per group.
Table 4.
Univariate and multivariate Cox regression for BOS. Shown in (A) are the various transformations of BALF biomarkers utilized in the model. In the adjusted models for CXCL9 and CXCL10 (B and C), variables were included if they changed the model by >10% in addition to the last biomarker measure. Shown are the HR for developing BOS with the 95% confidence intervals
| Univariate biomarker transformation | Hazard ratio (95% CI) | p-Value | |||
|---|---|---|---|---|---|
| (A) Unadjusted model | |||||
| CXCL9 last reading | 1.11 (1.04–1.19) | <0.01 | |||
| CXCL9 mean | 2.03 (1.28–3.24) | <0.01 | |||
| CXCL9 AUC | 1.9 (1.2–3.01) | 0.01 | |||
| CXCL9 sum | 1.92 (1.25–2.96) | <0.01 | |||
| CXCL9 maximum | 1 (0.99–1.01) | 0.62 | |||
| CXCL10 last reading | 3.8 (2.09–6.91) | <0.01 | |||
| CXCL10 mean | 2.24 (1.35–3.7) | <0.01 | |||
| CXCL10 AUC | 2.84(1.67–4.85) | <0.01 | |||
| CXCL10 sum | 2.2 (1.33–3.65) | <0.01 | |||
| CXCL10 maximum | 1 (0.99–1.01) | 0.58 |
| CXCL9 parameter | Crude hazard ratio (95% CI) | Hazard ratio adjusted for last measure (95% CI) | Hazard ratio adjusted for diagnosis (95% CI) | ||
|---|---|---|---|---|---|
| (B) Adjusted CXCL9 model | |||||
| CXCL9 AUC | 1.9 (1.2–3.08) | 1.78 (1.12–2.85) | 2.18 (1.31–3.65) | ||
| CXCL9 last measure | 1.04 (0.95– 1.13) | ||||
| Diagnosis (Obstructive vs. Restrictive) | 0.33 (0.1–1.16) |
| CXCL10 Parameter | Crude Hazard Ratio (95% CI) | Hazard ratio adjusted for last measure (95% CI) | Hazard ratio adjusted for gender (95% CI) | Hazard ratio adjusted for donor age (95% CI) | Hazard ratio adjusted for diagnosis (95% CI) |
|---|---|---|---|---|---|
| (C) Adjusted CXCL10 Model | |||||
| CXCL10 AUC | 2.84(1.67–4.85) | 1.92(0.97–3.80) | 3.32 (1.75–6.3) | 3.82 (1.89–7.78) | 4.00 (2.04–7.85) |
| CXCL10 last measure | 2.98 (1.55–5.75) | ||||
| Gender (Female) | 0.54 (0.17– 1.67) | ||||
| Donor age | 0.96 (0.92–1.01) | ||||
| Diagnosis (obstructive vs. restrictive) | 0.25 (0.07–0.97) |
Increased CXCL9 and CXCL10 exposure correlates with lung function obtained early after lung transplant
One of the determinants to longevity and quality of life after transplant is the stability and robustness of the lung function after transplantation. There are multiple factors that affect the degree of improvement following transplant, which include the type of disease for which the recipient is transplanted, the nature of the transplant (bilateral vs. single), the age and size of the transplant recipient and the number of episodes of acute rejection. We assessed the ability of the BALF biomarkers to correlate with FEV1 using both univariate and multivariate models. As shown in Table 5, multiple transformations of the biomarkers were associated with FEV1 function, with the running CXCL9 having the strongest negative association with FEV1. We were interested to test how other factors and biomarker transformations would alter the association between CXCL9 and CXCL10 AUC. In lung function multivariate models, we found no patient or transplant characteristics to be confounders of the association between biomarker AUC and FEV1. In the case of CXCL10 AUC, we found that the last measured biomarker taken closest to the FEV1 could significantly alter the AUC association with FEV1, but for CXCL9 AUC the last measured CXCL9 did not have a large impact. In the CXCL10 model, the AUC transformation was not even significant any longer when controlling for the last measure. This suggests for CXCL9, the biomarker was associated with both cumulative effects on lung function as well as instantaneous effects, but for CXCL10, the instantaneous effect was larger and more important than cumulative effects.
Table 5.
Univariate (Table 4A) and mutivariate (Tables 4B and C) association between BALF biomarker transformation and FEV1. The current measure for each biomarker refers to the closest biomarker measure at each given FEV1 measure. The AUC running tracks the accumulated AUC for each biomarker up until an FEV1 measure. The sum reflects the accumulated sum of a biomarker up until each FEV1 measure. For discrete measures (e.g. current measure), the parameter estimate reflects the change in FEV1 in mL for a 10-point change in that measure. For AUC, the parameter estimate reflects the change in FEV1 for 1000 change in AUC. Shown are unadjusted models and models for running CXCL9 and CXCL10 AUC controlling for the last measured CXCL9 or CXCL10. Table 4(A) shows the unadjusted univariate effects on FEV1
| Univariate biomarker transformation | Parameter estimate (mL of FEV1) and 95% CI | p-Value |
|---|---|---|
| (A) Unadjusted model | ||
| CXCL9 current measure | –2.7 (–5––0.4) | 0.02 |
| CXCL9 AUC running | –14.5 (–27.2––1.9) | 0.03 |
| CXCL9 sum running | –55.5 (–76.5––34.4) | <0.01 |
| CXCL10 current measure | –5.4 (–8––2.8) | 0.01 |
| CXCL10 AUC running | –2.8 (–10.1–4.5) | 0.45 |
| CXCL10 sum running | –42.6 (–78.2––6.7) | 0.02 |
| IL-17 current measure | –0.6 (–13.8–12.7) | 0.93 |
| IL-17 AUC running | 25.0 (5.1–45.6) | 0.01 |
| IL-17 sum running | 158.3 (35.9–280.9) | 0.01 |
| IL1-RA current measure | 0.15 (–0.1–0.4) | 0.24 |
| IL1-RA AUC running | 0.57 (0.11–1.04) | 0.02 |
| IL1-RA sum running | 2.7 (0.55–4.85) | 0.01 |
| MCP1 current measure | –0.7 (–1–– 0.4) | <0.01 |
| MCP1 AUC running | –0.6 (–1–0) | 0.06 |
| MCP1 sum running | –2.8 (–4.3––1.2) | <0.01 |
| RANTES current measure | –1.3 (–2.1––0.4) | <0.01 |
| RANTES AUC running | 0.5 (–1.2–2.2) | 0.54 |
| RANTES sum running | –13.3 (–17.3––9.4) | <0.01 |
| (B) Adjusted CXCL9 model Effect | ||
| CXCL9 AUC running | –14.5 (–28.3––0.8) | 0.04 |
| CXCL9 last measured | –2.5 (–4.3––0.7) | <0.01 |
| (C) Adjusted CXCL10 model Effect | ||
| CXCL10 AUC running | –4.8 (–11.7–2.2) | 0.18 |
| CXCL10 last measured | –5.9 (–8.6––3.2) | <0.01 |
Association between CXCL9 and CXCL10 and acute rejection
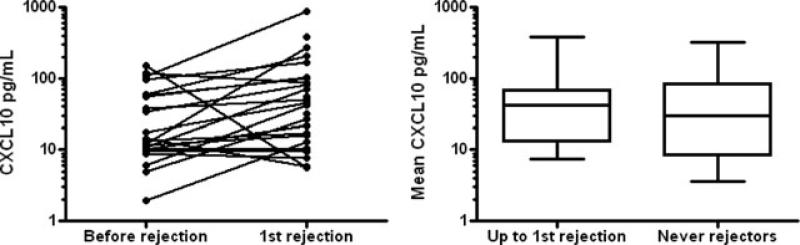
Because the presence of acute rejection is one predictor of the risk of developing BO, we next assessed how CXCL9 and CXCL10 BALF levels tracked in the setting of acute rejection compared to quiescence. Because of a relatively small number of rejection episodes, we defined rejection as any evidence of venulitis or bronchiolitis (A or B score) by ISHLT criteria as determined by the pulmonary pathologist. In total, 29 of 40 patients had, at least, one episode of rejection and 16 of 29 patients only had minimal (grade 1) rejection as their highest rejection grade. In a paired manner, we compared each patient who had a period of nonrejection to an episode of rejection. For the 22 patients who met this criterion, CXCL10 was elevated in the setting of rejection compared with the previous nonrejection period (median 43.4 pg/mL vs. 13.9, p < 0.01 by Wilcoxon Rank test, Figure 3A). We assessed CXCL10 levels in all the patients who had rejection by calculating the mean of all the samples leading up to and including the first episode of rejection. When compared to BALF from patients who never had rejection, the patients with rejection had slightly higher CXCL10 levels (median 41.3 vs. 29.9 pg/mL) but these differences were not statistically different (p = 0.67, Figure 3B). Using the same stringent analysis criteria did not detect a significant difference in CXCL9 values in rejection using either a paired or unpaired analysis (not shown). Further, we input acute rejection and biomarker data into a GEE model, which accounts for all the repeated observations and account for the data being correlated within patients. This GEE model also failed to find a significant association between CXCL9 or CXCL10 (or their transformations) and acute rejection.
Figure 3. Increased CXCL10 in the setting of acute rejection.
Panel A shows paired analysis of patients who had a period of nonrejection followed by the first episode of rejection. The “before rejection” represents the mean of all BALF CXCL10 before the first episode of rejection. A total of 22 patients met this criterion with median before rejection CXCL10 levels of 13.9 versus median first rejection levels of 43.4 (p < 0.01). Panel B shows the comparison of mean CXCL10 levels leading up to and including the first rejection in the 22 patients with at least one episode of rejection to mean CXCL10 levels in the 11 nonrejectors (41.3 vs. 29.9 pg/mL, p = 0.67).
CXCL9 and CXCL10 expression is localized to the bronchiolar epithelium
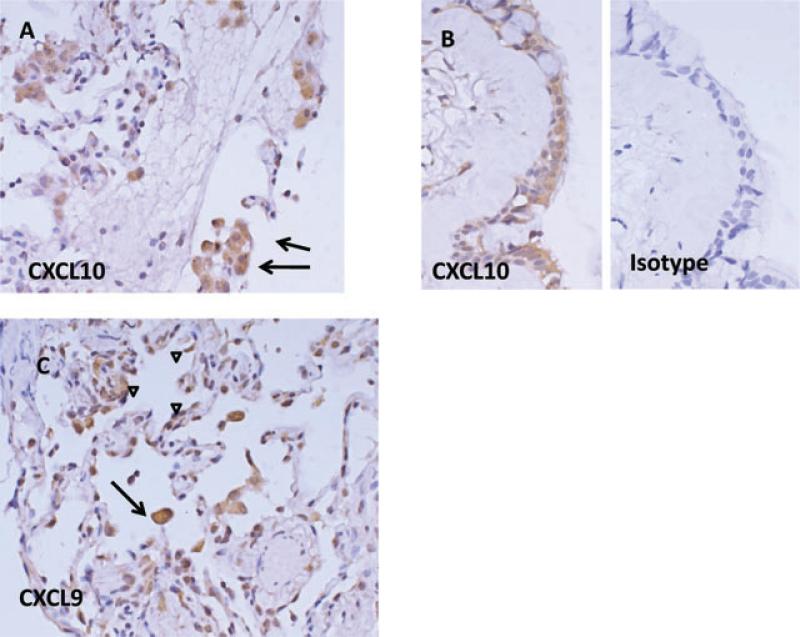
Given that CXCL9 and CXCL10 elevations were associated with graft failure, we sought to determine the source(s) of these chemokines. The alveolar macrophage is the predominant cell type recovered during bronchoalveolar lavage, and that this cell type is capable of secreting a large variety of proinflammatory chemokines. We predicted that staining would be localized to the macrophages present in the intra-alveolar spaces. We performed immunohistochemistry of transbronchial lung biopsy specimens obtained during routine surveillance bronchoscopies from 10 patients with the highest BALF levels of CXCL10 and CXCL9. Representative panels are shown in Figure 4. Consistent with our prediction, alveolar macrophages were found to stain readily for both CXCL9 and CXCL10. In addition, we noted in biopsy specimens that contained well-delineated bronchiolar epithelium significant staining for CXCL9 and CXCL10 and not for control isotype antibody. Further, in some specimens there was additional moderate staining found on the alveolar epithelium. We did not observe any significant staining of the bronchiolar submucosa or the pulmonary vasculature. Collectively these findings indicate that the elevated BALF CXCL9 and CXCL10 could be derived from both a hematopoietic source (macrophages) and a nonhematopoietic source-–the small airway epithelium itself, perhaps prompting lymphocyte egress into the epithelial-lined airways and accumulation in the BALF.
Figure 4. Anatomic compartment of staining for gamma-dependent chemokines.
Panel A shows positive staining for CXCL10 of alveolar macrophages. Panel B shows positive staining of bronchiolar epithelium compared to isotype control antibody. Panel C shows CXCL9 staining of both alveolar macrophages (arrows) and the alveolar epithelium (arrowheads).
Discussion
Our extensive analysis of sequential samples taken from BALF collected in the first year postlung transplant reveals a phenotype, which both correlates with lung function and is associated with a risk of death and chronic rejection.
Our work suggests that the IFNG-dependent chemokines CXCL9 and CXCL10 are elevated in the BALF of patients at risk for graft loss. These data are consistent with previously published data showing that these chemokines are elevated in patients with already established BOS and in animal models of chronic rejection (4–6). We believe that our findings lay the groundwork for clinical studies, which attempt to utilize these markers in real time to make clinical decisions. For example, patients found to have elevations of either or both CXCL9 and CXCL10, may be candidates for augmented immunosuppression. Further, patients with repetitive low levels of these chemokines may be candidates for targeted reduction of immunosuppression.
In this pilot analysis, we have used AUC analysis to approximate cumulative exposure over time and to give weight to persistent as opposed to transient inflammatory activity. The use of an AUC to more accurately estimate an exposure to a substance is a tool used extensively in pharmacokinetic drug studies. In addition, cumulative exposures to putative harmful substances or events has been described in environmental health with respect to toxins, such as pesticides and cigarette smoke, in cancer epidemiology, in lung injury and in cardiac function (9–13). In the field of transplantation, Harris et al. showed that renal transplant patients with elevated plasma transforming growth factor beta (TGFB) AUC had increased risk of chronic allograft nephropathy. These investigators were fortunate in that their sample size and number of repeated measures was large enough to conclude that TGFB AUC was an independent risk factor for chronic kidney failure beyond its association with acute rejection (14). Although our study of 40 patients with multiple samples measured per patient is extensive by clinical lung transplant standards, it is still insufficient in study size and sample number to conclude that CXCL9 and CXCL10 levels independently predict graft loss above and beyond the information gleaned from lung biopsy.
Going forward, the feasibility of using repetitive BALF sample analysis to make clinical decisions that impact future lung function will depend on the extent to which CXCL9 and CXCL10 are elevated before lung function starts to decline. In this study, we only observed 10 episodes of graft failure, but in those patients, 9 of 10 displayed significant elevations of CXCL10 from 3 to 9 months before death or retransplantation. These data are consistent with BO deriving from the cumulative effects of chronic inflammatory activity. Our findings prompt further investigation to determine the predictive ability of AUC on future graft function. We have used AUC to model cumulative exposure, but recognize that AUC alone may be imperfect. For example, the same value of AUC could represent different trends over time: a strictly increasing trend, multiple small peaks or a single very large peak. Each of these patterns would represent a very different underlying mechanism, which would not be revealed by analyzing AUC. Thus, future work carried out in a prospective fashion is required to determine if the pattern, in which a biomarker appears, carries as much weight, or even more than the sum total of its presence. In this regard, it is useful to highlight the fact that when BOS was the endpoint, the last measure of CXCL10 had the strongest association with chronic rejection. Although it is noteworthy that CXCL9, CXCL10 and, to a lesser extent, MCP-1 BALF levels did track with relevant clinically important readouts postlung transplant, it is also remarkable that several other biomarkers did not appear to correlate with meaningful outcomes. We did not see a significant relationship between graft failure, BOS, acute rejection or lung improvement posttransplant with respect to IL-13, IL-17, RANTES or IL1-RA. One general consideration relates to the direct production of CXCL9 and CXCL10 by respiratory epithelial cells. This may distinguish CXCL9 and CXCL10 from the other analytes in their enhanced proximity to the airways and suitability for collection in BALF. For the case of IL-17, there is growing data linking cellular Th17 responses driven by IL-17 to the subsequent development of BOS (15). Further, it is notable that Vanaudenaerde et al. have demonstrated elevated BALF protein levels in patients with acute rejection and BOS compared to stable patients (16,17). In those studies, the mean IL-17 concentration in the BALF in the setting of ACR and BOS was 3 and 2 pg/mL, respectively. With the multiplex ELISA platform utilized here, the lower limit of detection of IL-17 was 10 pg/mL. Hence, a significant number of samples were classified as not detected and this may have limited the ability to draw meaningful conclusions with respect to IL-17. IL1-RA represented an attractive potential biomarker as it demonstrated the most significant dynamic range of all the markers tested, prior work has demonstrated an inverse relationship between IL1-RA and renal function in kidney transplantation (18) and drug therapy harnessing the anti-inflammatory promise of IL1-RA has been demonstrated in rheumatoid arthritis (19). In spite of the relative detectability of BALF IL-1RA, its presence did not correlate with outcome in our study. This may reflect the fact that IL-1RA may be elevated in both the setting of inflammation as a counter regulatory cytokine in patients experiencing rejection and also may exist at high basal levels in patients with prolonged quiescence. Hence, our analytic approach may not have been sensitive enough to detect an effect of IL1-RA, even if one exits.
A central question to any clinical study utilizing biomarkers to assess a relevant clinical endpoint is the extent to which the markers studied are actually involved in the disease process. For the case of CXCL9 and CXCL10, we believe it is plausible that these IFNG-dependent chemokines are relevant for progression of BOS. For example, our histo-logic examination of transbronchial lung biopsy specimens in patients with very high levels of CXCL9 and CXCL10 demonstrated strong staining of the bronchiolar epithelium for these markers. Chronic rejection in lung transplant is characterized by a conversion of the bronchiolar lumen from a columnar epithelium to a stratified layer of fibroblastic tissue. Hence, the finding that the relevant pre-BO biomarker is found within the tissue compartment of interest adds to the biologic plausibility that these IFNG-dependent chemokines are actually participating in the disease process. CXCL9 and CXCL10 are chemoattractive for T cells expressing CXCR3. Therefore, one potential mechanism involved in the pathogenesis of BO is that these chemokines are attracting relevant T cells into the graft that bare the cognate receptor and can cause graft injury. Indeed, a growing body of work suggests that lymphocytic bronchitis (characterized by lymphocytes invading the bronchiolar wall) is a precursor toward BO (20). We are undertaking studies to determine if elevations of CXCL9 and CXCL10 are linked with concurrent increases in CXCR3 on CD4 and CD8 T cells.
In summary, persistently elevated levels of CXCL9 and CXCL10 strongly associate with graft and patient survival, and correlate with lung function in human lung transplant recipients. These data support the design and conduct of a prospective clinical trial investigating the use of BALF chemokine assessment as a tool to guide clinical immune management.
Acknowledgments
David C. Neujahr is supported by a grant from the Roche Organ Transplant Research Foundation and by an NIH Career Development Award (K08AI079 166). The investigators wish to thank the Atlanta Clinical and Translational Science Institute and the Emory Transplant Center Bioreposi-tory for supporting this research.
Abbreviations
- ACR
acute cellular rejection
- AUC
area under the curve
- BALF
bronchoalveolar lavage fluid
- BO
bronchiolitis obliterans
- BOS
bronchiolitis obliterans syndrome
- FEV1
forced expiratory volume in 1 second
- IFNG
interferon gamma
Footnotes
Disclosure
DC Neujahr and A Mohammed have conflicts of interest to disclose as described by the American Journal of Transplantation. These authors have submitted a provisional patent application to the US Patent office to utilize repeated measures of biomarkers in BALF in lung transplant patients as a diagnostic algorithm.
References
- 1.Christie JD, Edwards LB, Aurora P, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth official adult lung and heart-lung transplantation report–2009. J Heart Lung Transplant. 2009;28:1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Weigt SS, Wallace WD, Derhovanessian A, Saggar R, Lynch JP, Belperio JA. Chronic allograft rejection: Epidemiology, diagnosis, pathogenesis, and treatment. Semin Respir Crit Care Med. 2010;31:189–207. doi: 10.1055/s-0030-1249116. [DOI] [PubMed] [Google Scholar]
- 3.Glanville AR. Bronchoscopic monitoring after lung transplantation. Semin Respir Crit Care Med. 2010;31:208–221. doi: 10.1055/s-0030-1249117. [DOI] [PubMed] [Google Scholar]
- 4.Agostini C, Calabrese F, Rea F, et al. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 6.Belperio JA, Keane MP, Burdick MD, et al. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol. 2003;171:4844–4852. doi: 10.4049/jimmunol.171.9.4844. [DOI] [PubMed] [Google Scholar]
- 7.Keane MP, Gomperts BN, Weigt S, et al. IL-13 is pivotal in the fibro-obliterative process of bronchiolitis obliterans syndrome. J Immunol. 2007;178:511–519. doi: 10.4049/jimmunol.178.1.511. [DOI] [PubMed] [Google Scholar]
- 8.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flesch-Janys D, Steindorf K, Gurn P, Becher H. Estimation of the cumulated exposure to polychlorinated dibenzo-p-dioxins/furans and standardized mortality ratio analysis of cancer mortality by dose in an occupationally exposed cohort. Environ Health Perspect. 1998;106(Suppl 2):655–662. doi: 10.1289/ehp.98106655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost-Pineda K, Liang Q, Liu J, et al. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob Res. 2011;3:182–193. doi: 10.1093/ntr/ntq235. [DOI] [PubMed] [Google Scholar]
- 11.Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: The multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:732–739. doi: 10.1158/1055-9965.EPI-05-0798. [DOI] [PubMed] [Google Scholar]
- 12.Stevens TP, Dylag A, Panthagani I, Pryhuber G, Halterman J. Effect of cumulative oxygen exposure on respiratory symptoms during infancy among VLBW infants without bronchopulmonary dysplasia. Pediatr Pulmonol. 2010;45:371–379. doi: 10.1002/ppul.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling Y, Pong T, Vassiliou CC, Huang PL, Cima MJ. Implantable magnetic relaxation sensors measure cumulative exposure to cardiac biomarkers. Nat Biotechnol. 2011;3:273–237. doi: 10.1038/nbt.1780. [DOI] [PubMed] [Google Scholar]
- 14.Harris S, Coupes BM, Roberts SA, Roberts IS, Short CD, Brenchley PE. TGF-beta1 in chronic allograft nephropathy following renal transplantation. J Nephrol. 2007;20:177–185. [PubMed] [Google Scholar]
- 15.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 17.Vanaudenaerde BM, Dupont LJ, Wuyts WA, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi M, Daniel V, Naujokat C, et al. Decreasing plasma soluble IL-1 receptor antagonist and increasing monocyte activation early post-transplant may be involved in pathogenesis of delayed graft function in renal transplant recipients. Clin Transplant. 2010;24:415–423. doi: 10.1111/j.1399-0012.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 19.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: A systematic review. J Rheumatol. 2009;36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 20.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177:1033–1040. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]