Abstract
Background
Long-term success in lung transplantation is limited by obliterative bronchiolitis (OB). As of this time complete understanding of the mechanisms of OB has been elusive. Bone marrow-derived mesenchymal stem cells (MSC) have been shown to modulate repair of the injured lung in multiple disease models. We hypothesized that the injection of MSC would prevent the development of early airway obstruction (AO) in the heterotopic tracheal transplant model.
Methods
44 tracheas from BALB/c and C57BL/6 donors were transplanted into 22 C57BL/6 recipients. At the time of transplant 13 of the allogeneic recipient mice were injected with 5 × 105 MSCs from various murine sources. To confirm the role of the immune response in the generation of AO we used a permeable inhibitor of NF-κB in 11 recipients after been transplanted with 22 Balb/c tracheas
Results
After transplant, administration of MSC inhibited intraluminal obstruction by collagen in a 98% and TGF-β expression reduced to similar levels that the ones observed in the isograft controls. These effects were associated with a significant (p<0.05), increase in the expression of the anti-inflammatory cytokine IL-10. NF-κB inhibitor shows a decrease in the expression of TGF-β in the day 7 and day 14 groups resulting in a 60% reduction of luminal obstruction as well as the reduction of inflammatory cells to the airway.
Conclusions
Our observations suggest that the administration of MSC prevents the development of airway occlusion in a mouse model likely via modulation immune response altering TGF-β expression.
INTRODUCTION
To date there are many serious pulmonary conditions for which the only hope for survival is lung transplant. In spite of many advances in the care of lung transplant recipients, 1, 3, and 5 year survival are the lowest among transplanted organs at 84, 66, and 53% respectively1. The most common major long-term complication of lung transplant and the greatest limit to survival is obliterative bronchiolitis (OB) with its associated syndrome bronchiolitis obliterans syndrome (BOS). BOS is found in 27% of recipients at 2.5 years and 51% at 5.6 years post transplant2. Believed to be a form of chronic rejection, OB is characterized by chronic inflammation of the bronchial epithelium leading to the eventual obliteration of the distal airways by inflammatory infiltrates, fibroblasts, collagen, and matrix deposition3. The only current treatment consists of augmented immunosuppression which has had minimal impact on the overall disease-related mortality4 and is associated with increased complications, especially infectious.
Bone marrow-derived mesenchymal stem cells (MSC) are a group of plastic adherent cells, negative for CD45 and CD11b, which are capable of differentiation into a variety of cell types depending on culture conditions5–6. In the lung, MSC have been detected as multiple native cell types7 and, more importantly, have been shown to be potent modulators of the immune response in many pulmonary conditions including ALI, COPD, pulmonary hypertension, asthma, and fibrotic lung disease6. We have shown in other contexts that MSC migrate to the lung7–8 and decrease tissue damage and/or increase repair in response to injury9–11.
There are multiple animal models to study obliterative brochiolitis after lung transplantation, which each one has a unique role to understand the pathophysiology of OB. The common models include the orthotopic lung transplantation. This difficult technique mimics the surgical procedure of human transplant. This model had been used extensively to evaluate acute rejection, long term engraftment had fail to develop a typical OB lesion together with the low reproducibility reducing the use of this model to study OB. Other animal models, in which large animal are used, are only valid as part of preclinical trials. The mouse model of heterotopic trachea transplantation or HTT is a simple easy procedure, with high reproducibility which had been extensively used to study the immune aspects of early airway obstruction and the process of epithelial damage associated with tissue remodeling that result in OB. The fact that the tracheas are non-vascularized and no exposed to air, generates a form of obstructive airways disease and not exactly a form of OB that can reduce the significance of the model, however HTT had helped to understand the molecular mechanism involved in the development of the obstruction of the airways after lung transplant. Work done in our lab has shown the development of airway obstruction (AO) in the murine heterotopic tracheal transplant model and how other bone-marrow derived fibroblast progenitor cells, or fibrocytes, are in part responsible for the generation of AO. We have also delineated potential targets for prevention of its development9.
TGF-β is an essential mediator of the fibroproliferative response present in AO. Its expression is affected by the translocation of the transcription factor NF-κB from the cytoplasm to the nucleus. NF-κB translocation occurs when its dimers (mostly p50/p65 heterodimers) are liberated from sequestration in the cytoplasm by phosphorylation of an inhibitor protein, I-κBα. We have developed a non-phosphorylatable I-κBα, [I-κB α-(ΔN)], which is a dominant negative inhibitor of NF-κB activation and can be delivered into the cell as a fusion protein with a membrane-translocating sequence (MTS)12–13.
We hypothesized that injection of MSC would modulate the immune response and ameliorate the development of airway obstruction in a murine model of lung transplant. We predicted that TGF-β and its associated pathways would be important in the development of airway obstruction and would be impacted by the delivery of MSC.
MATERIALS AND METHODS
Animal Maintenance
Experiments were conducted using 10–14 week old, female C57BL/6, BALB/c, and CH3 mice (Jackson Laboratories®, Bar Harbor, ME). Mice were housed in cages and maintained on a 12-h light-12-h dark cycle at the Division of Animal Resources at Emory University. All animals had free access to food and water. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee
Trachea Grafting
Tracheas were transplanted as previously described6,13–14. Briefly, the mice were euthanized and the trachea was resected and immediately placed in ice-cold sterile PBS. C57BL/6 recipient mice were anesthetized with ketamine/xylazine (100 and 2 mg/kg intraperitoneally (Phoenix Pharmaceuticals®, St. Joseph, MO). Two 0.5 cm horizontal vertical incisions were made on the back of the recipient mouse, and subcutaneous pockets were formed by blunt dissection. One tracheal graft was placed heterotopically into each subcutaneous pocket and the wound was closed with suture. No immunosuppressive agents were given to any graft recipient. Summary of total number of tracheas transplanted are in Table 1A and 1B
Table 1.
A summary of the groups and number of grafts used in each experiment is presented
| A | |
|---|---|
| Number of Tracheal Transplants per MSC Treatment | |
| Experimental Treatment Groups | Number of Tracheas |
| Isograft | 8 |
| Allograft | 10 |
| Allograft, C57BL6-MSC | 14 |
| Allograft, CH3-MSC | 6 |
| Allograft, Balb/c-MSC | 6 |
| B | |||
|---|---|---|---|
| Number of Tracheal Transplants per Protein Treatment | |||
| Experimental Treatment Groups | Day 7 | Day 14 | Day 28 |
| Isograft, no protein | 4 | 4 | 4 |
| Allograft, no protein | 4 | 4 | 4 |
| Allograft, GST-MTS | 8 | 6 | 8 |
| Allograft, GST-IκBα(ΔN) | 8 | 6 | 8 |
| Allograft, GST-IκBα(ΔN)-MTS | 8 | 6 | 8 |
Generation of Administrations of Mesenchymal Stem Cells
A frozen vial of murine bone marrow-derived mesenchymal stem cells, obtained from the Tulane Center for Gene Therapy, New Orleans, Louisiana, was thawed and expanded as previously described15. MSC were generated using a protocol described previously6,16. Fresh bone marrow cells were isolated by flushing Dulbecco’s modified Eagle’s medium (DMEM; ATCC®, Manassas, MD) containing 1% penicillin-streptomycin (ATCC) through the medullary cavity of mouse femurs. The cells were washed once with DMEM (ATCC) and plated at 1×106 cells per 100-mm cell culture dish (Corning®, Corning, NY) in DMEM (ATCC) media containing 10% fetal calf serum (ATCC), nonessential amino acids (Cellgro®, Herndon, VA), and pyruvate (Invitrogen®, Carlsbad, CA), and cultured at 37°C in 5% CO2. After 48 hours, non-adherent cells were removed, fresh media was added, and the culture was maintained for 7 days. Cells were harvested and, using magnetic beads (Miltenyi Biotech®, Auburn, CA), macrophages were depleted with anti-CD11b (BD Biosciences®, Palo Alto, CA) antibody, and hematopoietic cells were removed using an anti-CD45 (BD) antibody. Before infusion, cells were washed twice with PBS and resuspended at a concentration of 5×106 cells/ml. MSC were harvested from three different strains of mice (C3H, C57Bl/6, and BALB/c). Mice were anesthetized by inhalation of isofluorane and 0.1 ml of cell suspension was injected retro-ocularly at the time of tracheal transplant.
Detection of Cytokines
Concentrations in blood were determined using a Luminex system (Luminex, Austin, TX) with an anti-mouse kit obtained from Millipore (Billerica, MA). Well filters were prewashed and 1:1 diluted samples were applied to each well. Specific antibody-coated beads were added to the wells and incubated for 18 h at 4°C temperature. After incubation, the plate was washed twice. Biotinylated antibodies were added and the mixture was incubated for 1 hour. Afterwards, the cytokine-antibody complexes were detected by adding streptavidin coupled to PE. The number of positive complexes was determined by reading each sample in a Luminex XYP platform.
Western Blot
Half of the harvested tracheas were homogenized in protein extract solution containing 0.1% Triton X-100, 100 mmol/liter NaCl, 10 mmol/liter HEPES (pH 7.9), 1 mmol/liter ethylene-diamine tetraacetic acid (EDTA) and 0.5 mmol/liter phenylmethanesulfonylfluoride (PMSF; Sigma-Aldrich® USA, St. Louis, MO) on ice, and centrifuged at 13,000 g for 10 min. at 4°C. The protein concentrations in the lysates were determined using the Bradford method (Bio-Rad® Laboratories USA, Hercules, CA). Samples were run using 10% sodium dodecylsuflate-polyacrylamide gel electrophoresis (SDS-PAGE; Invitrogen) and then transferred to nitrocellulose membranes. Blots were incubated with a rat anti-mouse TGF-β antibody (BD) or a mouse anti-β-actin antibody (Sigma-Aldrich), and recognized by horseradish peroxidase-conguated goat anti-rat and anti-mouse antibodies (Santa Cruz Biotechnologies®, Santa Cruz, CA). Finally, blots were visualized via chemiluminescence using the SuperSignal West Pico™ Kit (Pierce®, Rockford, IL). Expression of each band was normalized to its corresponding β-actin band.
Immunohistochemistry and Histology
Three to seven grafts from each group were used for immunohistochemistry. To determine the TGF-β positive cells in the grafts, paraffin sections were stained with rabbit anti-TGF-β antibodies (Santa Cruz). Slides were then treated with rhodamineconjugated donkey anti-rabbit antibody (Santa Cruz). Nuclei were detected with hematoxylin counter-staining. For experiments to determine obstruction, sections were stained with H&E for routine histologic examination and Masson’s trichrome to delineate collagen.
Intraluminal Collagen and TGF-β Immunohistochemistry Quantification
Images of trichrome-stained tracheal sections were taken with a high-resolution digital camera attached to a microscope. Adobe® Photoshop® 7.0 (©1999–2002 Adobe Systems Incorporated) was used to determine the percentage of luminal obstruction by collagen. This was derived by outlining each trachea from the subepithelial layer in, cutting it, and pasting it to a new image. All color within the lumen except the blue of the trichrome stain was removed. The relative amount of intraluminal collagen was quantified by measuring the mean pixel density using Scion Image Software (©2000–2001 Scion Corporation). To quantify the amount of cells stained positively by immunohistochemistry, Adobe® Photoshop® 7.0 was again used. The lumen of each section from the subepithelial layer in was selected out of the picture and pasted to a new image. The brown color of the IHC stain was isolated, copied, and pasted to a new image where it could be analyzed by Scion Image Software. To control for the potential effects of background staining, isograft control slides were included.
Computerized Morphometry
Computerized morphometry was determined as described by Farivar et al17. Images of Trichrome-stained tracheal sections were taken with a high-resolution digital camera attached to a microscope. Using H&E stained tracheas, the cursor was used to trace the inner surface of the actual residual lumen. The cross-sectional area within the actual residual lumen as then subtracted from the entire area contained within the cartilage. The percentage of airway obstruction was then calculated using the following formula: (area within cartilage − area within residual lumen)/area within cartilage × 100%.
Purification of NF-κB Inhibitor
GST fusion proteins were purified using Glutathione Sepharose affinity chromatography beads (Amersham Biosciences®). Thirty milligrams of 90% pure recombinant permeable and non-permeable proteins were obtained from 12L of bacterial culture. To reduce endotoxicity, proteins for this study were expressed in escherichia coli BL21 (DE3) cells inactive for the LPS gene. Recombinant cells were grown to optical density 0.8 and induced by the addition of 0.5 mM isopropylthiogalactoside (Sigma). Cells were allowed to grow at 25°C for 4 h and were harvested by centrifugation. The cells were incubated for 30 min at 4°C in lysis buffer [PBS containing 10 mM DTT, 1 mM EDTA, 1 mM PMSF, 0.02% lysozyme (Sigma)]. DNase I (10 ug/ml, Sigma) was added to the lysate and allowed to incubate at room temperature for 30 min. Triton X-100 was added to 1% final concentration, and the solution was allowed to incubate at room temperature for an additional 30 min. Affinity chromatography was performed by adding 5 mL equilibrated glutathione sepharose beads (Amersham) to the lysate, and allowed to bind overnight. The beads were washed with PBS and eluted by step gradient with PBS containing 20 mM glutathione. Fractions containing protein were pooled and analyzed by SDS-PAGE. Proteins requiring immediate use were dialyzed in 10,000 MW dialysis cassettes (Pierce) in a 0.2% Triton X-114 in PBS.
Recombinant protein treatment
GST-IκBα(ΔN)-MTS, GST-IκBα(ΔN) (control), and GST-MTS (control) proteins were administered intraperitoneally (100 µg/dose/mouse) at days relative to transplantation of −1, 0, 1, 2, 3, 4, 5, 7 and then every 48 hours from day 7 until day sacrifice. One group receiving isografts and three allograft groups, each receiving a different fusion protein, were sacrificed at day 7, 14, and 28.
Statistical Methods
For comparisons between groups, paired or unpaired t-test and 1-way analysis of variance (ANOVA) tests were used (p<0.05 considered significant). We used GraphPad Prism™ and GraphPad Instat™ (GraphPad Software®, Inc, San Diego, CA) to calculate the statistics.
RESULTS
MSC Attenuate Airway Obstruction in Allograft Transplant Recipients
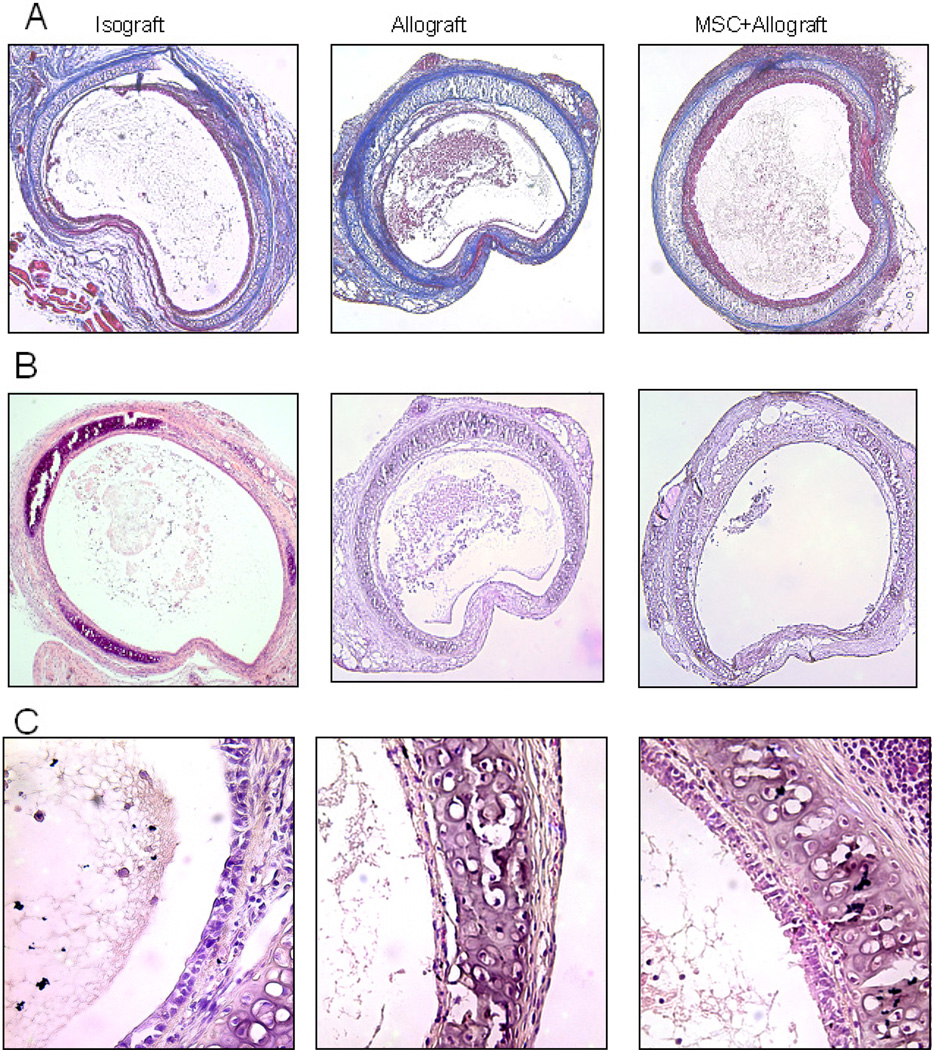
Donor tracheal segments from BALB/c and C57BL/6 were heterotopically transplanted into C57BL/6 recipients. Group assignment was defined by the donating species. Allograft controls were made up of BALB/c tracheas transplanted into C57BL/6 recipients. Isograft controls were made up of C57BL/6 tracheas transplanted into C57BL/6 recipients. Tracheas were harvested on day 10 and were homogenized for western blot analysis or fixed in formalin and then paraffin and sectioned for staining with H&E, Masson’s trichrome or immunohistochemistry. On harvesting, there was a qualitative difference in the amount of inflammation, collagen deposition, and epithelial disruption observed in the allograft tracheas as compared to the isograft. There was also noticeable damage to the epithelial layer. As illustrated in Figure 1, Masson’s trichrome staining for collagen and H&E staining showed increased intraluminal collagen deposition and total intraluminal obstruction in the allograft controls as compared to the isograft controls. These effects were prevented in the subjects that received MSC.
Figure 1.
MSC attenuates tracheal occlusion. C57BL/6 mice were transplanted heterotopically with C57BL/6 (isograft) or BALB/c (allograft) trachea. In the allograft group, a portion was injected with MSC on the day of transplant. Tracheal grafts were harvested on day 10 post-transplant and stained with Masson’s trichrome (A) or H&E (B). MSC decreased the total amount of tracheal occlusion as well as the amount of occlusion contributed by collagen. On higher magnification it can be seen that MSC preserved epithelial integrity in allograft recipients (C).
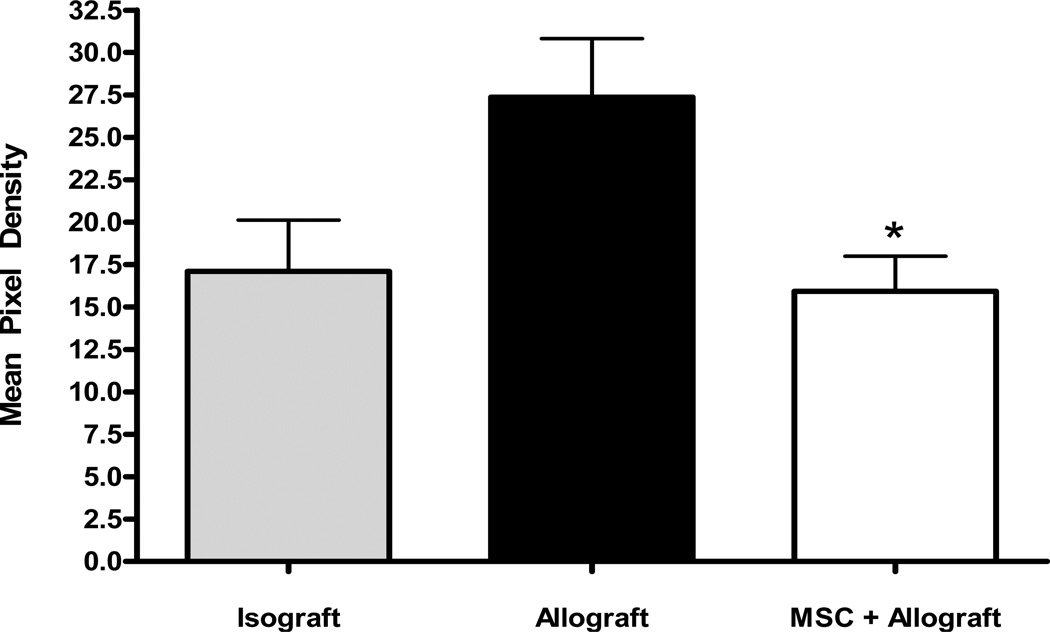
Quantification of intraluminal collagen in each group is shown in Figure 2. When the mean pixel density of the blue-stained collagen was measured there was a statistically significant increase in intraluminal collagen burden in the allograft control group as compared to the isograft control. This was reversed in the subjects that received MSC to a degree that achieved statistical significance.
Figure 2.
MSC attenuates tracheal occlusion. Intraluminal mean blue pixel density was measured and quantified for all groups showing a statistically significant decrease in the group injected with MSC at the time of transplant. *p<0.05 for MSC+allograft vs. allograft control.
MSC Decrease TGF-β Expression and Cytokine Expression Induced by Transplant
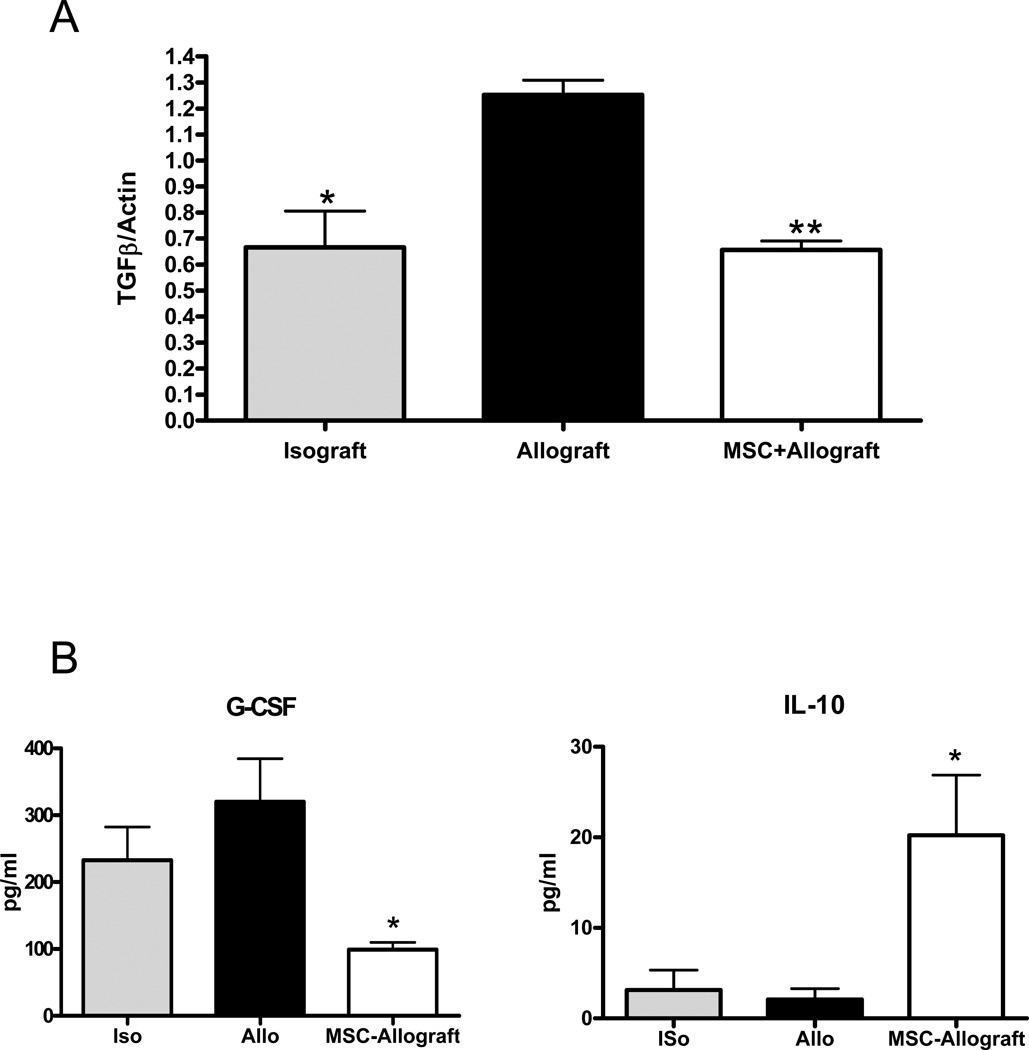
To help better delineate the underlying mechanisms of airway obstruction in the HTT model and the modulatory effects of MSC, we examined the effect of the tracheal transplant model on TGF-β expression. Western blot analysis showed a statistically significant increase in total TGF-β expression in the allograft group as compared to the isograft group. When MSC were injected at the time of transplant this rise TGF-β expression was prevented (Figure 3). We also determined cytokine levels in the serum of recipient mice after transplant using Luminex. G-CSF and IL-10 levels were significantly modified in allografts and the changes were blocked by stem cell infusion (Figure 3B). These results revealed that G-CSF and TGF-β1 levels were increased and IL-10 decreased in allografts and these changes were prevented by treatment with MSC.
Figure 3.
MSC block the rise in TGF-β induced and changes in cytokine expression by tracheal transplant. (A) Western blot analysis was performed with antibodies against TGF-β. Expression increased significantly in the allograft vs. isograft controls. This increase was blocked by injection of MSC at the time of transplant. *p<0.05 for isograft vs. allograft. **p<0.001 for MSC+allograft vs. allograft control. (B) Cytokine levels in the serum of recipient mice at day 10 post-transplant were determined using Luminex. Values represent mean ± standard error. *, **, p<0.05, n=4.
MSC Decrease TGF-β Airway Localization Induced by Transplant
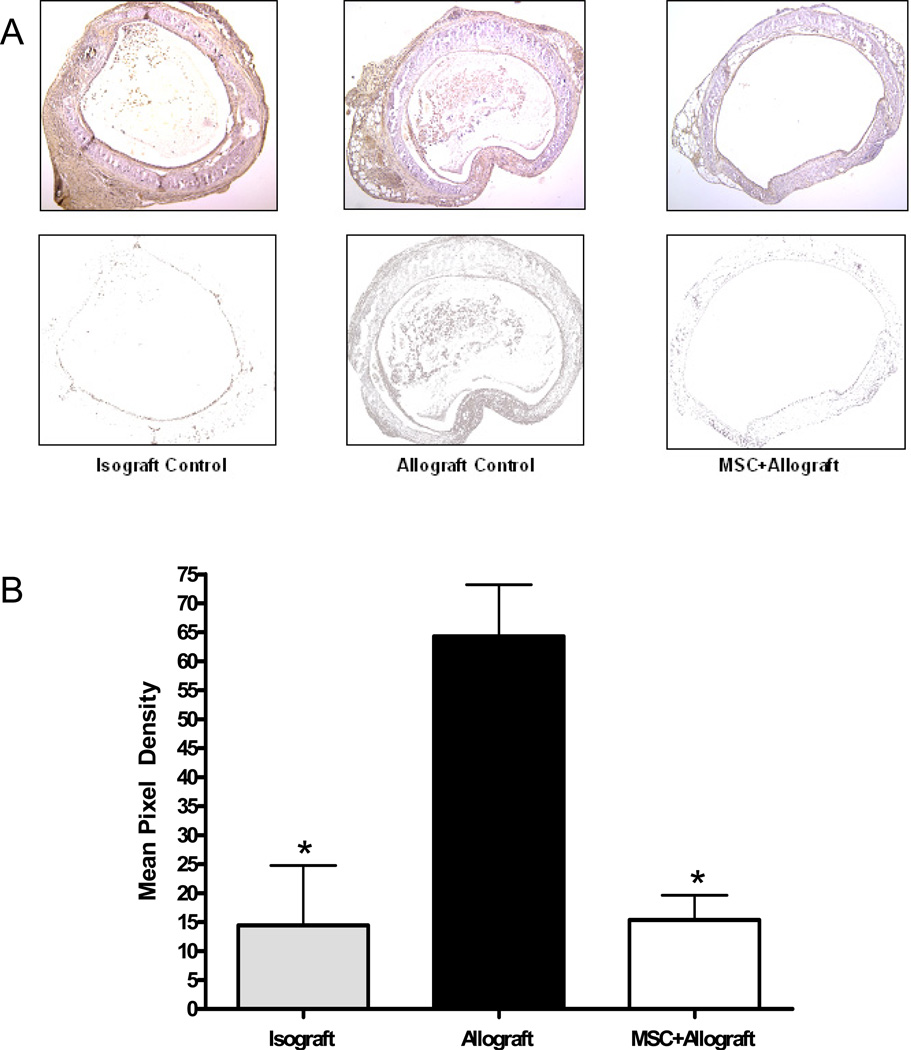
Immunohistochemistry with antibodies to TGF-β was performed on three samples from each subject group. The brown stain was isolated from each sample from the subepethilial layer in and the mean pixel density was calculated. There was a significant increase in TGF-β staining throughout the trachea and inside the lumen in the allograft vs. the isograft groups. This effect was blocked with the injection of MSC at the time of transplant (Figure 4).
Figure 4.
MSC block the rise in TGF-β induced by tracheal transplant. Immunohistochemistry was performed on paraffin sections from each group. Images were captured. Using Photoshop® the lumen and subepithelial layers of each trachea were selected out of the image. The brown color of the IHC stain was then isolated from the image (A). The mean pixel density of each was then analyzed with Scion© software (B). *p<0.05 compared to allograft control.
The Source of the MSC Did Not Impact the Results
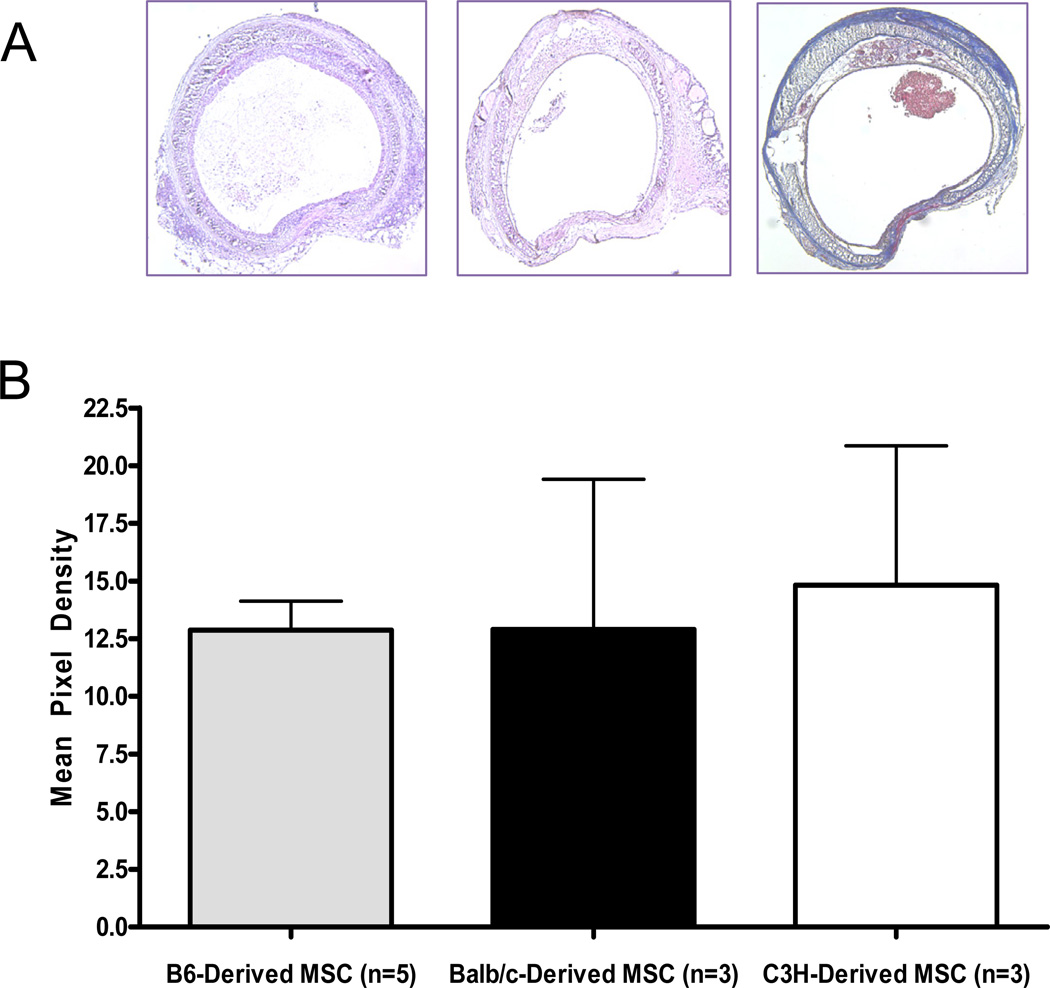
To compare the effect of MSC from different sources on tracheal occlusion, morphometry analysis was repeated with different sources of MSC. MSC harvested from C3H, BALB/c, and C57BL/6 mice were injected retro-ocularly at the time of transplant into three different groups of allograft recipients. All sources of MSC led to the same level of airway occlusion indicating that the source of the MSC does not diminish the inhibition of airway occlusion (Figure 5).
Figure 5.
Murine source of MSC does not affect tracheal occlusion by collagen. Qualitative observation of the amount of luminal occlusion by collagen did not differ by murine source of MSC (A). Morphometry was performed and there was no significant difference between the groups based on murine source of MSC (B).
Regulation of the immune response by NF-κB
We determined whether treating recipients with a protein inhibitor of NF-κB would affect development of AO. Morphometric analysis of the tracheas obtained from recipients treated systemically with GST-IκBα(ΔN)-MTS shows inhibition in airway obstruction compared to the animals inoculated with the control proteins. We saw activation of NF-κB in the transplanted tracheal epithelium in control animals but in animals treated with the inhibitor, p65 translocation was decreased associated with a decrease in accumulation of inflammatory cells in the sub-epithelium of the transplant. Immunohistochemistry and western blots showed reduced expression of TGF-β. These data implicate NF-κB activation as important in development of airway occlusion and a possible target of modulation by MSC.
Enhanced epithelial preservation in GST-IκBα(ΔN)-MTS protein treated allografts
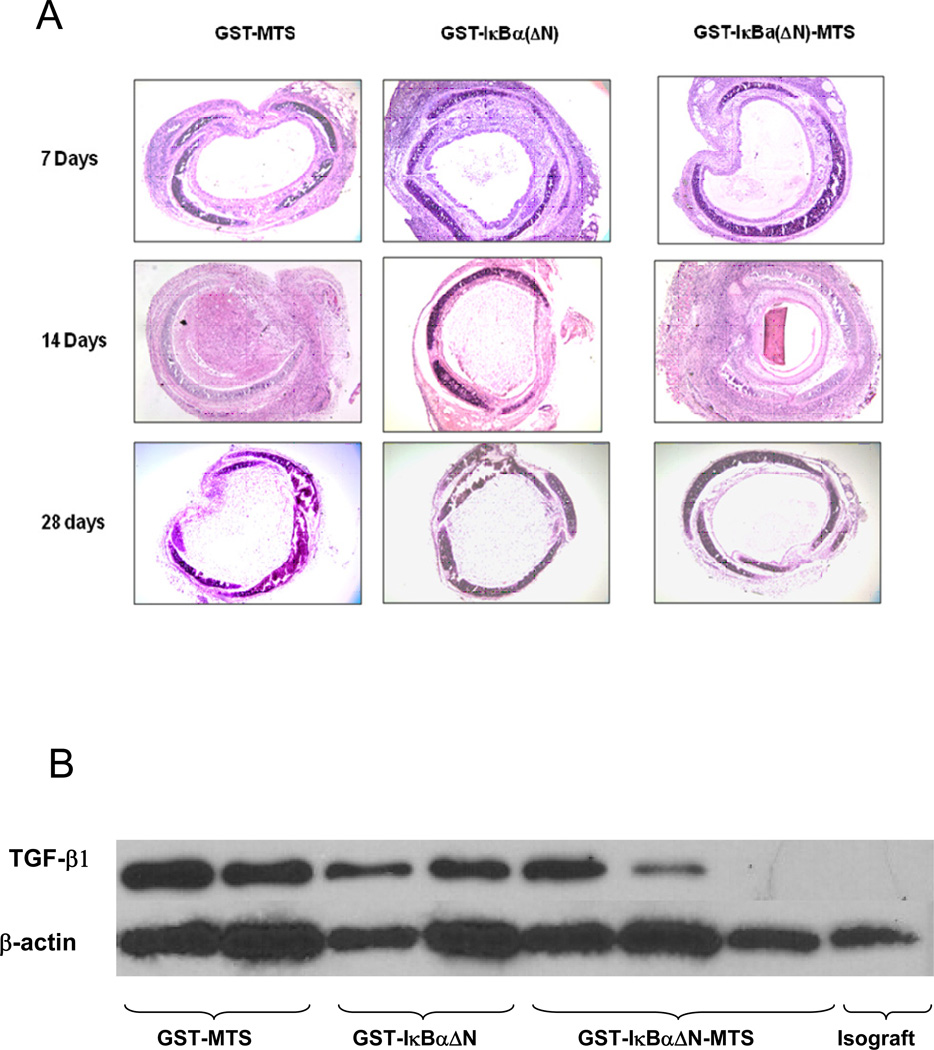
Recipients of heterotopic tracheal allografts and isografts were sacrificed at 7, 14, and 28 days post-transplantation. Isografts harvested from day 7, 14, and 28 demonstrate a patent tracheal lumen, intact airway epithelium, and minimal mononuclear cell infiltration within the lamina propria. Allograft recipients receiving no protein treatment demonstrate mononuclear cell infiltrations in the lamina propria into epithelium and lumen. Sections stained with hematoxylin and eosin demonstrate inflammation of the sub-epithelium at day 7, re-epithelialization at day 14, and major infiltration of the lumen by day 28. The allografts receiving no protein also show fibroproliferative obliteration of the airway by day 14, and major occlusion of the lumen caused by fibrosis by day 28. Similar findings were noted in allografts harvested from mice treated with GST-MTS and GST-IκBα(ΔN) control proteins. Once mononuclear cells infiltrate the sub-epithelium (day 7), the epithelium is targeted, sloughed off, and remodeled by day 14. Allografts harvested from recipients treated with GST-IκBα(ΔN)-MTS demonstrate decreased mononuclear cell infiltration of the sub-epithelium by day 7, while the epithelium remains intact. The lamina propria only begins to become infiltrated by day 14, when the sub-epitheliums of all other allograft recipients have lost their epithelium and re-epithelialized. By day 28, the tracheal allografts treated with GST-IκBα(ΔN)-MTS show preservation of the epithelium and minimal mononuclear cell infiltrations into the lumen as shown in histological analysis (Figure 6A).
Figure 6.
Infiltration of inflammatory cells in the lumen of the trachea (A). Hematoxylin & Eosin staining reveals the nuclei of inflammatory cells and lymphocytic infiltrations. The lumen of the trachea in the experimental groups injected with GST-IκBα(ΔN)-MTS are less occluded and show less inflammatory cells. Re-epithelialization is more evident in the GST-IκBα(ΔN)-MTS trachea versus the GST-MTS and GST-IκBα(ΔN) tracheas at day 14. (B). Western blot measuring TGF- β in trachea. TGF- β plays a key role in the immune response that culminates in the production of collagen, scarring, and ultimately fibrosis. Without TGF- β acting as part of the inflammatory cascade, fibrosis will not occur. TGF- β levels were significantly lower in the mice that received the NF-κB inhibitor.
NF-κB inhibition downregulates TGF-β expression in chronic allograft rejection
The translocation of the NF-κB (p50/p65) transcription factor into the nucleus regulates the downstream responses responsible for the fibroproliferation and remodeling characteristic of experimental early airway obstruction. In order to show that these key downstream responses to NF-κB activation were inhibited, we performed Western blotting of whole cell lysates of tracheal allografts and isografts. Allografts harvested from mice treated with the control proteins GST-MTS and GST-IκBα(ΔN) show TGF-β expression similar to allografts treated with no protein. Allografts harvested from mice treated with GST-IκBα(ΔN)-MTS protein show a decrease in the expression of TGF-β in the day 7 and day 14 groups as shown in figure 6B.
NF-κB inhibition reduces collagen deposition and luminal occlusion
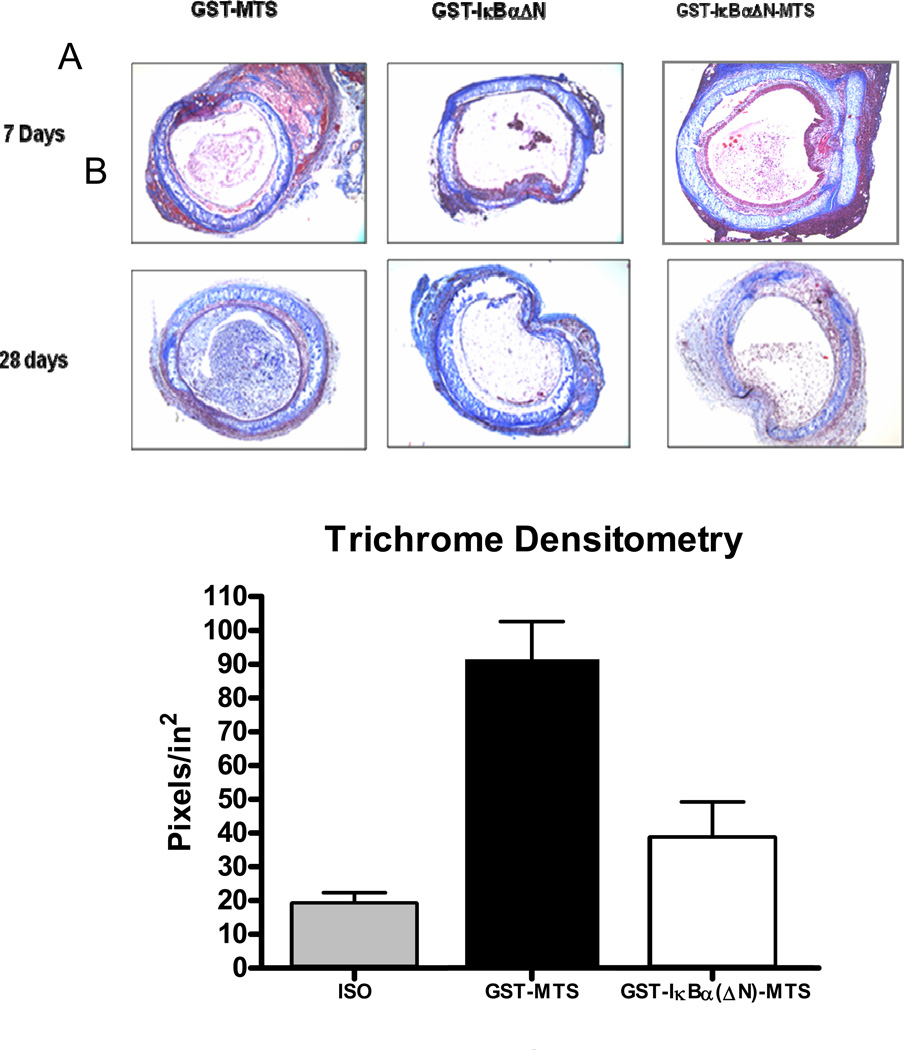
Fibrosis resulting from chronic tissue remodeling of the lumen of tracheal allografts causes occlusion of the airway. Collagen is the major component of fibrotic and matrix deposition in the lumen in tracheal allograft rejection. To identify collagen fibers (blue), sections from allografts harvested at day 7, 14, and 28 were stained with Masson Trichrome as shown in Figure 7A. Allografts harvested at day 7 show little collagen deposition as the process of occlusion remains in its early acute inflammatory episodes. In contrast, allografts from day 14 and day 28 showed a disparity in airway preservation among the groups. By day 14, the progression of AO causes fibrosis in the airway of allografts harvested from mice treated with GST-MTS control protein. Similar findings were observed in allografts harvested at day 14 from mice treated with GST-IκBα(ΔN), however, there was a reduction in collagen deposition as compared to allografts from mice receiving GST-MTS control protein. Allografts harvested at both day 14 and day 28 from mice receiving GST-IκBα(ΔN)-MTS showed a significant reduction in luminal occlusion as compared with sections from allografts from mice receiving GST-MTS and GST-IκBα(ΔN) control proteins. To quantify these findings, we averaged all shades of blue and displayed the photomicrographs as black. The non-luminal portions were subtracted (epithelium, lamina propria, cartilage), and the transformed images were imported into the Scion Image program, which allows for densitometry. As demonstrated in Figure 7B, the amount of fibrosis in tracheas harvested at day 28 from mice receiving GST-IκBα(ΔN)-MTS, indicated by blue-stained collagen, was reduced by greater than 40% compared to allografts harvested at day 28 from mice receiving GST-MTS or GST-IκBα(ΔN)-MTS control proteins.
Figure 7.
Collagen deposition in the tracheal lumen. (A). Masson Trichrome staining indicates collagen production as a blue color. While the trachea in both the acute and chronic models show collagen deposition, the GST-MTS and GST-IκBα(ΔN) mice in the chronic model shows much greater intra-luminal collagen deposition than the GST-IκBα(ΔN)-MTS mice. (B). Densitometry quantifies the color blue in the lumen of each photomicrograph
IκBα(ΔN)-MTS treatment Inhibits NF-κB translocation in the HTT murine model
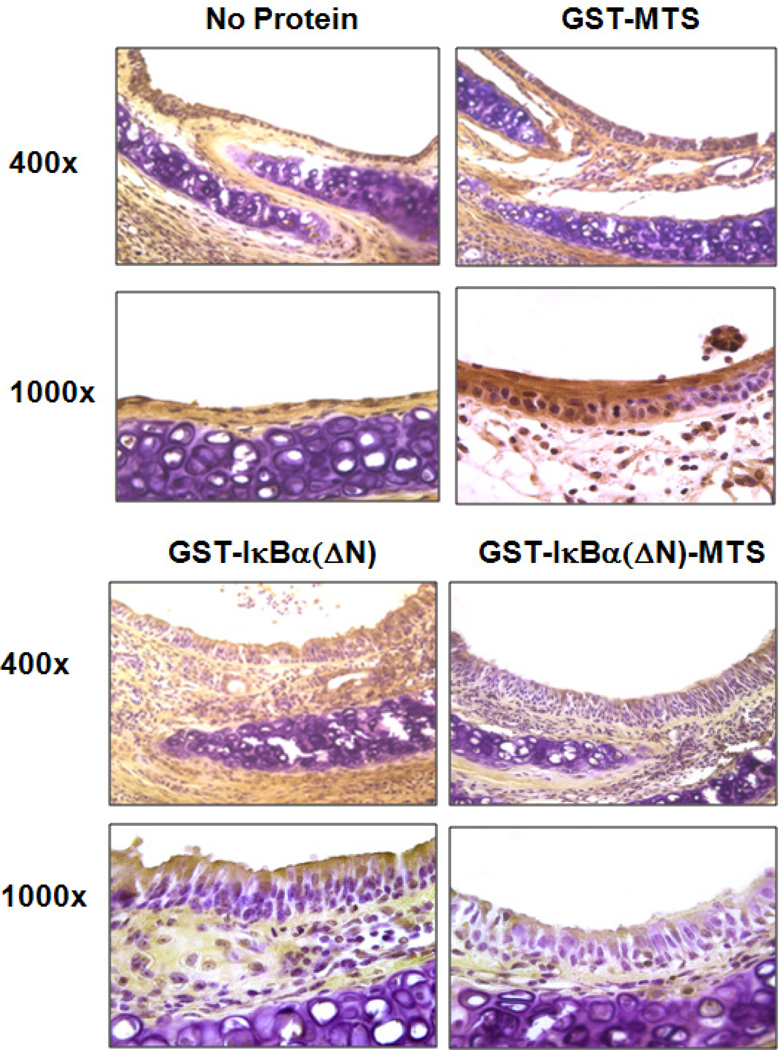
As stated earlier, NF-κB activation regulates several pro-inflammatory cytokines and fibroproliferative responses. We observed a downregulation of TGF-β in allografts receiving the cell permeable NF-κB inhibitor protein GST-IκBα(ΔN)-MTS. To confirm that the downregulation of TGF-β was a result of NF-κB inhibition upstream by GST-IκBα(ΔN)-MTS, we performed Immunohistochemistry for p65, part of the NF-κB p50/p65 heterodimer. The airway epithelium is the primary target of the alloimune response observed in murine chronic allograft rejection. Staining for p65 in allografts from mice receiving no protein shows nuclear translocation of p65 in epithelial cells. Similar phenomena were observed in sections of allografts from mice receiving GST-MTS control protein. However, some inhibition of nuclear translocation of p65 was shown in sections of allografts from mice receiving GST-IκBα(ΔN) control protein. This finding suggests that some GST-IκBα(ΔN) entered epithelial cells and blocked NF-κB activation. In contrast, sections from allografts from mice treated with the permeable inhibitor showed complete sequestration of NF-κB proteins to the cytoplasm as shown in Figure 8. P65 is shown to be highly expressed, yet remain in the cytoplasm. These results demonstrate that the delivered permeable protein inhibited translocation of the NF-κB complex from cytoplasm to nucleus. Indeed, these findings further confirm the epithelium’s role in directing the alloimmune response observed in early airway obstruction on the HTT murine model
Figure 8.
Immunohistochemistry for p65 protein Expression/Localization. The localization of the p65 (NF-κB) protein in the epithelium of day 7 tracheas shows p65 sequestration to the cytoplasm in mice receiving GST-IκBα(ΔN)-MTS. Tracheas harvested from control mice show greater p65 expression and nuclear localization
DISCUSSION
In this study we sought to investigate a potential therapeutic intervention to prevent the development of airway obstruction in a murine heterotopic model of lung transplant and to further characterize its mechanisms. Based on previous studies, we hypothesized that MSC would modulate the pro-fibrotic response of allogeneic transplant. We presumed this would be mediated through modulatory effects on TGF-β.
To demonstrate the impact of MSC on the development of AO we used the heterotopic tracheal transplantation model (HTT) as described by Hertz et al18 in which the transplantation of a trachea from a genetically discordant donor mouse into a subcutaneous pocket on the back of a recipient mouse resulted in rejection of the graft in a manner similar to that seen in human OB. Airway rejection in the HTT model is preceded by substantial peri-airway/sub-epithelial mononuclear cell infiltration similar to lymphocytic bronchiolitis that peaks between days 10–14 followed by lumen obliteration comparable to human OB by days 21–28. In contrast, genetically identical isografts have normal appearing airways with minimal luminal obstruction19.
Despite the many similarities between the morphological changes observed in the obstruction of the airways in the HTT model and human OB there some deficiencies in this mouse model. Most importantly, the transplanted trachea is not a functional airway and therefore may not adequately mimic the real-life milieu seen by transplanted human airways. Secondly, unlike a human transplanted lung, the mouse trachea has no primary blood supply which could significantly impact the immune response.
Our results show that significant airway occlusion can be observed as early as day 10 post-transplant. The material obstructing the airway consists largely of collagen. This response is likely mediated in large part by a rise in the expression of TGF-β in the lumen and its surrounding cells.
The injection of MSC at the time of transplant inhibited the development of significant airway occlusion, especially by collagen, as early as 10 days after transplant. There was also a relative preservation of the epithelial layer observed qualitatively in the MSC group as compared to the allograft control group. Injection of MSC also significantly reduced the observed rise in TGF-β seen in the allograft controls.
There was a significant rise in TGF-β expression in the allograft group compared to the isograft. This rise was blocked in the group where MSC were injected. We thus conclude that the inhibition of the fibroproliferative response underlying the development of OB in this model is mediated through modulation of TGF-β pathways in the airway.
We obtained MSC from three different strains of mice to determine if the strain of mice from which the cells were obtained impacted the results. When comparing the results from the three strains separately we found that the level of airway occlusion was comparable to the isograft group in all the MSC groups regardless of their original source.
To further characterize the pathways involved in experimental airway obstruction, we investigated the impact of an inhibitor of NF-κB. Numerous efforts have been made to develop regulators of NF-κB activity as a means of controlling the immune response. We have developed a dominant negative form of the cytoplasmic NF-κB binding protein IκBα, GST-IκBα(ΔN)-MTS, which can be delivered to cell cytoplasm using the membrane-translocating sequence (MTS). Our studies show that in vitro and in vivo administration of the permeable IκBα(ΔN) can inhibit nuclear translocation of NF-κB and prevent subsequent downstream responses. Thus, the objective was twofold; first, to block the NF-κB pathway in murine tracheal allografts, and second, to determine if inhibition of NF-κB inhibits AO. Amelioration of experimental AO in mice using this fusion protein points to a potential therapy for OB after lung transplantation.
Morphometric analysis, during airway occlusion, showed activation of NF-κB in the transplanted tracheal epithelium in control animals. Inhibition of NF-κB decreased translocation of p65, decreased accumulation of inflammatory cells in the sub-epithelium of the transplant and reduced expression of TGF-β.
In conclusion, this study shows that MSC inhibit the development of airway obstruction in a mouse model of HTT largely through modulation TGF pathways. Further NF-κB pathways are important in the development of AO and may be a crucial target of MSC modulation. This information adds to the growing body of knowledge of the pathogenesis of OB in lung transplant and raises the possibility of the future utility of MSC in the prevention of this devastating complication of lung transplant.
Acknowledgments
Disclosure Statement
This research was supported by grant number 5K01HL084683-02 from the National Heart Lung and Blood Institute, a grant from the American Federation for Aging Research, Emory University URC #2003100 and the McKelvey Center for Lung Transplantation at Emory University. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose
References
- 1.The Organ Procurement and Transplant Network. Available at: http://www.optn.org/data. [Google Scholar]
- 2.Christie JD, Edwards LB, Aurora P, et al. Registry of the international society for heart and lung transplantation: twenty-fifth official adult lung and heart/lung transplantation report—2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 3.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proc Am Thorac Soc. 2007;4:37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicod LP. Mechanisms of airway obliteration after lung transplantation. Proc Am Thorac Soc. 2006;3:444–449. doi: 10.1513/pats.200601-007AW. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 6.Iyer SS, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Medica. 2009;51:5–16. [PubMed] [Google Scholar]
- 7.Ortiz LA. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theise N, Henagariu O, Grove J, Jagirdar J, Koa P, Crawford J, Badve S, Saxena R, Krause D. Radiation pneumonitis in mice: A severe injury model or pneumocyte engraftement from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 9.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay M. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 12.Mora AL, LaVoy J, McKean M, Stecenko A, Brigham KL, Parker R, Rojas M. Prevention of NF-κB Activation In vivo by a Cell Permeable NF-κB Inhibitor Peptide. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L536–L544. doi: 10.1152/ajplung.00164.2005. [DOI] [PubMed] [Google Scholar]
- 13.Rojas M, Donahue J, Tan Z, Lin Y-Z. Genetic engineering of proteins with cell membrane permeability. Nature Biotechnology. 1998;26:370–376. doi: 10.1038/nbt0498-370. [DOI] [PubMed] [Google Scholar]
- 14.Kelly KE, Hertz MI, Mueller DL. T-cell and major histocompatiblity complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation. 1998;66:764–771. doi: 10.1097/00007890-199809270-00011. [DOI] [PubMed] [Google Scholar]
- 15.Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized Antigens Must Be Removed to Prepare Hypoimmunogenic Mesenchymal Stem Cells for Cell and Gene Therapy. Molecular Therapy. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 17.Farviar AS, Woolley SM, Naidu BF, et al. Roly (ADP) ribose synthetase inhibition reduces obliterative airway disease in rat tracheal allografts. J Heart Lung Transplant. 2004;23:993–1002. doi: 10.1016/j.healun.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. Am J Pathol. 1993;142:1945–1951. [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Torres E, Mora AL, Shim H, Ramirez A, Neujahr D, Brigham KL, Rojas M. Attenuation of obliterative bronchiolitis by a CXCR4 antagonist in the murine heterotopic tracheal transplant model. J Heart Lung Trasnplantation. 2008;27:1302–1310. doi: 10.1016/j.healun.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]