Summary
We have developed an integrated approach that combines empirical and computational methodologies to define an individual’s thrombin phenotype. We have evaluated the process of thrombin generation in healthy individuals and individuals with defined pathologies (hemophilia A and acute coronary syndrome) in order to develop general criteria relevant to assess an individual’s propensity for hemorrhage or thrombosis. Three complementary hypotheses have emerged from our work: 1) compensation by the ensemble of other coagulation proteins in individuals with specific factor deficiencies can “normalize” an individual’s thrombin generation process and represents a rationale for their unexpected phenotype; 2) individuals with clinically unremarkable factor levels may present thrombin generation profiles typical of individuals with hemostatic complications; and 3) in some hemostatic disorders a specific pattern of expression of a small ensemble of coagulation factors may be sufficient to explain the overall phenotype.
Introduction
Virchow’s triad[1] describes the factors influencing thrombosis: the vessel wall, blood and its flow. Of these, blood has been shown to be a clinical predictor of human health and disease. The coagulation and fibrinolytic systems are composed of a complex array of pro- and anti-coagulant and fibrinolytic components that maintain the balance of blood fluidity and vessel integrity. Numerous investigators over the past century have provided descriptions of the components associated with hemostasis (review see[2]). Extensive biochemical analyses, especially over the past forty years, have provided detailed molecular descriptions of the individual processes leading to thrombin generation, clot formation and vessel wall repair. Hemorrhage control begins with the exposure of blood to extravascular tissue factor (Tf) initiating a series of proteolytic reactions, formations of complex catalysts and cellular activations leading to the local formation of thrombin and the deposition of a fibrin platelet clot.
Qualitative or quantitative alterations in this hemostatic balance can result in hemorrhagic[3,4] or thrombotic diseases [5,6]. Even with current clinical screening techniques and available methodologies, high risk individuals are not easily recognized and events not accurately predicted [7]. One of the main reasons that this continues to be the case is that the vast majority of patients who suffer from hemostatic defects, but without obvious genetic defects have blood coagulation systems that are not clinically identified as abnormal by routine screening tools and factor assays. Identification of individuals who are at risk for hemorrhage or thrombosis is an area of research that could benefit from innovative technical methods.
Thrombin as a phenotypic discriminator
Background
Thrombin has long been recognized for its multiple functions in blood coagulation and platelet aggregation as well as its roles in tissue repair, development and pathogenic processes[8,9]. Thrombin has direct effects on coagulation through activation of platelets, formation and crosslinking of fibrin, and activation of various zymogens and cofactors in the coagulation cascade. Thrombin activity extends from the coagulation process, to anticoagulation and stimulation of fibrinolytic reactions and to effects on cell recruitment and proliferation essential for repair.
Our goal is to investigate how the process of thrombin formation relates to hemorrhagic and thrombotic disorders utilizing new approaches. Whether the balance of these thrombin functions in any individual can be related to their propensity for disease has not been clearly established. The need for methods that yield a more comprehensive description of thrombin generation has been generally recognized and a number of approaches have come into wider use in recent years. Some of these have shown promise in discriminating among major defects[10-15]. However, results have been mixed regarding the ability of these more robust thrombin assays to evaluate phenotype[16,17]. Interpretation of some studies has been difficult because of controversy regarding the impact of parameters such as contact pathway activation, calcium chelation and dilution on the assessment of the biological material used in different assays[18-21].
To address this point we have been developing an intergrated approach that combines empirical and computational methodologies to define an individual’s thrombin phenotype. We have focused on a systematic analysis of variations in the process of thrombin generation among individuals with specific pathologies inorder to develop general criteria relevant to assessing any individual’s propensity for hemorrhage or thrombosis.
Methods
The foundation of our computational modeling efforts has been its ability to adequately describe biochemical events in two closed empirical systems: a well-defined synthetic coagulation proteome and minimally altered phlebotomy whole blood. The symbiosis between these methods is essential.
Empirical
Two empirical methods are utilized to evaluate Tf-initiated coagulation: 1) a para vivo whole blood model in which the primary Tf pathway of coagulation is visualized without the interference of the contact pathway through the use of corn trypsin inhibitor (CTI), a suppressor of fXIIa. Whole blood is obtained by phlebotomy, maintained in CTI at 37°C and induced to clot by the addition of a fixed amount of membrane relipidated Tf [22,23]; and 2) a synthetic coagulation proteome model in which all of the procoagulant vitamin K dependent proteins (fVII/VIIa, fIX, fX, fII), their cofactors (fV and fVIII), the stoichiometric inhibitors (antithrombin (AT) and Tf pathway inhibitor (TFPI)) and a membrane source of either natural (i.e. platelets) or synthetic membranes (i.e. phosphatidylcholine/phosphatidylserine)[24] is initiated with a Tf source, most commonly a relipidated Tf reagent.
Computational
The reactions of Tf-initiated coagulation in a closed system are described using classical mechanistic based second order equations [25]. These time-based ordinary differential equations in the deterministic form are coupled via their shared species yielding a reaction network. The eight protein concentration inputs required to run the model (fII, fV, fVII, fVIII, fIX, fX, AT and TFPI) reflect either: 1) mean physiologic concentrations; or 2) an individual’s actual protein concentrations (which are routinely measured in clinical laboratories). FVIIa is estimated as 0.1% of the fVII concentration. Simulations are initiated with 5pM Tf, which is chosen based on empirical experiments that give a clot time or thrombin generation propagation phase onset of ~4 minutes. Overall, time courses are produced for 34 species representing reactants, intermediates or products. Simulation outputs for any given species are evaluated by summary measures that describe each curve, for example: the maximum level obtained, the time to the maximum level obtained, maximum rate generated and the area under the curve [26]. Specifically for thrombin profiles, we also determine the time to 2nM thrombin, which corresponds to the clot time in our empirical studies.
Results
Thrombin generation and pathology
Many pathologies of hemostasis appear to be multicausal in origin. In individuals with obviously compromised hemostatic systems, specific blood coagulation factor levels have been identified either signally or in combination as risk factors [27-31]. An integrative approach that applies insights gained from observing extremes of hemostatic function to individuals with less exaggerated alterations in a specific factor or ensemble of factors has been lacking. Mathematical modeling based on an individual’s blood composition and on biochemical mechanisms of blood coagulation potentially represents such an approach.
Utilizing our empirical and computational approaches, we evaluated healthy individuals and individuals with defined pathologies (hemophilia A and acute coronary syndrome).
“Apparently Healthy Populations”
When we looked at empirical thrombin generation in 13 healthy individuals over a 6-month time frame we determined that Tf-initiated whole blood thrombin generation was phenotypically individualized [32]. Results showed that thrombin levels, measured as thrombin-antithrombin complex (TAT), varied between individuals (Coefficient of variation (mean/standard deviation), CVg=25.2%) but were fairly consistent within individuals (CVi=11.6%)[32]. The results show that intrinsic to an individual’s blood, there is a defined propensity to respond with a characteristic level of thrombin for a constant Tf stimulus. Levels ranged from 270.6nM (SD19.6) TAT at 20 min (Subject 10) to 629.6nM (SD80.8) TAT at 20 min (Subject 6).
When empirical thrombin generation is compared with simulated thrombin generation by protein factor composition from three of these individuals, a general correspondence was observed between the magnitude of the empirical marker (whole blood TAT) for each individual and their respective numerical parameter (maximum level of thrombin)[32]. These data indicate that changes within the protein composition of these individuals, although all within the normal range, shift the thrombin generation curves to give an individualized effect. Overall, our empirical and computational assessments similarly discriminate among healthy individuals.
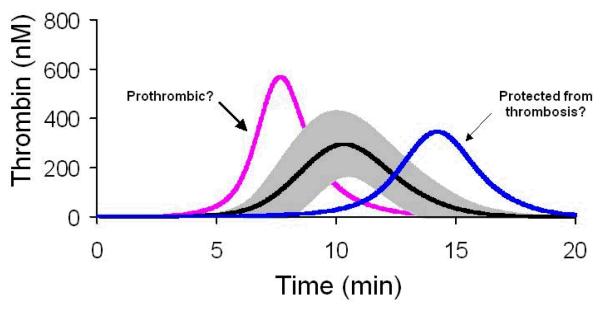
We then modeled thrombin generation in 473 healthy individuals using their individual factor levels[33]. When all of the individualized thrombin generation curves were grouped, the standard deviation of the curve was approximately 50% of the mean (Figure 2). The origins of this variability could not be reduced to one factor exerting a disproportionate effect on thrombin generation, implying that all of the pro- and anti-coagulant proteins act together. A further evaluation of defined thrombin generation subpopulations (n=13/group) based upon the time to reach maximum levels of thrombin, identified two groups that deviated by ~2 SD from the mean (Figure 2)[33].
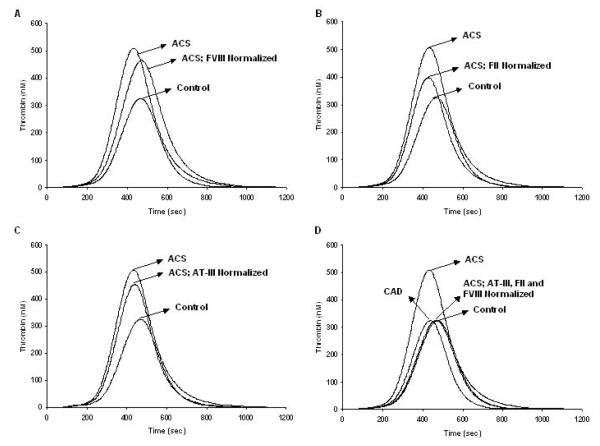
Figure 2. Thrombin generation in acute and stable coronary disease: normalization of the acute population.
Within the acute population, fVIII, prothrombin and AT were normalized by setting their factor levels to mean physiologic concentration (fVIII: 0.7nM (panel A), prothrombin: 1.4μM (panel B), AT: 3.4μM (panel C)). The normalized thrombin generation profile from the acute group is compared to the control (all factor levels at mean physiologic concentration). Panel D shows the thrombin generation output when all three factors (fVIII, prothrombin and AT) are normalized together and compared to the control and the stable group[36].
These analyses suggest that: 1) individuals can be distinguished by their thrombin generation parameters; 2) many coagulation factors contribute to the individualized thrombin generation profiles; and 3) embedded in the healthy population are individuals whose thrombin parameters are suggestive of altered states of hemostasis.
Thrombin generation in acute coronary syndrome
Coronary artery disease (CAD) is a leading cause of death for both men and women in the industrialized world. Thrombus formation following plaque rupture is the trigger for abrupt coronary artery occlusion and subsequent acute coronary syndrome (ACS)[34]. Therapies directed at reducing thrombin generation, using antiplatelet and anticoagulant regimens have become the mainstays of treatment for both acute and stable coronary disease[35].
We investigated whether computational thrombin generation profiles, dependent upon the composition of the major coagulation proteome proteins, can discriminate between individuals with acute and stable CAD[36]. Plasma coagulation factor compositions from 28 patients with acute disease were obtained after onset of chest pain. Similar data were obtained from 25 age- and sex-matched patients with stable coronary disease (>50% stenosis in at least one major coronary artery). All coagulation factors were in the clinically accepted normal range. When simulated thrombin generation profiles from individuals in each group were compared, maximum thrombin levels (p<0.01) and rates (p<0.01) were 50% higher in patients with acute syndromes than in those with stable disease while the initiation phases of thrombin generation were shorter.
To verify these results in an empirical system, the mean factor concentration of the patients were used in a synthetic coagulation proteome experiment [36]. These results show that the numerically derived thrombin generation profiles mimic the empirical results.
We also evaluated the contribution of each individual factor to the overall thrombin generation profile of the ACS population[36]. The goal was to identify those protein(s) which account for the difference in thrombin generation profiles between the two groups with coronary disease versus a physiologic control. These analyses indicated that fVIII, AT and prothrombin individually caused the largest change to the thrombin generation profile, however none by itself was sufficient to restore thrombin to a control level or even that of patients with stable coronary disease (Figure 2, panels A-C). Continuing this procedure by normalizing groups of factors simultaneously, we determined that the combination of fVIII, AT and prothrombin produced a thrombin profile that was congruent to our control (Figure 2, panel D).
Overall, this study suggests that the integration of blood composition data into an assessment of thrombin generation potential can discriminate between acute and stable coronary disease. Since it is well established that after an acute event all kinds of inflammatory and acute phase reactions occur, one can not extrapolate these findings to the situation just before the acute event. If it can be shown that changes in blood composition precede the onset of acute events, this approach could be useful as a diagnostic marker of prothrombotic risk.
Hemophilia A
Hemophilia A, an X-linked disease, is characterized by the decrease or absence of functional fVIII with an estimated prevalence of 1 in 10,000 males[4]. It is one of the most extensively studied hemorrhagic disorders, from clinical observations and treatment regimens[37], ranging from biochemical and molecular analyses[38] to investigative gene therapy[39-42]. Hemophilia A individuals have a wide spectrum of “normal” factor levels. From a literature review on the treatment of hemophilia individuals with fVIII concentrates versus clinical manifestations, one finds that in general, 30-50% of normal fVIII levels are required for treatment of most bleeding episodes of less severity, with one and occasionally two doses to control bleeding and prevent secondary hemorrhage[43]. Between 50-100% of fVIII activity levels are necessary to treat and prevent life-threatening hemorrhage or surgical bleeding. In fact, it is estimated that 10% of severe hemophilia A patients (≤1% fVIII) have only mild bleeding diathesis despite the biochemically undetectable levels of fVIII[44,45]. Thus, hemophilia A displays phenotypic heterogeneity with respect to clinical severity.
Thrombin generation and bleeding in hemophilia A
Using our CTI-inhibited whole blood model, we studied 11 mild (6-40%fVIII:C), 4 moderate (2-5% fVIII:C) and 12 severe (≤1%fVIII:C) fVIII deficient individuals with a well-characterized five-year bleeding history that included hemarthrosis, soft tissue hematoma and annual fVIII concentrate usage[46]. This clinical information was used to generate a bleeding score separated into three groups and compared to thrombin generation outputs. Our results showed that MaxL was significantly different (p<0.001) between the groups: 504nM (SD114), 315nM (SD117) and 194nM (SD91); with higher thrombin concentrations in the groups with lower bleeding scores. This empirical study in CTI-inhibited whole blood showed that thrombin generation appears to be associated with the bleeding phenotype of hemophilia A.
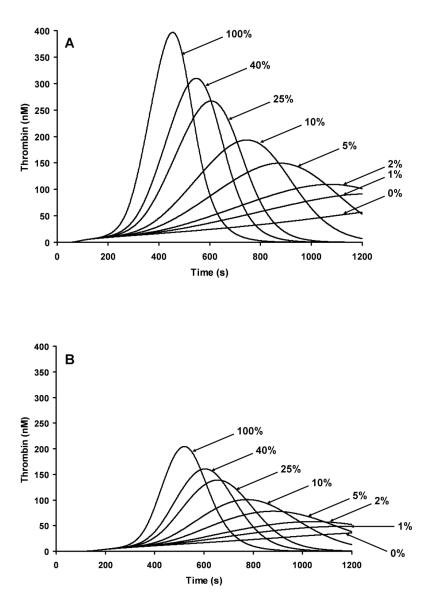
Since levels of thrombin generation in the empirical model have been shown to be related to overall factor levels, it would suggest that the bleeding phenotype of hemophilia A is also influenced by each individual’s ensemble of clotting factors. Therefore, we carried out a hypothetical analysis of the influence of factor levels by varying fVIII on thrombin generation. We modeled a fVIII titration of 0,1,2,5,10,25,40 and 100% fVIII, with all other factor values reflecting either clinically accepted high or low normal values for each procoagulant and anticoagulant. In Figure 3, panel A factor levels represent the high extreme of the normal range and panel B represents the low extreme of the normal range for each factor. For example when a fVIII deficient individual has 5% fVIII, if their other factor levels are at the high extreme of the normal range, they will be able to generate thrombin faster and reach maximum levels quicker than a 5% fVIII individual with their other factor levels at the low extreme of the normal range. This result suggests a rationale for why hemophiliacs with the same fVIII level, whether of therapeutic or natural origin, can differ hemostatically.
Figure 3. Factor VIII titration using numerical simulations at a 5pM Tf stimulus.
A fVIII titration of 0, 1, 2, 5, 10, 25, 40 and 100% fVIII are illustrated for individuals having their other factor levels (fII, fV, fVII, fIX, fX, AT and TFPI) set to the high range of normal (Panel A) or to the low range of normal (Panel B).
Conclusion
There is a growing consensus that global assessments of blood coagulation performance are essential to understanding individual variability. The limitation of empirical approaches in general is that while one can get a significant insight into an individual’s coagulant response via blood or plasma sample, the mechanism behind that individual response is not elucidated by these methods. Using a combination of empirical methods and a computational approach based upon a mechanistic description of Tf-initiated coagulation allows one to explore potential explanations for the observed variability among individuals.
The central idea is that in any individual, procoagulant and anticoagulant factor levels act together to generate a unique coagulation phenotype represented by their thrombin generation profile. Three complementary hypotheses have emerged from our work: 1) compensation by the ensemble of other coagulation proteins in individuals with specific factor deficiencies can “normalize” an individual’s thrombin generation process and that this effect represents a rationale for their unexpected phenotype; 2) individuals with clinically unremarkable factor levels may present thrombin generation profiles typical of individuals with hemostatic complications; and 3) in some hemostatic disorders a specific pattern of expression of a small ensemble of coagulation factors may be sufficient to explain the overall phenotype.
Figure 1.
Computational models of thrombin profiles from a healthy population shown as the mean±SD in grey (n=473)[33].
References
- (1).Dickson BC. Venous thrombosis:On the history of Virchow’s triad. University of Toronto Medical Journal. 2004;81:166–171. [Google Scholar]
- (2).Brummel-Ziedins K, Orfeo T, Jenny NS, Everse SJ, Mann KG. Blood coagulation and fibrinolysis. In: Greer JP, Foerster J, Lukens J, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe’s Clinical Hematology. Lippencott Williams & Wilkins; Philidelphia: 2003. pp. 677–774. [Google Scholar]
- (3).Mannucci PM. Tuddenham EGD. The hemophiliacs-from royal genes to gene therapy. N Engl J Med. 2001;344:1733–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- (4).Hoyer LW, Hemophilia A. N Engl J Med. 1994;330:38–47. doi: 10.1056/NEJM199401063300108. [DOI] [PubMed] [Google Scholar]
- (5).Reitsma PH. Protein C deficiency: from gene defects to disease. Thromb Haemost. 1997;78:344–350. [PubMed] [Google Scholar]
- (6).Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- (7).White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- (8).Davie EW, Kulman JD. An overview of the structure and function of thrombin. Semin Thromb Hemost. 2006;32(Suppl 1):3–15. doi: 10.1055/s-2006-939550. [DOI] [PubMed] [Google Scholar]
- (9).Jenny NS, Mann KG. Thrombin. In: Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Hemostasis and Thrombosis; Basic Principles & Clinical Practice. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 171–189. [Google Scholar]
- (10).Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, Lecompte T, Beguin S. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–253. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- (11).Sorensen B, Ingerslev J. Whole blood clot formation phenotypes in hemophilia A and rare coagulation disorders. Patterns of response to recombinant factor VIIa. J Thromb Haemost. 2004;2:102–110. doi: 10.1111/j.1538-7836.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- (12).Brummel-Ziedins K, Rivard GE, Pouliot RL, Butenas S, Gissel M, Parhami-Seren B, Mann KG. Factor VIIa replacement therapy in factor VII deficiency. J Thromb Haemost. 2004;2:1735–1744. doi: 10.1111/j.1538-7836.2004.00922.x. [DOI] [PubMed] [Google Scholar]
- (13).Dargaud Y, Luddington R, Baglin T. Platelet-dependent thrombography: a method for diagnostic laboratories. Br J Haematol. 2006;134:323–325. doi: 10.1111/j.1365-2141.2006.06188.x. [DOI] [PubMed] [Google Scholar]
- (14).Wolberg AS, Allen GA, Monroe DM, Hedner U, Roberts HR, Hoffman M. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. Br J Haematol. 2005;131:645–655. doi: 10.1111/j.1365-2141.2005.05820.x. [DOI] [PubMed] [Google Scholar]
- (15).Regnault V, Hemker HC, Wahl D, Lecompte T. Phenotyping the haemostatic system by thrombography--potential for the estimation of thrombotic risk. Thromb Res. 2004;114:539–545. doi: 10.1016/j.thromres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- (16).Beltran-Miranda CP, Khan A, Jaloma-Cruz AR, Laffan MA. Thrombin generation and phenotypic correlation in haemophilia A. Haemophilia. 2005;11:326–334. doi: 10.1111/j.1365-2516.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- (17).Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- (18).Mann KG, Whelihan MF, Butenas S, Orfeo T. Citrate anticoagulation and the dynamics of thrombin generation. J Thromb Haemost. 2007;5:2055–2061. doi: 10.1111/j.1538-7836.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- (19).DE Smedt E, Wagenvoord R, Coen HH. The technique of measuring thrombin generation with fluorogenic substrates: 3. The effects of sample dilution. Thromb Haemost. 2009;101:165–170. [PubMed] [Google Scholar]
- (20).Brummel-Ziedins K, Whelihan MF, Ziedins EG, Mann KG. The resuscitative fluid you choose may potentiate bleeding. J Trauma. 2006;61:1350–1358. doi: 10.1097/01.ta.0000235525.64176.01. [DOI] [PubMed] [Google Scholar]
- (21).Luddington R, Baglin T. Clinical measurement of thrombin generation by calibrated automated thrombography requires contact factor inhibition. J Thromb Haemost. 2004;2:1954–1959. doi: 10.1111/j.1538-7836.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- (22).Rand MD, Lock JB, van ’t Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–3445. [PubMed] [Google Scholar]
- (23).Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- (24).Butenas S, Brummel KE, Branda RF, Paradis SG, Mann KG. Mechanism of factor VIIa-dependent coagulation in hemophilia blood. Blood. 2002;99:923–930. doi: 10.1182/blood.v99.3.923. [DOI] [PubMed] [Google Scholar]
- (25).Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- (26).Brummel-Ziedins KE, Vossen CY, Butenas S, Mann KG, Rosendaal FR. Thrombin generation profiles in deep venous thrombosis. Journal of Thrombosis and Haemostasis. 2005;3:2497–2505. doi: 10.1111/j.1538-7836.2005.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).van Boven HH, Lane DA. Antithrombin and its inherited deficiency states. Semin Hematol. 1997;34:188–204. [PubMed] [Google Scholar]
- (28).Lu D, Bovill EG, Long GL. Molecular mechanism for familial protein C deficiency and thrombosis in protein C Vermont (Glu20-->Ala and Val34-->Met) J Biol Chem. 1994;269:29032–29038. [PubMed] [Google Scholar]
- (29).Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- (30).van Hylckama V, van der Linden I, Bertina RM, Rosendaal FR. High levels of factor IX increase the risk of venous thrombosis. Blood. 2000;95:3678–3682. [PubMed] [Google Scholar]
- (31).Lavigne G, Mercier E, Quere I, Dauzat M, Gris JC. Thrombophilic families with inheritably associated high levels of coagulation factors VIII, IX and XI. J Thromb Haemost. 2003;1:2134–2139. doi: 10.1046/j.1538-7836.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- (32).Brummel-Ziedins KE, Pouliot RL, Mann KG. Thrombin generation: phenotypic quantitation. J Thromb Haemost. 2004;2:281–288. doi: 10.1046/j.1538-7933.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- (33).Brummel-Ziedins K, Vossen CY, Rosendaal FR, Umezaki K, Mann KG. The plasma hemostatic proteome: thrombin generation in healthy individuals. J Thromb Haemost. 2005;3:1472–1481. doi: 10.1111/j.1538-7836.2005.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- (35).Harrington RA, Becker RC, Ezekowitz M, Meade TW, O’Connor CM, Vorchheimer DA, Guyatt GH. Antithrombotic therapy for coronary artery disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:513S–548S. doi: 10.1378/chest.126.3_suppl.513S. [DOI] [PubMed] [Google Scholar]
- (36).Brummel-Ziedins K, Undas A, Orfeo T, Gissel M, Butenas S, Zmudka K, Mann KG. Thrombin generation in acute coronary syndrome and stable coronary artery disease: dependence on plasma factor composition. J Thromb Haemost. 2008;6:104–110. doi: 10.1111/j.1538-7836.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- (37).Mannucci PM. Hemophilia: treatment options in the twenty-first century. J Thromb Haemost. 2003;1:1349–1355. doi: 10.1046/j.1538-7836.2003.00262.x. [DOI] [PubMed] [Google Scholar]
- (38).Goodeve AC, Peake IR. The molecular basis of hemophilia A: genotype-phenotype relationships and inhibitor development. Semin Thromb Haemost. 2003;29:23–30. doi: 10.1055/s-2003-37936. [DOI] [PubMed] [Google Scholar]
- (39).High KA. Gene transfer as an approach to treating hemophilia. Semin Thromb Haemost. 2003;29:107–120. doi: 10.1055/s-2003-37945. [DOI] [PubMed] [Google Scholar]
- (40).Lillicrap D. Gene expression: overview and clinical implications. Vox Sang. 2002;83(Suppl 1):77–79. doi: 10.1111/j.1423-0410.2002.tb05272.x. [DOI] [PubMed] [Google Scholar]
- (41).VandenDriessche T, Collen D, Chuah MK. Viral vector-mediated gene therapy for hemophilia. Curr Gene Ther. 2001;1:301–315. doi: 10.2174/1566523013348508. [DOI] [PubMed] [Google Scholar]
- (42).VandenDriessche T, Collen D, Chuah KL. Gene therapy for the hemophilias. J Thromb Haemost. 2003;1:1550–1558. doi: 10.1046/j.1538-7836.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- (43).Arun B, Kessler CM. Clinical manifestations and therapy of the hemophilias. In: Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Hemostasis and Thrombosis; Basic Principles & Clinical Practice. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 815–824. [Google Scholar]
- (44).Aledort L. Why thrombin generation? From bench to bedside. Pathophysiol Haemost Thromb. 2003;33:2–3. doi: 10.1159/000071635. [DOI] [PubMed] [Google Scholar]
- (45).Ahlberg A. Haemophilia in Sweden.VII. Incidence, treatment, and prophylaxis of arthropathy and other musculoskeletal manifestations of hemophilia A and B. Acta Orthop Scand. 1965;77:3–132. doi: 10.3109/ort.1965.36.suppl-77.01. [DOI] [PubMed] [Google Scholar]
- (46).Brummel-Ziedins KE, Whelihan MF, Gissel M, Mann KG, Rivard GE. Thrombin generation and bleeding in hemophilia A. Haemophilia. 2009 doi: 10.1111/j.1365-2516.2009.01994.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]