Abstract
The D2 dopamine receptor (D2R) is important in the pathogenesis of essential hypertension. We have already reported that systemic deletion of the D2R gene in mice results in reactive oxygen species (ROS)-dependent hypertension, suggesting that the D2R has antioxidant effect. However, the mechanism of this effect is unknown. DJ-1 is a protein which has antioxidant properties. D2R and DJ-1 are expressed in the mouse kidney and colocalize and co-imunoprecipitate in mouse renal proximal tubule cells. We hypothesized that D2Rs regulate renal ROS production in the kidney through regulation of DJ-1 expression or function. Heterozygous D2+/− mice have increased blood pressure, urinary 8-isoprostanes, and renal Nox 4 expression, but decreased renal DJ-1 expression. Silencing D2R expression in mouse renal proximal tubule cells increases ROS production and decreases the expression of DJ-1. Conversely, treatment of these cells with a D2R agonist increases DJ-1 expression and decreases Nox 4 expression and NADPH oxidase activity, effects that are partially blocked by a D2R antagonist. Silencing DJ-1 expression in mouse renal proximal tubule cells increases ROS production and Nox 4 expression. Selective renal DJ-1 silencing by the subcapsular infusion of DJ-1 siRNA in mice increases blood pressure, and renal Nox 4 expression and NADPH oxidase activity. These results suggest that the inhibitory effects of D2R on renal ROS production are at least, in part, mediated by a positive regulation of DJ-1 expression/function and that DJ-1 may have a role in the prevention of hypertension associated with increased ROS production.
Keywords: DJ-1, oxidative stress, dopamine D2 receptor, kidney, hypertension
Introduction
Dopamine synthesized in the kidney has an important role in the regulation of fluid and electrolyte balance and systemic blood pressure (1-3). Dopamine exerts its actions via two families of G protein-couple receptors D1-like receptors (D1R and D5R) and D2–like receptors (D2R, D3R, and D4R). Several lines of evidence show that an intact dopaminergic system is necessary to maintain normal blood pressure and that genetic hypertension is associated with alterations in dopamine production and receptor function (1-4).
Deletion of any dopamine receptor in mice results in increased blood pressure by mechanisms that are receptor dependent. In particular, mice lacking the D2R gene have reactive oxygen species (ROS)-dependent hypertension (4). Moreover, dopamine and D2R agonists have been shown to have antioxidant activity (5, 6). D2R agonists have free radical scavenging and antioxidant activities both in vitro and in vivo (7,8). In vitro and in vivo studies have also shown that the protective effects of the D2R are abolished in the presence of D2R antagonists, indicating receptor specificity (9, 10). However the mechanisms involved in the antioxidant effects of the D2Rs are not known.
DJ-1 (also known as Park 7) is a protein originally described as an oncogene (11). It is present in most rodent and human tissues, such as the brain, heart, kidney, liver, pancreas, and skeletal muscle (11). DJ-1 was also identified as an autosomal recessive gene of Parkinson's disease. DJ-1 is a multifunctional oxidative stress response protein that defends cells against ROS and mitochondrial damage (12). Its protective role against oxidative stress has been demonstrated in several pathological disease models both in vitro and in vivo (13-16). However, the physiological role of the DJ-1 in the kidney is unknown.
We hypothesized that DJ-1 is involved in the antioxidant activity of renal D2R. In this study, we found that D2R physically interacts with DJ-1 and regulates the expression of the DJ-1 in the kidney. Our in vitro and in vivo studies show that the inhibitory effect of D2R on renal ROS production is at least, in part, mediated by regulating DJ-1 expression and function. These findings indicate an essential role of DJ-1 in the increase in blood pressure associated to oxidative stress.
Materials and methods
D2R-deficient Mice (D2+/−)
The original F2 hybrid strain (129/SvXC57BL/6J, Oregon Health Sciences University) that contained the mutated Drd2 allele (D2+/−) was backcrossed to wild-type C57BL/6J for >20 generations and genotyped. All mice were bred in the Animal Care Facility of the Children's Research Institute (CRI), Children's National Medical Center (CNMC). D2+/− mice and wild-type littermates (D2+/+) were studied at 6 to 8 months of age; D2+/− mice were used because similar to D2−/− mice they have high blood pressure (17) and increased oxidative stress but do not have increased aldosterone production as do D2−/−mice (4). We wanted to study the role of DJ-1 and D2R on renal oxidative stress without the confounding effect of increased aldosterone levels. All studies were approved by the Animal Care and Use Committee of the CRI/CNMC. Mice were housed in metabolic cages the day before blood pressure measurement for collection of 24-h urine samples. Systolic blood pressures were measured (Cardiomax II, Instruments) from the aorta, via the femoral artery, under pentobarbital anesthesia (50 mg/kg IP). Blood pressures were recorded 1 h after the induction of anesthesia and when the blood pressures were stable. The mice were euthanized (pentobarbital 100 mg/kg) at the conclusion of the study. The organs were harvested and flash- frozen, prior to their preparation for specific studies.
Acute renal-specific downregulation of DJ-1
Renal cortical DJ-1 was silenced by the subcapsular infusion of DJ-1-specific siRNA, via an osmotic minipump. In brief, adult male C57BL/6J mice were uninephrectomized one week prior to the implantation of osmotic minipumps. For implantation of the minipumps, the mice were anesthetized with pentobarbital (50 mg/kg body weight, intraperitoneally). Osmotic minipumps (ALZET® Osmotic Pump, 100 μl; flow rate 0.5 μl/hr for 7 days) were filled with previously validated DJ-1-specific siRNA (delivery rate 3 μg/day) or non-silencing siRNA as control. The siRNAs were dissolved in an in vivo transfection reagent (TransIT® In Vivo Gene Delivery System, Mirus) under sterile conditions. The minipumps were fitted with polyethylene delivery tubings (Alzet #0007701) and the tip of the tubing was inserted within the subcapsular space of the remaining kidney. Surgical glue was applied at the puncture site to hold the tube in place and prevent extrarenal leakage. The osmotic pump was sutured to the abdominal wall to prevent excessive movement of the pump.
Urinary isoprostane
Urinary 8-isoprostane, an index of oxidative stress, was determined by enzyme immunoassay (Cayman Chemical Company). Values were corrected for urinary creatinine.
Determination of NADPH oxidase activity
NADPH oxidase activity was determined by measuring NADPH-induced chemiluminescence in the presence of lucigenin (5 μmol/L, Invitrogen) and NADPH (100 mol/L, ICN Biomedicals) (18). The specificity of the NADPH-dependent superoxide anion production was verified by treatment with diphenylene iodinium (DPI, Sigma).
Cell culture
Undifferentiated mouse renal proximal tubule cells were cultured from progenitor kidney cells (kindly supplied by Dr Ulrich Hopfer, Case Western Reserve University, School of Medicine), isolated from mouse embryo kidneys, following the procedure described by Woost et al. (19). Differentiated cells were cultured to 60-70% confluence and transfected using Hyperfect (Qiagen,) with non-silencing siRNA (30 nmol/L, Qiagen) or Drd2 siRNA (30 nmol/l, Qiagen) and studied after 72 h. In additional experiments, cells cultured to 90-95 % confluence were serum-starved for 2 h and treated for 24 h with 1 μmol/L quinpirole (D2R/D3R agonist, Sigma-Aldrich), or 1 μmol/L quinpirole plus 1 μmol/L L-741,262 (selective D2R antagonist, Sigma-Aldrich)(20,21).
Immunofluorescence and confocal analysis
Thin sections (3 μm) of formalin-fixed paraffin-embedded mouse kidney were deparaffinized in xylene and rehydrated with step-down concentrations of ethanol. DJ-1 was visualized using a polyclonal mouse anti-DJ-1 antibody (Santa Cruz Biotech), followed by Alexa Fluor 488-goat anti-mouse IgG antibody (Molecular Probes). D2R was visualized using a polyclonal rabbit anti-D2R antibody (Millipore), followed by Alexa Fluor 568-goat anti-rabbit IgG antibody (Molecular Probes). As a negative control, the primary antibodies were replaced with normal rabbit serum at an appropriate dilution. Colocalization of D2R and DJ-1 was identified by the yellow color in the merged images
Immunoblotting
Mouse kidney homogenates and cell lysates were subjected to immunoblotting, as reported previously (4, 17, 18). The primary antibodies used were polyclonal rabbit anti-DJ-1 (NOVUS, #NB300-270), polyclonal rabbit anti-D2R (Millipore, #AB5084P), polyclonal rabbit anti-Nox4 (Epitomics, #3187-1) and monoclonal mouse anti-GAPDH (Millipore,#MAB374). The densitometry values were corrected by the expression of GAPDH.
Detection of ROS
Intracellular ROS were assayed through the oxidation of 2′, 7′-dichlorofluorescein diacetate (DCFDA, Molecular Probes). Briefly, cells were incubated with fresh DCFDA (10 μM) in medium for 30 min at 37°C. DCFDA fluorescence was measured using a microplate reader in 96-well plates at an excitation wavelength of 485 nm and emission wavelength of 530 nm. ROS production was expressed in arbitrary units (AU), corrected for protein concentration (AU/per mg protein). All assays were performed in duplicate.
Co-immunoprecipitation
Serum-starved mouse renal proximal tubule cells were lyzed using RIPA lysis buffer. Equal amounts of cell lysates (500 μg of protein) were mixed with polyclonal rabbit anti-D2R antibody (Millipore Laboratories), or normal rabbit IgG (Santa Cruz Biotechnology) as negative control, or polyclonal rabbit anti-DJ-1 antibody (Novus) as positive control. The immune complexes were pelleted out, and the bound proteins were eluted using 30 μl of Laemmli buffer. The samples were subjected to immunoblotting and probed with the rabbit anti-DJ-1 antibody.
Statistical Analysis
Data are mean ± SEM. Comparisons between two groups used the Student's t test. One-way ANOVA was followed by post-hoc analysis using the Holm-Sidak multiple comparison test to assess significant differences among three or more groups. P<0.05 was considered statistically significant.
Results
DJ-1 is expressed in the mouse kidney and physically interacts with D2R
DJ-1 is expressed in the mouse kidney, as shown by immunofluorescence staining and a specific band in western blots (Figures 1 and 2). In the mouse renal cortex, DJ-1 is expressed mainly in the brush border and the cytosol of proximal tubule cells with minimal expression in distal tubule cells. In the proximal tubule, DJ-1 partially colocalizes with the D2R (Figure 1A). Furthermore, DJ-1 and D2R physically interact in the mouse kidney, as shown by the bands corresponding to the molecular size of DJ-1 in the mouse kidney samples after immunoprecipitation with an anti-D2R antibody and immunoblotting with an anti-DJ-1 antibody (Figure 1B).
Figure 1. Colocalization and co-inmunoprecipitation of D2R and DJ-1 in mouse kidney.
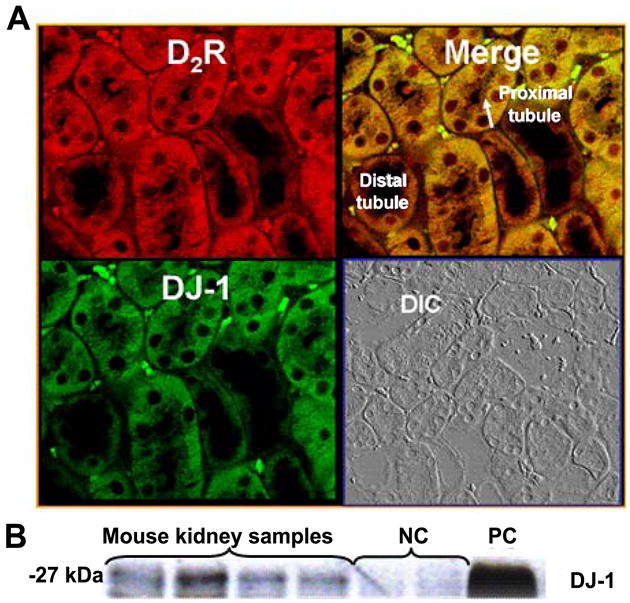
(A) Formalin-fixed, paraffin-embedded kidney sections of mouse were prepared to determine in vivo colocalization of D2R (pseudocolored red) and DJ-1 (pseudocolored green) by confocal microscopy. Differential interference contrast (DIC) images were also obtained to show cell integrity and boundaries. The arrow indicates the brush border membrane. The colocalization of D2R and DJ-1 (shown as yellow in merge images) was found in the cytoplasm and brush border of proximal tubules. Scale bar, 10 μm, ×600 magnification, n = 3–5 independent experiments.
(B) The D2R and DJ-1 physically interact in mouse kidney. Total mouse kidney lysates were immunoprecipitated with anti-D2R antibody and immunoblotted with anti-DJ-1 antibody. Negative control (NC), immunoprecipitant is rat IgG and positive control (PC); immunoprecipitant is anti-DJ-1 antibody. The bands that appear in the mouse kidney samples show physical interaction between D2R and DJ-1.
Figure 2. Expression of renal D2R, systolic blood pressure, renal DJ-1 expression, urinary excretion of 8-isoprostane, and renal Nox4 expression in D2+/− mice and their wild-type littermates.
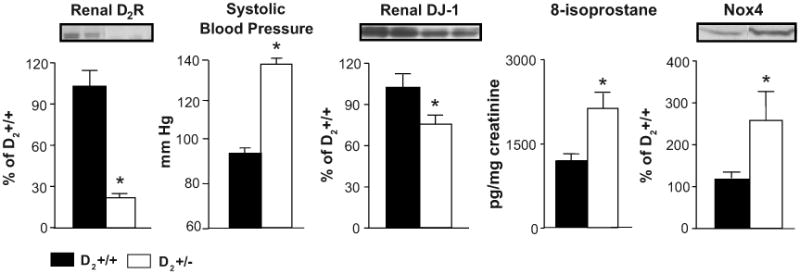
Renal cortical homogenates were immunoblotted using D2R, DJ-1, or Nox4 specific antibodies; GAPDH was used for normalization of the data. Urinary 8-isoprostane was determined by enzyme immunoassay and values were corrected for urinary creatinine. Systolic blood pressure was measured from the aorta, via the femoral artery, under pentobarbital anesthesia. Data are expressed as mean ± S.E. n=6 in each group. *, P< 0.05 vs. wild-type, t-test,
The expression of DJ-1 is increased in mice with decreased D2R expression
To determine the role of DJ-1 in the antioxidant effect of D2R we studied mice with heterozygous deletion of D2R allele (D2+/−). As mentioned above D2+/− mice were used to avoid the confounding effect of increased aldosterone since they have high blood pressure and increased oxidative stress but urinary aldosterone excretion in D2+/− mice is similar to those in wild-type littermates (4.3±1.5 vs 5.2±1.3 ng/day, respectively) and lower than those in D2−/− mice (4), indicating that increased oxidative stress in D2+/− mice is independent of aldosterone. The expression of D2R in the renal cortex of D2+/− mice is decreased about 75% relative to wild-type littermates. This is in agreement with previous reports indicating that in the brain the mutated Drd2 allele is dominant negative (22) Both systolic and diastolic blood pressures, measured under anesthesia, were increased in D2+/− mice compared to their wild-type littermates (systolic, 126±6 vs. 92±4 mmHg; diastolic, 97±5 vs. 60±3 mmHg) (Figure 2), in agreement with our previous report (17) and similar to homozygous D2−/− mice (4). Also in agreement with our previous results in D2−/− mice (4), the urinary excretion of 8-isoprostane was increased by 71% and the renal cortical expression of the NADPH-oxidase subunit Nox 4 was increased by 125% in D2+/− mice. In contrast the DJ-1 expression in the renal cortex was decreased by 30% in D2+/− mice, suggesting that D2R may regulate DJ-1 expression.
Silencing the D2R in mouse renal proximal tubule cells decreases DJ-1 expression and increases NADPH oxidase activity
To confirm the results obtained in D2+/− mice, we silenced D2R expression in mouse renal proximal tubule cells using D2R siRNA. D2R expression was decreased by 70% while DJ-1 expression was decreased by 60%. This was associated with an increase (180%) in ROS production (Figure 3A).
Figure 3. Effect of silencing or stimulation of D2R on the expression of DJ-1, production of ROS, Nox4 expression, and NADPH oxidase activity in mouse renal proximal tubule cells.
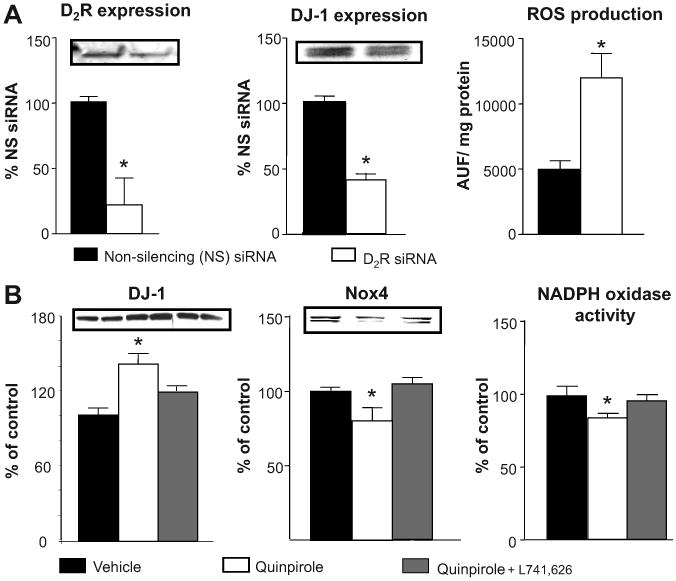
(A) Mouse renal proximal tubule cells were studied 72 h after transfection with D2R specific siRNA or non-silencing siRNA.
(B). Mouse renal proximal tubule cells were treated with a D2R agonist, quinpirole (1 μM), in the presence or absence of a D2R antagonist, L741,626 (1 μM), for 24 h. Total cell lysates were immunoblotted using D2R, DJ-1, or Nox4 specific antibodies. GAPDH was used for normalization of the immunoblots. ROS production was measured using dichlorofluorescein diacetate (DCFDA). NADPH oxidase activity was quantified by measuring NADPH-induced chemiluminescence in the presence of lucigenin (5 μmol/L) and NADPH (100 μmol/L). Data are expressed as mean ± S.E. *, P< 0.05, vs. others, t-test or one-way ANOVA and Holm-Sidak post hoc test, n = 3-5 independent experiments
D2R stimulation increases DJ-1 expression and decreases Nox4 expression and NADPH oxidase activity in mouse renal proximal tubule cells
In mouse renal proximal tubule cells, treatment with the D2R agonist quinpirole increased DJ-1 expression by about 40%. This effect was partially blocked by a selective D2R antagonist. The treatment also modestly decreased NADPH oxidase activity (15%) and Nox4 expression (21%). These data suggest that D2R may have a role in regulating DJ-1 expression that may affect Nox4 expression and NADPH oxidase activity (Figure 3B). The greater effect of D2R silencing than D2R stimulation on ROS production, Nox4 expression, and NADPH oxidase activity could be related to difference in degree of change in DJ-1 expression (70% vs. 40%).
Silencing DJ-1 expression increases ROS production and Nox4 expression in mouse renal proximal tubule cells
To determine if part of the antioxidant effect of the D2R is through regulation of DJ-1 expression/function, we silenced DJ-1 expression in mouse renal proximal tubule cells using DJ-1 siRNA. The treatment decreased DJ-1 expression by 60% and increased ROS production by 70%, as well as the expression of Nox 4 by 61%, supporting a role of DJ-1 in the regulation of renal Nox4 expression and ROS production (Figure 4). The lesser increase in ROS production with DJ-1 silencing (60%) relative to D2R silencing (180%) could be taken to suggest that the antioxidant effect of D2R can only be partially explained by DJ-1.
Figure 4. Effect of DJ-1 silencing on ROS production and Nox4 expression in mouse renal proximal tubule cells.
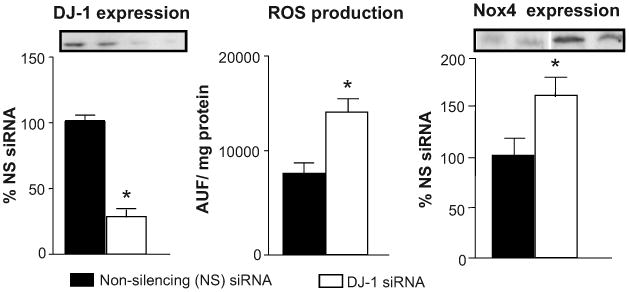
Mouse renal proximal tubule cells were transfected with DJ-1-specific siRNA or non-silencing siRNA. Total cell lysates were immunoblotted using DJ-1 or Nox4 specific antibodies. GAPDH was used for normalization of the immunoblotting data. ROS production was measured using dichlorofluorescein diacetate (DCFDA). Data are expressed as mean ± S.E. *, P< 0.05, t –test, n = 3-5 independent experiments.
Selective renal silencing of DJ-1 expression in mice increases renal Nox4 expression, renal NADPH oxidase activity, and systolic blood pressure
To determine the renal effects of DJ-1 on oxidative stress and systemic blood pressure, we selectively silenced DJ-1 expression in the mouse kidney by the subcapsular infusion of DJ-1 siRNA for 7 days. The infusion decreased DJ-1 expression by 30% and increased Nox4 expression by 50%, which was associated with a 380% increase in NADPH oxidase activity. It should be noted that prolonged (7 d) siRNA treatment had a greater effect in decreasing DJ-1 expression and increasing Nox4 expression and NADPH oxidase activity than with acute (24 h) treatment. The infusion also resulted in a 20% increase in systolic blood pressure measured under anesthesia (Figure 5), suggesting that deficient DJ-1 expression or function may result in increased renal ROS production and subsequently in increased blood pressure. The percent increase in renal NADPH oxidase activity was greater than the percent increase in blood pressure. This could be taken to suggest that the anti-hypertensive effect of D2R can only be partially explained by its inhibitory effect on ROS production. Indeed, the D2R can negatively regulate sodium transport (23) that may or may not be related to antioxidant mechanisms.
Figure 5. Effect of renal subcapsular infusion of DJ-1 siRNA on DJ-1 and Nox4 expression in the kidney, renal NADPH oxidase activity, and systolic blood pressure.
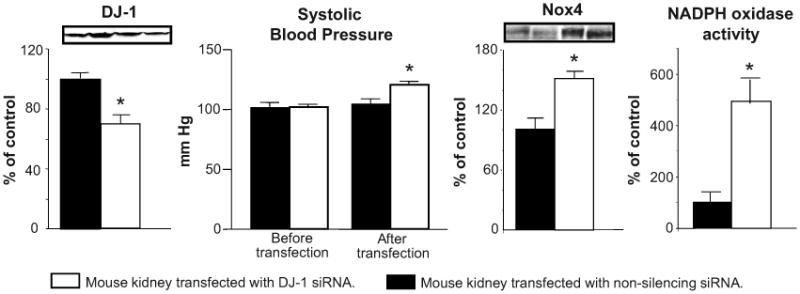
Renal cortical DJ-1 was silenced by a 7-day subcapsular infusion of DJ-1-specific siRNA via an osmotic minipump. Adult male C57BL/6J mice were uninephrectomized one week before implantation of the minipump. Systolic blood pressures were measured from the aorta, via the femoral artery, under anesthesia. Kidney tissue homogenates were immunoblotted using DJ-1 and Nox4 specific antibodies. GAPDH was used for normalization of the immunoblotting data. NADPH oxidase activity was determined by measuring NADPH-induced chemiluminescence, in the presence of lucigenin (5 μmol/L) and NADPH (100 μ mol/L). Data are expressed as mean ± S.E. *P< 0.05, -test or one-way ANOVA and Holm-Sidak post hoc test, n = 3-4 independent experiments
Discussion
The present results provide evidence that DJ-1 is expressed in the kidney, mainly in proximal tubules, and is regulated by D2R. We have already reported that disruption of D2R in mice causes hypertension that is associated with increased ROS production and oxidative stress suggesting that D2R negatively regulates ROS production (4). We now report that DJ-1 mediates at least, in part, the antioxidant effects of the D2R in the kidney.
Our results provide new evidence that lack of just one allele of the D2R results in an increase in the expression of the NADPH oxidase isoform Nox4 and excretion of 8-isoprostane, similar to what we have already reported in mice lacking both D2R alleles (4). We have also reported that the lack of one or both D2R alleles increases blood pressure to a similar extent (17).
Stimulation of D2Rs is associated with increased DJ-1 expression. Although we have used the mixed D2R-D3R agonist, quinpirole, to determine the effects of D2R stimulation on the expression of DJ-1 we have ruled out a significant effect of the D3R for two reasons: 1) the effects of quinpirole on DJ-1 expression are almost completely blocked by the specific D2R antagonist L741,626, with minimal D3R antagonism, and 2) we have evidence that the D3R is not involved in the regulation of oxidative stress in mice; mice with deletion of the D3R do not have increased oxidative stress in the kidney, increased excretion of 8-isoprostane or production of ROS (unpublished observation)
The mechanisms by which D2R regulates the expression of DJ-1 are unknown. The D2R may directly increase DJ-1 expression by activation of the MAP kinase pathway through activation of ERK1/2 (24), a pathway that has been shown to upregulate DJ-1 expression both in vivo and in vitro (25). Nevertheless, the negative regulation of ROS by D2R may be related to its positive regulation of DJ-1.
DJ-1 belongs to a protein superfamily which includes archetypical bacterial Thij and Pfpl (26) and in vertebrates is expressed in a variety of tissues, including the brain, kidney, liver, pancreas, and skeletal muscle (11). DJ-1 is a ubiquitous redox-responsive cytoprotective protein that has been associated with oncogenesis, control of gene transcription, and regulation of mRNA stability (27), and acts as an antioxidant and antiapoptotic transcriptional modulator (26). Downregulation of DJ-1 expression in renal proximal tubule cells is associated with increased Nox4 expression and ROS production suggesting that in these cells DJ-1 also has antioxidant effects. DJ-1 exerts its antioxidant effects at several levels, although it may have intrinsic activity as an atypical peroxiredoxin-like peroxidase that plays a role in scavenging mitochondrial ROS (28). However, most of the antioxidant effects of DJ-1 are due to its ability to increase the expression of other antioxidant genes, such as superoxide dismutase and heme oxygenase-1 during oxidative stress (29) and the modulation of Akt activation and ERK1/2 signalling, key signalling pathways in the modulation of the oxidative response (30-32). Therefore, the increase in ROS production in renal proximal tubule cells when DJ-1 is silenced may be related to decreased activity of antioxidant enzymes and also increased expression/activity of prooxidant enzymes, e.g., Nox4. DJ-1 silencing in renal proximal tubule cells increases ROS production to a lesser extent than does downregulation of D2R suggesting that mechanisms other than that related to DJ-1 are involved in the antioxidant effect of D2R in the kidney.
The role of oxidative stress in the pathogenesis of hypertension has been extensively studied (33, 34). The present study shows that renal selective downregulation of DJ-1 expression in mice is associated with increased Nox4 expression, NADPH oxidase activity, and a 20% increase in blood pressure. The greater percent increase in renal NADPH oxidase activity than the percent increase in blood pressure could be taken to suggest that the anti-hypertensive effect of D2R can only be partially explained by its inhibitory effect on ROS production.
Several enzymes and signalling pathways are involved in the antioxidant function of DJ-1 in tissues other than the kidney (30, 35-37). However, there are no reports on the effect of DJ-1 on NADPH oxidase expression or function. Our results suggest that DJ-1 may directly or indirectly act as an inhibitor of Nox 4 transcription/translation; the mechanisms by which these occur remain to be determined. It is possible that changes in the redox state of the cells could indirectly be responsible for changes in Nox4 expression since increases in ROS are known to increase Nox4 transcription (38, 39).
In summary our results show that the inhibitory effects of D2R on renal ROS production are at least, in part, mediated by positive regulation of DJ-1 expression or function which in turn is involved in decreasing NADPH oxidase expression/activity. This is the first report providing evidence of a role of renal DJ-1 in the regulation of oxidative stress and ROS-dependent hypertension.
Perspectives
The results of the present study show that DJ-1 may have an important role in the regulation of the antioxidant activity in the kidney. DJ-1 appears to have protective effects in the kidney by dampening oxidative stress that can cause hypertension and kidney disease. Further studies are needed to establish whether or not modulation of renal DJ-1 function is a therapeutic approach in hypertension.
Acknowledgments
Sources of founds: This work was supported in part by grants from National Institutes of Health (HL023081, DK039308, HL074940, HL068686, and HL092196).
Footnotes
Diclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jose PA, Eisner GM, Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 2002;11:87–92. doi: 10.1097/00041552-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 3.Jose PA, Eisner GM, Felder RA. Regulation of blood pressure by dopamine receptors. Nephron Physiol. 2003;95:19–27. doi: 10.1159/000073676. [DOI] [PubMed] [Google Scholar]
- 4.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–678. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa N, Tanaka K, Asanuma M, Kawai M, Masumizu T, Kohno M, Mori A. Bromocriptine protects mice against 6-hydroxydopamine and scavenges hydroxyl free radicals in vitro. Brain Res. 1994;657:207–213. doi: 10.1016/0006-8993(94)90969-5. [DOI] [PubMed] [Google Scholar]
- 6.Zou L, Xu J, Jankovic J, He Y, Appel SH, Le W. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neurosci Lett. 2000;281:167–170. doi: 10.1016/s0304-3940(00)00853-3. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa N, Tanaka K, Asanuma M, Kawai M, Masumizu T, Kohno M, Mori A. Bromocriptine protects mice against 6-hydroxydopamine and scavenges hydroxyl free radicals in vitro. Brain Res. 1994;657:207–213. doi: 10.1016/0006-8993(94)90969-5. [DOI] [PubMed] [Google Scholar]
- 8.Zou L, Xu J, Jankovic J, He Y, Appel SH, Le W. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine in C57BL/6 mice. Neurosci Lett. 2000;281:167–170. doi: 10.1016/s0304-3940(00)00853-3. [DOI] [PubMed] [Google Scholar]
- 9.Takashima H, Tsujihata M, Kishikawa M, Freed WJ. Bromocriptine protects dopaminergic neurons from levodopa-induced toxicity by stimulating D2 receptors. Exp Neurol. 1999;159:98–104. doi: 10.1006/exnr.1999.7122. [DOI] [PubMed] [Google Scholar]
- 10.Iida M, Miyazaki I, Tanaka K, Kabuto H, Iwata-Ichikawa E, Ogawa N. Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain Res. 1999;838:51–59. doi: 10.1016/s0006-8993(99)01688-1. [DOI] [PubMed] [Google Scholar]
- 11.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J, Kim SY, Cha GH, Lee SB, Kim S, Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Aleyasin H, Rousseaux MW, Phillips M, Kim RH, Bland RJ, Callaghan S, Slack RS, During MJ, Mak TW, Park DS. The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci USA. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension. 2001;38:303–308. doi: 10.1161/01.hyp.38.3.303. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Asico L, Yu P, Wang Z, Jones JE, Escano C, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase and blood pressure. Am J Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 19.Woost PG, Kolb RJ, Finesilver M, Mackraj I, Imboden H, Coffman TM, Hopfer U. Strategy for the development of a matched set of transport-competent, angiotensin receptor-deficient proximal tubule cell lines. In Vitro Cell Dev Biol Anim. 2006;42:189–200. doi: 10.1290/0511076.1. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson SM, Norton CS, Watson SJ, Akil H, Robinson TE. Amphetamine-evoked c-fos mRNA expression in the caudate-putamen: the effects of DA and NMDA receptor antagonists vary as a function of neuronal phenotype and environmental context. J Neurochem. 2003;86:33–44. doi: 10.1046/j.1471-4159.2003.01815.x. [DOI] [PubMed] [Google Scholar]
- 21.Lokhandwala MF, Steenberg ML. Evaluation of the effects of SKF 82526 and LY 171555 on presynaptic (DA2) and postsynaptic (DA1) dopamine receptors in rat kidney. J Auton Pharmacol. 1984;4:273–277. doi: 10.1111/j.1474-8673.1984.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 22.Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- 23.Ozono R, Ueda A, Oishi Y, Yano A, Kambe M, Katsuki M, Oshima T. Dopamine D2 receptor modulates sodium handling via local production of dopamine in the kidney. J Cardiovasc Pharmacol. 2003;1:S75–S79. doi: 10.1097/00005344-200312001-00017. [DOI] [PubMed] [Google Scholar]
- 24.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 25.Lev N, Ickowicz D, Barhum Y, Lev S, Melamed E, Offen D. DJ-1 protects against dopamine toxicity. J Neural Transm. 2009;116(2):151–160. doi: 10.1007/s00702-008-0134-4. [DOI] [PubMed] [Google Scholar]
- 26.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Bonifati V, Oostra BA, Heutink P. Linking DJ-1 to neurodegeneration offers novel insights for understanding the pathogenesis of Parkinson's disease. J Mol Med (Berl) 2004;82:163–174. doi: 10.1007/s00109-003-0512-1. [DOI] [PubMed] [Google Scholar]
- 28.Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan X, Kelsen SG, Merali S. Proteomic analysis of oxidative stress-responsive proteins in human pneumocytes: insight into the regulation of DJ-1 expression. J Proteome Res. 2008;7:4955–4961. doi: 10.1021/pr800295j. [DOI] [PubMed] [Google Scholar]
- 30.Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, Slack RS, Mak TW, Park DS. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci USA. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu L, Cui T, Fan C, Zhao H, Zhao C, Lu L, Yang H. Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochem Biophys Res Commun. 2009;383:469–474. doi: 10.1016/j.bbrc.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–35. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 34.Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 35.Batelli S, Albani D, Rametta R, Polito L, Prato F, Pesaresi M, Negro A, Forloni G. DJ-1 modulates α-synuclein aggregation state in a cellular model of oxidative stress: relevance for Parkinson's disease and involvement of HSP70. PLoS One. 2008;3:e1884. doi: 10.1371/journal.pone.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Im JY, Lee KW, Junn E, Mouradian MM. DJ-1 protects against oxidative damage by regulating the thioredoxin/ASK1 complex. Neurosci Res. 2010;67:203–208. doi: 10.1016/j.neures.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Liu J, Chen S, Wang Y, Cao L, Zhang Y, Kang W, Li H, Gui Y, Chen S, Ding J. DJ-1 modulates the expression of Cu/Zn-superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann Neurol. 2011;70:591–599. doi: 10.1002/ana.22514. [DOI] [PubMed] [Google Scholar]
- 38.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 39.Nava M, Quiroz Y, Vaziri N, Rodriguez-Iturbe B. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284:F447–F454. doi: 10.1152/ajprenal.00264.2002. [DOI] [PubMed] [Google Scholar]


