SUMMARY
Biomarker evidence and clinical observations support the hypothesis that there is a diagnosable condition termed preclinical Alzheimer’s disease (AD). Recently, a workgroup convened under the auspices of the National Institute on Aging and the Alzheimer’s Association proposed a framework for defining preclinical AD. The definition was based on the presence of biomarkers that are indicative of the AD pathophysiological process. In the context of abnormal AD biomarkers, the workgroup postulated that ‘subtle cognitive changes’ occurred as well. Based on studies of genetically at-risk individuals and those destined to become demented, who were observed while still cognitively normal, low performance on learning and memory functions may be the earliest cognitive manifestations of preclinical AD, at the group level at least. It is not clear whether subtle cognitive decline can be detected reliably on an individual basis. Preclinical AD cognitive changes could be diagnosed by traditional neuropsychological testing, computerized testing, assessments of subjective memory loss, assessments of levels of participation in cognitively stimulating activities and direct measurement of activity using recently developed monitoring technology. Confounding effects of normal aging, test–retest variability, variations in educational attainment, as well as the presence of other brain diseases make diagnosing cognitive decline due to preclinical AD challenging.
The concept of preclinical Alzheimer’s disease
Preclinical Alzheimer’s disease (AD) is not a new concept. Longitudinal observational studies have demonstrated, at a group level, that people destined to develop dementia due to AD have lower cognitive test scores while still being asymptomatic compared with the group who do not develop dementia [1–8]. Longitudinal examination of asymptomatic individuals who appear normal and who were genetically predisposed to AD dementia – either because of carriage of the e4 allele of APOE [9,10] or because of carriage of mutations in APP, PS1 or PS2 genes [11–13] – also showed group-wise cognitive differences compared with appropriately matched noncarriers. With the advent of amyloid imaging and evermore refined structural imaging, identifying asymptomatic individuals at-risk for AD dementia is close to reality. It is imperative to understand the prospects and limitations of cognitive assessment in asymptomatic individuals who are at-risk for becoming demented in the future.
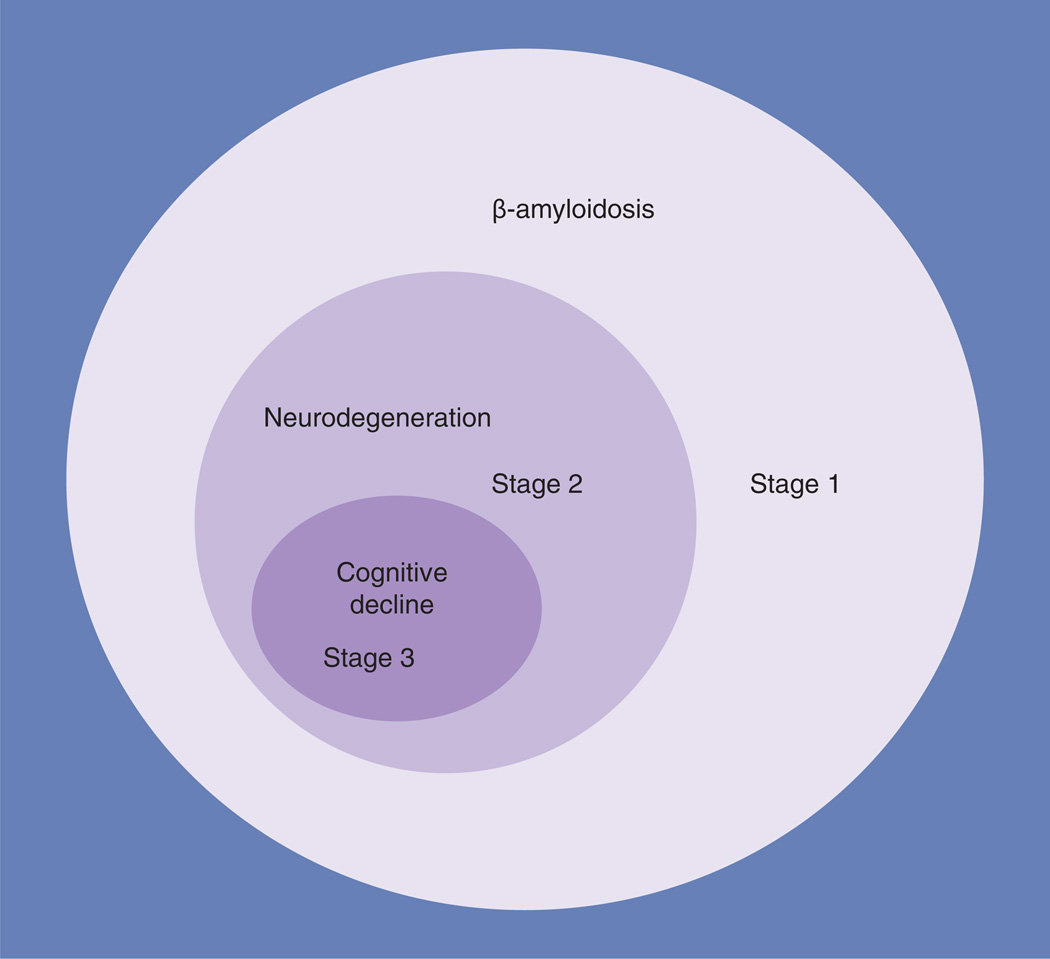
In 2011, a workgroup convened under the auspices of the National Institute on Aging and the Alzheimer Association (NIA-AA) proposed a framework for defining ‘preclinical’ AD based on three stages (Figure 1) [14]. The conceptual framework of preclinical AD was based on a hypothetical model of the pathophysiology of AD [15]. The model posits that, in cognitively normal individuals, there are a set of pathophysiological processes in AD that are observable with imaging and cerebrospinal fluid (CSF) biomarkers. Although the complete evolution from biomarkers to dementia has yet to be demonstrated, the model fits the existing data on the transition from cognitively normal to mild cognitive impairment (MCI) [14,15]. The first stage of preclinical AD is defined by the presence of an abnormal level of β-amyloid by amyloid imaging or by CSF assay. The second stage of preclinical AD includes abnormal β-amyloid levels plus evidence of neuronal neurodegeneration from structural imaging, metabolic imaging or CSF tau levels. In the third stage of preclinical AD, ‘subtle cognitive changes’ accompany the Stage 2 changes. The NIA-AA work group purposely left their formulation of the cognitive features of preclinical AD to be defined by future empirical investigation, but they suggested that memory dysfunction was likely to be a key element. To quote the document: “…these individuals may demonstrate evidence of decline from their own baseline … even if they still perform within the ‘normal’ range on standard cognitive measures. There is emerging evidence that more sensitive cognitive measures, particularly with challenging episodic memory measures, may detect subtle cognitive impairment in amyloid-positive individuals.” [14]. As defined in the workgroup document, the cognitive changes are asymptomatic and only have meaning in the context of the abnormal biomarkers [14].
Figure 1. A graphic depiction of the National Institute on Aging and the Alzheimer Association model of preclinical Alzheimer’s disease.
The size of the circles should not be construed as representing the relative proportions of subjects meeting the criteria. Stage 1 represents β-amyloidosis. Stage 2 represents neurodegeneration in the setting of β-amyloidosis. Stage 3 represents cognitive decline in the setting of both β-amyloidosis and neurodegeneration. The Venn diagram depicts how neurodegeneration in Stage 2 is conditioned on the presence of abnormal levels of β-amyloidois, and how cognitive decline denoting Stage 3 is conditioned on the joint presence of abnormalities of both amyloid and neurodegeneration biomarkers.
Data taken from [14].
The intention of the NIA-AA workgroup on preclinical AD was to call attention to individuals with biomarker evidence of preclinical AD who had cognitive decline. Thus, at the high-functioning end of the preclinical spectrum, those with preclinical AD will have, at most, some cognitive decline compared with their prior level of functioning, even though their test scores would still fall into the normal range. At the lower functioning end of the spectrum, but still in the ‘normal range’, the cognitive decline would approach, but be less than that seen in MCI. The concept of subtle cognitive decline in preclinical AD is a relatively new one and its operationalization is a work in progress. Ideally, longitudinal observations would be used for the diagnosis [10] but if the concept is to have broad use, a cross-sectional definition must also be available. As of 2012, however, the concept of preclinical AD is strictly for research purposes. Much work, including major therapeutic break-throughs, needs to be done before the concept of preclinical AD is ready for general clinical use.
Decreasing levels of cognitive performance from superior levels to lower levels probably represents a continuum of risk in which any cutoff point represents a convenience for researchers and clinicians, not a biologically relevant inflection point. Nonetheless, while the distinction between preclinical AD-related cognitive decline and that of MCI may seem arbitrary, there is an increased risk for decline to dementia once the threshold for MCI is reached [16]. Short of that threshold, and within the group of individuals defined as cognitively normal, there is a subgroup that is experiencing changes in cognition that will eventually manifest as dementia. Thus, just as designating an individual as having MCI provides increased certainty of future decline, designating a person as having “subtle cognitive decline consistent with preclinical AD” is meant to convey increased risk.
The prevalence of preclinical AD
As yet, there are no epidemiological studies in preclinical AD. Current estimates of the prevalence of preclinical AD in 70–80-year-old cohorts, based on amyloid imaging, average approximately 30% [17–21]. This value can be shown to correspond with evidence from other sources. Based largely on the amyloid imaging literature, an estimate of 15–20 years from the onset of abnormal amyloid accumulation to the time of cognitive symptom onset has been suggested [17,22]. It is not known how much of that period includes cognitive decline. The long lag between the appearance of the earliest pathological changes and the clinical manifestations is supported by neuropathological findings in young autopsied APOE e4 carriers [23], as well as on neuropsychological and clinical follow-up of APOE e4 homozygotes in whom declines in memory test performance begin between age 55 and 60 years, with the clinical emergence of MCI by approximately age 70 years [9].
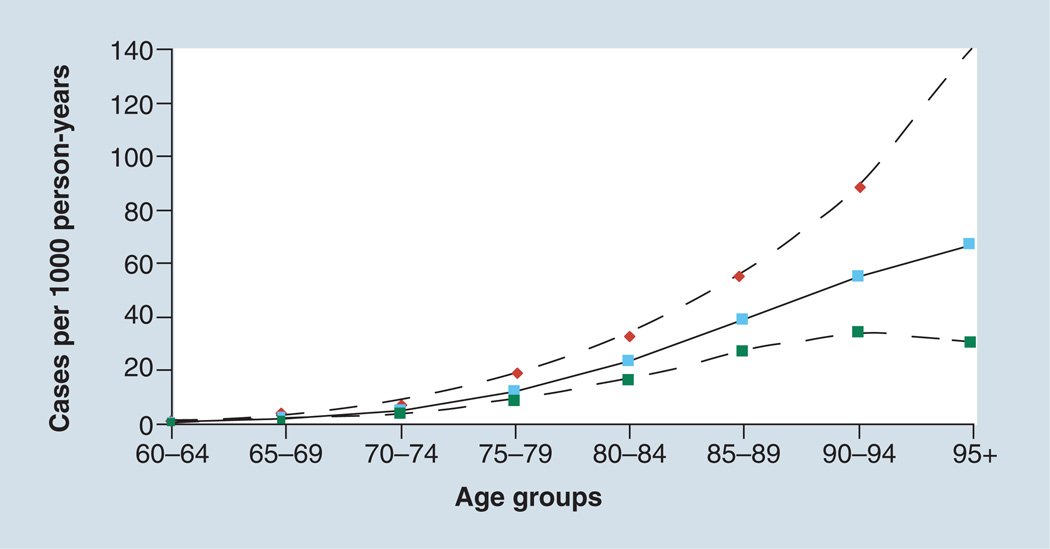
The prevalence of preclinical AD must be highly age-dependent. Based on estimates of the incidence of AD, and reflecting them back by 15 years to the presumed onset of AD pathophysiological changes, the incidence of preclinical AD at age 50 would be in the range of 5–10 per 10,000 person-years (Figure 2) [24]. By 70 years of age, the incidence of preclinical AD would be approximately 1–3 per 100 person-years. If the duration of time spent in the preclinical phase of the disease is 15–20 years, this would mean that the prevalence of preclinical AD at age 70 years may range from 15 to as high as 60 per 100 individuals. Because of competing mortality, not all those with preclinical AD will become demented. Thus, with some assumptions, the prevalence of preclinical AD estimated from amyloid imaging studies is in the same range that could be computed from traditional epidemiological sources.
Figure 2. Incidence of Alzheimer’s disease dementia.
Solid line shows incidence rates; dashed lines represent the 95% CIs. The same rates of preclinical Alzheimer’s disease would be seen 15–20 years earlier. The point estimates of incidence (per 1000 person-years) by 5-year age brackets are: 0.58 (60–64 years); 1.86 (65–69 years); 5.06 (70–74 years); 11.74 (75–79 years); 23.1 (80–84 years); 38.58 (85–89 years); 54.88 (90–94 years); and 66.85 (95+ years).
Data taken from a meta-analysis [24].
In our experience, in a group of subjects with a median age of 78 years (interquartile range: 74–82 years) from a population-based study of cognitive aging [25], the Mayo Clinic Study of Aging found that 30% met the criteria for preclinical AD by the NIA-AA criteria [25].
Cognitive profile of preclinical AD
Based on the view that the amnestic form of MCI and the dominant presentation of AD dementia are characterized by deficits in learning and (episodic) memory, an a priori conceptual model of the dominant cognitive deficits in preclinical AD has focused on impairments in learning and memory. However, the few longitudinal studies and studies of genetically at-risk subjects suggest a more diverse set of cognitive changes in the preclinical stage of AD (Table 1). Deficits in learning and memory are certainly present, but executive dysfunction is also implicated. The question of great interest is what other areas are equally impaired? Or, alternatively, are there cognitive functions that are more impaired than learning and memory?
Table 1.
Findings in asymptomatic at-risk individuals.
| Study population | Findings | Ref. |
|---|---|---|
| Genetically at-risk subjects | ||
| APOE e4 carriers (n = 317) | Declines in delayed recall on word-list learning (auditory verbal learning test); no change in letter fluency or spatial estimation in judgment of line orientation | [9] |
| APOE e4 carriers (n = 265) | Only minimal evidence of frontal dysfunction (on paced auditory serial attention task) in preclinical subjects | [30] |
| E280A PS1 carriers (n = 30) | Novel memory test of ‘feature binding’ – learning associations between features of to-be-remembered material – on a visual short-term memory task | [13] |
| A431E or L235V PSI carriers (n = 30) | All tests impaired; did not separate by age versus age of onset in affected members of kindred | [11] |
| Retrospective studies of individuals who became demented | ||
| Case–control (IL, USA) | 5–6 years prior to diagnosis: global decline accelerated; working and semantic memory slightly earlier in persons destined to become demented | [1] |
| Case–control (France) | 12 years prior to diagnosis: impairment in semantic memory and concept formation in persons destined to become demented | [2] |
| Case–control (Canada) | 5–10 years follow-up short delayed verbal recall, animal fluency, information predicted dementia | [4] |
| Case–control (NY, USA) | >4 years follow-up: selective reminding test + Fuld Object Memory test + digit symbol substitution test + category (semantic) fluency predicted dementia | [3] |
| Case–control (MA, USA) | 22-year follow-up: measures of new learning, recall, retention, abstract reasoning predicted dementia. Lower scores for measures of abstract reasoning and retention predicted AD dementia after a dementia-free period of 10 years | [5] |
| Case–control (MO, USA) | Up to 27 years of observation of cohort: a decline in cognitive function observed at 3 years (visuospatial), 2 years (global composite) and 1 year (verbal and working memory) | [6] |
| Case–control (MD, USA) | Up to 15 years of observation: declines in performance on tests of episodic memory accelerated 7 years before diagnosis. Declining performance on tests of executive function accelerated 2–3 years before diagnosis | [7] |
| Case–control (PA, USA) | 8-year follow-up: poorer scores in all cognitive domains in those subsequently diagnosed with AD dementia compared with subjects who remained nondemented | [8] |
AD: Alzheimer’s disease.
Although the duration of observation was quite long for all of the studies selected for Table 1, some of the studies might have included subjects who, at entry into the studies, may be regarded as having MCI because of the magnitude of their deficits. However, a critical reader might question whether a distinction between cognitively normal but low-scoring and MCI is a feasible distinction to make. Taking into account the biases that went into test selection for the studies listed in Table 1, measures of learning and memory were often abnormal but many other nonmemory domains also demonstrated impairments.
Studies of cognitive function in elderly persons with abnormal levels of β-amyloid have sometimes shown cognitive deficits and sometimes not. Table 2 describes these studies [18,20,21,26–29]. Because the NIA-AA model of preclinical AD posits that abnormal brain amyloidosis and neurodegeneration could occur without cognitive correlates (i.e., Stages 1 and 2), the variable findings are not unexpected.
Table 2.
Results of cognitive assessments in cognitively normal persons with abnormal amyloid imaging or cerebrospinal fluid amyloid.
| Subject group | Findings | Ref. |
|---|---|---|
| 32 subjects (mean age: 72 years) | Participants with a PIB-positive scan performed 0.8 standard deviations worse on the composite episodic memory score than PIB-negative participants | [18] |
| 20 subjects (mean age: 72 years) from University of California, Berkeley (CA,USA) and 17 subjects from the ADNI (mean age: 78 years) | PIB levels correlated with episodic memory score in Berkeley but not ADNI subjects | [26] |
| 43 subjects (age range: 65–88 years) | No cognitive changes in PIB-positive groups | [20] |
| 66 subjects (mean age: 73 years) | There was little or no overall relationship between amyloid burden and performance on neuropsychological tests, but in persons with low cognitive reserve there was a strong inverse relationship of amyloid burden to memory | [21] |
| 40 subjects with low CSF β-amyloid from the ADNI (mean age: 76 years) | No differences in any cognitive test score except a difference on Trailmaking part B | [27] |
| 51 subjects (mean age: 79 years) who had been followed prior to PET | Higher PIB levels were associated with prior longitudinal decline on MiniMental State Examination, learning and delayed free recall on California verbal learning test | [28] |
| 28 subjects (mean age: 64 years), 8 APOE e4 homozygotes, 8 heterozygotes | Despite higher levels of PIB binding in the homozygotes, there were no cognitive differences in homozygotes, heterozygotes and noncarriers | [29] |
ADNI: Alzheimer’s Disease Neuroimaging Initiative; CSF: Cerebrospinal fluid; PIB: Pittsburgh compound B.
Taking the observations in Tables 1 & 2 together, we believe that declines in episodic memory are the earliest and most consistent observation in preclinical AD [9]. Later in the process, some executive functions might be impaired [30].
Challenges to detecting preclinical AD in behavior & cognition
There is no guarantee that a cognitive or behavioral profile of preclinical AD can be developed that has suitable specificity and sensitivity for clinical use, especially because all of the findings of preclinical change reported to date have required group analyses. No measure has shown sufficient sensitivity and specificity in individuals. Competing causes of poor cognitive performance in middle-aged and elderly adults make the job of detection of preclinical cognitive changes very difficult (Table 3). In addition, there are many nonspecific threats to detecting cognitive changes from preclinical AD, such as altered vision and hearing, which occur with aging, depression and cultural variations in approaching cognitive assessment. There are other phenomena, described below, that cause cognitive impairment that pose even more difficult challenges for detecting preclinical AD.
Table 3.
Factors that add to difficulty in detecting preclinical Alzheimer’s disease.
| Factors | Putative mechanisms of interaction with AD pathophysiology |
Putative mechanisms of interaction with cognitive decline |
|---|---|---|
| Cognitive aging | Age increases AD pathophysiology | Age is associated with decline in many cognitive functions |
| Innate intelligence (education) | Higher education or high childhood socioeconomic status might protect against AD pathophysiology or cerebrovascular pathology | Lower life-long intellect and lower educational attainment depress cognitive test scores |
| Non-AD pathologies | High burdens of non-AD pathologies lower threshold for AD pathophysiology-induced cognitive decline | Non-AD pathologies might affect cognitive domains not ordinarily impacted by AD pathophysiology |
| Vascular risk factors | High burdens of non-AD pathologies lower threshold for AD pathophysiology-induced cognitive decline | Vascular pathologies might affect cognitive domains not ordinarily impacted by AD pathophysiology |
| MCI due to AD, not correctly diagnosed | A more advanced stage of AD pathophysiology | Diagnostic imprecision |
| Hearing and visual loss | None | Impair test performance independently of level of cognitive function |
AD: Alzheimer’s disease; MCI: Mild cognitive impairment.
Test–retest variability
Human performance on cognitive testing is inherently noisy, and variability from test session to test session is considerable [31]. Delayed recall test scores are particularly variable [32]. In the context of screening for cognitive impairment, there are risks depending upon results from one test session. Low performance on any psychometric test after a single administration can reflect chance, and this poor performance may revert to normal on subsequent administration, reflecting nothing more than the well-known phenomenon of regression toward the mean. Serial testing should exhibit less noise, although the costs in time and effort for acquiring multiple assessments could be daunting.
Aging
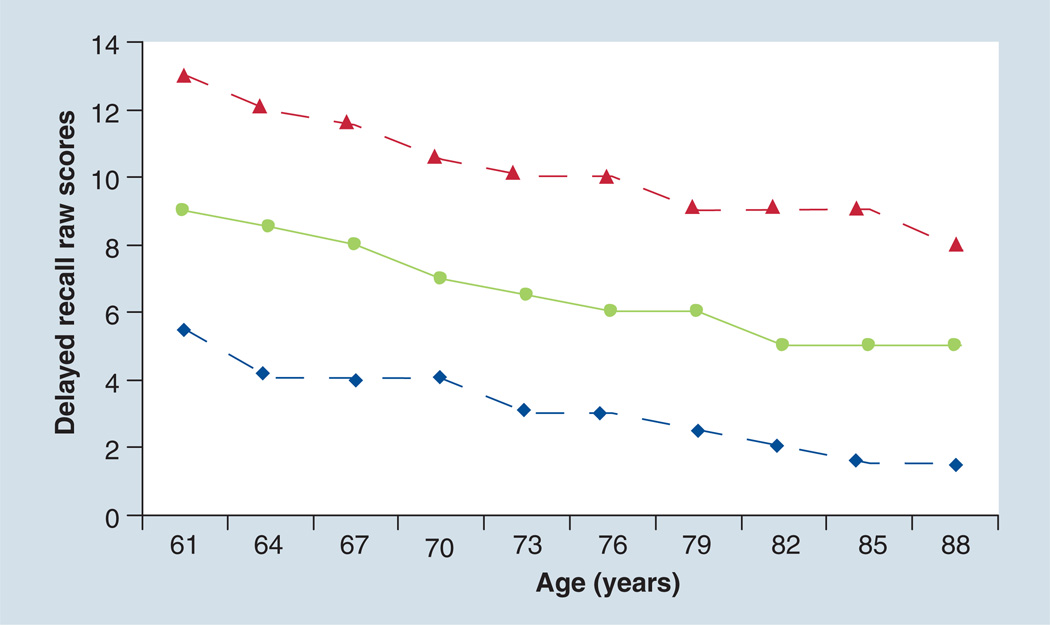
A major competing cause of poor cognitive performance is aging itself. The changes in test performance over the sixth–ninth decades of life are very large. For example, normative data from memory testing show dramatic declines with age on all aspects of learning and memory (Figure 3) [33,34]. In fact, by the ninth decade of life, delayed recall in persons considered normal is nearly half of that of persons in their fifties [33]. If cognitive assessments for preclinical AD do not take age into account, a very large number of people over 80 years of age would meet the criteria for the diagnosis of preclinical AD. On the other hand, to the extent that the prevalence of dementia in the tenth decade of life is very high, the high proportion of persons over 80 years of age with low test scores may be a valid indicator of the numbers at risk.
Figure 3. Normative data from the delayed recall assessment of the auditory verbal learning test from Mayo’s Older Adults Normative Study.
The solid line represents the mean of delayed recall raw scores, and the dashed lines represent one standard deviation.
Adapted with permission from [33].
There are some authors, experts in cognitive neuropsychology, who believe that most cognitive aging is due to underlying nascent neurodegenerative and cerebrovascular changes [35]. However, there are changes in adult cognition that seem to occur well before the appearance of AD pathophysiology or other late-life processes. Some cognitive functions show a decline that is evident as early as age 30 or 40 years compared with the paragon of age 20 years. Except for vocabulary knowledge, performance on tests of reasoning, spatial visualization, memory and speed all decline by the fourth decade of life [36]. To quote Salthouse, “there are nearly monotonic age-related declines beginning in early adulthood … results are consistent with the interpretation that the cross-sectional age differences reflect a shift in the entire distribution, rather than an increase in the breadth of the distribution as would be expected if only some people declined whereas others remained stable. [37].”
Education
The marked variability in cognitive performance as a function of native intellect, as indexed by educational attainment, is another important confound to the detection of preclinical AD [38–42]. Across the range of educational attainment, the range in performance on most neuropsychological tests is considerable. For example, in the experience of a large epidemiological study in the USA, median scores on the digit symbol substitution test, a letter fluency test and a delayed word recall test were nearly 50% lower among individuals with < ninth-grade education compared with those with >12 years of education [43]. Individuals with lower educational attainment might be inappropriately overdiagnosed as having preclinical AD, if the criteria relied solely on cognitive testing. On the other hand, lower educational attainment itself may be a true risk factor for future dementia. Education appears to offer a ‘protection’ or at least a delay in the appearance of cognitive impairment [42,44]. That is, for two individuals with the same level of AD (or other) pathology, the one with the higher education will experience cognitive decline at a later time point than a person with lower educational attainment. This is of crucial importance for interpreting the risks conferred by results of cognitive testing. Therefore, factoring out education in cognitive assessments for preclinical AD may lead to underdetection of those at risk for AD dementia for those with lower educational attainment, but may aid in the detection of at-risk persons with high educational attainment [45].
Non-Alzheimer pathologies
A third competing process is the presence of non-AD pathology. Neuropathological studies of persons who were cognitively normal within 18 months prior to death often had AD and non-AD-type pathological changes [46–48]. While some or most pathology causing dementia in the elderly may indeed be AD, other pathologies certainly play a role. The neuropathological changes of cerebrovascular disease, synucleinopathies and non-AD tauopathies, among others, also accumulate with advancing age. There is very-little-to-no-work on preclinical cognitive aspects of synucleinopathy and non-AD tauopathies. It seems highly implausible that the cognitive consequences of these pathophysiologies are so specific as to allow differentiation of AD-related and non-AD-related causes.
Because vascular risk factors in midlife may act as a proxy for preclinical disease, the non-AD pathophysiology that is best studied is vascular. There is extensive literature on the cognitive consequences of diabetes mellitus, hypertension and other vascular risk factors in cognitively normal individuals [49–57]. Deficits in both attentional/psychomotor/executive and learning domains have been observed with diabetes and hypertension. There is also a large literature on cognitive impairment that is not dementia associated with white matter hyperintensities [58–60] and lacunar infarcts [61–64]. To make matters more complicated, AD and vascular risk factors may interact with the APOE genotype. One study showed that the presence of cardiovascular risk accelerated the memory decline of e4 homozygotes but not the heterozygotes or noncarriers [65]. Conceptually, one might have expected the vascular risk factors to show associations with only attentional or psychomotor domain impairment, but actual observations are not as clear. Sometimes, memory decline is evident in individuals with a history of stroke [53,66,67]. The cognitive consequences of cerebrovascular disease on cognition overlap with those of AD pathophysiology and complicate efforts to detect changes that are specific for AD.
Assessment of preclinical AD
Traditional cognitive testing
Brief mental status examinations are not likely to be useful in preclinical AD. Their brevity, ceiling effects and lack of discrimination at the highest end of the measurement scale make them unlikely to be useful in asymptomatic individuals at risk for AD dementia. On the other hand, more detailed but standard neuropsychological test instruments that are employed in clinical practice have been shown to be useful for assessment of preclinical AD. As outlined in Tables 1 & 2, a number of test instruments are sensitive to disease in persons at risk genetically for AD dementia, or in persons who subsequently developed AD dementia over a long-term follow-up. Standard paper-and-pencil learning and memory tests, such as the Rey Auditory Verbal Learning test [9] or the Free and Cued Selective Reminding test [68], possess suitable psychometric properties for use in asymptomatic individuals. These properties include a lack of a ceiling effect and the ability to discriminate performance at the higher end of the performance range. There are several other examples of list-learning procedures, such as the California Verbal Learning Task [69], and the paragraph learning tasks, such as the Wechsler Memory Scale stories [70], or variations on tasks of visual associative learning (‘binding’ [13] or ‘pattern separation’ [71]), but there is insufficient experience currently to indicate that one is superior to the others.
All of the above examples in Tables 1 & 2 of ‘success’ in detecting preclinical changes were at the group level. Whether any traditional tests have the precision to assess risk on an individual basis is largely unknown at this time. In one instance, where the experiment was carried out, the Free and Cued Selective Reminding test was shown to have good sensitivity for predicting subsequent dementia among nondemented individuals in a population-based study, but rather poor specificity [68].
Serial testing using traditional neuropsychological tests offers the prospect for improving the reliability of traditional testing, which might improve specificity. Unfortunately, practice effects, in which subjects learn the stimuli, is a concern if different test versions are not used. Additionally, if multiple test versions are used, nonequivalency between versions would add variability to scores. Furthermore, serial testing adds an additional level of logistical complexity to assessment.
For the purpose of detecting preclinical AD, automation of cognitive assessment is highly desirable because of the large number of individuals that would need to be screened. Unfortunately, high-throughput screening using traditional cognitive testing may not be feasible for these reasons. The availability of computerized approaches that take advantage of technological advances seems to be a promising solution.
Computerized batteries
The potential of computerized cognitive assessments in the elderly has yet to be fully exploited [72]. Computerized testing may be more interesting to subjects and offers several conceptual advantages over traditional testing. The ability to measure reaction time and to develop tasks that minimize practice effects, and the opportunity to tailor testing to an individual, depending upon performance, could be transformative. The use of virtual reality for testing of visual cognition has been carried out, and offers some promise in preclinical AD [72–74]; real-time natural language analysis is another evolving technology that holds promise [75]. However, there are limitations to computerized testing. A lack of access to computers, visual or motor impairment in a substantial fraction of elders, cost and reliability are concerns. However, it seems inevitable that electronic cognitive assessment methodology will gradually become ubiquitous. There are many computerized batteries for cognitive assessment that are now available [76]. Despite the potential, it has yet to be demonstrated that the conceptual advantages of computerized testing translate into greater diagnostic accuracy or predictive ability compared with traditional means of cognitive assessment.
Subjective appraisal of self-functioning
Self-rating of daily functioning might be a way of detecting subtle cognitive changes in the preclinical stage of AD. While self-report in individuals with overt cognitive impairment suffers because of the anosognosia that accompanies emerging AD dementia, self-rating might be a feasible strategy before loss of insight occurs. Instruments designed for informants, such as the Functional Activities Questionnaire [77] or the Everyday Cognition Scale [78], could be used for this purpose. A number of instruments exist to quantify self-rating of cognitive functioning [79]. Scores on some of these instruments appear to be associated with AD-related biomarkers [80,81] and with risk for future dementia [82–85]. It seems possible, therefore, that low self-appraisal of one’s cognitive abilities might be a suitable proxy for subtle cognitive impairment. On the other hand, work in the Sydney Memory and Aging Study has found that 95.5% of nondemented participants answered at least one question regarding their own cognitive functioning as abnormal [86]. The nature of the appraisal of one’s own memory is inherently variable. The appraisal may be influenced by cultural norms, mood state and social network values. Nonetheless, there are enough studies showing that subjective memory complaints correlate with neurodegenerative changes and with risk for future dementia, that appraisal of self-functioning ought to be seriously considered in any battery for detecting preclinical AD.
Inventories of cognitively engaging activities
Engaging in cognitively stimulating activities has been associated with lower rates of cognitive impairment [87–90]. While the studies conceptualized the inventories as representations of protective activities in mid-life, one could also look upon a change in participation in late life as an early sign of cognitive decline. As part of the process of preclinical AD, it is possible that loss of interest in prior pastimes and loss of ability to perform some cognitively stimulating activities as well as one did in the past, may be as good proxies as the other options described above [88]. Combining technology with assessment of daily activities is another experimental approach being considered.
Measuring activity in real-time
A research group at Oregon Health and Sciences University (OR, USA) have installed in-home sensors for detecting activity levels [91]. If some of the earliest changes in preclinical AD are apathy and loss of initiative, subtle declines in activity might be detectable with quantitative in-home monitoring. Unless subjects were already experiencing declines, serial measurement of activity levels, whether by questionnaires or sensors, could detect meaningful changes. However, the specificity of the changes would have to be established by other means, because other health issues also affect mobility and activity levels.
Defining cutoff points for preclinical AD: a dilemma with two possible choices
Defining the cutoff points for identifying individuals at-risk for preclinical AD on any given test will be a challenge. The cutoff point that distinguishes normal cognition from that to be considered as abnormal for the preclinical AD definition was not discussed by the preclinical AD task force [14]. Use of different cutpoints for different levels of age and education are a potential solution, but the use of a series of cutpoints introduces considerable complexity. The same problem applies to both cross-sectional and longitudinal testing. There are probably no conceptual insights that will solve the problem; only empirical longitudinal investigations will reveal what approaches work best.
For selecting cross-sectional cutoff points, there are two issues. First, what is the cutoff point for defining where risk for preclinical AD begins (i.e., the cutoff point on the normal side of the distribution)? Second, what is the cutoff point for defining where preclinical AD ends and, instead, MCI should be diagnosed? For the latter cutoff point, it would seem obvious that the often-cited ‘−1.5 standard deviation’ point for defining where MCI begins would be the value for the cutoff point where an individual would no longer meet the criteria for preclinical AD [16]. Unfortunately, although the nature of clinical practice and use of diagnostic criteria force us to use cutoff points, risk for future dementia across the spectrum of cognitive function is more likely continuous, so any cutoff points are arbitrary and subject to imprecision in risk prediction.
If serial testing were available, it would be possible, in principle, to define a rate of decline that exceeded a ‘normal’ age-related rate. Analysis of slope requires at least three observations over time. A very large sample size and many years of observation would be needed to have sufficient data on individuals who eventually became demented.
The Mayo Clinic Study of Aging has begun to evaluate different cognitive cutoff points [25]. They examined cutoff points of <5, <10 or <15% on a global cognitive variable, based on a cognitive test score distribution from nine standard neuropsychological tests given to a group of subjects adjudicated by a consensus clinical process to be cognitively normal. No adjustments for age or education were used. Using either more stringent or looser definitions changed the number of cognitively normal subjects who would be considered to meet criteria for Stage 3 of preclinical AD. Using the unadjusted tenth percentile, they [25] found that out of a group of 450 normal subjects, only 13 (3%) met criteria for Stage 3, preclinical AD [14]. Longitudinal follow-up will be required to determine what cutoff points, as well as the choice of the cognitive assessment used, are best for predicting future MCI or dementia.
Role of cognitive testing in preclinical AD therapeutic trials
Cognitive assessment will play a role in characterizing the asymptomatic subjects who participate in trials for preclinical AD [92]. Our view is that cognitive function – as defined by one or more of the approaches mentioned above – should be an inclusion criteria for trials of preclinical AD as long as they are part of a set of criteria that also include biomarkers of AD pathophysiology, such as abnormal amyloid imaging and evidence for neurodegeneration. As a general principle, lower levels of cognitive functioning, on any measure, in subjects with abnormal AD biomarkers are likely to increase the probability of cognitive decline over the course of a trial.
One strategy might be to use tests of cognitive functioning to exclude persons who are too cognitively intact. Alternatively, low cognitive functioning could serve as an inclusion criterion. Choosing the proper test instruments, the proper cutoff points and the best way to account for different levels of age and education are going to be difficult issues. A third option, recruiting subjects and following them for 2 years to select those who decline before administering therapy, would be a very novel and innovative approach but one for which actual implementation would be challenging.
Detecting preclinical AD will need to take place on several different levels, including the clinical trial situation and the routine clinical practice setting. The two will have to have some similar structure for the former to match the latter for regulatory purposes. APOE genotyping and biomarker determinations will almost certainly be part of the process. Whether cross-sectional or longitudinal evaluation of potential at-risk individuals will be the common approach will be determined by feasibility and experience.
Ethics & the diagnosis of preclinical AD
At the present time, the term preclinical AD should not be used in clinical practice. There are too many uncertainties surrounding its prognosis. Nonetheless, genetic and biomarker tests are being directly marketed to consumers, and so the question of clinical significance is being forced upon us. Those with a family history of AD often perceive themselves to be at increased risk (the stronger the family history, the greater the risk perception [93]) and so may be more likely to be attracted by such marketing. Insights gained regarding the efficacy and safety of such testing, and specifically, APOE genotype disclosure, derive from the REVEAL study.
REVEAL has taught us that levels of distress related to disclosure or nondisclosure are similar, and post-test levels of distress correlate strongly with pretest levels [94]. A total of 9% of participants with no significant psychiatric problems developed one within a year [95], and among the three e4 homozygotes included, two developed clinically significant psychological distress (although the relationship to genetic disclosure itself was not certain) [96]. As to its perceived value, among those receiving information about their APOE genotype and its implications for AD risk, 40–50% forgot this information after a year [94], and in a more recent analysis, only 41% of REVEAL participants agreed they would be willing to pay at least US$100 for AD-related genetic testing if it were offered clinically [97]. We tend to overestimate our risk for AD, so such programs might help patients worry less, but individuals discovering they are e4-positive experience far greater levels of distress and revise their pretest misperceptions of their own risk far less than those discovering they are e4-negative [94,96]. Those discovering they are e4-positive are more likely to alter their lifestyle in favor of AD-related healthy habits [98], although it is unclear whether such lifestyle changes offer any AD-related benefits.
Future perspective
We believe that the concept of preclinical AD will be a major focus of research in late-life cognitive disorders in the future. There are many challenges ahead before preclinical AD becomes a valid target of diagnosis and therapeutics, but we see it as inevitable because of the difficulties of treating diseases, such as AD, once they become symptomatic. Because of a growing research focus, operationalizing the concept of preclinical AD will soon occur [99,100].
Practice Points.
-
■
The newly developed framework for preclinical Alzheimer’s disease (AD) also includes the recognition that some individuals with preclinical AD may have subtle cognitive decline.
-
■
The subtle cognitive decline in preclinical AD includes alterations in learning and delayed recall.
-
■
Detecting preclinical AD will be very difficult in individual cases because of the many variations in cognitive performance caused by educational background, the effects of other brain diseases and inherent variability in neuropsychological test performance.
-
■
Traditional neuropsychological testing may be able to detect subtle cognitive decline in preclinical AD.
-
■
Newer approaches, including computerized testing and measurement of daily activities using wireless monitoring, are interesting new approaches for detecting subtle cognitive decline but require further testing in selected cohorts before their proper role is known.
-
■
The ethics of disclosing a diagnosis of preclinical AD is an area that requires further thought and consensus building.
Acknowledgements
The authors thank N Silverberg for her review of an earlier version of the manuscript.
DS Knopman was supported by NIH grants P50 AG16574, U01 AG06786 and R01 AG11378, and the Robert H and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation. RJ Caselli was supported by NIH grants P30 AG19610, R01 AG031581, and the Arizona Alzheimer’s Disease Research Consortium. DS Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lilly Pharmaceuticals and is an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals and Forest Pharmaceuticals. RJ Caselli serves as Medical Editor for Clinical Neurology News®.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1. Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch. Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. ■ Showed that the rate of cognitive decline accelerates once the individual meets the criteria for mild cognitive impairment.
- 2.Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann. Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 3.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- 4.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 5.Elias MF, Beiser A, Wolf PA, et al. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham cohort. Arch. Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch. Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grober E, Hall CB, Lipton RB, et al. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxton J, Lopez OL, Ratcliff G, et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 9. Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE ε4 effect. N. Engl. J. Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. ■■ In an analysis of APOE e4 carriers, the trajectory of decline in memory performance was demonstrated, while subjects were still cognitively normal.
- 10.Caselli RJ, Reiman EM, Locke DE, et al. Cognitive domain decline in healthy apolipoprotein E e4 homozygotes before the diagnosis of mild cognitive impairment. Arch. Neurol. 2007;64:1306–1311. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 11.Ringman JM, Diaz-Olavarrieta C, Rodriguez Y, et al. Neuropsychological function in nondemented carriers of presenilin-1 mutations. Neurology. 2005;65:552–558. doi: 10.1212/01.wnl.0000172919.50001.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol. 2011;10:213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 13. Parra MA, Abrahams S, Logie RH, et al. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain. 2010;133:2702–2713. doi: 10.1093/brain/awq148. ■ Novel paradigm for assessing learning and memory.
- 14. Sperling RA, Aisen P, Beckett L, et al. Towards defining the preclinical stage of Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s association workgroup. Alzheimer’s Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. ■■ The criteria for preclinical Alzheimer’s disease (AD) and their rationale are described.
- 15. Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. ■■ Model of AD pathogenesis that describes the sequential and dynamic processes involved.
- 16.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 17.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol. Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Pike KE, Savage G, Villemagne VL, et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 19.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Arch. Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kok E, Haikonen S, Luoto T, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Arch. Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- 24.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch. Gen. Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 25. Jack CR, Jr, Knopman DS, Weigand SD, et al. Preliminary assessment of new criteria for preclinical Alzheimer’s disease. Ann. Neurol. 2011;20:20. ■ In a population-based sample, the frequency of different preclinical stages is described.
- 26.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schott JM, Bartlett JW, Fox NC, Barnes J. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1–42. Ann. Neurol. 2010;68:825–834. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- 28.Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-β measured by [11C] PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caselli RJ, Dueck AC, Locke DE, et al. Longitudinal modeling of frontal cognition in APOE ε4 homozygotes, heterozygotes, and noncarriers. Neurology. 2011;76:1383–1388. doi: 10.1212/WNL.0b013e3182167147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tractenberg RE, Pietrzak RH. Intra-individual variability in Alzheimer’s disease and cognitive aging: definitions, context, and effect sizes. PLoS ONE. 2011;6(4):e16973. doi: 10.1371/journal.pone.0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s disease assessment scale that broaden its scope. the Alzheimer’s disease cooperative study. Alzheimer Dis. Assoc. Disord. 1997;11(Suppl. 2):S13–S21. [PubMed] [Google Scholar]
- 33.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s older americans normative studies updated AVLT norms for ages 59–97. Clin. Neuropsychol. 1992;6(Suppl.):83–104. [Google Scholar]
- 34.Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. Rey’s verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J. Int. Neuropsychol. Soc. 2005;11:290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salthouse TA. Decomposing age correlations on neuropsychological and cognitive variables. J. Int. Neuropsychol. Soc. 2009;15:650–661. doi: 10.1017/S1355617709990385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salthouse TA. Selective review of cognitive aging. J. Int. Neuropsychol. Soc. 2010;16:754–760. doi: 10.1017/S1355617710000706. ■ Discussion of cognitive neuroscience of aging, from an expert in the field.
- 38.Hall CB, Derby C, LeValley A, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- 39.Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 40.Callahan CM, Hall KS, Hui SL, et al. Relationship of age, education, and occupation with dementia among a community-based sample of African Americans. Arch. Neurol. 1996;53:134–140. doi: 10.1001/archneur.1996.00550020038013. [DOI] [PubMed] [Google Scholar]
- 41.Qiu C, Backman L, Winblad B, Aguero-Torres H, Fratiglioni L. The influence of education on clinically diagnosed dementia incidence and mortality data from the Kungsholmen project. Arch. Neurol. 2001;58:2034–2039. doi: 10.1001/archneur.58.12.2034. [DOI] [PubMed] [Google Scholar]
- 42.Brayne C, Ince PG, Keage HA, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133:2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 43.Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Atherosclerosis risk in communities (ARIC) study investigators. Gerontology. 1998;44:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 44.Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain. 2011;134:1479–1492. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rentz DM, Huh TJ, Faust RR, et al. Use of IQ-adjusted norms to predict progressive cognitive decline in highly intelligent older individuals. Neuropsychology. 2004;18:38–49. doi: 10.1037/0894-4105.18.1.38. ■ Graphic demonstration of the interaction of premorbid intellect and tolerance of brain amyloidosis.
- 46.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J. Neuropathol. Exp. Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 47.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N. Engl. J. Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 48.White L. Brain lesions at autopsy in older Japanese–American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu–Asia aging study. J. Alzheimers Dis. 2009;18:713–725. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 49.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu Asia aging study. Neurobiol. Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 50.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 51.Carmelli D, Swan GE, Reed T, et al. Midlife cardiovascular risk factors, APOE, and cognitive decline in elderly male twins. Neurology. 1998;50:1580–1585. doi: 10.1212/wnl.50.6.1580. [DOI] [PubMed] [Google Scholar]
- 52.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu–Asia aging study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 53.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 54.Tzourio C, Dufouil C, Ducimetiere P, Alperovitch A. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. EVA study group. Epidemiology of Vascular Aging. Neurology. 1999;53:1948–1952. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- 55.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE ε4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 56.Glynn RJ, Beckett LA, Hebert LE, et al. Current and remote blood pressure and cognitive decline. JAMA. 1999;281:438–445. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- 57.Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, cognitive function in older women. Arch. Neurol. 2002;59:378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 58.Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch. Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 59.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- 60.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the cardiovascular health study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 61.van der Flier WM, van Straaten EC, Barkhof F, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 62.Saczynski JS, Sigurdsson S, Jonsdottir MK, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke. 2009;40:677–682. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the atherosclerosis risk in communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 64.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the cardiovascular health study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 65.Caselli RJ, Dueck AC, Locke DE, et al. Cerebrovascular risk factors and preclinical memory decline in healthy APOE ε4 homozygotes. Neurology. 2011;76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reitz C, Luchsinger JA, Tang MX, Manly J, Mayeux R. Stroke and memory performance in elderly persons without dementia. Arch. Neurol. 2006;63:571–576. doi: 10.1001/archneur.63.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petrovitch H, White L, Masaki KH, et al. Influence of myocardial infarction, coronary artery bypass surgery, and stroke on cognitive impairment in late life. Am. J. Cardiol. 1998;81:1017–1021. doi: 10.1016/s0002-9149(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 68.Auriacombe S, Helmer C, Amieva H, et al. Validity of the Free and Cued Selective Reminding test in predicting dementia: the 3C study. Neurology. 2010;74:1760–1767. doi: 10.1212/WNL.0b013e3181df0959. [DOI] [PubMed] [Google Scholar]
- 69.Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the california verbal learning test. J. Consult. Clin. Psychol. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 70.Wechsler DA. Wechsler Memory Scale-Revised Psychological Corporation. NY, USA: 1987. [Google Scholar]
- 71.Yassa MA, Lacy JW, Stark SM, et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 doi: 10.1002/hipo.20808. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverberg NB, Ryan LM, Carillo MC, et al. Assessment of cognition in early dementia. Alzheimer’s Dement. 2011;7:e60–e76. doi: 10.1016/j.jalz.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haley GE, Berteau-Pavy F, Parkv B, Raber J. Effects of ε4 on object recognition in the non-demented elderly. Curr. Aging Sci. 2010;3:127–137. doi: 10.2174/1874609811003020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE ε4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007;147:6–17. doi: 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Roark B, Mitchell M, Hosom JP, Hollingshead K, Kaye J. Spoken language derived measures for detecting mild cognitive impairment. IEEE Trans. Audio Speech Lang. Process. 2011;19(7):2081–2090. doi: 10.1109/TASL.2011.2112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Snyder PJ, Jackson CE, Petersen RC, et al. Assessment of cognition in mild cognitive impairment: a comparative study. Alzheimers Dement. 2011;7:338–355. doi: 10.1016/j.jalz.2011.03.009. ■ Broad review of computerized cognitive assessment batteries.
- 77.Teng E, Becker BW, Woo E, et al. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2010 doi: 10.1097/WAD.0b013e3181e2fc84. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tomaszewski Farias S, Mungas D, Harvey DJ, et al. The measurement of everyday cognition: development and validation of a short form of the everyday cognition scales. Alzheimers Dement. 2011;7:593–601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts JL, Clare L, Woods RT. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dement. Geriatr. Cogn. Disord. 2009;28:95–109. doi: 10.1159/000234911. [DOI] [PubMed] [Google Scholar]
- 80.Stewart R, Dufouil C, Godin O, et al. Neuroimaging correlates of subjective memory deficits in a community population. Neurology. 2008;70:1601–1607. doi: 10.1212/01.wnl.0000310982.99438.54. [DOI] [PubMed] [Google Scholar]
- 81.Stewart R, Godin O, Crivello F, et al. Longitudinal neuroimaging correlates of subjective memory impairment: 4 year prospective community study. Br. J. Psychiatry. 2011;198:199–205. doi: 10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- 82.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch. Gen. Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 83.Dufouil C, Fuhrer R, Alperovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. J. Am. Geriatr. Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 84.Jorm AF, Masaki KH, Davis DG, et al. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology. 2004;63:1960–1961. doi: 10.1212/01.wnl.0000144348.70643.f2. [DOI] [PubMed] [Google Scholar]
- 85.Geerlings MI, Jonker C, Bouter LM, Ader HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am. J. Psychiatry. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 86.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of ‘subjective cognitive complaints’ in the Sydney memory and ageing study. Am. J. Geriatr. Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 87.Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the three-city study. Neurology. 2009;73:854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- 88.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 89.Helzner EP, Scarmeas N, Cosentino S, Portet F, Stern Y. Leisure activity and cognitive decline in incident Alzheimer disease. Arch. Neurol. 2007;64:1749–1754. doi: 10.1001/archneur.64.12.1749. [DOI] [PubMed] [Google Scholar]
- 90.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kaye JA, Maxwell SA, Mattek N, et al. Intelligent systems for assessing aging changes: home-based, unobtrusive, and continuous assessment of aging. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2011;66(Suppl. 1):i180–i190. doi: 10.1093/geronb/gbq095. ■ Innovative approach to assessment using home-based monitoring.
- 92.Aisen PS, Andrieu S, Sampaio C, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hiraki S, Chen CA, Roberts JS, Cupples LA, Green RC. Perceptions of familial risk in those seeking a genetic risk assessment for Alzheimer’s disease. J. Genet. Couns. 2009;18:130–136. doi: 10.1007/s10897-008-9194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N. Engl. J. Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. ■ Analysis of the consequences of disclosing APOE genotype: a paradigm for the approach to preclinical AD.
- 95.Eckert SL, Katzen H, Roberts JS, et al. Recall of disclosed apolipoprotein E genotype and lifetime risk estimate for Alzheimer’s disease: the REVEAL study. Genet. Med. 2006;8:746–751. doi: 10.1097/01.gim.0000250197.44245.a3. [DOI] [PubMed] [Google Scholar]
- 96.Ashida S, Koehly LM, Roberts JS, et al. The role of disease perceptions and results sharing in psychological adaptation after genetic susceptibility testing: the REVEAL Study. Eur. J. Hum. Genet. 2010;18:1296–1301. doi: 10.1038/ejhg.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kopits IM, Chen C, Roberts JS, Uhlmann W, Green RC. Willingness to pay for genetic testing for Alzheimer’s disease: a measure of personal utility. Genet. Test Mol. Biomarkers. 2011;15:871–875. doi: 10.1089/gtmb.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chao S, Roberts JS, Marteau TM, et al. Health behavior changes after genetic risk assessment for Alzheimer disease: the REVEAL study. Alzheimer Dis. Assoc. Disord. 2008;22:94–97. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reiman EM, Langbaum JB, Tariot PN. Alzheimer’s prevention initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomark. Med. 2010;4:3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bateman RJ, Aisen PS, De Strooper B, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 2011;3(1):1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]