Abstract
BACKGROUND & AIMS
Expression of the tight junction protein claudin-1 is dysregulated in colon tumors and associates with their progression. Up-regulation of claudin-1 reduces expression of E-cadherin. We investigated the mechanisms by which claudin-1 regulates E-cadherin expression and its effects in colon cancer cells.
MATERIALS AND METHODS
We used gene expression analysis, immunoblotting, and reverse transcription polymerase chain reaction to associate expression of the repressor of transcription Zinc Finger E-box binding homeobox-box1 (ZEB-1) with claudin-1. We analyzed SW480 colon cancer cells that overexpressed claudin-1, or SW620 cells in which claudin-1 expression was repressed, to determine the effects on ZEB-1 and E-cadherin expression, invasive activity, and resistance to anoikis. We studied cells that expressed constitutively active or dominant negative forms of factors in the Wnt or phosphotidylinositol-3-kinase signaling pathways and used pharmacologic inhibitors of these pathways to study their role in claudin-1-dependent regulation of ZEB-1. We used microarray analysis to examine gene expression patterns in 260 colorectal tumor and normal colon samples.
RESULTS
Claudin-1 down-regulates E-cadherin expression by up-regulating expression of ZEB-1. Claudin-1 activates Wnt and phosphotidylinositol-3-kinase/Akt signaling. ZEB-1 mediates claudin-1-regulated changes in cell invasion and anoikis. Expression of claudin-1 correlated with that of ZEB-1 in human colon tumor samples. In the progression from normal colonic epithelium to colon adenocarcinoma, levels of E-cadherin decreased, whereas levels of claudin-1 and ZEB-1 increased. Down-regulation of E-cadherin and up-regulation of ZEB-1 in colon tumors were associated with shorter survival times.
CONCLUSIONS
Claudin-1 up-regulates the repressor ZEB-1 to reduce expression of E-cadherin in colon cancer cells, increasing their invasive activity and reducing anoikis. This pathway is associated with colorectal cancer progression and patient survival.
Keywords: Colorectal Cancer, Transcription Factor, Signal Transduction, Prognostic Factor, Gene Regulation
The claudin family of proteins is the principal constituent of the tight junction.1 Claudins are dynamic proteins and help regulate cellular functions such as proliferation, migration, and differentiation in addition to their tight junction function.2–4 In addition, claudin family members are expressed in tissue-specific manner and appear to be regulated differentially in cancers of diverse tissue origin.5–7 We have reported that claudin-1 expression is highly up-regulated and mislocalized in colon cancer and correlates with tumor progression.5 Other groups have observed similar correlation of claudin-1 expression with the advanced stage of oral squamous cell carcinoma and with angiolymphatic and perineural invasion, consistent with an aggressive tumor phenotype.8
Invasion and metastasis are hallmarks of malignant tumor progression. Notably, loss of E-cadherin expression is a key event during epithelial to mesenchymal transition (EMT) and associates with cell invasion and metastasis. We have shown that modulation of claudin-1 expression in colon cancer cells affects E-cadherin expression in an inverse manner.5 Several transcriptional repressors of E-cadherin gene expression have been identified and include Snail1 (zinc finger protein), Snail2 (Slug), zinc finger E-box binding homeobox 1 (ZEB-1), and zinc finger E-box binding homeobox 2 (ZEB-2).9–111 In addition to Snail1 and Snail2, ZEB-1 was recently identified as a key transcription factor involved in the regulation of EMT and cancer progression in colon and breast cancer.12,13
In the current study, we have validated claudin-1-dependent changes in E-cadherin expression using global gene expression analysis in response to genetic manipulation of claudin-1 expression in colon cancer cells. We have further confirmed the specific role of ZEB-1 in claudin-1-dependent changes in E-cadherin expression, cell invasion, and anoikis. Wnt and phosphotidylinositol-3-kinase (PI-3K)/Akt activation help mediate claudin-1-dependent regulation of ZEB-1 expression. Furthermore, we show positive correlation between claudin-1 and ZEB-1 expressions in colon cancer patients and their potential association with the patient survival. Taken together, our current findings support a key role of claudin-1/ZEB-1/E-cadherin axis in the regulation of malignant colon cancer phenotype.
Materials and Methods
Cell Lines, Human Tissues, and Microarray Analysis
Total RNA from SW480control, SW480claudin-1 (stably over-expressing claudin-1), SW620control, and SW620siRNA (stably inhibited claudin-1 expression) cells was used for microarray analysis using Affymatrix (Santa Clara, CA) 3′-gene expression array. RNA from human samples was hybridized to Affymetrix Human Genome U133 Plus 2.0 GeneChip Expression Array. For details please see Supplementary Materials and Methods.
Plasmids, Reagents, and Luciferase Reporter Assay
For details on plasmids, reagents, and luciferase reporter assay, please see Supplementary Materials and Methods.
Anoikis
Cells were grown in polyHEMA-coated dishes to induce anoikis. Apoptosis was determined using Cell death detection ELISAPLUS kit (Roche Diagnostics Corp (Indianapolis, IN)) as previously described.5
Invasion Assay
The invasion assay was done as described.14
RNA Isolation and Semiquantitative Reverse-Transcription Polymerase Chain Reaction
Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA). One microgram RNA was reverse transcribed using RETROscript Kit (Ambion, Austin, TX), and polymerase chain reaction was done as described.5,14
Statistical Analysis
For statistical analysis, please see Supplementary Materials and Methods.
Results
In Colon Cancer Cells, Modulation of Claudin-1 Expression Alters E-cadherin and ZEB-1 Expression
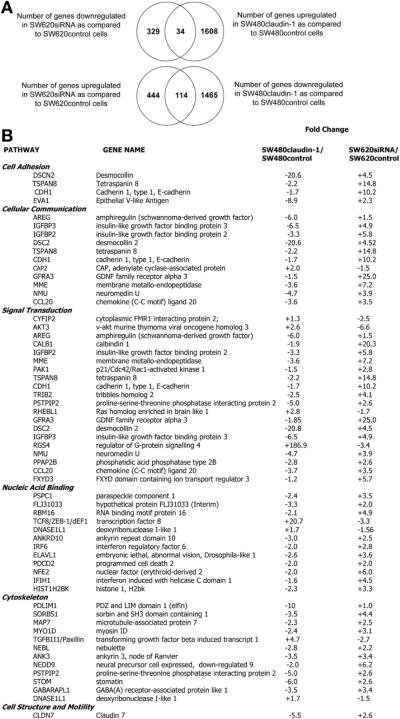
To determine the genes involved in claudin-1–dependent changes in the tumorigenic ability of colon cancer cells, we performed microarray analysis using cells genetically manipulated for claudin-1 expression. Changes in gene expression were analyzed in response to stable overexpression (SW480claudin-1 vs SW480control cells) or silencing (SW620siRNA vs SW620control cells) of claudin-1 expression. Analysis was tightly controlled, and only those genes were selected that showed greater than 2-fold change and were altered in reciprocal manner with the overexpression and silencing of claudin-1 expression compared with respective controls. We identified 34 genes that were significantly up-regulated in SW480claudin-1 cells, however were down-regulated in SW620siRNA cells compared with respective controls. Conversely, we identified 114 genes that were significantly down-regulated in SW480claudin-1 cells and were up-regulated in SW620siRNA cells compared with control cells (Figure 1A, Venn diagram). A list of the above selected genes is provided in a pathway-specific manner in Figure 1B and includes genes associated with cell cytoskeleton, focal adhesion complex, growth factor receptor, and associated signaling such as Desmocollin15 and Insulin-like growth factor binding protein (IGFBP)–2 and –3,16,17 Paxillin,18 and claudin-7,19 which are important in the regulation of colon cancer progression.
Figure 1.
Microarray analysis using claudin-1 manipulated cells. (A) Venn diagram depicting genes up-regulated upon overexpression of claudin-1 in SW480 cells or down-regulated upon silencing of claudin-1 expression in SW620 cells compared with respective control cells (top panel). Bottom panel shows genes down-regulated with claudin-1 overexpression and up-regulated upon silencing of its expression. (B) Table showing pathway specific analysis of genes that were significantly modulated in claudin-1-manipulated cells (P < .001). Gene symbols and associated fold change in the gene expressions are as determined by microarray analysis. Known relationships (connectedness) between up-regulated and down-regulated genes were determined independently, and, when networks existed, functional enrichment within the networks was determined in PubMed and on the Affymetrix Web site.
Our microarray analysis demonstrated that E-cadherin expression also decreased (~2-fold) in SW480claudin-1 vs SW480control cells and increased (~10-fold) in SW620siRNA vs SW620control cells (Figure 1B). These data supported our earlier finding that claudin-1 expression affects E-cadherin expression in colon cancer cells.5 Overexpression of claudin-2, yet another claudin family member, in SW480 cells did not affect E-cadherin expression (data not shown).
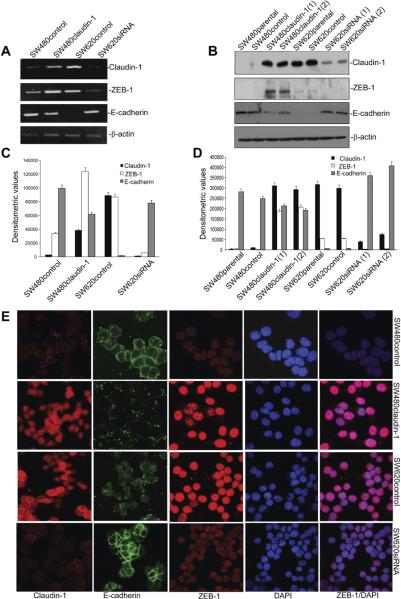
Importantly, neither Snail1 nor Snail2, 2 well-characterized transcriptional repressors of E-cadherin expression, were among the genes significantly altered in claudin-1–manipulated cells (Figure 1B), which was further in accordance with our previous findings.5 However, expression of ZEB-1/TCF-8/δEF-1, yet another E-cadherin transcriptional repressor, was markedly up-regulated (~20.7-fold) in SW480claudin-1 vs SW480control cells and was decreased (~3.3-fold) in SW620siRNA vs SW620control cells. Together, our microarray analysis supported our previous findings and demonstrated positive correlation between claudin-1 and ZEB-1 expressions. We further validated results of our microarray analysis for genes under investigation at messenger RNA (mRNA) and protein levels using semiquantitative reverse-transcription polymerase chain reaction and immunoblot analysis (Figure 2A–D). Immunofluorescent analysis demonstrated predominant nuclear localization of ZEB-1, which colocalized with 4′,6-diamidino-2-phenylindole, a nuclear stain. Results confirmed higher ZEB-1 expression in SW480claudin-1 and SW620control cells compared with SW480control and SW620siRNA cells. In contrast, E-cadherin expression was low and punctate in SW480claudin-1 and SW620control cells but, however, was largely membrane localized in SW480control and SW620siRNA cells (Figure 2E). Thus, our data demonstrated that claudin-1 expression in colon cancer cells correlates positively with ZEB-1 expression and inversely with E-cadherin expression.
Figure 2.
Modulation of claudin-1 expression induces ZEB-1 expression and inhibits E-cadherin expression. (A) Semiquantitative reverse-transcription polymerase chain reaction. (B) Immunoblot analysis. Total RNA or protein lysates from SW480control, SW480claudin-1, SW620control, and SW260siRNA cells were used. (C and D) Densitometric analysis. Bars represent mean ± standard deviation (n = 3). (E) Immunofluorescent staining to determine cellular localization of claudin-1, E-cadherin, and ZEB-1. 4=,6-Diamidino-2-phenylindole (DAPI) served as nuclear marker.
Claudin-1–Dependent Regulation of ZEB-1 Expression Is Unidirectional
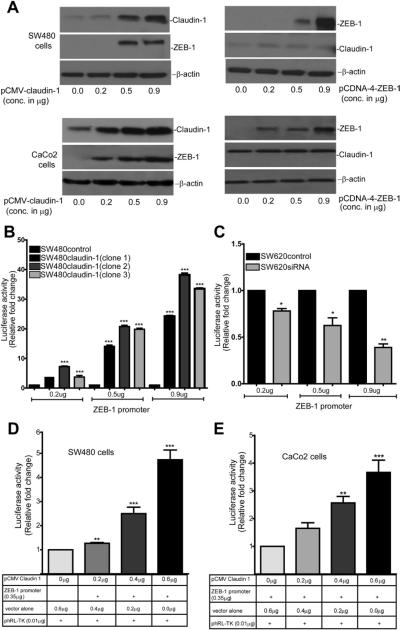
In normal epithelial cells, claudin-1 is membrane localized and is a target of the transcriptional repressors of E-cadherin.20 However, in our current study, not only claudin-1 expression was dysregulated, but its modulation also resulted in altered ZEB-1 and E-cadherin expression. Therefore, we examined whether claudin-1 and ZEB-1 expressions were interdependent. For this, we overexpressed human claudin-1 or ZEB-1 in SW480 and CaCo2 cells and determined the effect on endogenous ZEB-1 and claudin-1 expression respectively. Notably, similar to the effects in SW480claudin-1 vs SW480control cells, transient overexpression of claudin-1 also increased ZEB-1 expression in both cell lines in dose-dependent manner. In contrast, ZEB-1 overexpression in the same cells did not affect claudin-1 expression (Figure 3A).
Figure 3.
Claudin-1 regulates ZEB-1 expression through the regulation of mRNA transcription. (A) SW480 and CaCo2 cells were transiently transfected with claudin-1 or ZEB-1 expression plasmids (progressively increasing amount). Immunoblot analysis was done to examine respective expressions. β-Actin served as loading control. Effect of claudin-1 overexpression on ZEB-1 promoter activity in (B) SW480control and SW480claudin-1 (3 independent clones) cells or (C) SW620control and SW620siRNA cells. ZEB-1 promoter (0.2, 0.5, or 0.9 μg) was transiently transfected in each cell type. Values represents mean ± standard deviation from 3 independent studies (***P < .001, **P <.01). Effect of transient cotransfection of human ZEB-1 promoter with claudin-1 expression plasmid (0.2, 0.4, or 0.6 μg) (D) in SW480 and (E) CaCo2 cells. Values represents mean ± standard deviation of 3 independent experiments (**P < .01, ***P < .001).
Claudin-1 Regulates ZEB-1 mRNA Transcription
The above data suggested claudin-1-dependent regulation of ZEB-1 protein and mRNA expression in colon cancer cells. Therefore, we assessed the possibility of claudin-1-dependent regulation of ZEB-1 mRNA transcription. To test, we performed luciferase reporter assay using a human ZEB-1 promoter-luciferase reporter (referred in this manuscript as pGL3/ZEB-1, a gift from Dr Nakshatri21). SW480control, SW480claudin-1, SW620control, and SW620siRNA cells were transfected with pGL3/ZEB-1 (progressively increasing amounts) along with the renilla reporter plasmid. Resultant luciferase activity was significantly higher (P < .001) in SW480claudin-1 cells compared with SW480control cells (Figure 3B). In contrast, pGL3/ZEB-1-luciferase activity was inhibited in SW620siRNA cells compared with SW620control cells (Figure 3C). We further determined whether changes in ZEB-1 transcription in claudin-1-manipulated cells were direct effect of the changes in claudin-1 expression. For this purpose, we performed transient cotransfection of pGL3/ZEB-1 (constant amount) and pCMV-claudin-1 (full-length claudin-1 complementary DNA; increasing concentration) in SW480 and CaCo2 cells. Similar to our observations in SW480claudin-1 cells, transient overexpression of claudin-1 also increased ZEB-1 promoter activity (4- to 5-fold, P < .001; Figure 3D and E). Thus, our data suggested that claudin-1 regulates ZEB-1 expression through the regulation of mRNA transcription.
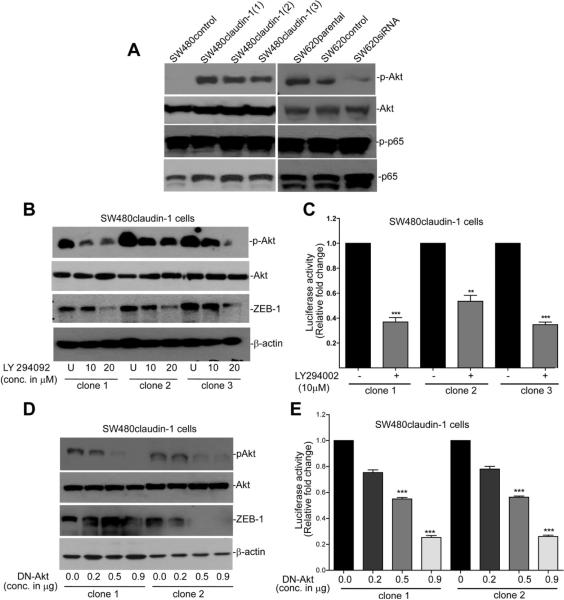
PI-3Kinase/Akt Signaling Mediates Claudin-1-Dependent Regulation of ZEB-1 Expression
Nuclear factor (NF)-κB and PI-3K/Akt signaling, key players in the regulation of EMT and tumorigenesis, have emerged as important regulators of ZEB-1 transcription.21,22 Therefore, we determined whether claudin-1-dependent regulation of ZEB-1 expression was dependent on potential modulation of PI-3K/Akt and/or NF-κB activation. We determined p65-phosphorylation to detect NF-kB activation and Akt-phosphorylation (Ser-473) to evaluate PI-3K/Akt activation. Modulation of claudin-1 expression had no obvious effect on p65-phosphorylation; however, Akt phosphorylation was highly up-regulated in SW480claudin-1 vs SW480control cells and decreased in SW620siRNA vs SW620control cells (Figure 4A). Therefore, we further examined causal association between Akt phosphorylation and ZEB-1 expression in claudin-1-manipulated cells. We used LY294002, a PI-3K selective inhibitor for this purpose SW480claudin-1 (3 independent clones) cells were treated with LY294002 (10 or 20 μmol/L) for 24 hours in regular culture medium, and the effect on ZEB-1 expression was determined. As expected, LY294002 inhibited Akt phosphorylation; however, it also inhibited ZEB-1 expression (Figure 4B). We then determined whether LY294002 treatment also inhibits ZEB-1 mRNA transcription. As shown in Figure 4C, inhibition of Akt phosphorylation indeed inhibited pGL3/ZEB-1-luciferase reporter activity (P < .05) and thus suggested the role of PI-3K/Akt signaling in claudin-1-dependent regulation of ZEB-1 transcription. We obtained similar decrease in ZEB-1 protein expression and promoter activity upon expression of dominant negative (DN)-Akt (Figure 4D and E). Taken together, our data supported the role of PI-3K/Akt signaling in claudin-1-dependent regulation of ZEB-1 expression.
Figure 4.
Claudin-1 regulates ZEB-1 expression in PI-3K/Akt-dependent manner. (A) Immunoblot analysis using antigen-specific phospho or nonphospho antibodies. (B) Effect of LY294092 upon ZEB-1 expression. SW480claudin-1 (clones 1, 2, and 3) cells were grown in the presence of vehicle (U) or LY294092 (10 or 20 μmol/L) for 24 hours. Resultant cell lysates were used to determine ZEB-1, p-Akt, Akt, or β-actin expressions. (C) Effect of LY294092-treatment upon ZEB-1 promoter activity. Cells were cotransfected with PGL3/ZEB-1 reporter and phRL-TK constructs and were treated with the vehicle (U) or LY294092 (10 μmol/L) for 24 hours. Effect of DN-Akt expression upon (D) ZEB-1 protein expression. (E) ZEB-1 promoter activity. Cells were cotransfected with PGL3/ZEB-1 and DN-Akt constructs. Promoter activity is presented as fold change relative to renilla activity. Mean ± standard deviation of at least 3 independent experiments (*P < .05, **P < .01). DN, dominant negative; p-Akt, phospho-Akt.
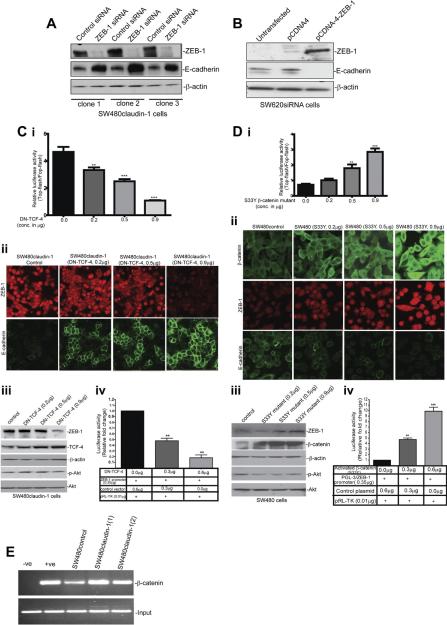
ZEB-1 Mediates Claudin-1-Dependent Regulation of E-Cadherin Expression
Next, we determined causal role of ZEB-1 expression in claudin-1-dependent changes in E-cadherin expression. In this regard, we silenced ZEB-1 expression in SW480claudin-1 cells (high ZEB-1 and low E-cadherin expression) using human ZEB-1-specific siRNA. Inhibition of ZEB-1 expression resulted in increased E-cadherin expression compared with SW480claudin-1 cells transfected with the control siRNA small interfering, despite stable claudin-1 overexpression, and thus supported the role of ZEB-1 in claudin-1-dependent changes in E-cadherin expression. To further validate, we overexpressed ZEB-1 full-length complementary DNA in SW620siRNA cells (claudin-1 expression is stably repressed, ZEB-1 expression is low, and E-cadherin expression is high) (Figure 5B, lane 3). Control cells received empty vector (Figure 5B, lane 2). Forced ZEB-1 expression in SW620siRNA cells resulted in decreased E-cadherin expression despite repression of claudin-1 expression. Taken together, the above studies supported a causal role of ZEB-1 in claudin-1-dependent changes in E-cadherin expression.
Figure 5.
ZEB-1 expression helps mediate effect of claudin-1 expression upon E-cadherin expression and is regulated by Wnt signaling. (A) Effect of human ZEB-1 siRNA upon ZEB-1 and E-cadherin expression in SW480claudin-1 cells (3 independent clones). (B) Effect of the forced expression of ZEB-1 upon E-cadherin expression in SW620siRNA cells. (C) Effect of the forced expression of DN-TCF-4 upon (i) Top-flash activity, (ii) cellular localization of ZEB-1 and E-cadherin, (iii) ZEB-1 expression, and (iv) ZEB-1 promoter activity. (D) Effect of the forced expression of β-catenin (S33Y) CA-mutant upon (i) Top-flash activity; (ii) cellular localization of ZEB-1, β-catenin, and E-cadherin; (iii) ZEB-1 expression; and (iv) ZEB-1 promoter activity. (E) Chromatin immunoprecipitation (ChIP) analysis to determine binding of β-catenin to human ZEB-1 promoter. −ve, IgG control; +ve, Anti-Pol II antibody. CA, constitutively active.
Modulation of Wnt Signaling Alters ZEB-1 Expression in Claudin-1-Manipulated Cells
Loss of E-cadherin expression results in the translocation of β-catenin to cell cytoplasm and nucleus. Nuclear β-catenin, in turn, induces gene expression program favoring tumor invasion, and mounting evidence indicates reciprocal interaction of E-cadherin and β-catenin with EMT-inducing transcriptional repressors. We have reported that claudin-1 overexpression in colon cancer cells results in the loss of E-cadherin expression and nuclear localization of β-catenin.5 Because our current data demonstrated positive influence of claudin-1 expression upon ZEB-1 expression, we further determined the potential role of β-catenin activation in claudin-1-dependent regulation of ZEB-1 expression. In this regard, we overexpressed DN-TCF-4 in SW480claudin-1 cells (high claudin-1 expression, high Wnt activity). Conversely, we overexpressed constitutively active β-catenin mutant (S33Y) in SW480 cells (low claudin-1 expression, low Wnt activity). Top-flash reporter assay, an index of β-catenin activity, was used to ensure the functional activation of the transfected DN-TCF-4. As expected, expression of DN-TCF-4 inhibited Top-flash activity in a dose-dependent manner (Figure 5C, i). DN-TCF-4 expression also resulted in the inhibition of ZEB-1 expression, whereas E-cadherin expression increased (Figure 5C, ii and iii; Supplementary Figure 3). In contrast, expression of S33Y-β-catenin mutant in SW480 cells resulted in dose-dependent increase in Top-flash activity, nuclear β-catenin, and ZEB-1 expression, whereas E-cadherin expression decreased (Figure 5D, i–iii). This increase in ZEB-1 expression was predominantly nuclear while E-cadherin expression was mislocalized/punctate (Figure 2E, Supplementary Figures 2 and 3).
We further evaluated effect of Wnt signaling upon ZEB-1 promoter activity. For this, pGL3/ZEB-1 was cotransfected with the S33Y-β-catenin mutant in SW480 cells or with the DN-TCF-4 in SW480claudin-1 cells. The ZEB-1 promoter activity increased with the expression of S33Y-β-catenin (Figure 5C, iv) and decreased with the expression of DN-TCF-4 (Figure 5D, iv). Thus, our data supported the role of Wnt activation in claudin-1-dependent regulation of ZEB-1 expression.
Direct activation of Wnt signaling by TCF/β-catenin transcription factors requires presence of TCF-binding site(s) in the promoter of targeting gene. Therefore, we analyzed the 801-base pair human ZEB-1 promoter (used in our studies) using the software “Matinspector” (Genomatix software, Munich, Germany) and found a consensus TCF/LEF binding site (5′-TCAAT-3′). To test binding of β-catenin to this promoter and effect of claudin-1 expression upon such binding, we performed chromatin immunoprecipitation assays using β-catenin-targeting monoclonal antibody. Immunoglobulin G served as negative antibody control. We used SW480control and SW480claudin-1 cells for this purpose. The quantitative polymerase chain reaction analysis using primers encompassing the TCF/LEF site in the ZEB-1 promoter demonstrated efficient pull down in SW480control cells and thus supported binding of β-catenin to ZEB-1 promoter. This binding further increased in SW480claudin-1 cells (Figure 5E). Importantly, further determination of potential correlation between ZEB-1 and β-catenin expressions in colon cancer patients (using a large patient database; Supplementary Material) demonstrated a significant and positive correlation (P < .001; Supplementary Figure 4).
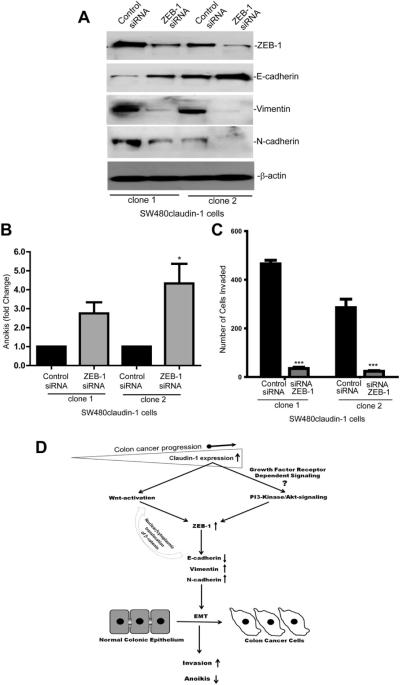
ZEB-1 Expression Helps Regulate Claudin-1-Dependent Changes in the Tumorigenic Ability of Colon Cancer Cells
Claudin-1 expression correlates with colon cancer progression and metastasis.5,8 In addition, claudin-1 overexpression in colon cancer cells enhances cell invasion and resistance to anoikis.5 ZEB-1 expression associates with similar cancer phenotype.12,13 Therefore, we examined whether ZEB-1 helps regulate claudin-1-dependent changes in colon cancer cell invasiveness and resistance to anoikis. For this, we performed siRNA-dependent silencing of ZEB-1 expression in SW480claudin-1 cells. Notably, silencing of ZEB-1 expression resulted in increased E-cadherin expression, whereas expression of mesenchymal markers vimentin and N-cadherin decreased (Figure 6A). This inhibition of ZEB-1 expression also resulted in a significant increase in anoikis compared with the cells that received control siRNA (P < .01, Figure 6B). We also observed a ~10-fold decrease in cell invasion in SW480claudin-1 cells where ZEB-1 expression was silenced compared with cells transfected with control siRNA (P < .001, Figure 6C). Taken together, our data suggested central role of ZEB-1 expression in claudin-1-dependent changes in invasion and anoikis in colon cancer cells. A postulated model depicting claudin-1-dependent regulation of ZEB-1 expression, associated signaling, and cancer-related functions is presented in Figure 6D.
Figure 6.
ZEB-1 helps regulate claudin-1-dependent changes in the tumorigenic ability of colon cancer cells. (A) SW480claudin-1 cells were transfected with the control or human ZEB-1-specific siRNA. Resultant lysates were subjected to immunoblot analysis to determine ZEB-1, E-cadherin, Vimentin, N-cadherin, or β-actin expression. (B) Effect of ZEB-1 expression upon claudin-1-dependent resistance to anoikis (*P < .05). (C) Effect of ZEB-1 expression upon claudin-1-mediated effects on cell invasion (***P < .001). Bars represent mean ± standard deviation of 3 different experiments. (D) Model depicting claudin-1-dependent regulation of ZEB-1 expression, associated signaling pathways, and cancer-related cellular functions.
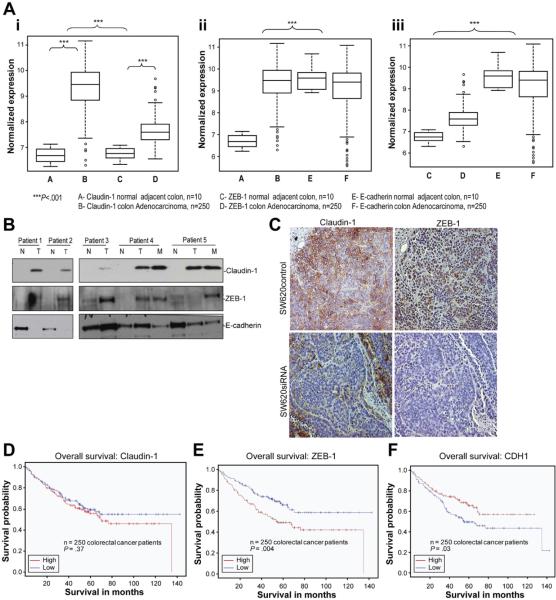
In Colon Cancer Patient Samples, ZEB-1 Expression Correlates Positively With Claudin-1 Expression, Whereas Both Claudin-1 and ZEB-1 Expression Correlate Inversely With E-Cadherin Expression
Based on our novel findings, we assessed potential clinical relationship among ZEB-1, claudin-1, and E-cadherin expressions. We used microarray analysis using 250 colon cancer patient tumor samples and 10 normal adjacent tissue samples (demographics in Supplementary Figure 1). We noted significant up-regulation of both claudin-1 and ZEB-1 expression in colorectal tumors compared with normal colon mucosa (Figure 7A, i; P <.001). Immunohistochemical analysis demonstrated predominant nuclear expression of ZEB-1 in the colon cancer samples and supported increase in its expression in the cancer samples compared with normal adjacent colon (Supplementary Figure 5). Furthermore, when claudin-1 and ZEB-1 expression levels were compared with E-cadherin, we observed significant inverse expression patterns for both claudin-1 vs E-cadherin expression (Figure 7A, ii; P < .001) and ZEB-1 vs E-cadherin expression (Figure 7A, iii; P < .001) between colorectal adenocarcinomas and normal adjacent mucosal specimen. These clinical data show significant association between low E-cadherin expression and parallel up-regulation of claudin-1 and ZEB-1 expressions in the progression from normal colonic epithelium to colon adenocarcinoma. We also determined claudin-1, ZEB-1, and E-cadherin expression in tumor specimen from 5 colorectal cancer patients compared with normal adjacent colon and metastatic lesions (if available) using immunoblot analysis. We noted increase in claudin-1 and ZEB-1 expressions in all tumor samples compared with normal colon and down-regulation of E-cadherin in 4 of the tumors and 1 of the metastatic lesions compared with normal tissues (Figure 7B). These data supported our findings by expression analysis. To confirm further this correlation, we examined ZEB-1 expression in xenografts resulting from the use of SW620control and SW620siRNA cells in vivo in athymic nude mice. Immunohistochemical analysis showed that, similar to claudin-1 expression, ZEB-1 expression was high in SW620control xenografts, however, was decreased in the xenografts resulting from the use of SW620siRNA cells (Figure 7C).
Figure 7.
Determination of Claudin-1, E-cadherin, and ZEB-1 expression in human colorectal cancer: correlation and outcome analysis. (A) Normalized expression for claudin-1, E-cadherin, and ZEB-1 in 250 colon adenocarcinoma and 10 normal adjacent specimens using microarray analysis: (i) Claudin-1 and ZEB-1 expressions were significantly up-regulated in the adenocarcinoma group compared with the adjacent normal group (***P < .001). Median and standard deviations are shown. (ii) Claudin-1 and E-cadherin expressions show inverse expression pattern in colorectal cancer patients compared with normal adjacent tissue specimens (***P < .001), and (iii) ZEB-1 and E-cadherin expressions show inverse expression pattern in colorectal cancer patients compared with normal adjacent tissues (***P < .001). (B) Claudin-1, ZEB-1, and E-cadherin expression in matched normal (N), primary (T), and metastasis (M) human colon tumor samples. (C) Xenograft tumors resulting from the use of SW620control or SW620siRNA cells in vivo in nude mice were immunostained to detect claudin-1 or ZEB-1 expression. (D) Comparison of the overall survival in colon cancer patients in correlation with claudin-1 expression. Patients with lower claudin-1 expression (blue line) show a trend toward better survival than patients with high claudin-1 expression (red line) (P = .37). (E and F) Comparison of overall survival in colorectal cancer patient data for ZEB-1 and E-cadherin (CDH1) expression. High expression in this group of colorectal cancer patients was defined as greater than median expression and was compared with patients with low expression (less than the median expression value). Kaplan–Meier analysis was performed, comparing patients with high ZEB-1 expression (red line) to low ZEB-1 expression (blue line) (E) or patients with high E-cadherin expression (red line) to low E-cadherin expression (blue line) (F). Higher expression of ZEB-1 (P = .004) and lower expression of E-cadherin (P = .03) correlated with significantly worse survival in this group of colorectal cancer patients.
High Claudin-1, ZEB-1 Expression and Low E-cadherin Expression Associate With Poor Prognosis and Patient Survival
Given the inverse expression patterns noted among claudin-1, ZEB-1, and E-cadherin expression, we asked whether high claudin-1 and ZEB-1 expression, and low E-cadherin expression would identify high-risk colorectal cancer patients. Survival estimates based on claudin-1, ZEB-1, and E-cadherin expression were determined in above described colorectal cancer patient database. We used a median cut-off for claudin-1, ZEB-1, and E-cadherin expression (higher-than-median = high expression and lower-than-median = low expression). We noted a trend toward better overall survival for patients with lower-than-median claudin-1 expression (Figure 7D, P = .37). We also observed significant overall survival differences for patients according to ZEB-1 and E-cadherin expression (P = .004 and P = .03, respectively, Figure 7E and F). Specifically, patients exhibiting high ZEB-1 expression had worse overall survival than low ZEB-1 expressing patients, whereas high E-cadherin expressing patients had better overall survival than low E-cadherin expressing patients. We also noted significant differences in the outcomes using disease-free survival for both ZEB-1 and E-cadherin (data not shown). These data provide in vivo evidence that ZEB-1 and E-cadherin downstream of claudin-1 may be useful biomarkers in colorectal cancer to indicate invasive tumor biology and poor prognosis.
Discussion
Metastasis to vital organs is the major cause of mortality among colon cancer patients. Thus, for effective disease management, it is important that we understand mechanism(s) that help colon cancer cells acquire invasive/metastatic potential. In this regard, data from our and other laboratories support association of claudin-1 with cancer growth and progression in colon cancer along with liver, oral squamous, and melanoma cancers.3,5,23,24
Importantly, a key finding in our earlier studies was that manipulation of claudin-1 expression in colon cancer cells affects E-cadherin expression in an inverse manner.5 This finding was novel and clinically important because E-cadherin is an established epithelial marker and indicator of poor prognosis.25 Our data gain support from the findings of Lioni et al, which demonstrated similar inverse effect of claudin-7 expression, a claudin family member, upon E-cadherin expression in esophageal carcinoma cells.26 Notably, similar to our findings, claudin-7-dependent regulation of E-cadherin was unidirectional because forced E-cadherin expression in the same cells did not affect claudin-7 expression.26 Inhibition of junctional adhesion molecule A, yet another tight junction protein, induces E-cadherin transcription in hepatic cells.27 However, no study has yet determined the mechanism(s) that may underlie the regulation of E-cadherin by the tight junction proteins including claudin-1.
Transcriptional repression is a key regulatory mechanism of the regulation of E-cadherin expression. In our studies, claudin-1 regulates E-cadherin mRNA expression. Interestingly, Snail1 and Snail2, transcriptional repressors often implicated in the transcriptional repression of E-cadherin, were not affected by modulation of claudin-1 expression.5 However, we did identify ZEB-1/TCF-8/δEF-1, yet another transcriptional repressor of E-cadherin, among genes that were up-regulated upon overexpression of claudin-1 and were decreased with the inhibition of claudin-1 expression. However, modulation of claudin-1 expression in colon cancer cells also has positive influence on EMT. Therefore, we determined whether observed changes in ZEB-1 and E-cadherin expression are not simply effects of the differentiation status of the cells under study. The fact that transient overexpression of claudin-1 resulted in increased ZEB-1 expression in 2 different colon cancer cell lines support a causal association between claudin-1 and ZEB-1 expression. Transient overexpression of ZEB-1 in the same cells did not affect claudin-1 expression and thus excluded the possibility of reciprocal regulation.
Our studies further demonstrated the importance of ZEB-1 expression in claudin-1-dependent regulation of cell invasion and anoikis in colon cancer cells. Invasion and resistance to anoikis are characteristics associated with the aggressiveness of cancer cells and thus support a key role of claudin-1/ZEB-1/E-cadherin axis in the regulation of colon cancer progression. Such a proposition gains strong support from our data from patient samples and potential correlation of patient survival with ZEB-1 expression. Notably, epithelial carcinogen-esis associates with the loss of tissue architecture (loss of organized cell-cell and cell-matrix adhesions) allowing migration of malignant cells from their immediate niche. Thus, we postulate that claudin-1-dependent regulation of E-cadherin expression is one of the mechanisms that may contribute to the underlying loss of epithelial architecture and thereby help initiate invasion. Importantly, loss of membrane localized E-cadherin expression would result in the translocation of β-catenin to the nucleus where it will activate a target gene expression program, linking EMT to Wnt signaling. However, Wnt signaling might also be linked to EMT, by direct/indirect activation of snail2 or ZEB-1 via other Wnt target genes (eg, cyclooxygenase-2 or insulin-like growth factor-I).28 This suggests a feed-forward loop of dedifferentiation in invading cancer cells in the colorectal carcinoma.
Our data that show inhibition of Wnt signaling in SW480claudin-1 cells decreases ZEB-1 expression and promoter activity and restores E-cadherin expression while its activation in SW480control cells results in reciprocal outcome support the notion of such a feed-forward loop. Thus, claudin-1 overexpression in colon cancer cells increases ZEB-1 expression, which, in turn, decreases E-cadherin expression. Loss of membrane anchored E-cadherin expression would then lead to nuclear translocation of β-catenin and thereby Wnt activation. Wnt signaling, in turn, will increase binding of activated β-catenin with the ZEB-1 promoter, increasing ZEB-1 mRNA transcription and thus induce dedifferentiation, invasion, and malignant behavior. Importantly, published studies of ours and other groups suggest that claudin-1 itself is a target of Wnt signaling in colon cancer; however, it also regulates cellular distribution of β-catenin and thus potentially its activation.5,29 Outcome of our studies using ZEB-1 promoter support transcriptional regulation as the major mechanism underlying claudin-1-dependent changes in ZEB-1 expression, which involves Wnt and PI-3K/Akt signaling. Importantly, oncogenic signaling pathways including growth factor (epidermal growth factor, insulin-like growth factor) receptor-dependent signaling as well as survival mechanisms including NF-κB and PI-3K/Akt pathways help regulate ZEB-1 expression.21,30 It should be noted that the above regulatory pathways are constitutively activated during tumorigenesis including colon carcinogenesis and are associated with cancer progression In this regard, PI-3K/Akt signaling represses E-cadherin transcription by stabilizing the function of transcriptional repressors Snail and Slug.30 It is possible that similar mechanism may operate in claudin-1-dependent regulation of ZEB-1 expression in colon cancer cells, however, remains to be determined. Notably, manipulation of Wnt signaling in our current study altered ZEB-1 expression without affecting Akt phosphorylation and thus suggests that PI-3K/Akt-mediated regulation of ZEB-1 is either distinct or upstream of the Wnt signaling. Taken together, our data suggest complex regulation of ZEB-1 expression in response to the changes in claudin-1 expression and supports its key role in the regulation of claudin-1-dependent changes in colon cancer.
In summary, our current findings demonstrate novel role of claudin-1 in the reduction of E-cadherin expression through specific up-regulation of ZEB-1 in colon cancer. Most importantly, our analysis using gene-specific manipulation along with data from colon cancer patient samples support important role of claudin-1/ZEB-1/E-cadherin axis in the regulation of colon cancer progression and metastasis. Moreover, we demonstrate that increased ZEB-1 expression and decreased E-cadherin expression are independent predictors of poor clinical outcome in colon cancer. The fact that claudin-1 did not rate to the same extent as ZEB-1 as the marker of clinical outcome suggests that cellular localization (membrane vs cytoplasm and/or nucleus) of claudin-1 protein along with its cellular content may help define the fate of colon cancer cells. Of interest, cytoplasmic/nuclear localized claudin-1 is more evident in advanced colon cancer cases.5 Irrespective, our studies confirm a modulatory effect of claudin-1 protein upon ZEB-1 expression. Further investigations are required to understand better the roles of claudin-1, ZEB-1, and E-cadherin in colon cancer progression and malignancy and to determine how dysregulation of these genes/proteins may alter responses to therapeutic intervention.
Supplementary Material
Acknowledgments
A.B.S. and A.S. contributed equally to the work.
Funding Supported by NIH grant CA124977 (to P.D.); 5P50DK044757 and P30DK058406 Pilot projects, DK088902 (to A.B.S.); the Society of University Surgeons-Ethicon Scholarship Award (to J.J.S.)CA112215 (to T.J.Y.); DK052334, CA069457, and the GI Cancer SPORE grant CA95103 (to R.D.B.); the Vanderbilt-Ingram Cancer Center grant P30CA68485; and the NIH grant supporting the Digestive Diseases Center, DK58404.
Abbreviations used in this paper
- DN
dominant negative
- EMT
epithelial to mesenchymal transition
- LEF
lymphoid-enhancer binding factor
- mRNA
messenger RNA
- NF-κB
nuclear factor-κB
- PI-3K
phosphotidylinositol-3-kinase
- ZEB-1
Zinc Finger E-box binding homeobox-box1
Footnotes
Supplementary data Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.08.038.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Tsukita S, Furuse M. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol. 2002;14:531–536. doi: 10.1016/s0955-0674(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 2.Ikari A, Takiguchi A, Atomi K, et al. Decrease in claudin-2 expression enhances cell migration in renal epithelial Madin-Darby canine kidney cells. J Cell Physiol. 2011;226(6):1471–1478. doi: 10.1002/jcp.22386. [DOI] [PubMed] [Google Scholar]
- 3.Yoon C-H, Kim M-J, Park M-J, et al. Claudin-1 acts through c-Abl-protein kinase CI (PKCI) signaling and has a causal role in the acquisition of invasive capacity in human liver cells. J Biol Chem. 2010;285:226–233. doi: 10.1074/jbc.M109.054189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JW, Hsiao WT, Chen HY, et al. Up-regulated claudin-1 expression confers resistance to cell death of nasopharyngeal carcinoma cells. Int J Cancer. 2010;126:1353–1366. doi: 10.1002/ijc.24857. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan P, Singh AB, Deane NG, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda H, Pazin MJ, D'Souza T, et al. Regulation of the CLDN3 gene in ovarian cancer cells. Cancer Biol Ther. 2007;6:1733–1742. doi: 10.4161/cbt.6.11.4832. [DOI] [PubMed] [Google Scholar]
- 7.Michl P, Barth C, Buchholz M, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265–6271. [PubMed] [Google Scholar]
- 8.dos Reis PP, Bharadwaj RR, Machado J, et al. Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer. 2008;113:3169–3180. doi: 10.1002/cncr.23934. [DOI] [PubMed] [Google Scholar]
- 9.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–787. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolos V, Peinado H, Perez-Moreno MA, et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 11.Comijn J, Berx G, Vermassen P, et al. The two-handed E Box Binding Zinc Finger Protein SIP1 down-regulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 12.Aigner K, Dampier B, Descovich L, et al. The transcription factor ZEB1 ([δ]EF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spaderna S, Schmalhofer O, Wahlbuhl M, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 14.Shiou SR, Singh AB, Moorthy K, et al. Smad4 regulates claudin-1 expression in a transforming growth factor-β-independent manner in colon cancer cells. Cancer Res. 2007;67:1571–1579. doi: 10.1158/0008-5472.CAN-06-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan K, Hardy R, Haq A, et al. Desmocollin switching in colorectal cancer. Br J Cancer. 2006;95:1367–1370. doi: 10.1038/sj.bjc.6603453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl D, Hessel E, Oesterle D, et al. IGFBP-2 overexpression reduces the appearance of dysplastic aberrant crypt foci and inhibits growth of adenomas in chemically induced colorectal carcinogenesis. Int J Cancer. 2009;124:2220–2225. doi: 10.1002/ijc.24193. [DOI] [PubMed] [Google Scholar]
- 17.Williams AC, Smartt H, H-Zadeh AM, et al. Insulin-like growth factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-[κ]B. Cell Death Differ. 2006;14:137–145. doi: 10.1038/sj.cdd.4401919. [DOI] [PubMed] [Google Scholar]
- 18.Sachdev S, Bu Y, Gelman IH. Paxillin-Y118 phosphorylation contributes to the control of Src-induced anchorage-independent growth by FAK and adhesion. BMC Cancer. 2009;9:12. doi: 10.1186/1471-2407-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darido C, Buchert M, Pannequin J, et al. Defective claudin-7 regulation by Tcf-4 and Sox-9 disrupts the polarity and increases the tumorigenicity of colorectal cancer cells. Cancer Res. 2008;68:4258–4268. doi: 10.1158/0008-5472.CAN-07-5805. [DOI] [PubMed] [Google Scholar]
- 20.Marta–nez-estrada OM, Culleracs A, Soriano FX, et al. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-[κ]B represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2006;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 22.Hong KO, Kim JH, Hong JS, et al. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J Exp Clin Cancer Res. 2009;28:28. doi: 10.1186/1756-9966-28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leotlela PD, Wade MS, Duray PH, et al. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene. 2006;26:3846–3856. doi: 10.1038/sj.onc.1210155. [DOI] [PubMed] [Google Scholar]
- 24.Oku N, Sasabe E, Ueta E, et al. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006;66:5251–5257. doi: 10.1158/0008-5472.CAN-05-4478. [DOI] [PubMed] [Google Scholar]
- 25.Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24:73–76. doi: 10.1016/s0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 26.Lioni M, Brafford P, Andl C, et al. Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am J Pathol. 2007;170:709–721. doi: 10.2353/ajpath.2007.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konopka G, Tekiela J, Iverson M, et al. Junctional adhesion molecule-A is critical for the formation of pseudocanaliculi and modulates E-cadherin expression in hepatic cells. J Biol Chem. 2007;282:28137–28148. doi: 10.1074/jbc.M703592200. [DOI] [PubMed] [Google Scholar]
- 28.Stemmer V, de Craene B, Berx G, et al. Snail promotes Wnt target gene expression and interacts with [β]-catenin. Oncogene. 2008;27:5075–5080. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- 29.Miwa N, Furuse M, Tsukita S, et al. Involvement of claudin-1 in the β-catenin/Tcf signaling pathway and its frequent up-regulation in human colorectal cancers. Oncol Res. 2000;12:469–476. doi: 10.3727/096504001108747477. [DOI] [PubMed] [Google Scholar]
- 30.Grille SJ, Bellacosa A, Upson J, et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Research. 2003;63:2172–2178. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.