Abstract
Introduction
Idiopathic pulmonary fibrosis (IPF) is a devastating progressive lung disease with an average survival of only 3 to 5 years. The mechanisms underlying the initiation and progression of IPF are poorly understood, and treatments available have only modest effect on disease progression. Interestingly, the incidence of IPF is approximately 60 times more common in individuals aged 75 years and older, but the mechanism by which aging promotes fibrosis is unclear. The authors hypothesized that aged lungs have a profibrotic phenotype that render it susceptible to disrepair after injury.
Methods
Young and old mice were treated with bleomycin to examine disrepair in the aged lung. In addition, uninjured young and old mouse lungs were analyzed for transforming growth factor-beta 1 (TGF-β1) production, extracellular matrix composition and lung fibroblast phenotype. Lung fibroblasts were treated with a DNA methyltransferase inhibitor to examine the potential epigenetic mechanisms involved in age-associated phenotypic alterations.
Results
The lungs of old mice showed worse fibrosis after bleomycin-induced injury compared with the lungs from young mice. At baseline, aged lungs expressed a profibrotic phenotype characterized by increased mRNA expression for fibronectin extracellular domain A (Fn-EDA) and the matrix metalloproteinases (MMPs) MMP-2 and MMP-9. Old lungs also expressed higher levels of TGF-β receptor 1 and TGF-β1 mRNA, protein and activity as determined by increased Smad3 expression, protein phosphorylation and DNA binding. Lung fibroblasts harvested from aged lungs showed reduced expression of the surface molecule Thy-1, a finding also implicated in lung fibrosis; the latter did not seem related to Thy-1 gene methylation.
Conclusion
Altogether, aged lungs manifest a profibrotic phenotype characterized by enhanced fibronectin extracellular domain A and MMP expression and increased TGF-β1 expression and signaling and are populated by Thy-1–negative fibroblasts, all implicated in the pathogenesis of lung fibrosis.
Key Indexing Terms: Lung fibrosis, Aging, Injury, Extracellular matrix, Lung fibroblast
Idiopathic pulmonary fibrosis (IPF) is a devastating chronic progressive pulmonary disease with high morbidity and mortality. Its median survival has been reported to be between 3 and 5 years.1 The pathogenic mechanisms involved in the initiation and progression of IPF are poorly understood, and there are no effective treatments.1,2 Interestingly, the incidence of IPF increases with age, being approximately 60 times higher in patients aged 75 years and older,3 but the factors responsible for this increased incidence remain unclear.
Aging has been associated with impaired organ function and increased susceptibility to injury and development of fibrosis.2– 4 In lungs, aging has been associated with enlarged airspaces reminiscent of tobacco-related emphysema, and this is believed to be caused by increased expression of proteases.5 Several explanations have been given for these changes, including chronic inflammation, increased free radical damage, a decline in immune responses and alterations in stem cell/progenitor cell differentiation potential,5 but the mechanisms that predispose the aged or old lung to disrepair and fibrosis after injury are not completely elucidated.
Several factors have been associated with tissue disrepair and scarring after injury, including the exaggerated production of profibrotic growth factors such as transforming growth factor-beta 1 (TGF-β1) and the expression of fibronectin (Fn), a matrix glycoprotein expressed early after tissue injury and implicated in wound healing and tissue repair.6 Aberrant expression of matrix-degrading proteases such as the matrix metalloproteinases (MMPs) has also been implicated in tissue disrepair.7 Furthermore, specific subpopulations of fibroblasts, characterized by decreased expression of the Thy-1 surface marker, have also been implicated in the development of lung fibrosis.8
We hypothesized that aging renders the lung susceptible to ineffective repair by altering the expression of the above factors, thereby promoting exaggerated repair responses after injury that promote the development of fibrosis rather than normal recovery. To test this hypothesis, we examined the expression of extracellular matrices and related profibrotic factors in aged and young lungs harvested from rodents. Our studies reveal that the aged lung exhibits a profibrotic phenotype that may contribute to its increased susceptibility to injury and disrepair and that this increased susceptibility might be related to alterations in lung fibroblast phenotype.
MATERIALS AND METHODS
Animals
Young (3 months) and old (24 months) wild-type and green fluorescent protein– expressing C57BL/6 mice were obtained from Jackson Laboratories. Pilot studies were conducted with green fluorescent protein mice, which demonstrated no differences compared with wild-type mice. All studies were subject to Institutional Animal Care Use and Committee review at Emory University and conformed to institutional and Association for Assessment and Accreditation of Laboratory Animal Care International standards for humane treatment of laboratory animals.
Bleomycin Administration
Mice were anesthetized by ketamine and xylazine intra-peritoneal injection, and the trachea was exposed using sterile technique. Bleomycin (Sigma-Aldrich, St Louis, MO) in phosphate-buffered saline (PBS) at 3.5 units/kg or PBS vehicle alone was injected into the tracheal lumen. After inoculation, the incision was closed, and the animals were allowed to recover. Fourteen days after bleomycin injection, the mice were euthanized by isoflurane inhalation, and lungs were harvested for mRNA and protein isolation. The left lobes of the lungs were inflated to a constant pressure of 20 cm of water.
Hydroxyproline Assay
Hydroxyproline content in whole mouse lungs was used to quantify lung collagen content and was measured colorimetrically as previously described.9 A standard curve was generated for each experiment using a hydroxyproline standard. Results were expressed as micrograms (μg) of hydroxyproline per lung.
Messenger RNA Expression Analysis
Messenger RNA from whole lung was isolated as previously described using RNeasy kit (Qiagen, Valencia, CA).10 First-strand cDNA was synthesized. Quantitative polymerase chain reaction (PCR) was performed with primers set for 18s, Fn, Fn extracellular domain A (Fn-EDA), Fn extracellular domain B, collagen type I, TGF-β1, Smad3, plasminogen activator inhibitor-1 (PAI-1), TGF-β receptor 1 (TGF-βR1) and TGF-βR2 (Table 1) using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA); the real-time iCycler sequence detection system (Bio-Rad) was used for the real-time PCR analysis. The level of target mRNA expression was normalized to 18s housekeeping gene levels, and relative target mRNA levels were determined according to the comparative cycle threshold method (Applied Biosystems 7900HT Sequence Detection System, User Bulletin No. 2; Applied Biosystems, Grand Island, NY), and relative expression values were calculated as previously described.10 Similarly, mRNA was isolated from primary lung fibroblasts (PLF) passage 3 to 5 and analyzed for Thy-1 mRNA expression.
TABLE 1.
Mouse primer sequences
| Gene | Forward | Reverse |
|---|---|---|
| 18s | 5′ GGA CCA GAG CGA AAG CA | 5′ ACC CAC GGA ATC GAG AAA |
| Fn | 5′ AAT GGA AAA GGG GAA TGG AC | 5′ CTC GGT TGT CCT TCT TGC TC |
| Fn-EDA | 5′ ATC GCC CTA AAG GAC TGG | 5′ CAT CCT CAG GGC TCG AGT AG |
| Fn-EDB | 5′ AGG TGG ACC CCG CTA AAC T | 5′ CAT TAA TGA GAG TGA TAA CGC |
| PAI-1 | 5′ TCA TCA GAC AAT GGA AGG GC | 5′ ACT GTG CCG CTC TCG TTT AC |
| Smad3 | 5′ GCA TGG ACG CAG GTT CTC | 5′ TTG CAT CCT GGT GGG ATC |
| Smad3 for qPCR | 5′ GCA TGG ACG CAG GTT CTC | 5′ AGG AGA TGG AGC ACC AGA AG |
| TGF-β1 | 5′ CCC ACT CCC GTG GCT TC | 5′ GTT CCA CAT GTT GCT CCA C |
| TGF-βR1 | 5′ CCC AAC TAC AGG ACC TTT TTC A | 5′ CAG TGG TAA ACC TGA TCC AGA |
| TGF-βR2 | 5′ TTG GAT TGC CAG TGC TAA | 5′ AAG AAG CCA CAG TAA CAT GAC A |
| Thy-1 | 5′ GCT CTC AGT CTT GCA GGT G | 5′ ACG TGC TTC CTC TTC TCT CG |
Fn-EDA, fibronectin extracellular domain A; PAI-1, plasminogen activator inhibitor-1; TGF-β1, transforming growth factor-beta 1; TGF-βR1, TGF-β receptor 1; Fn-EDB, fibronectin extracellular domain B.
Protein Expression Analysis
Total proteins were isolated from frozen murine lungs as previously described.11 Total protein concentrations were determined by a Bradford assay.12 Equal amounts of lung samples were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)gels and then transferred to nitro-cellulose membranes. The blots were blocked and then incubated with a rat anti-TGF-β1 antibody (1:500; Abcam, Cambridge, MA), a rabbit anti-Fn antibody (1:5000; Sigma-Aldrich), TGF-βR1 (1:200 dilution) and TGF-βR2 antibodies (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), Smad2/3 (1:1000; Cell Signaling, Danvers, MA) and phosphorylated Smad3 antibodies (1:1000; Cell Signaling), a mouse anti-Fn-EDA antibody (1:200; Abcam) or β-actin antibody (1:500; Sigma-Aldrich, St. Louis, MO) at 4 °C overnight. The blots were washed and incubated with an appropriate horseradish peroxidase– conjugated secondary antibody (at 1:2000 dilution for all blots; Amersham Biosciences, Pittsburgh, PA), washed and visualized through enzyme-linked chemiluminescence using the SuperSignal West Pico kit (Pierce Biotechnology, Rockford, IL).
Electrophoretic Mobility Shift Assay
Whole lungs from young or old mice were isolated, washed with ice-cold PBS and nuclear binding proteins were extracted according to a previously published protocol.13 Double-stranded Smad3/4 consensus oligonucleotides (5′-TCGAGAGCCAGACAA AAAGCCAGACATTTAGCCAGACAC; Santa Cruz Biotechnology) were radiolabeled with 32P-ATP using T4 polynucleotide kinase enzyme. Nuclear protein was incubated with radiolabeled oligonucleotide (2–300,000 CPM/ng) in a reaction mixture. For competition reactions, 100-fold molar excess of nonradiolabeled Smad3/4 consensus oligonucleotides (100× Smad) or nonradiolabeled mutated Smad3/4 (5′-TCGAGAGCTACATAAAAAGCTACATATTTAGCTACATAC, 100× mSmad) oligonucleotides were added to the reaction mixture. For loading control, extracts were loaded onto a SDS-PAGE gel and stained with coomassie blue. The DNA-protein complexes were separated on 6% native polyacrylamide gels (20:1 acrylamide/bis ratio) in low ionic strength buffer at 10 V/cm2. Gels were fixed in a 10% acetic acid/10% methanol solution for 10 minutes, dried under vacuum and exposed to x-ray film.
Analysis of Gelatinolytic Activity (Gelatin Zymography)
Gelatin zymography was performed using a 9% SDS-PAGE gel saturated with 1 mg/mL gelatin (Sigma-Aldrich) to determine gelatinolytic activity in young and old mouse lungs as previously described.14 A densitometry analysis was performed for determination of gelatinolytic activity.
Isolation of PLF
PLF were harvested from young and old C57BL/6 mice as previously described.15 Cells were cultured in fibroblast culture medium (Dulbecco’s modified eagle’s medium with 4.5 g/L glucose supplemented with 20% fetal bovine serum, 100 U/mL penicillin G sodium, 100 U/mL streptomycin and 0.25 μg/mL amphotericin B). PLF between passages 3 and 5 were used for Thy-1 expression analysis and treatment of cells.
Evaluation of Thy-1 Methylation
Passage 3 to 5 PLF isolated from lung of old mice were plated in 6-well plates at 2500 cells/cm2 for 24 hours before treatment. Culture medium with or without 5 μM 5-aza-2′-deoxycytidine (AZA, Sigma) was added to the cells. The medium was changed every 24 hours with freshly added AZA. After 3 days, cells were harvested for total RNA isolation to examine for Thy-1 expression by real-time PCR (RT-PCR) as previously described.16
Flow Cytometry Analysis
PLF from young and old mice (passage 3–5) were stained with 1:50 anti-mouse antibody against Thy-1.2 conjugated with phycoerythrin fluorescent (BioLegend), followed by washing with fluorescence-activated cell sorting buffer and fixed with 5% formalin. Samples and appropriate controls were analyzed on a FACSCalibur (Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed using the FlowJo 7.2.5 software (Tree Star, San Carlos, CA).
Histology
Hematoxylin and eosin and Masson’s trichrome staining (for collagen deposition) were performed on nonadjacent 5 μm paraffin-embedded lung sections. Morphometric analyses for collagen deposition quantification were performed using ImageJ 1.42 (http://rsbweb.nih.gov/ij/). One of the injured young lungs was used for data normalization.
Statistical Analysis
All data are expressed as mean ± standard mean error. Unpaired 2-tailed t tests and 1-way analysis of variance tests were used for single and multiple comparisons, respectively (P values <0.05 were considered significant). Post-test analysis was performed using Dunnett’s multiple comparison test to compare between groups. GraphPad Prism and GraphPad InStat version 4 were used to calculate the statistics.
RESULTS
Old Lungs Develop More Fibrosis After Injury
To test whether age affects susceptibility to fibrosis after lung injury, we used the bleomycin injury model in 3- (young) and 24 (old)-month-old C57BL/6 mice. A PBS control group (vehicle only) was included for comparison, given that saline instillation could potentially result in inflammation, which could also lead to increased extracellular matrix expression. Data were analyzed at 14 days postinjury. We did not observe differences between the PBS treatment group and the nontreatment group in terms of histologic findings or gene expression analysis (data not shown). Lung histology showed a marked increase in the severity of the injury and in collagen deposition seen in old mice compared with the age-matched PBS-treated and young bleomycin-treated animals using Masson’s trichrome staining and morphometric analysis software (Image J) (Figure 1A and B). We also found a significant increase in Col1A1 mRNA expression and in hydroxyproline content in old lungs treated with bleomycin compared with young animals and age-matched PBS-treated controls (Figure 2). These studies reveal that old lungs show increased fibrosis in response to bleomycin-induced lung injury.
FIGURE 1.
Aging increases bleomycin-induced lung injury and fibrosis. Three-month-old and 24-month-old C57BL/6 mice were treated with 3.5 units/kg of bleomycin or PBS intratracheally. Lungs were harvested at 14 days. (A) Histologic sections were stained with Masson’s trichrome to evaluate for collagen deposition. Magnification is 2× (inset 10×). (B) Graph represents the intensity of Masson’s trichrome staining in young (open bar) and old (close bar) lungs at 14 days after bleomycin treatment as analyzed by ImageJ 1.42 software. One of the lungs from young mice was used as a standard sample (value set as 1). *P < 0.05 compared with young group.
FIGURE 2.
Aging increases collagen mRNA expression and deposition after bleomycin-induced lung injury. Three-month-old and 24-month-old C57BL/6 mice were treated with 3.5 units/kg of bleomycin (close bars) or PBS (open bars) intratracheally. Lungs were harvested at 14 days and processed for quantitative PCR and hydroxyproline content analysis. Graphs depict type 1 collagen mRNA expression (A) and hydroxyproline content (B) in the lungs harvested from young and old animals 14 days after treatment. One of the samples from the young group was used as standard sample for quantitative PCR analysis. *P < 0.05 and #P = 0.05. PBS, phosphate buffer saline-treated group; Bleo, bleomycin-treated group.
Old Lungs Show Evidence of Increased TGF-β1 and TGFβR1 Expression
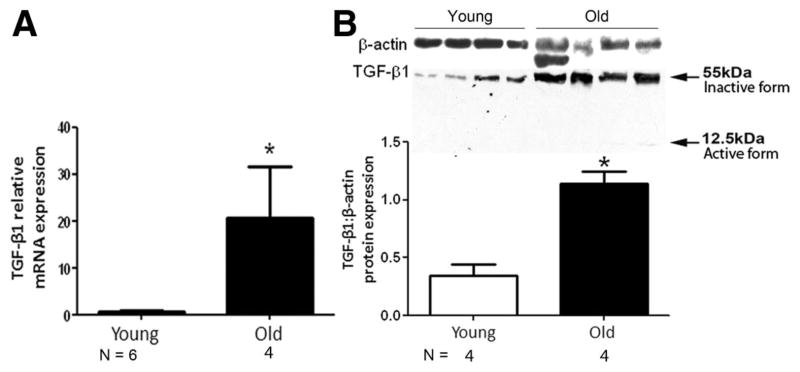
To examine the mechanisms responsible for increased susceptibility to fibrosis in the old lung, lungs harvested from uninjured young and old animals were processed for TGF-β1 mRNA and protein expression. As shown in Figure 3, old lungs showed increased TGF-β1 mRNA expression, which was associated with increased expression of inactive TGF-β1 protein (Figure 3B). An insignificant amount of the active form of TGF-β1 was seen in some of the old lung samples (Figure 3B).
FIGURE 3.
Aging is associated with an increase in TGF-β1 expression. Lungs were harvested from uninjured 3-month-old and 24-month-old C57BL/6 mice and analyzed for TGF-β1 expression. (A) Graph depicts TGF-β1 mRNA expression measured by quantitative PCR. (B) Graph depicts relative densitometry analysis of Western blots evaluating TGF-β1 protein. The inactive form of TGF-β1 migrated at 55 kDa, and the active form migrated at 12.5 kDa in SDS-PAGE gel. One of the samples from young group was used as standard sample for quantitative PCR analysis. β-actin protein expression was used for normalization for protein expression. *P < 0.05 compared with young group.
We also found increased expression of TGF-βR1 mRNA and protein in old lung, whereas the expression of TGF-βR2 was not altered (Figure 4A and B).
FIGURE 4.
TGF-βR1 and Smad3 expression increase with age. Lungs were harvested from uninjured 3-month-old and 24-month-old C57BL/6 mice and analyzed for TGF-βR1, TGF-βR2 and Smad3 expression. (A) Lung samples were evaluated for TGF-βR1, TGF-βR2 and Smad3 by RT-PCR as depicted in the upper panel showing an RT-PCR gel electrophoresis. Graphs represent quantitative PCR analysis for Smad3, TGF-βR1 and TGF-βR2 mRNA expression in young (open bars) and old (closed bars) lungs. One of the samples from the young group was used as standard sample for quantitative PCR analysis. (B) Lung samples were evaluated for TGF-βR1, TGF-βR2, Smad3 and phospho-Smad3 protein expression using Western blot. Upper panel shows representative Western blot gel. Graphs represent relative densitometric analysis of samples from young (open bar) and old (close bars) lungs. β-actin protein expression was used for normalization. *P < 0.05 compared with young group.
Old Lungs Show Evidence of Increased Smad3-Dependent TGF-β1 Signaling
Having found increased TGF-β1 and TGF-βR1 expression in old lungs, we assessed the TGF-β1/Smad3 signaling pathway. First, we demonstrated increased Smad3 mRNA and protein expression as determined by quantitative RT-PCR and Western blot analysis, respectively, in old lungs (Figure 4A and B). Furthermore, we detected Smad3 phosphorylation by Western blot in aged lungs, but not in young lungs (Figure 4B). There was also a trend toward increased total Smad3 protein expression in aged lungs, but this was not statistically significant (Figure 4B). Second, we used electrophoretic mobility shift assay to evaluate for evidence of activation of this pathway as determined by increased DNA binding by Smad3. As shown in Figure 5A, we found increased Smad3 DNA binding in old lungs compared with young lungs. Note that in competition reactions, 100-fold molar excess of nonradiolabeled Smad3/4 oligonucleotide was able to compete for binding of Smad3/4, indicating specificity of the DNA-protein interaction. The nonradiolabeled mutated Smad3/4 oligonucleotide was not able to compete for the binding of Smad3/4. Figure 5B shows an SDS-PAGE gel loaded with nuclear extracts from young and old mouse lungs stained with coomassie blue to indicate equal loading, including protein loading for competitive reaction for electrophoretic mobility shift assay.
FIGURE 5.
Aged lungs show increased TGF-β1/Smad3 signaling. Lungs were harvested from uninjured 3-month-old and 24-month-old C57BL/6 mice. (A) Top gel [electrophoretic mobility shift assay (EMSA)] showing increased Smad3 DNA binding in noninjured old lungs compared with young lungs. For competition reactions, 100-fold molar excess of nonradiolabeled Smad3/4 (100 × Smad) or mutated Smad3/4 (100× mSmad) oligonucleotide was added to the reaction mixture containing nuclear extracts isolated from the lungs of old mice. (B) Bottom gel showing an SDS-PAGE gel loaded with nuclear extracts and stained with coomassie blue to indicate equal loading, including protein loading for competitive reaction (last 2 lanes, upper gel). (C) Representative reverse transcriptase PCR gel and quantitative PCR of whole lung showing increased PAI-1 mRNA expression in old lungs (close bars) compared with young lungs (open bars). *P < 0.05 compared with young group.
Considering that Smad3 expression, phosphorylation and DNA binding were increased, we evaluated the expression of downstream targets of TGF-β1/Smad3 signaling such as PAI-1. We found increased mRNA expression of PAI-1 in old lungs (Figure 5C).
Old Lungs Show Alterations in the Expression of Extracellular Matrices and MMPs
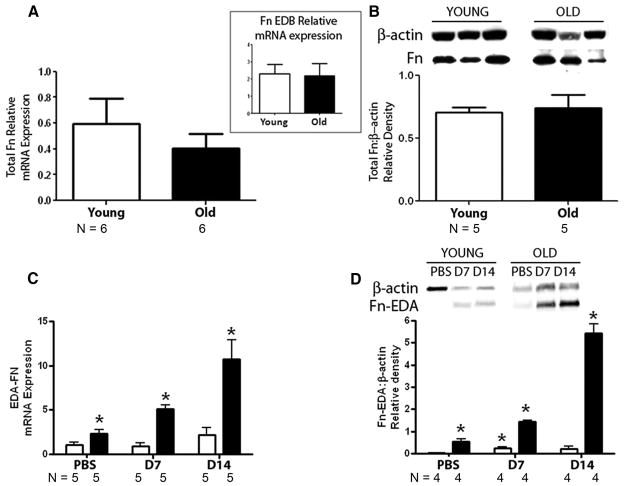
In view that old lungs manifest increased expression of downstream targets of TGF-β1/Smad3 signaling, we set out to test the expression of extracellular matrices and MMPs. We tested for Fn and found that aging did not alter both total Fn mRNA and protein expressions (Figure 6A and B). However, old lungs showed increased expression of Fn-EDA mRNA and protein at baseline and an increase in the expression of Fn-EDA mRNA and protein after bleomycin that seemed time dependent (0, 7 and 14 days after bleomycin injection) (Figure 6C).17 Interestingly, no alterations were found in the expression of mRNA coding for another Fn splice variant, Fn extracellular domain B in uninjured lungs (Figure 6A, inset).
FIGURE 6.
Aging is associated with alterations in fibronectin-EDA splice variant expression in lung. (A) Lungs were harvested from uninjured 3–month-old and 24-month-old C57BL/6 mice. Graphs depict quantitative PCR for mRNA expression of Fn. Insert depicts mRNA expression of Fn-EDB. (B) Graph depicts Western blot analysis (for protein expression) in young and old lungs for total Fn. (C and D) Three-month-old (open bars) and 24-month-old (close bars) C57BL/6 mice were treated with 3.5 units/kg of bleomycin or PBS intratracheally, and lungs were harvested at 7 and 14 days tested for Fn-EDA mRNA (C) and protein (D) expression. One of the samples from the young group was used as a representative example of quantitative PCR analysis. β-actin protein expression was used for normalization. P < 0.001 across all groups by 1-way analysis of variance. P < 0.05 compared with PBS-treated young group.
We then assessed the expression of MMP-2 and MMP-9 and their inhibitors tissue inhibitor of metalloproteinases-1 (TIMP-1) and TIMP-2, respectively. In Figure 7A, we found increased mRNA expression of MMP-2, MMP-9 and TIMP-2, but not TIMP-1 with age. Gelatin zymography showed increased gelatinolytic activity related to MMP-9 in old lungs compared with young lungs (Figure 7B). Gelatinolytic activity of MMP-2 was not altered.
FIGURE 7.
MMP-9 mRNA expression and activity are increased in aged lungs. Lungs were harvested from uninjured 3-month-old and 24-month-old C57BL/6 mice for MMP mRNA expression and activity. (A) Graphs depict quantitative PCR analysis of young and old lungs for MMP-2, MMP-9, TIMP-2 and TIMP-1 mRNA expression. (B) Representative gel for gelatin zymography analysis of MMP-2 and MMP-9 activity. Graphs show densitometry analysis of gelatin zymography from young (open bars) and old (close bars) lungs. *P < 0.05 compared with young group.
Primary Fibroblasts Harvested From Old Lungs Show Decreased Expression of Thy-1
We were interested in evaluating lung fibroblasts obtained from uninjured young versus old lungs for their expression of Thy-1. Thy-1 is a surface protein that regulates TGF-β1 activation and Fn expression.18 Furthermore, Thy-1 null mice show increased fibrosis in response to bleomycin-induced lung injury compared with wild-type mice.8 We found that fibroblasts harvested from both young and old lungs have similar morphology with a slight increase in rectangle/round cells in the lungs of old mice, which is suggestive of Thy-1–negative fibroblasts (white arrows)19 (Figure 8A and B). However, fibroblasts from aged lungs showed a significant decrease in Thy-1 mRNA expression (Figure 8C). Consistent with this, fibroblasts isolated from old lungs were submitted to flow cytometry analysis, which showed a decrease in the percent of Thy-1–positive cells and mean fluorescence intensity for Thy-1 expression per cell (Figure 8D–F). To determine whether epigenetic mechanisms were responsible for the loss of Thy-1 expression with age, we treated lung fibroblasts isolated from the lungs of old mice with AZA, a DNA methyltransferase inhibitor.16 We found no significant change in Thy-1 mRNA expression in lung fibroblasts after treatment with AZA (Figure 8C).
FIGURE 8.
Aging is associated with increase in number of Thy-1–negative fibroblasts in the lung, and this is not influenced by DNA methylation. Lungs were harvested from uninjured 3-month-old and 24-month-old C57BL/6 mice, and primary fibroblasts were isolated for analysis of Thy-1. (A and B) Phase contrast microscopy of passage 3 young (A) and old (B) primary lung fibroblasts in culture. Magnification, 20×. (C) Quantitative PCR to assess Thy-1 expression in lung fibroblasts isolated from young (open bars) and old (closed bar) mice and in lung fibroblasts from old mice treated with 5′AZA to induce DNA demethylation (gray bar). P < 0.001 as analyzed by 1-way analysis of variance. (D) Representative histogram of flow cytometry for Thy-1 performed in young (gray line) and old (black line) lungs. (E and F) Percent (E) and mean (F) fluorescent intensity of Thy-1 expressed by lung fibroblasts isolated from young and old lungs as analyzed by flow cytometry. *P < 0.05 compared with young group by t test and post-test analysis.
DISCUSSION
Aged or old lungs show increased susceptibility to injury and development of fibrosis, but the mechanisms responsible for this remain poorly understood. We hypothesize that aging is associated with a profibrotic phenotype that leads to increased susceptibility to disrepair and fibrosis after lung injury. We found that old mice develop more pronounced fibrosis after bleomycin-induced lung injury compared with young mice. Interestingly, this susceptibility to fibrosis in old lungs was associated with increased expression of the profibrotic growth factor TGF-β1, the matrix glycoprotein Fn-EDA and MMPs and activation of the TGF-β1/Smad3 pathway with increased Smad3 phosphorylation and DNA binding. Furthermore, these changes were associated with alterations in lung fibroblast phenotype as highlighted by a decrease in Thy-1 expression. Of note, the age-related decrease in lung fibroblast Thy-1 expression did not seem related to gene promoter methylation, as others have found in other systems.16
Acute inflammatory responses are triggered after injury and include the recruitment of leukocytes and disruption of epithelial and endothelial cells. As a result, a variety of chemokines, cytokines and growth factors are released, including the profibrotic growth factor TGF-β1.20,21 In the normal homeostasis state, TGF-β1 is present in the lungs as an inactive form bound to the extracellular matrix and becomes activated through cleavage by newly released enzymes/proteases and other mechanisms in response to injury.20 Activated TGF-β1 binds to its receptors (eg, TGF-βR1), resulting in the activation of downstream intracellular signaling pathways, for example, Smads, leading to a cascade of events that promotes fibrosis.20 We found increased TGF-β1 mRNA expression in old lungs, but when protein was analyzed, we found that it was mainly in the inactive form. The latter is consistent with the observation that old lungs are histologically normal. Nevertheless, we documented alterations in the TGF-β1 signaling pathway that may predispose the old lung to disrepair after injury, including an increase in the expression of its receptor TGF-βR1. However, no differences were found in the expression of TGF-βR2, a receptor needed to form the receptor complex capable of activating Smad3 signaling.22 We also observed increased Smad3 expression and Smad 3 phosphorylation, increased DNA binding by Smad3 and increased mRNA expression of PAI-1, a downstream target of TGF-β1/Smad3 signaling. Although we believe that most of the TGF-β1 signaling is through Smad3 activation, it is possible that other signaling such as through p38 and MEK1/MAPK activation could play a role in this process.23 Furthermore, it is important to note that we analyzed the expression using whole lung, which contains multiple type of cells. Future studies to identify the specific site of these changes (ie, lung epithelium, endothelium) would be important.
Considering the above evidence for the, at least, partial activation of TGF-β1/Smad signaling, we tested for alterations in the expression of other downstream genes such as those coding for extracellular matrices and MMPs. In clinical and experimental forms of acute and chronic lung injury, there is increased expression of extracellular matrix components such as Fn.24 Fn is a matrix glycoprotein that affects many cellular processes, including adhesion, migration, proliferation and differentiation and is a sensitive marker of activation of tissue remodeling.6,24,25 In injured lungs, deposition of Fn is believed to accelerate the re-epithelialization of denuded basement membranes25 and increase the proliferation of fibroblasts.26 In addition, Fn, together with TGF-β1, promotes myofibroblast differentiation27 and facilitates the deposition of collagens.28 There are more than 21 splice variants of Fn, which result from alternative splicing of a single Fn gene.29 Of these splicing variants, Fn-EDA has received the most attention lately, considering that its expression is decreased in terminally differentiated cells,27 that it is necessary for myofibroblast transdifferentiation by TGF-β1,30 that animals deficient in Fn-EDA are protected against bleomycin-induced lung injury15 and that there is an increase in Fn-EDA in the lungs of patients with IPF.15 Alterations in extracellular matrix composition could influence cellular differentiation, including stem cell differentiation in response to injury.30 –32 Whether these changes lead to disrepair by skewing lung progenitor cell differentiation toward a myofibroblast phenotype or affecting the type of inflammatory cells recruited (eg, recruitment and homing of fibrocytes) will need to be determined. However, these mechanisms are interesting considering that we found increased Fn-EDA expression in old lungs. Thus, it is reasonable to propose that excessive expression of Fn-EDA in lung might promote fibrogenic responses in the setting of lung injury, but this needs experimental validation. Although we did not investigate the mechanisms responsible for Fn-EDA expression in old lungs, we postulate that this is partly due to TGF-β1 because this growth factor upregulates fibroblast Fn-EDA expression through activation of the PI3K/Akt/mTOR and Smad3 signaling pathways.33,34
We also tested for the expression and activity of common collagenases of the MMP family, MMP-2 and MMP-9, and their inhibitors, TIMP-2 and TIMP-1, respectively. MMP-9, among other MMPs, has been reported to be increased in experimental lung fibrosis.9 We found a significant increase in MMP-2, MMP-9 and TIMP-2 mRNA expression in old lungs, but TIMP-1 expression did not change significantly with age. Gelatin zymography showed no change in the MMP-2 activity in old lungs compared with young lungs. We believe that this is caused by the balance of both MMP2 and TIMP-2 mRNA expression observed. However, MMP-9 –related activity was significantly increased with age, which could be explained by the observed increase in its mRNA expression without a concomitant increase in its inhibitor TIMP-1. The increase in MMPs may be viewed as another manifestation of the profibrotic phenotype found in old lungs given evidence suggesting that MMP2/MMP9 could activate TGF-β in vitro.35 In view of increased MMP9 activity along with increased TGF-β expression and signaling, it is intriguing that these changes did not result in increased collagen type 1 expression or changes leading to the development of fibrosis in the aged lung. The latter could be explained by the fact that we evaluated whole lungs for analysis of mRNA and protein expression; the fibrotic change might be limited and compartmentalized preventing it from being detected. Another explanation is that small alterations in TGF-β signaling in aged lung might be offset by an increase in MM9 activity because it is conceivable that MMPs and other proteases degrade newly deposited extracellular matrices, thereby preventing the accumulation of aberrant matrices in old lungs, a mechanism that is overcome only after injury. On the other hand, the increase in MMP expression could lead to increased susceptibility to injury leading to increased leukocyte migration and more tissue damage in the injured lung.
Although we have not identified the exact mechanisms involved in the abnormalities described above, we postulate that phenotypic alterations in lung fibroblasts might be critical for these events. In lung, there are heterogeneous populations of fibroblasts characterized by the differential expression of one of its surface molecules, Thy-1. Interestingly, only Thy-1–negative fibroblasts have been shown to be capable of activating TGF-β1, whereas Thy-1–positive fibroblasts failed to do so.18 In addition, Thy-1 null mice show increased fibrotic responses to bleomycin-induced lung injury compared with wild-type mice.8 This suggests that phenotypic changes in fibroblasts resulting in downregulation of Thy-1 might promote fibroproliferation in lung. This mechanism might be important in IPF because these patients show accumulation of Thy-1–negative fibroblasts in fibroblastic foci.16 In this study, we demonstrate the downregulation of Thy-1 mRNA and protein expression in PLF isolated from old lungs. Alterations in cell morphology were not analyzed in detail. However, the images obtained suggested a slight increase in the number of cells with round morphology, which is suggestive of Thy-1–negative fibroblasts as previously described, but this requires further exploration.36 However, it should be highlighted that previous data describing such morphologic changes in Thy-1–negative cells were obtained in rat cells, not mouse cells, as is the case here. Differences in morphology between Thy-1–positive and Thy-1–negative cells seem to be subtle in mouse cells.36 Although the expression of Thy-1 protein may have been altered during cell culture and may not reflect the phenotype of fibroblasts in vivo, the decrease of Thy-1 mRNA expression suggests a true decrease in the expression of this surface marker.
The exact mechanisms controlling fibroblast phenotype during aging are unknown. Several mechanisms have been proposed, including the epigenetic control of Thy-1 gene transcription through DNA methylation, which has been shown to increase during aging.16,37 To examine this, we treated fibroblasts isolated from old lungs with a demethylating agent, AZA. We did not find changes inThy-1 mRNA expression with this treatment. These data suggest that age-related alterations in lung fibroblast Thy-1 expression may not be a result of hypermethylation of Thy-1 gene promoter regions as previously described.16 On the other hand, it has been shown that inflammation could alter endothelial cell Thy-1 expression and vice versa. These cells secrete different levels of inflammatory cytokines such as interleukin-1α (IL-1α), Prostaglandin E2 and IL-1.38 – 40 IL-1β stimulated PGE2 expression in orbital Thy-1–positive fibroblasts, whereas stimulating IL-8 expression in Thy-1–negative fibroblasts.40 More recently, after stimulation with tumor necrosis factor-alpha, Thy-1–positive fibroblasts showed reduction in MMP-9 and intercellular adhesion molecule 1, and this is believed to be caused by interference with Src kinase activation.41 This is interesting given that we found increases in Thy-1–negative fibroblasts in the lungs of old mice in conjunction with an increase in MMP-9 expression. In addition, it is not clear whether the change in lung fibroblast Thy-1 expression leads to all the profibrotic changes described in aged lung or whether the relative changes in lung extracellular matrix composition (by residential lung cells or recruited cells) lead to alterations in lung fibroblast phenotype. The latter is supported by a study showing that changes in culture surface composition (ie, basement membrane matrix versus collagen type I) could influence epithelial cell phenotype and TGF-β1 expression in vitro.42 Whether the changes we found here are directly linked to each other is unknown, and the exact mechanisms of loss of Thy-1 expression with age will require further investigation.
In summary, we found that old lungs are more susceptible to development of injury and fibrosis in the bleomycin-induced lung injury model. We believe that this is caused by a profibrotic phenotype present in old lungs, which is characterized by increased expression of TGF-β1, TGF-βR1 and Smad3 and increased expression of the Fn-EDA splice variant and MMPs. Other potential mechanisms involved in the development of fibrosis in the bleomycin model relate to the tissue expression of bleomycin hydrolase (BH).43 Currently, there are no published studies examining the activity or level of BH in aged tissues. One study showed a change of BH levels during development with an increase in BH levels in a variety of rat tissues (brain, liver, kidney, skin, etc.) up to the age of 6 weeks; afterward, the levels decreased. Unfortunately, that particular study did not evaluate BH levels in older animals (ie, 1–2 years).44 Others have shown alterations in BH associated with Alzheimer’s disease, which is a disease of older individuals.45,46 Therefore, presumably, there could be some changes of BH in lungs of old mice compared with young ones. However, the predisposition to injury in elderly murine lungs is not unique to bleomycin because ventilator-induced lung injury, lipopolysaccharide and cigarette smoking have been shown to cause increased injury in aging animals.47– 49 We believe that alterations shown in current study were associated with an increase in the relative amount of Thy-1– deficient fibroblasts present in old lungs. Interestingly, these changes did not seem associated with fibrosis at baseline probably because the majority of the TGF-β1 present in old lungs was in the inactive form. However, we did find evidence for at least partial activation of TGF-β1/Smad3 signaling. We believe that the above changes prime the old lung to disrepair after injury, thereby rendering it susceptible to fibrosis. In this model, aging represents a first hit that stretches the reparative mechanisms of the lung without causing fibrosis. However, under these circumstances, a second hit caused by any of many risk factors for lung injury (eg, infection, toxic inhalation) may overwhelm these processes, thereby unleashing exuberant repair responses that lead to fibrosis. Although this relationship seems well established, to our knowledge, this is the first in-depth exploration of the phenotype of aged lungs because it relates to tissue remodeling. Further work is required to determine the mechanisms responsible for establishing this profibrotic phenotype in old lungs in the hope of identifying potential targets for intervention.
Acknowledgments
The authors thank Valerie Mac for technical assistant on hydroxyproline analysis; Edilson Torres for technical assistant on gelatin zymography; Allan M. Ramirez, MD, for generously providing TGF-βR1 and 2 and PAI-1 primers for qPCR; and Vasantha Kolachala, PhD, for scientific discussions.
This study was supported by Cystic Fibrosis Foundation Program for Adult Care Excellence (to VS), Emory Center for Respiratory Health (to VS and JR), American Foundation for Aging (to MR and JR) and Unrestricted grant, McKelvey Lung Transplant Center (to DCN).
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mora AL, Rojas M. Aging and lung injury repair: a role for bone marrow derived mesenchymal stem cells. J Cell Biochem. 2008;105:641–7. doi: 10.1002/jcb.21890. [DOI] [PubMed] [Google Scholar]
- 3.Lawson WE, Crossno PF, Polosukhin VV, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–26. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Collard HR, Raghu G, et al. Does chronic microaspiration cause idiopathic pulmonary fibrosis? Am J Med. 2010;123:304–11. doi: 10.1016/j.amjmed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–80. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 6.Limper AH, Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest. 1992;101:1663–73. doi: 10.1378/chest.101.6.1663. [DOI] [PubMed] [Google Scholar]
- 7.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–31. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagood JS, Prabhakaran P, Kumbla P, et al. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–79. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Mora A, Shim H, et al. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol. 2007;37:291–9. doi: 10.1165/rcmb.2006-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sueblinvong V, Loi R, Eisenhauer PL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–11. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Mora AL, LaVoy J, et al. Increased bleomycin-induced lung injury in mice deficient in the transcription factor T-bet. Am J Physiol Lung Cell Mol Physiol. 2006;291:L658–67. doi: 10.1152/ajplung.00006.2006. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–89. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unemori EN, Pickford LB, Salles AL, et al. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest. 1996;98:2739–45. doi: 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman J, Ritzenthaler JD, Bechara R, et al. Ethanol stimulates the expression of fibronectin in lung fibroblasts via kinase-dependent signals that activate CREB. Am J Physiol Lung Cell Mol Physiol. 2005;288:L975–87. doi: 10.1152/ajplung.00003.2004. [DOI] [PubMed] [Google Scholar]
- 16.Sanders YY, Pardo A, Selman M, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:610–8. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muro AF, Moretti FA, Moore BB, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–45. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am J Pathol. 2004;165:659–69. doi: 10.1016/s0002-9440(10)63330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntosh JC, Hagood JS, Richardson TL, et al. Thy1 (+) and (−) lung fibrosis subpopulations in LEW and F344 rats. Eur Respir J. 1994;7:2131–8. doi: 10.1183/09031936.94.07122131. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–21. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 22.Wrana JL, Attisano L, Carcamo J, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–14. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez AM, Shen Z, Ritzenthaler JD, et al. Myofibroblast trans-differentiation in obliterative bronchiolitis: tgf-beta signaling through smad3-dependent and -independent pathways. Am J Transplant. 2006;6:2080–8. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 24.Roman J. Extracellular matrices in lung injury and repair. In: Schwarz M, King T, editors. Interstitial Lung Disease. London: B.C. Decker, Inc; 2003. pp. 276–99. [Google Scholar]
- 25.Roman J. Extracellular matrix and lung inflammation. Immunol Res. 1996;15:163–78. doi: 10.1007/BF02918505. [DOI] [PubMed] [Google Scholar]
- 26.Bitterman PB, Rennard SI, Adelberg S, et al. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983;97:1925–32. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muro AF, Chauhan AK, Gajovic S, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–60. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition: Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extra-cellular matrix. J Cell Biol. 1982;92:485–92. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarzbauer JE, Patel RS, Fonda D, et al. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987;6:2573–80. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma W, Tavakoli T, Derby E, et al. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Evseenko D, Schenke-Layland K, Dravid G, et al. Identification of the critical extracellular matrix proteins that promote human embryonic stem cell assembly. Stem Cells Dev. 2009;18:919–28. doi: 10.1089/scd.2008.0293. [DOI] [PubMed] [Google Scholar]
- 33.Magnuson VL, Young M, Schattenberg DG, et al. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991;266:14654–62. [PubMed] [Google Scholar]
- 34.White ES, Sagana RL, Booth AJ, et al. Control of fibroblast fibronectin expression and alternative splicing via the PI3K/Akt/mTOR pathway. Exp Cell Res. 2010 doi: 10.1016/j.yexcr.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 36.Phipps RP, Baecher C, Frelinger JG, et al. Differential expression of interleukin 1 alpha by Thy-1+ and Thy-1− lung fibroblast subpopulations: enhancement of interleukin 1 alpha production by tumor necrosis factor-alpha. Eur J Immunol. 1990;20:1723–7. doi: 10.1002/eji.1830200815. [DOI] [PubMed] [Google Scholar]
- 37.Shames DS, Minna JD, Gazdar AF. DNA methylation in health, disease, and cancer. Curr Mol Med. 2007;7:85–102. doi: 10.2174/156652407779940413. [DOI] [PubMed] [Google Scholar]
- 38.Silvera MR, Sempowski GD, Phipps RP. Expression of TGF-beta isoforms by Thy-1+ and Thy-1− pulmonary fibroblast subsets: evidence for TGF-beta as a regulator of IL-1-dependent stimulation of IL-6. Lymphokine Cytokine Res. 1994;13:277–85. [PubMed] [Google Scholar]
- 39.Fries KM, Blieden T, Looney RJ, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994;72:283–92. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- 40.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002;32:477–85. doi: 10.1002/1521-4141(200202)32:2<477::AID-IMMU477>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 41.Shan B, Hagood JS, Zhuo Y, et al. Thy-1 attenuates TNF-alpha-activated gene expression in mouse embryonic fibroblasts via Src family kinase. PLoS ONE. 2010;5:e11662. doi: 10.1371/journal.pone.0011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streuli CH, Schmidhauser C, Kobrin M, et al. Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol. 1993;120:253–60. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filderman AE, Lazo JS. Murine strain differences in pulmonary bleomycin metabolism. Biochem Pharmacol. 1991;42:195–8. doi: 10.1016/0006-2952(91)90702-7. [DOI] [PubMed] [Google Scholar]
- 44.Kamata Y, Itoh Y, Kajiya A, et al. Quantification of neutral cysteine protease bleomycin hydrolase and its localization in rat tissues. J Biochem. 2007;141:69–76. doi: 10.1093/jb/mvm005. [DOI] [PubMed] [Google Scholar]
- 45.Suszynska J, Tisonczyk J, Lee HG, et al. Reduced homocysteine-thiolactonase activity in Alzheimer’s disease. J Alzheimers Dis. 2010;19:1177–83. doi: 10.3233/JAD-2010-1311. [DOI] [PubMed] [Google Scholar]
- 46.Higuchi M, Iwata N, Saido TC. Understanding molecular mechanisms of proteolysis in Alzheimer’s disease: progress toward therapeutic interventions. Biochim Biophys Acta. 2005;1751:60–7. doi: 10.1016/j.bbapap.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Nin N, Lorente JA, De Paula M, et al. Aging increases the susceptibility to injurious mechanical ventilation. Intensive Care Med. 2008;34:923–31. doi: 10.1007/s00134-007-0960-0. [DOI] [PubMed] [Google Scholar]
- 48.Lin SP, Sun XF, Chen XM, et al. Effect of aging on pulmonary ICAM-1 and MCP-1 expressions in rats with lipopolysaccharide-induced acute lung injury. J Southern Med Univ. 2010;30:584–7. [PubMed] [Google Scholar]
- 49.Matulionis DH. Chronic cigarette smoke inhalation and aging in mice: 1. Morphologic and functional lung abnormalities. Exp Lung Res. 1984;7:237–56. doi: 10.3109/01902148409087916. [DOI] [PubMed] [Google Scholar]