Abstract
Accurate prognosis prediction in oncology is critical. In patients with hepatocellular carcinoma (HCC), unlike most solid tumors, coexistence of two life-threatening conditions such as cancer and cirrhosis difficult prognostic assessments. Despite the usefulness of clinical staging systems for HCC in routine clinical decision-making (e.g., Barcelona-Clinic Liver Cancer algorithm), there is still need to refine and complement outcome predictions. Recent data suggest the ability of gene signatures from the tumor (e.g., EpCAM signature) and adjacent tissue (e.g., poor-survival signature) to predict outcome in HCC (either recurrence or overall survival), although independent external validation is still required. In addition, novel information is being produced by alternative genomic sources such as miRNA (e.g., miR-26a) or epigenomics, areas in which promising preliminary data is thoroughly explored. Prognostic models need to contemplate the impact of liver dysfunction and risk of subsequent de novo tumors in patient’s life expectancy. The challenge for upcoming years will be to precisely depict genomic predictors (e.g., gene signatures, miRNA or epigenetic biomarkers) at each stage of the disease and their specific influence to determine patient prognosis.
Keywords: Liver cancer, personalized medicine, genomic profiling, prognostic modeling, biomarkers, gene signatures, miRNA, epigenetics
BACKGROUND
Hepatocellular carcinoma (HCC) accounts for more than 90% of liver cancers, and is a major health problem with 700,000 new cases per year worldwide. Most HCCs arise within a previously damaged liver; chronic hepatitis (B and C) and alcohol abuse being as the leading environmental causes for the underlying liver disease1. Approximately 20-30% of the estimated 170 million hepatitis C (HCV)infected individuals worldwide will develop cirrhosis. The annual incidence of HCC in cirrhotic patients is 3-5%, and one third of them will develop a tumor over their lifespan. In addition, due to its high prevalence, hepatitis B virus (HBV) lies beneath most HCC cases in Africa and Asia, being the main etiological factor worldwide. Different strategies aimed to reduce HBV infection, including universal nationwide vaccination programs and antiviral therapies have demonstrated a significant reduction in the HBV-related HCC burden2-4.
HCC is the leading cause of death among cirrhotic patients, and the third cause of cancer-related mortality5. In Western countries, less than 30% of newly diagnosed patients are eligible for curative therapies such as resection, liver transplantation or local ablation1. Moreover, 15-20% of early stage tumors present with dismal outcome leading to prompt neoplastic dissemination and short-term survival. According to the National Cancer Institute levels of evidence, there are no level I studies demonstrating survival benefits of conventional chemotherapy in HCC patients. However, the unprecedented results of a phase III trial (level IA) showing that sorafenib, a multi-kinase inhibitor, improves survival in patients with advanced disease, opened a new era in the therapeutic approach to this cancer6. Results from this trial established sorafenib as the new standard of care, demonstrated the benefits of molecular targeted therapies, and underscored the importance of oncogene addiction discovery in HCC7. Unlike other solid tumors (e.g., GIST), there are no oncogenic addiction loops identified in HCC, but considering the excellent therapeutic results obtained in other addicted tumors, its discovery and selective blockade could significantly impact patient prognosis. This seminal step has changed the landscape of clinical and translational research in the field. A number of molecular compounds are currently moving to late clinical developmental phases, clearly highlighting several unmet needs remaining in the primary and adjuvant settings. Accurate prediction of patient therapeutic response based on tumor molecular singularities will further improve overall efficacy of molecular therapies in HCC.
Two major advancements could critically improve the outcome of patients with this neoplasm: First, the identification of critical molecular subclasses with different prognostic implications. Prognosis prediction still relies exclusively on clinical parameters and molecular data is not guiding therapeutic decision-making, which represents a critical bottleneck for improving patient outcome. Identification of biomarkers able to define sub-groups of patients with dismal prognosis will translate into better therapeutic strategies and allocation of resources8. Second, the identification of key genetic or epigenetic drivers of specific subclasses will enable development of more personalized treatment algorithms. Both challenges are hampered by the complexity of the molecular basis of liver cancer. This review will briefly analyze novel advances in genomic-based prognosis assessment in HCC, reviewing mRNA gene signatures reported and summarizing data on miRNA and epigenomic biomarkers. We will also present an overview of an integrated model of outcome prediction in HCC combining clinical and genomic data coded in the tumor and non-tumor adjacent cirrhotic tissue.
ON THE HORIZON
Modeling prognosis integrating clinical and genomic data
The last decade has witnessed a revolution in the way scientists characterize the human genome. Most of these advances relied on an exponential increase in the throughput capacity of new genomic technologies. Researchers are no more restricted to analyze a limited number of genes, but instead, the whole genome can be thoroughly scrutinized. Moreover, different functional aspects of the genome can be simultaneously evaluated, including DNA structural damage (e.g., point mutations, chromosomal rearrangements), functional genomics (e.g., mRNA and miRNA dysregulation), epigenetics (e.g., aberrant methylation and histone deacetylation), metabolic profiles, etc. The quantity of information generated with these new technologies still surpasses our capacity to assimilate it. This vast amount of molecular data, multidimensional in nature, demand an integrated analytic approach within systems biology frameworks9. Unlike other malignancies, HCC remains somehow orphan to such innovative and complex investigational initiatives.
Cancer classification aims to establish prognosis, select the adequate treatment for the best candidates, and aid researchers to design clinical trials with comparable criteria. Different staging systems are currently available in HCC, but none has so far incorporated molecular data. Cirrhosis, another life-threatening condition, is present in more than 80% of patients with HCC, what renders prognosis prediction a major challenge. Some clinical-based staging systems (e.g., Barcelona-Clinic Liver Cancer (BCLC) algorithm7), have addressed both components, establishing a road-map for routine clinical decision-making. Nevertheless, performance of clinical-based systems requires further refinement to address some daily clinical situations. For example, current systems are unable to accurately detect HCC dropouts from the waiting list for liver transplantation, or to preoperatively identify patients that will develop a tumor recurrence after surgical resection. Inaccurate predictions can cause unnecessary harm to patients, preclude application of curative therapies, and significantly increase healthcare costs.
The unprecedented high-throughput capacity of newly developed array-based genomic platforms favors the assumption that genome-wide approaches could help to improve prognostic estimations achieved by clinical systems. Genomic profiling has already demonstrated its prediction benefits in other malignancies10, 11. In fact, some signature-based chips are currently under evaluation as predictors of therapeutic response in oncology (e.g., breast). Although initially restricted to fresh frozen samples, current technologies allow genomic profiling of partially degraded samples, such as formalin-fixed paraffin-embedded tissue12, 13. In addition, the performance of current array-hybridization-based technologies continues to improve while their cost decreases14, 15. These multi-gene-based assays are now classified according to the U.S. Food and Drug Administration as potential diagnostic devices (In vitro diagnostic multivariate index assays (IVDMIA)16). Many studies have proposed molecular classifications of HCC using mRNA-based gene expression profiling, obtained from tumor or non-tumoral adjacent cirrhotic tissue and are reviewed elsewhere8, 9, 17. Gene signatures from the tumor capturing biological signals related to proliferation and cell cycling (e.g., “proliferation class”18, “G3”19, “class A”20) seem to identify patients with more aggressive disease. Moreover, patients with tumors supposedly derived from progenitor cells tend to have worse prognosis (e.g., “hepatoblast signature”21, “EpCAM”22, “CK19 signature”23) (Table 112, 19-22, 24-38). In this regard, mRNA profiling appears to indicate tumor cellular lineage. Poor prognostic signatures generated so far have not been specifically associated with any risk factor, such as HCV or HBV, or underlying pre-neoplastic condition. This would indicate that genes predicting poor outcome might be common regardless of etiology, an area of research that should be pursued with further studies. Also, genomic profiling of the adjacent non-tumoral cirrhotic tissue allowed the development of signatures able to accurately identify patients with poor prognosis12, 38. This is probably due to their ability to identify risk of developing de novo tumors, progression of liver dysfunction and detection of micro-environmental favoring conditions for intrahepatic metastasis. In fact, one of the signatures from adjacent tissue indicating poor prognosis has been recently validated in a different scenario. We tested this signature in a cohort of compensated cirrhotic patients with a median follow-up of 10 years, one third of which died due to liver complications. The poor prognosis gene signature identified 20% of patients at high risk of developing complications (ascites, bleeding, HCC) and poor outcome39. Thus, this signature identifies risk of progression of cirrhosis, and might be a relevant tool for trial enrichment in chemopreventive studies. However, all these signatures were frequently ill-defined, generated in patients at different stages, and with distinct etiologies for their underlying liver disease. Hence, they require independent external validation on a patient-by-patient basis. Once validated, next step will comprise design of physical devices (e.g., chips) including the key prognostic genes. These devices will require prospective evaluation in routine clinical conditions prior to their definitive implementation and inclusion in guidelines.
Table 1.
Relevant miRNA-based and epigenetic alterations, which prognostic impact in HCC patients needs to be tested or confirmed.
| Molecular alteration | Clinical significance | REMARK recommendations* | Status† |
|---|---|---|---|
| mRNA based (gene signatures)¶ | |||
| Poor-survival signature (186 genes)12Φ | Poor survival | OK | EV |
| EpCAM signature22 | Poor survival | OK | EV |
| Venous metastasis signature38 | Hepatic metastasis | OK | EV |
| Class A20 / Hepatoblast signature21 | Poor survival | OK | IV, EV |
| G3 subclass19 | Poor survival | - | IV, EV |
| miRNA-based | |||
| Down-regulation miR-26a26 | Poor survival | OK | EV |
| 20-miRNA signature31 | Venous metastasis, overall survival |
OK | EV |
| Down-regulation miR-12224 | Poor survival | - | IV, EV |
| Down-regulation Let-7 members25 | Early recurrence | - | IV, EV |
| Up-regulation miR-125a27 | Better survival | - | IV, EV |
| 19-miRNA signature30 | Poor survival | - | IV, EV |
| Up-regulation miR-22128 | Multinodularity; reduced time to recurrence |
- | T, IV, EV |
| Up-regulation miR-92, miR-20, miR-1829 | Poor differentiation | - | T, IV, EV |
| Epigenetics | |||
| Genome wide hypomethylation32 | Tumor Progression, Survival | - | IV, EV |
| Hypermethilation of E-cadherin or GSTP135 | Poor survival | - | IV, EV |
| Degree of hypomethylation33 | Advanced histological grade; larger tumor size |
- | T, IV, EV |
| 264-gene hypermethylation profile34 | Moderately/poor differentiated tumors |
- | T, IV, EV |
| Hypermethilation of E-cadherin36 | Microvascular invasion; tumor recurrence |
- | T, IV, EV |
| Hypermethilation of RIZ137 | Correlation with disease-free survival |
- | T, IV, EV |
Reasonable compliance with REMARK recommendations for tumor marker prognostic studies44
Current status in terms of clinical implementation (T: need further preliminary prognostic evaluation, IV: lacks internal validation, EV: lacks external validation)
Molecular classifications (mRNA-based) with prognostic impact are thoroughly discussed elsewhere8,9,17
Genomic signature obtained from early HCC
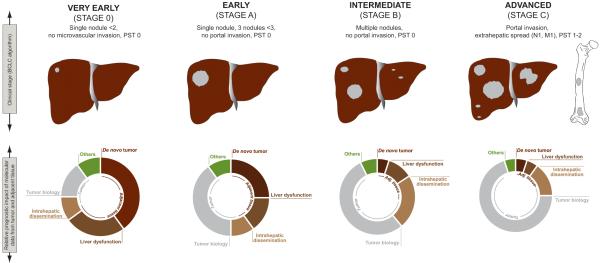
In principle, any genomic-based staging system in HCC should incorporate information related to tumor aggressiveness, hepatic dysfunction, and risk of de novo HCC development. Hence, such prognostic model should consider genomic data coded in the tumor itself and the adjacent non-tumoral cirrhotic tissue. By genomic data we mean an integrative vector obtained upon assimilation of genetic, transcriptomic and epigenetic data. Figure 1 summarizes the core structure of a versatile prognostic model that combines genomic information from each tissue compartment (i.e., tumor and adjacent cirrhosis) at different stages of HCC. In patients with very early HCC (i.e., tumors less than 2 cm without vascular invasion or extrahepatic spread) treated with surgical resection in which tumor is likely removed before dissemination, prognosis will be mostly determined by the risk of developing a de novo primary tumor and the risk of liver dysfunction. Both risks are coded in the surrounding cirrhotic tissue and framed within the “field effect” concept12. The scientific challenge is set to identify which is the genomic vector that determines patient prognosis at each stage of the disease. Once discovered, current strategies on treatment allocation, clinical trial design and chemoprevention will probably require re-evaluation. As cancer progresses, genomic data from the tumor will be increasingly informative because, at this point, tumor dissemination will govern patient survival. This is the case of the majority of gene signatures with prognostic significance reported so far. As depicted in table 1, most of them have been obtained from tumors resected at intermediate or even advanced tumoral stage, and this explains their prognostic implication.
Figure 1.
Model of HCC prognosis combining clinical and genomic data. Stages follow the BCLC algorithm7. Circles represent an educated guess of the relative influence of each causality component in patient prognosis, at different stages of the disease. In patients with early tumors, survival is mostly determined by genomic data coded in non-tumoral cirrhotic tissue (“field effect”) since it determines risk of liver dysfunction and development of a de novo HCC. As cancer progresses, genomic data from the tumor (in grey) increases its prediction capacity since cancer-related death limits survival in patients with advanced disease.
Lessons learned from genome-based prognostic assessment: Priming study design
Accumulated experiences of translational genomics studies teach us that the reported prognostic signatures are often not reproducible or valid due to problems with study design (e.g., sample size, process of validation, relevance to actual clinical context), or differences in assay platform/protocol40-42. Most of the published microarray studies were conducted on retrospectively collected tissue samples, which were initially non-intentionally obtained for these studies. This could result in biased representation of patient population and cause low reproducibility by increasing clinical heterogeneity across studies, which could be further enhanced by small sample size. This can be critical in HCC research since there is considerable diversity in demographic and clinical variables such as etiology, disease stage, or patient ethnicity according to geographic site43.
There are crucial methodological issues when dealing with microarray-based biomarker discovery investigations: (1) estimation of appropriate sample size, (2) adjustment for multiple significance testing to reduce the risk of false discovery, (3) assessment of the statistical confidence of classification or prediction for each patient considering actual clinical application of the biomarker, and (4) validation of biomarker on a completely independent set of patients, preferably from different institutions. Moreover, calculated sample size may not assure the clinical relevance of the molecular biomarker if the effect size (i.e., extent of differential expression measured as statistic, fold change, etc.) varies across assay platforms. Lastly, all such microarray studies should adhere to the standard requirement for diagnostic/prognostic biomarker research summarized in the REMARK statement44. Table 1 summarizes the analysis of gene signatures according to these statements and the type of validation required by them to be incorporated in clinical guidelines. For instance, there are several biomarker studies in advanced phases of preclinical development including mRNA signatures from the tumor (e.g., EpCAM22) and the adjacent tissue (e.g., poor survival12), although they still require independent external validation for its definitive clinical implementation. Regarding miRNA, only one study that evaluated miR-26a26 has reached advanced developmental phases whereas epigenetic biomarkers are still on exploratory phases.
A key issue when searching for a prognostic biomarker (i.e., “supervised” analysis using outcome information as a guide), is to use a uniform definition for clinical endpoints to enable inter-study comparisons7. This endpoint is preferably correlated with more homogeneous biological property in order to achieve reproducible results, less affected by variation in clinical practice across institutions. For example, in discovering a tumor recurrence-predictive biomarker, time-to-recurrence is preferable to disease-free survival, which is the composite of recurrence and death that may not be attributable to single biological characteristic captured by the biomarker7. In HCC, it is important to note that there are two types of recurrence that can arise from distinct biological background: true metastasis as dissemination of primary tumor cells (usually within 2 years after curative treatment) and de novo metachronic tumors occurring in a diseased liver45. The proportion of each type of recurrence greatly varies across tumor stages46.
Beyond mRNA-based predictions: prognostic role of miRNA and epigenetics
Besides mRNA-based gene signatures, recent data suggest the prognostic capacity of genome-wide miRNA and methylation profiling in cancer47, being plausible that their predictions will complement those obtained with mRNA arrays. Currently, more than 700 human miRNAs have been discovered and annotated in the miRBase registry (miRBase version 12.0). A growing number of studies indicate that miRNAs are frequently deregulated in cancer. A recent study showed how few miRNAs (approximately 200) accurately reflected the tissue of origin and developmental history of human cancers, and provided a more accurate tumor classification compared with the expression profiling of 16,000 mRNAs48. Several studies support the ability of miRNA profiling to classify cancer patients according to their clinical outcome (e.g., leukaemia49, colon50, 51, lung52, breast 53, 54 and pancreatic55 cancer). In HCC, a number of studies have highlighted the prognostic predictive power of miRNA profiling (Table 1). Data coming from these studies, even those at high-end level, need to be externally validated prior being incorporated into clinical guidelines. This is the case of the most relevant study so far published, low expression of miR-26a was reported as an independent predictor of survival in a large cohort of hepatitis B related Chinese HCC patients26. Another study showed that the expression of more than 200 precursor and mature miRNAs in HCC and adjacent benign livers provided a 19-miRNA signature significantly associated with disease outcome30. Similarly, tumors with metastatic HCC had a distinctive 20-miRNA signature compared with non-metastatic disease after analyzing 241 patients31. Also, there is evidence indicating that miR-122 is frequently down-regulated in HCC patients with poor prognosis24. It seems that loss of miR-122 could be associated with suppression of hepatic phenotype and the acquisition of malignant and invasive properties. Down-regulation of members of Let-7 family, that target important oncogenes such as RAS and MYC, has been found significantly associated with tumor early recurrence25. Interestingly, these patients were also clustered in a molecular subclass (i.e., “proliferation subclass”) identified upon unsupervised analysis of mRNA-based array data18. From a therapeutic standpoint, expression restoration of certain miRNA resulted in suppression of cancer cell proliferation, induction of tumor-specific apoptosis, and dramatic protection from disease progression in experimental models56. This study demonstrates that miRNA modulation is a feasible alternative in HCC therapeutics, deserving initial exploration in the early clinical setting57. In addition, cholesterol-modified antisense oligonucleotides, termed ‘antagomirs’ and locked-nucleic acids (LNA) have been developed to inhibit the expression of complementary miRNA and their use in in vivo models is currently under investigation58.
DNA hypermethylation is a highly prevalent molecular lesion in cancer cells and it has been long correlated to transcription inactivation. Unlike genetic alterations, they are potentially reversible and lead to heritable states of gene expression that are not caused by changes in the DNA sequence59. DNA-methylation mapping revealed cancer-specific profiles of hypermethylated CpG islands (hypermethylomes) able to distinguish between different tumor types60 and to predict patient outcome. Studies reporting epigenetic biomarkers are summarized in Table 1. DNA is methylated by methyltransferases (DNMTs), enzymes which over-expression is associated to poor survival in HCC61, 62. Furthermore, inactivation of tumor suppressor genes (TSG) involved in cell cycle, apoptosis, and proliferation have been described in HCC. APC, p16, SFRP1, IGFBP3, RASSF1 and SOCS1 show the highest frequency of promoter hypermethylation and consequent gene silencing63. Additionally, a methylation index of 105 TSGs showed a significant inverse correlation with HCC patient survival64. A very recent study showed that a methylation profile present in surgically margins of HCC patients treated with resection significantly impacted survival, indicating that epigenetic alterations, similarly to mRNA profiling12, of non-tumor adjacent tissue is of great potential to predict patient prognosis37. On the contrary, low methylation levels in certain promoter regions are also linked to advanced histopathological grade in HCC33. Pharmacoepigenetics, an emerging discipline, can eventually aid physicians to anticipate treatment response. For example, hypermethylation of the DNA-repair gene MGMT is the best-known independent predictor of response to chemotherapy in glioblastomas65. DNA-demethylating agents and histone deacetylase inhibitors (HDACis) are entering clinical practice. Recently, the HDACi suberoylanilide hydroxamic acid has been approved for the treatment of cutaneous T-cell lymphoma66. However, there is not a single demethylating agent currently under advanced clinical evaluation in HCC.
In summary, it is difficult to predict which of the above approaches will make the first, significant impact in clinical practice. This is in part due to continued technical improvements of mRNA, miRNA, and epigenetic data collection and analyses. However, it can be expected that integrative analyses of the data obtained with all three approaches will potentially constitute more informative prognostics than either of the approaches alone.
FUTURE PROSPECTS
The so-called postgenomics era has highly enriched translational research. Data at the transcriptomic and epigenetic levels are easily obtained from large series of samples, being both great sources to generate hypothesis. More comprehensive studies are now feasible, and there are tools that allow studying biological systems as a whole, far beyond a mere description of its parts. Even though this is an old concept (i.e., systems biology), the technology necessary to address these studies is gradually becoming accessible. Despite nowadays it mostly focuses on simple organisms, it is expected that it will soon develop a more translational perspective, undertaking clinical practice challenges. In HCC, three areas will substantially benefit from this approach: prognosis assessment, prediction of treatment response, and identification of novel targets for molecular therapies.
Regarding prognosis assessment, recently reported prognostic gene signatures and miRNA can enter and complement clinical variables in staging systems, once they have been externally validated by independent studies. On the other hand, promising data is currently under development with epigenetic biomarkers, which are expected to refine prognostic stratification. Finally, predictors of treatment response will emerge along with novel drugs in the treatment of HCC. Sorafenib positive results6 have opened a new era in HCC research. Future trends in drug development will pivot on accurate assessment of genetic traits in human disease on an individual basis (i.e., personalized medicine). In HCC, the identification of these singularities will allow maximizing therapeutic response by selecting the best drug for the ideal candidate. In this scenario, precise segregation between driver and passenger events is a major challenge. Additionally, personalized approaches may facilitate population enrichment in clinical trials, what could ultimately decrease futility rate among investigational drugs.
Acknowledgments
Grant Support: AV is a recipient of a Sheila Sherlock fellowship (European Association for the Study of the Liver). AL is the recipient of a postdoctoral fellowship grant from the German Research Foundation (DFG). ST is supported by a fellowship from Istituto Nazionale dei Tumori, Fondazione IRCCS, Milan (Itay). HC is supported by a grant from Spanish National Health Institute (SAF-2007-61898). CA is supported by a grant of Instituto de Salud Carlos III. JML has grants from National Institute of Health -NIDDK 1R01DK076986-01, National Institute of Health (Spain) grant I+D Program (SAF-2007-61898) and Samuel Waxman Cancer Research Foundation.
REFERENCES
- 1.Llovet J, Burroughs A, Bruix J. Hepatocellular carcinoma. The Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Chang MH, Chen CJ, Lai MS, et al. Taiwan Childhood Hepatoma Study Group Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med. 1997;336:1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 3.Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–55. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 4.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 5.Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–14. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 6.Llovet J, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Llovet J, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva A, Toffanin S, Llovet JM. Linking molecular classification of hepatocellular carcinoma and personalized medicine: preliminary steps. Curr Opin Oncol. 2008;20:444–53. doi: 10.1097/CCO.0b013e328302c9e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med. 2010;61:317–28. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mi S, Lu J, Sun M, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci U S A. 2007;104:19971–6. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Ross K, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 12.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimsza LM, Leblanc ML, Unger JM, et al. Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2008;112:3425–33. doi: 10.1182/blood-2008-02-137372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan JB, Yeakley JM, Bibikova M, et al. A versatile assay for high-throughput gene expression profiling on universal array matrices. Genome Res. 2004;14:878–85. doi: 10.1101/gr.2167504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Payton JE, Grieselhuber NR, Chang LW, et al. High throughput digital quantification of mRNA abundance in primary human acute myeloid leukemia samples. J Clin Invest. 2009;119:1714–26. doi: 10.1172/JCI38248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draft Guidance for Industry Clinical Laboratories, and FDA Staff - In Vitro Diagnostic Multivariate Index Assays. http://wwwfdagov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm079148htm.
- 17.Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis. 2010;30:35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–88. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyault S, Rickman DS, de RA, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Chu I, Heo J, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–76. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–6. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita T, Forgues M, Wang W, et al. EpCAM and -Fetoprotein Expression Defines Novel Prognostic Subtypes of Hepatocellular Carcinoma. Cancer Res. 2008;68:1451–61. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 23.Andersen JB, Loi R, Perra A, et al. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology. 2009 doi: 10.1002/hep.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–36. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viswanathan SR, Powers JT, Einhorn W, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–8. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–47. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Xie L, He X, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–22. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 28.Gramantieri L, Fornari F, Ferracin M, et al. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res. 2009;15:5073–81. doi: 10.1158/1078-0432.CCR-09-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami Y, Yasuda T, Saigo K, et al. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–45. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Gusev Y, Aderca I, et al. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–27. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budhu A, Jia HL, Forgues M, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 32.Calvisi DF, Ladu S, Gorden A, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Lin CH, Hsieh SY, Sheen IS, et al. Genome-wide hypomethylation in hepatocellular carcinogenesis. Cancer Res. 2001;61:4238–43. [PubMed] [Google Scholar]
- 34.Gao W, Kondo Y, Shen L, et al. Variable DNA methylation patterns associated with progression of disease in hepatocellular carcinomas. Carcinogenesis. 2008;29:1901–10. doi: 10.1093/carcin/bgn170. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ, Kang GH. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 2003;163:1371–8. doi: 10.1016/S0002-9440(10)63495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon GY, Yoo BC, Koh KC, Cho JW, Park WS, Park CK. Promoter methylation of E-cadherin in hepatocellular carcinomas and dysplastic nodules. J Korean Med Sci. 2005;20:242–7. doi: 10.3346/jkms.2005.20.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lou C, Du Z, Yang B, Gao Y, Wang Y, Fang S. Aberrant DNA methylation profile of hepatocellular carcinoma and surgically resected margin. Cancer Sci. 2009;100:996–1004. doi: 10.1111/j.1349-7006.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Budhu A, Forgues M, Ye Q, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Hoshida Y, Villanueva A, Sangiovanni A, et al. Gene expression signature predicts outcome of liver cirrhosis. Hepatology. 2009;50:312A. [Google Scholar]
- 40.Michiels S, Koscielny S, Hill C. Prediction of cancer outcome with microarrays: a multiple random validation strategy. The Lancet. 2005;365:488–92. doi: 10.1016/S0140-6736(05)17866-0. [DOI] [PubMed] [Google Scholar]
- 41.Ntzani EE, Ioannidis JP. Predictive ability of DNA microarrays for cancer outcomes and correlates: an empirical assessment. Lancet. 2003;362:1439–44. doi: 10.1016/S0140-6736(03)14686-7. [DOI] [PubMed] [Google Scholar]
- 42.Shi L, Reid LH, Jones WD, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151–61. doi: 10.1038/nbt1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elserag H. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 44.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 45.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045–7. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 46.Hoshida Y, Villanueva A, Llovet JM. Molecular profiling to predict hepatocellular carcinoma outcome. Expert Rev Gastroenterol Hepatol. 2009;3:101–3. doi: 10.1586/egh.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 48.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 49.Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 50.Schepeler T, Reinert JT, Ostenfeld MS, et al. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–24. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 51.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–36. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 53.Foekens JA, Sieuwerts AM, Smid M, et al. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc Natl Acad Sci U S A. 2008;105:13021–6. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–52. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 56.Kota J, Chivukula RR, O’Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toffanin S, Villanueva A, Llovet J. miRNA delivery: emerging therapy for hepatocellular carcinoma. Gastroenterology. 2010;138:1202–4. doi: 10.1053/j.gastro.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 59.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–98. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 60.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–9. [PubMed] [Google Scholar]
- 61.Oh BK, Kim H, Park HJ, et al. DNA methyltransferase expression and DNA methylation in human hepatocellular carcinoma and their clinicopathological correlation. Int J Mol Med. 2007;20:65–73. [PubMed] [Google Scholar]
- 62.Saito Y, Kanai Y, Nakagawa T, et al. Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int J Cancer. 2003;105:527–32. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- 63.Calvisi D, Ladu S, Gorden A, et al. Ubiquitous Activation of Ras and Jak/Stat Pathways in Human HCC. Gastroenterology. 2006;130:1117–28. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Calvisi D, Ladu S, Gorden A, et al. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713–22. doi: 10.1172/JCI31457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 66.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]