Abstract
Background
Single-lung transplantation (SLT) and bilateral lung transplantation (BLT) are both good options for patients with end-stage lung disease secondary to idiopathic pulmonary fibrosis. It is, however, unclear whether BLT offers any survival advantage over SLT. The purpose of our study was to evaluate a large group of patients to determine if either SLT or BLT officered a long-term survival advantage for patients with IPF.
Methods
This was an Institutional Review Board-approved retrospective analysis of the United Network of Organ Sharing database from 1987 to 2008. Survival was determined using Kaplan-Meir estimates and the effect of laterality was determined by Cox proportional hazards and propensity analyses.
Results
Lung transplantation for idiopathic pulmonary fibrosis was performed in 3,860 patients (2,431 SLTs and 1429 BLTs). Multivariate and propensity analysis failed to show any survival advantage for BLT (hazard ratio = 0.90, 95% confidence interval = 0.78 to 1.0, p = 0.11). One-year conditional survival favored BLT (hazard ratio 0.73, 95% confidence interval 0.60 to 0.87, p = 0.00064). Risk factors for early death included recipient age over 57 and donor age over 36 years.
Conclusions
Bilateral lung transplantation should be considered for younger patients with idiopathic pulmonary fibrosis and results may be optimized when younger donors are used.
Over the past ten years the number of lung transplants performed annually in the United States has nearly doubled, and in that same time the number of transplants performed for idiopathic pulmonary fibrosis (IPF) has more than tripled (133 in 1998 to 485 in 2007). This increase in lung transplants performed for IPF was most dramatic after 2004 and resulted in IPF surpassing chronic obstructive pulmonary disease, in 2007, as the most common indication for transplant [1]. This change was due to the change in the organ allocation system from a time on the wait list system to a recipient acuity system. Not as easily explainable is the increase in the number of double-lung transplants performed for IPF over the same time. In 1998 only 20% of transplants for IPF were bilateral lung transplants (BLT) yet by 2007 this number had increased to nearly 50% [2].
In order to gain insight into the reason as to why programs were deciding to perform more BLTs, we sent a web-based poll to all lung transplant surgical directors. The poll asked whether the programs favored BLT over single (S)LT for IPF and why they chose their specific approach. The results showed widely varying responses. The SLT was identified as the operation of choice by 30% of programs while BLT was seen as the optimal procedure in 55% of programs, and both SLT and BLT were selected by 15% of the responders. The majority of responders who favored either SLT or BLT selected data in the literature and the International Society of Heart and Lung Transplant (ISHLT) database and clinical acumen as the major reasons for selecting what they felt was the ideal operation for patients with IPF.
This survey leads one to believe that there must be ambiguity in the current available data. Data from the 2009 ISHLT registry did show a significant improvement in long-term survival for patients who underwent a BLT for IPF. However, this analysis looked at survival as a single variable and was not adjusted for any donor or recipient characteristics and therefore likely introduced significant bias into the analysis [2].
The data in the current literature are equally difficult to analyze and support arguments for both SLT and BLT as the ideal operation for IPF. Mason and colleagues [3] looked at 82 patients who underwent transplantation for IPF at the Cleveland Clinic, and found better one-year and five-year survival rates in patients who underwent BLT. Meyer and colleagues [4], on the other hand, analyzed 821 patients who were transplanted for IPF and found improved survival rates for patients less than 60 years of age who underwent SLT. Nwakanma and colleagues [5] looked at patients on the other end of the IPF age spectrum and found no difference in survival between SLT and BLT for patients over 60 years of age.
The lack of convincing data in the literature led us to analyze the current available data to attempt to shed some light on the following question: Does SLT or BLT offer a survival advantage for patients with IPF?
Material and Methods
Study Design
This retrospective cohort study sought to compare the long-term survival of patients receiving two different transplantation surgeries. The United Network of Organ Sharing (UNOS) database was queried for all pulmonary fibrosis cases eligible for lung transplantation between October 1, 1987 and August 15, 2008. The primary classification variable was procedure type (unilateral or bilateral transplantation). Patients receiving living lobar transplants were omitted from the study. This represented 3,860 surgeries on 3,830 patients. For patients who are represented twice in the database due to retransplantation, only the record from their first surgery was considered and survival measurements were indexed to this primary surgery. The study was approved by the Emory University Institutional Review Board in compliance with Health Insurance Portability and Accountability Act regulations and the Declaration of Helsinki. The Institutional Review Board reviewed and approved this study. Long-term follow-up and survival was retrieved from the UNOS database and therefore dependent on accurate entry by individual transplant programs.
Covariates of Interest
In addition to laterality, 25 potential preoperative risk factors were identified that were potential covariates of long-term survival. These variables were chosen because of their potential association with survival and their availability. These variables included donor and recipient characteristics and are listed in Table 1.
Table 1.
Recipient and Donor Characteristics (25 Covariates)
| Predictor Variable | Single Lung (n = 2,431) | Double Lung (n =1,429) | p Value |
|---|---|---|---|
| Recipient age (SD) | 56.5 (9.5) | 51.8 (12.2) | <0.001 |
| Donor age (SD) | 32.1 (13.7) | 32.4 (15.2) | 0.67 |
| Donor CMVa | 1434 (59.4) | 909 (64.1) | 0.004 |
| Recipient CMV by IgGa | 952 (63.1) | 712 (62.9) | 0.91 |
| Donor cigarette historya | 404 (47.4) | 217 (61.1) | <0.001 |
| Recipient diabetesa | 275 (12.9) | 179 (13.1) | 0.85 |
| Recipient on ECMO | 1 (0.04) | 9 (0.63) | <0.001 |
| Recipient on ECMO after transplant | 8 (0.33) | 13 (0.91) | 0.018 |
| Recipient Caucasian racea | 2024 (83.3) | 1109 (77.6) | <0.001 |
| Donor Caucasian racea | 1693 (69.8) | 910 (63.7) | <0.001 |
| Recipient pretransplant FEV1 (SD)a | 52.0 (17.2) | 48.3 (17.5) | <0.001 |
| Recipient pretransplant FVCa | 49.2 (16.5) | 47.5 (17.3) | 0.014 |
| Recipient female gender | 829 (34.1) | 487 (34.1) | 0.99 |
| Donor female gender | 889 (36.6) | 644 (45.1) | <0.001 |
| Recipient cardiac output | 5.51 (1.54) | 5.43 (1.47) | 0.17 |
| Recipient mean pulmonary artery pressure | 23.7 (9.0) | 29.1 (12.4) | <0.001 |
| Donor cigarette historya | 511 (23.5) | 260 (18.8) | <0.001 |
| Donor cocaine historya | 164 (10.2) | 131 (10.9) | 0.53 |
| Recipient pretransplant Pao2a | 2.75 (2.18) | 3.18 (2.65) | <0.001 |
| Recipient pretransplant Pco2a | 40.95 (7.8) | 41.85 (9.1) | 0.030 |
| Recipient pretransplant ventilator | 15 (0.6) | 41 (2.9) | <0.001 |
| CMV mismatch (D+,R–)a | 1,160 (77.2) | 893 (79.1) | 0.25 |
| Trauma to donora | 1,280 (56.1) | 714 (51.2) | 0.003 |
| Race mismatcha | 828 (34.2) | 572 (40.1) | <0.001 |
| Gender mismatch | 716 (29.5) | 507 (35.5) | <0.001 |
Contains some missing data. Mean values in parentheses.
CMV = cytomegalovirus; ECMO = extracorporeal membrane oxygenation; FEV1 = forced expiratory volume in the first second of expiration; FVC = forced vital capacity; IgG = immunoglobulin G; Pao2 = arterial difference in partial pressure of oxygen.
Missingness
The UNOS database exists in several versions and later versions collect more variables than previous editions. Thus, for patients treated earlier in the study period the missingness is due to the fact that certain variables were not systematically collected. Thus, by definition, most of the missing data in this study are considered missing at random (MAR) as the missingness likelihood is directly conditional upon an extraneous variable (year). Variables that contained too much missing data (more than 40%) were omitted from consideration. Data were 100% complete for the primary study endpoint-survival time. Data were missing for the following preoperative covariates: donor CMV [cytomegalovirus] status (n = 29, 0.8%), patient's CMV immunoglobulin G (IgG) (n = 1,220, 31.6%), patient's diabetes status (n = 367, 9.5%), patient's race (n = 2, 0.1%), donor's race (n = 6, 0.2%), preoperative forced expiratory volume in the first second of expiration (n = 475, 12.3%), preoperative forced vital capacity (n = 470, 12.2%), recipient cardiac output (n = 1,242, 32.2%), recipient mean pulmonary artery pressure (n = 1,096, 28.4%), donor history of cigarette use (n = 303, 7.9%), donor history of cocaine use (n = 1,044, 27.1%), recipient arterial carbon dioxide (n = 1,484, 38.5%), partial pressure of oxygen (n = 1,112, 28.8%), CMV mismatch between patient and donor (n = 1,229, 31.8%), related trauma (n = 185, 4.8%), and race mismatch between patient and donor (n = 8, 0.2%).
A multiple imputation algorithm was employed to impute missing values so that the whole sample could be analyzed. This was done in an effort to avoid selection bias that can occur by deleting cases with missing variables of interest. Ten datasets were imputed and estimates from the datasets were combined using methods originally described by Schafer [6] and Molenberghs and Kenward [7]. Data were assumed to be missing at random.
Kaplan-Meier survival curves were computed to compare the survival between unilateral and bilateral transplantations. Cox proportional hazards regression models were used to estimate the hazard ratio associated with bilateral transplantation compared with unilateral transplantation. The proportional hazards assumption, which is that the effect of bilateral transplantation is constant over time, was evaluated by visual inspection of the log negative log survivor function and by examining the correlation between Schoenfeld residuals and survival time.
Risk Adjustment
Patients were classified according to the surgery type (unilateral or bilateral) they received. To control for potential selection bias, a variety of risk adjustment approaches were performed to estimate the independent effect of bilateral transplantation. These approaches represent competing approaches to estimating this effect. The approaches include direct adjustment, propensity adjustment, and propensity matching.
The direct adjustment method assessed the effect of the variable representing bilateral treatment in the presence of the 25 covariates, which served to control for the confounding effects of these variables. This was achieved using a Cox proportional hazards model with 26 terms; bilateral treatment and the 25 covariates. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were computed to estimate the relative instantaneous hazard of death with respect to the unilateral group. A reduced associative model was employed, when necessary, to control for significant covariates.
The propensity score approach, described by Blackstone [8] and D'Agostino [9], was formulated for each patient based on 25 risk factors available preoperatively (see Table 1). For the propensity score calculation, a multiple logistic regression model was constructed non-parsimoniously to estimate the probability of being treated with bilateral surgery as a function of all 25 preoperative risk factors. The resulting conditional probability of a patient receiving bilateral surgery is the propensity score. The propensity score was then used as a covariate in proportional hazard regression models that also contained the bilateral term. The assumption of a linear relationship between the propensity score and the logit of the predicted probabilities of each outcome was verified by plotting these quantities in deciles.
A propensity matching algorithm was designed to pair the bilateral patients (n = 1,429) with 1,429 similar unilateral patients. This was achieved using optimal matching, which minimizes the propensity differences across bilateral patients and the matched unilateral patients. Once a homogeneous set of patients was identified, the survival of the groups was compared using a Cox model and an HR estimate was computed.
Lastly, a substudy was performed in bilateral patients to determine which age threshold best portended at least one year of survival posttransplant. Three approaches to identifying the age cutoff were performed. First, a logistic regression model was used to relate one-year survival (Y/N) to different cutoffs of age at surgery. Each cutoff was modeled univariately as a function of the dichotomous age cutoff and the AUROC [area under the receiver operator characteristic] curve), a measure of variable discrimination, was noted for each model. The threshold value is the cutoff that maximizes the AUROC. Second, this process was repeated only adjusting for other covariates identified as independently being related to one-year survival. Third, a visual inspection of the ROC curve that results from the univariate logistic model that employs continuous age as a predictor was qualitatively assessed. The identification of the point where the curve “stops rising and starts running” was gathered and the associated predicted probability was backfit to the model to find the age threshold.
All tests were evaluated at the 0.05 alpha level. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Univariate group comparisons of continuous and categoric variables were performed using two-sample t tests and χ2 tests, respectively.
Results
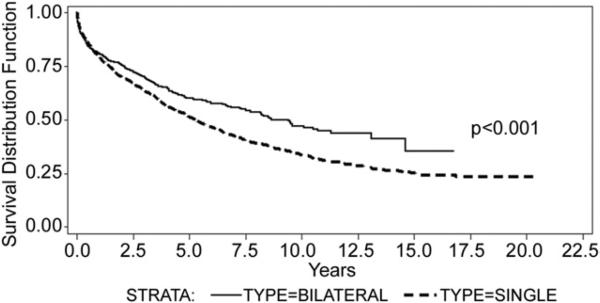
Unadjusted analysis, comparing survival based solely on BLT versus SLT, revealed a significant survival advantage for the BLT group with a mean survival of 7.37 years for the SLT group and 8.34 years for the BLT group (p < 0.001, HR = 0.798; 95% CI 0.716 to 0.889) (Fig 1). Next, a multivariate analysis was performed on recipient age alone because age has been shown, in previous studies, to be an important factor in long-term survival. This analysis showed a continued survival advantage for patients undergoing BLT (p = 0.01). However, the advantage was significantly lessened compared with the unadjusted model, as demonstrated by the hazard ratio that increased to 0.866 (95% CI 0.755 to 0.968).
Fig 1.
Unadjusted (crude) survival: bilateral lung transplant (—) versus single-lung transplant (---).
Twenty-five covariates were chosen for the study based on the rationale detailed in the methods section of the manuscript (Table 1). The direct adjustment model, utilizing the 25 covariates chosen for the study, failed to show any survival advantage based on type of procedures (p = 0.11, HR = 0.904; 95% CI 0.800 to 1.024). The effect of the type of procedure on survival was then evaluated by a propensity analysis, and again no difference was found in long-term survival for either BLT or SLT (p = 0.11, HR = 0.904; 95% CI 0.798 to 1.024).
Because recipient age appeared to have such a significant effect on survival the propensity analysis was performed again and adjusted for age (propensity matching optimal). This analysis also failed to show a survival advantage based on type of procedure (p = 0.27, HR = 0.904; 95% CI 0.825 to 1.054). The results from all of the statistical analysis discussed are summarized in Table 2. Next we calculated survival based on age groups. Patients were separated in age less than 40 years, age 41 to 60 years, and age greater than 61 years. There was no significant difference in long-term survival, by procedure type, or in any of the age groups (p = 0.91, 0.18, and 0.56, respectively).
Table 2.
Adjusted Effect of Bilateral Transplant
| Analysis Type | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Crude estimate (no adjustment) | 0.798 (0.716–0.889) | <0.001 |
| Age estimate (only age adjusted) | 0.866 (0.775–0.968) | 0.011 |
| Direct adjustment (25 covariates) | 0.904 (0.800–1.024) | 0.11 |
| Propensity regression adjustment | 0.933 (0.825–1.054) | 0.27 |
| Propensity matching optimal | 0.904 (0.798–1.024) | 0.11 |
CI = confidence interval.
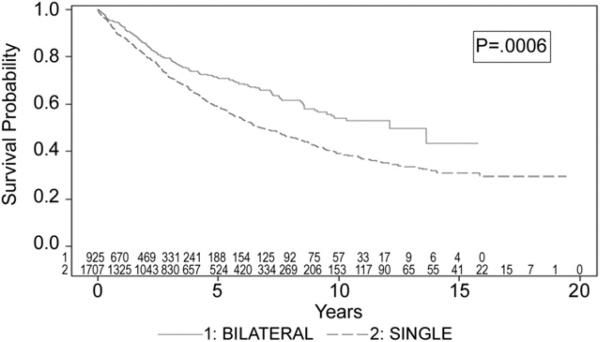
Finally, survival based on one-year conditional survival was evaluated in order to determine long-term survival rates unaffected by early deaths. When the adjusted model was rerun for survival, for all patients who were alive at one year, bilateral transplantation was found to be a significant predictor of improved long-term survival (HR 0.73, 95% CI 0.60 to 0.87, p = 0.00064). The BLT group was found to have a significantly better long-term survival compared with the SLT group (12.08 years versus 6.8 years, p = 0.0006) (Fig 2).
Fig 2.
Long-term survival conditional on survival at one year; bilateral lung transplant (—) versus versus single-lung transplant (—).
The adjusted analysis, utilizing the 25 covariates from the earlier model and year of transplantation, was then performed to determine risk factors for death at one year for the bilateral group. Recipient age, donor age, and year of transplantation and were found to be significant predictors of one-year survival in this group (Table 3.) The ROC analysis revealed that BLT one-year mortality increased significantly for recipients over age 57 and when donors older than 36 were used.
Table 3.
Predictors of One-Year Survival in Bilateral Patients
| Effect | Hazard Ratio | Lower | Upper | p Value |
|---|---|---|---|---|
| Transplant year | 0.93608 | 0.91177 | 0.96103 | 0.000001 |
| Recipient age | 1.02137 | 1.01215 | 1.03067 | 0.000005 |
| Donor age | 1.00785 | 1.00153 | 1.01420 | 0.014839 |
Comment
The first successful lung transplant, performed by Dr. Joel Cooper and the Toronto Lung Transplant Group in 1983 [10], was a single-lung transplant for pulmonary fibrosis. Three years later the group published a paper reporting on the good functional results and early survival of two patients transplanted for pulmonary fibrosis. Since that time the transplant literature has taken opposing sides on the preference for BLT or SLT for pulmonary fibrosis.
Our data show that survival after BLT is better than SLT for pulmonary fibrosis, when no recipient or donor characteristics are considered in the analysis. However, when covariates representing possible risk factors for donor and recipients were entered into the analysis the survival advantage for BLT was no longer. When the two groups were further “balanced” using propensity analysis, BLT also failed to show a survival advantage over SLT.
Some authors [11] have suggested that BLT should be reserved for younger patients, yet our initial data failed to show a survival advantage for BLT in any age group. Other studies [4, 5] have shown no survival benefit for BLT in older patients and even a survival disadvantage for younger patients. We were unable to find a survival advantage any age group in patients undergoing BLT.
Other authors have also suggested that high-risk recipients benefit from BLT. A recent article by Weiss and colleagues [12] found that high-risk patients (those with a high LAS [lung acuity score]) who underwent BLT actually had an improved one-year survival compared with SLT patients. The survival advantage, however, was modest at 14% and it is unclear how factors such as shorter wait-list times for higher LAS patients improved results for higher volume programs and a bias toward BLT for higher LAS patients factored into the results.
Other studies have found an increased mortality in patients undergoing BLT for IPF. The 2009 ISHLT Transplant Registry [2], in fact, showed a greater relative risk of death, at one year, for BLT as compared with SLT. Meyer and colleagues’ [4] analysis of the UNOS database revealed a higher one-month mortality in younger patients (30 to 49 years) after BLT. A study similar to ours, by Thabut and colleagues [13], found results similar to the ones that we have reported, and also found a higher risk of one-year mortality in the bilateral group. This group found that BLT did confer a long-term survival advantage for all patients who survived the first year after transplant. Our data confirmed this finding. Furthermore, we were able to show that recipient age and donor age were significant predictors of one-year mortality in the BLT group and that the age cutoff for this survival advantage was 57 in the recipients and 36 in the donors. This information could be used to help define protocols for which patients should receive BLT for IPF.
Patients who present with IPF and secondary pulmonary hypertension should also be considered for BLT. Although this study was not designed to evaluate the effect of pulmonary hypertension on survival, pulmonary hypertension was found to be a predictor of survival in the adjusted model. One manuscript has addressed this topic. Whelan and colleagues [14] found increased pulmonary artery pressure to be a risk factor for early death in patients undergoing SLT.
When considering what type of transplant to perform on patients with IPF, programs should consider the potential benefits of BLT and SLT. The SLT allows for maximal resource utilization and may be better tolerated in older patients or patients who have had previous heart surgery. The BLT, on the other hand, appears to offer a survival advantage at a cost of a higher one-year mortality. Utilizing the data from our results, one could consider trying to optimize long-term survival by only performing BLT on patients younger than 57 years.
Our study does not address the possible reason for improved survival in the BLT group. One explanation could be lower risk for developing chronic rejection. Long-term survival for patients undergoing transplant for emphysema has been shown to be better for BLT than SLT. This survival advantage has been thought to be largely due to the increased freedom from bronchiolitis obliterans (BOS) in the BLT patients over time [15]. This same philosophy may explain the long-term survival advantage for patients undergoing BLT for IPF. The Munich Lung Transplant Group [16] found a significant increase in BOS-free survival in patients undergoing BLT for IPF compared with SLT patients. They also found SLT to be a significant predictor for death and BOS in their patients. Haider and colleagues [17] compared groups of patients who underwent SLT for IPF and emphysema and found that, despite a similar incidence of BOS, mortality was greater in the IPF group after the onset of chronic rejection.
This study is limited by the fact that it is a retrospective review of a large database over many years. Many of the data points were incomplete, which necessitated the use of a multiple imputation algorithm. Additionally, many variables that could have affected survival, such as donor pneumonia or recipient debilitation, are not available in the UNOS database and therefore are not accounted for in our study. Also, survival was affected by transplant year and most likely by experience of the individual programs. It is possible that more experienced programs utilized BLTs more often than less experienced ones and this could have biased the results. Finally, the UNOS database does not pair recipients with their specific donor. This is a unique relationship and outcomes can be dependent not only on the individual factors but also on the interaction between recipient and donor.
The study is not without limitations due to the use of a large incomplete database but we were able to analyze several thousand patients and include many donor and recipient characteristics and thus decrease error due to bias. The optimal study would be a prospective randomized trial but this would necessitate enrollment by every transplant program to obtain the necessary number of patients and require years to complete. This study will, almost assuredly, never happen, and therefore we must utilize the data that we have to formulate the best treatment options for patients. Based on our findings it seems reasonable to consider BLT for younger patients or when younger donors can be utilized. Transplanting patients with a combination of these two variables could possibly allow for lower postoperative morbidity and therefore improved early and late survival. This may represent an interesting concept for future studies.
References
- 1. [October 1, 2009];Health Resources and Service Administration OPTN/SRTR Annual Report. Table 12.4a. Available at: www.hhs.gov.
- 2.Christie JD, Edwards LB, Aurora P, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J Heart Lung Transplant. 2009;28:1031–49. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Mason DP, Brizzio ME, Alster JM, et al. Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg. 2007;84:1121–8. doi: 10.1016/j.athoracsur.2007.04.096. [DOI] [PubMed] [Google Scholar]
- 4.Meyer DM, Edwards LB, Torres F, Jessen ME, Novick RJ. Impact of recipient age and procedure type on survival after lung transplantation for pulmonary fibrosis. Ann Thorac Surg. 2005;79:950–8. doi: 10.1016/j.athoracsur.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 5.Nwakamna LU, Simpkins CE, Williams JA, et al. Impact of bilateral versus single lung transplantation on survival in recipients 60 years of age and older: analysis of United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2006;133:541–7. doi: 10.1016/j.jtcvs.2006.09.062. [DOI] [PubMed] [Google Scholar]
- 6.Schafer JL. Analysis of incomplete multivariate data. Chapman & Hall/CRC; Boca Raton, FL: 1997. [Google Scholar]
- 7.Molenberghs G, Kenward MG. Missing data in clinical studies. 1st Ed. John Wiley and Sons; New York, NY: 2007. pp. 105–17. [Google Scholar]
- 8.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123:8–15. doi: 10.1067/mtc.2002.120329. [DOI] [PubMed] [Google Scholar]
- 9.D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Toronto Lung Transplant Group Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med. 1986;314:1140–5. doi: 10.1056/NEJM198605013141802. [DOI] [PubMed] [Google Scholar]
- 11.Rinaldi M, Sansone F, Boffini M, et al. Single versus double lung transplantation in pulmonary fibrosis: a debated topic. Transplant Proc. 2008:2010–2. doi: 10.1016/j.transproceed.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Survival after single versus bilateral lung transplantation for high-risk patients with pulmonary fibrosis. Ann Thorac Surg. 2009;88:1616–26. doi: 10.1016/j.athoracsur.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Thabut G, Christie JD, Ravaud P, Castier, et al. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Ann Intern Med. 2009;151:767–74. doi: 10.7326/0003-4819-151-11-200912010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Whelan TPM, Dunitz JM, Kelly RF, et al. Effect of preoperative pulmonary artery pressure on early survival after lung transplantation for idiopathic pulmonary fibrosis. J Heart Lung Transplant. 2005;24:1269–74. doi: 10.1016/j.healun.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Cassivi SD, Meyers BF, Battafarano RJ, et al. Thirteen-year experience in lung transplantation for emphysema. Ann Thorac Surg. 2002;74:1663–70. doi: 10.1016/s0003-4975(02)04064-x. [DOI] [PubMed] [Google Scholar]
- 16.Neurohr C, Huppmann P, Deuschner W, et al. Potential functional and survival benefit of double over single lung transplant for selected patients with idiopathic pulmonary fibrosis. Transpl Int. 2010;23:887–96. doi: 10.1111/j.1432-2277.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 17.Haider Y, Yonan N, Mogulkoc N, Carroll KB, Egan JJ. Bronchiolitis obliterans syndrome in single lung transplant recipeints – patients with emphysema versus patients with idiopathic pulmonary fibrosis. J Heart Lung Transplant. 2002;21:327–33. doi: 10.1016/s1053-2498(01)00398-9. [DOI] [PubMed] [Google Scholar]