Abstract
Purpose
Gastric fundoplication (GF) for gastroesophageal reflux disease (GERD) may protect against the progression of chronic rejection in lung transplant (LT) recipients. However, the association of GERD with acute rejection episodes (ARE) is uncertain. This study sought to identify if ARE were linked to GERD in LT patients.
Methods
This single-center retrospective observational study, of patients transplanted from January 1, 2000, to January 31, 2009, correlated results of pH probe testing for GERD with ARE (≥International Society for Heart and Lung Transplantation A1 or B1). We compared the rates of ARE among patients with GERD (DeMeester Score > 14.7) versus without GERD as number of ARE per 1,000 patient-days after LT. Patients undergoing GF prior to LT were excluded.
Results
The analysis included 60 LT subjects and 9,249 patient-days: 33 with GERD versus 27 without GERD. We observed 51 ARE among 60 LT recipients. The rate of ARE was highest among patients with GERD: 8.49 versus 2.58, an incidence density ratio (IDR) of 3.29 (P = .00016). Upon multivariate negative binomial regression modeling, only GERD was associated with ARE (IDR 2.15; P = .009). Furthermore, GERD was associated with multiple ARE (36.4% vs 0%; P < .0001) and earlier onset compared with patients without GERD: ARE proportion at 2 months was 0.55 versus 0.26 P = .004).
Conclusion
In LT recipients, GERD was associated with a higher rate, multiple events, and earlier onset of ARE. The efficacy of GF to reduce ARE among patients with GERD needs further evaluation.
Despite advances in the understanding of the mechanisms and management of immune-mediated injury, acute cellular rejection episodes (ARE) continue to be a prevalent complication after lung transplantation (LT). Its consequences are relevant; repeated ARE are strongly linked to the development of bronchiolitis obliterans syndrome (BOS).1,2 Even minimal ARE have been shown to be a risk factor for BOS.3,4 Some evidence implicates gastroesophageal reflux disease (GERD) after LT as a nonalloimmune factor in the development of chronic rejection and BOS.5–7 Earlier studies have suggested that surgical correction of reflux in LT patients with BOS may improve lung function and that early fundoplication results in a decreased severity of BOS.8,9 Although the associations of BOS with ARE and GERD have been established, the link between GERD with ARE is still unclear. Furthermore, surgical correction of GERD is not well documented as a treatment modality for ARE.
Studies evaluating the associations and mechanisms by which nonalloimmune phenomena such as GERD result in ARE are sparse and inconsistent. Experiments in rats have shown that lung allografts develop ARE after aspiration of gastric contents.10 Additionally, human lung allografts with ARE have been shown to display higher levels of pepsin in bronchoalveolar lavage (BAL) compared with controls.11 Hartwig et al12 reported that gastric fundoplication (GF) <45 days after LT reduced the incidence of ARE by 66%. However, an earlier, larger retrospective analysis from the same center showed no difference in the rate of ARE between patients without or, with reflux but no surgery, or with reflux treated with fundoplication.8 The association of GERD with ARE in LT merits further examination, because GERD is potentially treatable and ARE are strongly linked to an increased morbidity of BOS. The aim of the present study was to clarify the limited body of knowledge regarding this poorly defined association. We hypothesized that the rate of ARE among patients with GERD was higher than that among those without GERD. Furthermore, we believed that GERD in LT recipients increased the severity and rate of ARE in the early period after LT.
METHODS
Design and Subjects
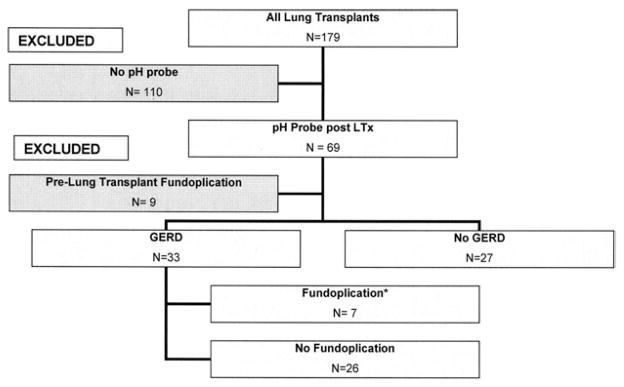
This retrospective observational analysis includes lung transplantation recipients who underwent dual-channel (proximal and distal esophagus) pH probe testing for presence of GERD from January 2000 to January 2009. In addition, patients underwent esophageal manometry with impedance. The study was approved by our Investigational Review Board. We recorded the times after transplantation, fundoplication, death, or each ARE from scheduled surveillance transbronchial biopsies (TBB) at 6 months. The 6-month time course was chosen because most ARE occur during this timeframe. Subsequently, patients were divided into 2 groups: lung transplant allografts with GERD versus those without GERD. Only the episodes and the time at risk for ARE before GF (if performed) in patients with GERD were incorporated into the analysis (Fig 1). We excluded from the study patients with GF before LT who had been shown to have GERD around the time of transplantation.
Fig 1.
Selection criteria. *Episodes of AR and patient-days after gastric fundoplication in this group were excluded.
The rate, also known as the incidence density (ID), of ARE for each group was the summation of all events of ARE per cumulative time of each member in the subgroup. For ease of comparison, the ID of acute rejection was converted into units of events per 1,000 patient-days.
Definitions of GERD and ARE
Starting in 2007, patients were evaluated with pH probe manometry at 3 months after LT or with the first episode of ARE. Additionally, other patients transplanted before implementation of this new protocol were evaluated by pH probe for the presence of GERD either because of symptoms or for evaluation of BOS. A composite DeMeester score of upright and supine readings >14.7 defined the presence of GERD by the pH study. This degree of reflux corresponds to the 95th percentile of reflux in a random cohort of subjects.13,14 In addition, we recorded by manometry and impedence, simultaneous as well as retrograde contractions. Excluding the time before pH probe testing, all patients received a proton pump inhibitor or H2 blocker treatment during the study period. Surgical treatment for GERD was performed by laparoscopic Nissen fundoplication.
The diagnosis of ARE required pathologic confirmation from TBB. Vasocentric lymphocytic inflammation (A) or bronchiolar lymphocytic inflammation (B) ≥1 according to the International Society of Heart and Lung Transplant (ISHLT) severity criteria indicated the presence of ARE.15 Only scheduled surveillance TBBs were included in the analysis.
Acute Rejection Management
After January 2007, all patients received basiliximab induction therapy after lung transplantation and tacrolimus for maintenance immunosuppression as opposed to cyclosporine. As previously published, maintenance therapy involved a combination of prednisone, tacrolimus, or cyclosporine combined with mycophenolate or azathioprine.16 Every recipient underwent surveillance bronchoscopy with TBB at 2 weeks as well as 1, 2, 3, 6, 9 and 12 months after LT. To minimize measurement bias between groups, only surveillance TBBs were recorded during 6 months of follow-up. Therefore, an LT patient without complications would be expected to have 5 surveillance TBBs. Surveillance TBB was not performed in the setting of acute illness or if there was a risk for significant complications.
When ARE ≥A2 occurred, patients were treated with 3 daily doses of methylprednisolone (10 –15 mg/kg intravenously) before return to their baseline steroid dose. Each documented ARE was followed by another TBB at 4 – 6 weeks after therapy.
Statistical Analysis
We calculated the 95% confidence interval of ARE IDs, including ARE with certain severity or within a time window, among the GERD or non-GERD cohort by using exact Poisson confidence intervals. To compare the IDs between cohorts, we used exact conditional test for the ratio of the 2 Poisson rates. Because of the nonrandom distribution of patients and imbalances in cohort characteristics, we identified 5 covariates a priori that were most likely to affect the outcomes. Using univariate negative binomial regression analysis, crude ARE rates were adjusted for: GERD versus non-GERD cohort; number of HLA mismatches, highest primary graft dysfunction (PGD) severity at 48 or 72 hours, type of native lung disease, cytomegalovirus (CMV) status of the recipient, and use of basiliximab and tacrolimus. Multivariate analysis was performed on the covariates identified on univariate analysis to have significant effects, by using negative binomial regression analysis. The probability distributions of time to the first ARE after lung transplantation were estimated by using Kaplan-Meier estimators for comparisons with log-rank tests. All tests were 2 sided. A P value of ≤.05 was considered to be significant. All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
The 60 lung allograft recipients who underwent pH probe testing were divided into 2 groups: GERD (n = 33) versus non-GERD (n = 27) subjects (Fig 1). Forty-three subjects underwent pH probe testing after the 2007 implementation of our institution’s new GERD screening protocol: 21 with GERD versus 22 without GERD. Among the patients with GERD, 12 TBBs and 790 patient-days occurred after GF in 7 patients who were excluded from the analysis. After omitting biopsies and time after GF, patients with GERD showed a similar frequency of completed surveillance TBBs (82.3% vs 83.7%) but slightly shorter median follow-up (160.5 vs 182 days). No significant differences were present between the groups in age, gender, race, native lung disease, recipient CMV status, severity of PGD, use of basiliximab and tacrolimus, or HLA mismatch (Table 1).
Table 1.
Baseline Characteristics
| GERD (n = 33) | No GERD (n = 27) | P Value | |
|---|---|---|---|
| Median age, (y) [95% CI] | 56.0 (29.0–66.0) | 60.0 (18.0–67.0) | .09 |
| Male (%) | 51.51 | 63.0 | .79 |
| Native lung disease (%) | |||
| ILD | 30.3 | 40.7 | .69 |
| CF | 3.0 | 3.7 | |
| COPD | 48.5 | 33.3 | |
| Other | 18.2 | 22.2 | |
| Caucasian (%) | 75.8 | 74.1 | .88 |
| PGD highest at 48 or 72 h (%) | |||
| 0–1 | 60.6 | 66.7 | .63 |
| 2–3 | 39.4 | 33.3 | |
| Recipient CMV status (%) | |||
| R+/D+, R+/D− | 69.7 | 85.2 | .16 |
| R−/D+, R−/D− | 30.3 | 14.8 | |
| Basiliximab and tacrolimus (%) | 60.6 | 81.5 | .08 |
| HLA mismatches (%) | |||
| 0–4 | 24.2 | 18.5 | .59 |
| 5–6 | 75.8 | 81.5 | |
Abbreviations: ILD, interstitial lung disease; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; CI, confidence interval; PGD, primary graft dysfunction; CMV, cytomegalovirus.
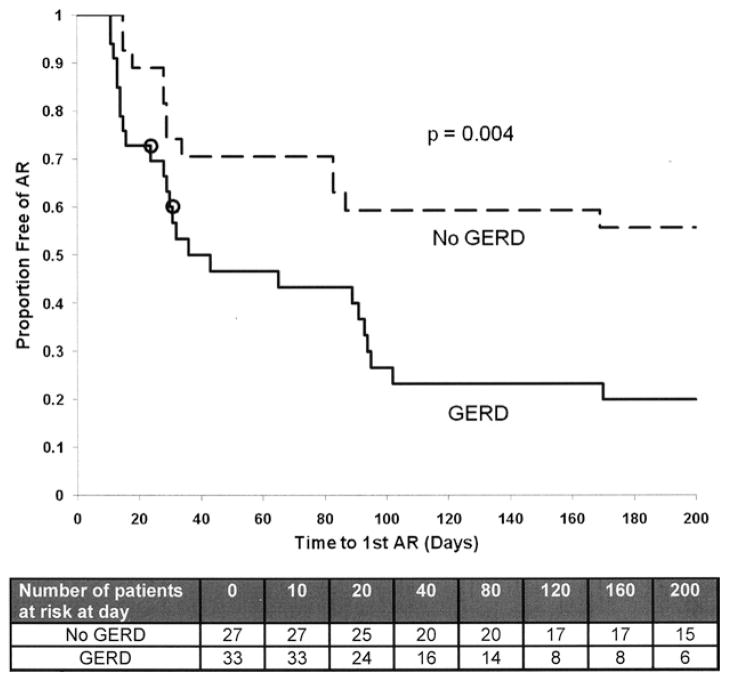
Among the 60 subjects, 51 ARE occurred in 36 LT recipients during 9,249 patient-days: 5.51 ARE per 1,000 patient-days. The allocation of ARE and times at risk to develop ARE are presented in Table 2. GERD was associated with a higher unadjusted rate of ARE compared with patients without GERD (ID ratio [IDR], 3.29; P = .00016). Furthermore, patients with GERD more frequently developed ≥2 ARE than patients without GERD: 36.4% vs 0% (P < .0001). Although patients with GERD developed more severe ARE, it was proportionate to the increased rate of ARE for all severities (Table 3). In time-to-event analysis, GERD was associated with earlier onset of ARE after LT (Fig 2). The proportion of patients with GERD developing ARE at 6 months was 80% versus 44% in patients without GERD (P = .004). The 2 patients with GERD who did not develop ARE before undergoing surgical treatment were censored at the time of GF.
Table 2.
Crude and Multivariate Analysis
| GERD (n = 33) (95% CI) | No GERD (n = 27) (95% CI) | P Value | |
|---|---|---|---|
| Median # of ARE | 1 (0–3) | 0 (0–1) | |
| Episodes of ARE | 39 | 12 | |
| # of surveillance TBBs | 126 | 113 | |
| Patient-days | 4,593 | 4,656 | |
| Incidence density (1,000 patient-days) | 8.49 (6.03–11.61) | 2.58 (1.33–4.50) | .00016 |
| Crude incidence density ratio | 3.29 | .00016 | |
| Adjusted* incidence density ratio | 2.15 | .009 | |
Abbreviations: ARE, acute rejection episodes; TBB, transbronchial biopsy; others as in Table 1.
GERD, recipient CMV status, and use of basiliximab and tacrolimus were identified to have significant effects by univariate analysis and were used for multivariate analysis by negative binomial method.
Table 3.
Secondary Analysis
| GERD (n = 33) (95% CI) | No GERD (n = 27) (95% CI) | P Value | |
|---|---|---|---|
| ≥2 ARE | 12 (36.4%) | 0 | <0.0001 |
| Incidence density by severity of ARE | |||
| A1 | 3.48 (1.99–5.66) | 0.86 (0.23–2.20) | .011 |
| A2 | 4.14 (2.49–6.46) | 1.29 (0.47–2.80) | .013 |
| A3 | 0.44 (0.05–1.57) | 0 | .49 |
| A4 | 0 | 0 | .35 |
| B1 | 1.31 (0.48–2.84) | 0.86 (0.23–2.20) | .74 |
| B2 | 0.44 (0.05–1.57) | 0 | .49 |
Fig 2.
Time to first acute rejection episodes (ARE). P = .004 calculated by log-rank test; GERD = gastroesophageal reflux disease; O = censored if fundoplication before to ARE.
It is important to mention that 31.7% of lung transplant patients showed abnormal liquid bolus peristalsis and 52.5% abnormal viscous bolus peristalsis. There was a 17.7% incidence of retrograde peristalsis on the impedance study.
From 2000 to 2006, 17 patients were evaluated by pH probe testing based on symptoms and clinical suspicion of GERD. The 12 patients with GERD developed 18 ARE during 1,819 patient-days compared with 4 ARE during 868 patient-days among the 5 patients without GERD (9.89 vs 4.61 per 1,000 patient-days; IDR 2.14). After implementation of the new GERD screening protocol in 2007, 21 patients showed GERD and 22 patients did not. The former group developed 21 ARE during 7,089 patient-days compared with ARE in 7,750 patient-days in the latter group: 2.96 vs 1.03 per 1,000 patients-days; IDR 2.87.
The covariates use of basiliximab and tacrolimus, severity of PGD, recipient CMV status, HLA mismatches, and native lung disease were evaluated by univariate analysis (Table 4). Only GERD, use of basiliximab and tacrolimus, and negative recipient CMV status showed significant effects on the ID of ARE namely IDR 2.51 (P = .001), IDR 0.51 (P = .007), and IDR 1.86 (P = .016) respectively. After including these covariates with GERD in a multivariate model, only GERD maintained an association with ARE (IDR 2.15; P = .009; Table 2).
Table 4.
Univariate Analysis
| Covariate | Incidence Density Ratio for ARE (95% CI) | P Value |
|---|---|---|
| GERD | 2.51 (1.45–4.35) | .001 |
| Basiliximab and tacrolimus | 0.51 (0.32–0.84) | .007 |
| PGD score ≥2 | 0.69 (0.38–1.24) | .22 |
| Recipient CMV (−) | 1.86 (1.12–3.09) | .016 |
| Native lung disease | 1.00 | .54 |
| CF | 2.19 (0.30–14.83) | .42 |
| COPD | 2.01 (0.27–13.71) | .48 |
| ILD Other | 1.31 (0.29–13.71) | .79 |
| HLA mismatch (≥5) | 1.07 (0.56–2.06) | .83 |
DISCUSSION
The principle finding of this investigation was that patients with GERD showed a 3.29-fold increase in the rate of ARE compared with patients without GERD (P = .00016). After adjustment for confounding variables, GERD remained associated with ARE by 2.15 (P = .009). Additionally, 36.4% of patients with GERD developed ≥1 ARE compared with no patients without GERD (P < .0001). Finally, GERD was associated with earlier onset of ARE: by 6 months, 80% of patients with GERD developed, ARE compared with 44% of patients without GERD (P = .004).
This study supported the hypothesis that GERD may be linked to acute injury in lung allografts accentuating the rate, frequency, and onset of ARE. This observation is relevant to transplant clinicians because multiple ARE of all severities shows strong associations with the development of BOS.3,17–19 The present study supports previous investigations using molecular analysis and rat models to link ARE in lung transplant allografts to reflux.10,11
In contrast, Cantu et al8 failed to observe an association between GERD and the time of fundoplication with the development of ARE; In that retrospective investigation, patients with early fundoplication, namely, <90 days after LT, showed a higher rate of ARE compared with patients without GERD or those with GERD treated by late fundoplication. Evaluating our data, ~54% of patients with GERD developed ARE within the initial 45 days, whereas only 26% of patients without GERD were affected. This assumption is supported by the findings published by Hartwig et al.12 Although their methodology is unpublished, their lung transplantation patient who underwent GF within 45 days show a higher freedom from ARE. Perhaps the lack of association between GERD and ARE in the Cantu et al8 investigation may reflect a false negative conclusion, because 90 days is beyond the time frame of the majority of ARE.
The retrospective nature of the present study warrants recognition of some limitations. First, we acknowledge that selection bias might affect the validity of our results. Not all patients underwent pH probe testing before 2007. Perhaps during that time, patients who were more symptomatic tended more to undergo pH probe testing. Yet, it is well established that patients with end-stage lung disease often have frequent and asymptomatic reflux.20 –22 Despite this potential bias, the IDRs of ARE did not differ before and after implementation of the new protocol. Additionally, some patients never underwent pH probe testing after implementation of an aggressive protocol to evaluate GERD in all lung transplant subjects, because of severe illness or death. However it seems unlikely that selection of healthier patients for pH probe testing would results in a type I error.
Second, we attempted to avoid measurement bias by using only surveillance TBBs and 6 months of follow-up among both groups. The proportion of TBBs was similar in both groups, but the follow-up was slightly shorter in patients with GERD, The unequal follow-up time was unavoidable, because the time after GF for 7/33 patients with GERD was excluded from the analysis, which tended to make the IDR slightly higher in favor of GERD. However, the difference in patient-days between the 2 groups was minimal and unlikely to affect the linkage between the rate of ARE and GERD. Additionally, the disparity would not affect the association with multiple episodes and an earlier onset of ARE.
Finally, in this comparison of unmatched groups, the measured effect might be influenced by associated cofounders. We attempted to adjust for the most important covariates that were determined a priori by statistical modeling. However, patients without GERD tended to get more basiliximab and tacrolimus treatment, which have been advocated to decrease the rate of ARE mediated by alloimmune mechanisms.23,24 Despite differences in the unmatched cohorts, these covariates did not significantly influence the association of GERD with ARE upon multivariate analysis.
Our analysis supported the hypothesis that the development of ARE was augmented by nonimmune mechanisms, such as GERD. Surgical correction for GERD is commonly performed to mitigate the late complications of lung transplantation, BOS. Yet our findings suggested that lung injury from GERD starts within weeks after LT. Although aggressive scrutiny for the presence of GERD in this population is justified to identify patients at high risk for ARE and BOS, the timing and benefit of GF for ARE remain uncertain. A prospective evaluation comparing the timing of GF in relation to LT is necessary to determine whether surgical treatment can diminish the rate of ARE.
References
- 1.Estenne M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 2.Sharples LD, et al. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins PM, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2004;170:1022. doi: 10.1164/rccm.200302-165OC. [DOI] [PubMed] [Google Scholar]
- 4.Khalifah AP, et al. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant. 2005;5:2022. doi: 10.1111/j.1600-6143.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 5.Ward C, et al. Pepsin like activity in bronchoalveolar lavage fluid is suggestive of gastric aspiration in lung allografts. Thorax. 2005;60:872. doi: 10.1136/thx.2004.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blondeau K, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 7.D’Ovidio F, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Cantu E, 3rd, et al. J Maxwell Chamberlain memorial paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142. doi: 10.1016/j.athoracsur.2004.04.044. discussion 1142. [DOI] [PubMed] [Google Scholar]
- 9.Davis RD, Jr, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 10.Hartwig MG, et al. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg. 2006;131:209. doi: 10.1016/j.jtcvs.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 11.Stovold R, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med. 2007;175:1298. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 12.Hartwig MG, Appel JZ, Davis RD. Antireflux surgery in the setting of lung transplantation: strategies for treating gastroesophageal reflux disease in a high-risk population. Thorac Surg Clin. 2005;15:417. doi: 10.1016/j.thorsurg.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 14.Streets CG, DeMeester TR. Ambulatory 24-hour esophageal pH monitoring: why, when, and what to do. J Clin Gastroenterol. 2003;37:14. doi: 10.1097/00004836-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Pelaez A, et al. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant. 2009;28:67. doi: 10.1016/j.healun.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bando K, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110(4) doi: 10.1016/S0022-5223(05)80003-0. discussion 13. [DOI] [PubMed] [Google Scholar]
- 18.Girgis RE, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15:1200. [PubMed] [Google Scholar]
- 19.Kroshus TJ, et al. Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114:195. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 20.Sweet MP, et al. The prevalence of distal and proximal gastroesophageal reflux in patients awaiting lung transplantation. Ann Surg. 2006;244:491. doi: 10.1097/01.sla.0000237757.49687.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweet MP, et al. Gastroesophageal reflux in patients with idiopathic pulmonary fibrosis referred for lung transplantation. J Thorac Cardiovasc Surg. 2007;133:1078. doi: 10.1016/j.jtcvs.2006.09.085. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 23.Borro JM, et al. Comparative study of basiliximab treatment in lung transplantation. Transplant Proc. 2005;37:3996. doi: 10.1016/j.transproceed.2005.09.192. [DOI] [PubMed] [Google Scholar]
- 24.Hachem RR, et al. A comparison of basiliximab and anti-thymocyte globulin as induction agents after lung transplantation. J Heart Lung Transplant. 2005;24:1320. doi: 10.1016/j.healun.2004.09.002. [DOI] [PubMed] [Google Scholar]