Abstract
Tissue factor plays a primary role in both hemorrhage control and thrombosis depending upon whether its presentation is extravascular or intravascular. The molecular architecture and function of the tissue factor molecule and its role in the activations of factor IX and factor X have been elegantly elucidated but controversies prevail with respect to distinctions between tissue factor sources and tissue factor “activity.” This presentation will review data on the architecture and functions of the tissue factor-factor VIIa complex and discuss the elements of the controversies associated with tissue factor presentation in both normal and pathologic milieu.
Ancient History
The observation that tissue “juice” accelerated coagulation was published over 150 years ago and formalized with the terms “thrombokinase” and “thromboplastin” as the initiator of extrinsic pathway of coagulation over a century ago [1]. Subsequently it was observed that thromboplastic activity could be divided into lipid and protein components; the latter termed tissue factor, was isolated by Nemerson and Bach [2]. The isolated protein enabled the cloning of the protein and gene sequencing [3] and expression of recombinant (r) forms of the protein corresponding to residues 1-263 [4], 1-243 and 1-219 [5] which have been the principle subjects utilized in protein chemistry and functional experiments. A soluble tissue factor-factor VIIa complex was crystallized and a high resolution structure obtained [6]. However, in spite of this level of molecular detail, multiple controversies have swirled regarding the structure and function of tissue factor.
Structure
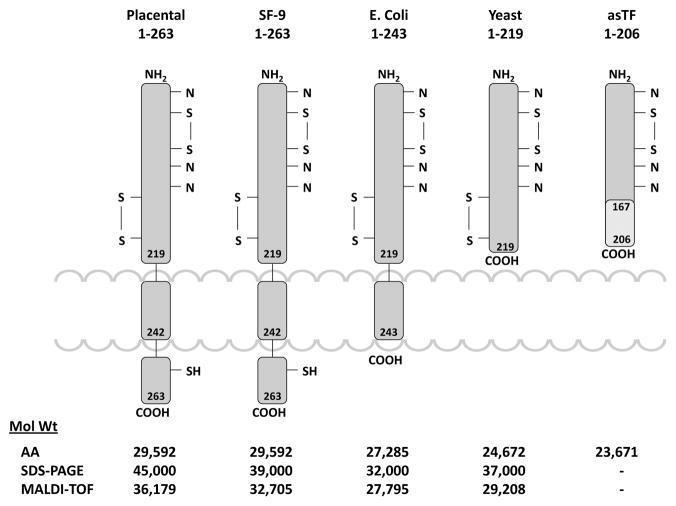
Two forms of tissue factor are present in recombinant and natural tissue factor, one of which has the two first amino acids deleted, thus the mature proteins contain either 261 or 263 amino acids [3]. The molecule is described as having three domains: an extracellular domain, residues 1-219, composed of two fibronectin type 3 domains; a transmembrane domain, residues 220-242, and a cytoplasmic domain, residues 243-263 [3]. The various forms of the protein which have been studied are represented in Figure 1 which illustrates the source and structures of the tissue factor species and their structures of the tissue factor species and their molecular weights. Posttranslational modifications include disulfide bond pairing (Cys49 - Cys57 and Cys186 - Cys209)[7]. The protein is palmitoylated by thioester formation at Cys245 [8]. Natural and r1-263 tissue factor are glycosylated at a number of sites while tissue factor r1-243 is produced in bacteria and is not glycosylated [7, 9, 10]. The Cys186 – Cys209 disulfide bridge is of particular interest with respect to the “deencryption” of tissue factor [11].
Figure 1.
Models illustrating the organizational structures of the tissue factor molecules extending from the placental protein to the expressed recombinant proteins and the soluble tissue factor observed in plasma. Cytoplasmic, inter-membrane and extra-cellular domains are illustrated with the locations of the disulfide bonds and potential sites of glycosolation. The molecular weights of each of these species based upon amino acid composition, SDS gel and mass spectroscopy are also illustrated.
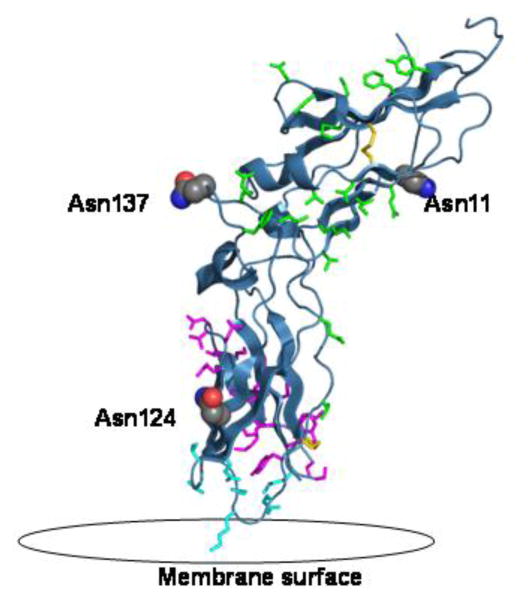
There are three potential glycosylation sites on the extracellular domain, Asn11 and Asn124 and Asn137 [7]. These are represented in the crystal structure of tissue factor, Figure 2, with the residues important for tissue factor interaction with factor VIIa, and the disulfide bridges Cys49–Cys57 and Cys186–Cys209. Asn124 is the closest to the membrane and in the proximity of binding of factor X to tissue factor [6].
Figure 2.
The extracellular domain of tissue factor (PBD 2HFT) on a modeled lipid membrane. The figure shows in red the three sites of glycosylation on TF including Asn11, Asn124 and Asn137. Highlighted in green are residues important for TF interaction with FVIIa including Thr17, Lys20, Ile22, Glu24, Gln37, Asp44, Lys46, Lys48, Asp58, Thr60, Phe76, Tyr78, Gln110, Leu133, Arg135, Phe140, Val207. Highlighted in magenta are residues important for the interaction with FX including Thr154-Glu174 and Tyr185. Highlighted in aqua are the residues important for TF interaction with the membrane including Gln118, Val119, Thr121, Lys159, Asp180, Lys181, Glu183. Also shown in yellow are the two disulfide bridges of TF at positions Cys49-Cys57 and Cys186-Cys209.
Function
Following vascular perforation the complex of extravascular tissue factor, anionic phospholipids, and plasma factor VIIa (approximately 1% of factor VII is present as cleaved protease form, factor VIIa) initiates the hemostatic process. The tissue factor-factor VIIa membrane complex (the extrinsic factor Xase) activates factor X to factor Xa. Since bleeding is not associated with defects in the proteins of the intrinsic (contact) pathway (factor XII, prekallikrein, high molecular weight kininogen), a biological conundrum ensued since the bleeding pathologies of hemophilia A and hemophilia B could not be explained by factor Xa generated through the extrinsic pathway. Osterud and Rapaport [12], however, showed that tissue factor-factor VIIa can activate factor IX to factor IXa, providing a pathway that would include the congenital hemophilias. The rate of factor IX activation by tissue factor-factor VIIa is enhanced by partial feedback activation by factor Xa cleavage [13]. Factor IXa is produced at about the same rate as factor Xa by the tissue factor-factor VIIa complex when both substrates are presented. Thus, both the “extrinsic” and “intrinsic” factor X activators participate in the extrinsic pathway of coagulation. The factor VIIIa-factor IXa complex is more efficient than tissue factor-factor VIIa and the latter is inhibited by the tissue factor pathway inhibitor (TFPI) [14]. The dynamics of the factor X activation process by the two complexes provides a logical explanation of why individuals with hemophilia A or hemophilia B display a hemorrhagic phenotype even though factor Xa can be produced from tissue factor-factor VIIa alone [15] The latter mechanism was invoked in the development of recombinant factor VIIa for the treatment of hemophilia individuals with inhibitors [16]. In that instance, superphysiologic concentrations of factor VIIa are provided which together with endogenous tissue factor provides sufficient factor Xa to overcome many hemorrhagic conditions [17].
The generation of thrombin, the enzyme responsible for clot formation, as well as other procoagulant and anticoagulant functions during the blood coagulation process occurs in a distinctly non-linear fashion in closed systems [18]. During an initiation phase, tiny amounts of thrombin are generated, platelets, zymogens and procofactors are activated and the complex enzymes responsible for most thrombin generation are assembled. Subsequently, during a propagation phase, a dramatic increase in both the rate and extent of thrombin generation is observed. The duration of the initiation phase, which roughly corresponds to the clotting time of blood and plasma, is predominantly dependent upon the concentrations of tissue factor and TFPI. The propagation phase generation of factor Xa is primarily driven by the factor VIIIa-factor IXa complex.
The observations of circulating soluble tissue factor antigen [19] and tissue factor on microparticles [20] raised the hypothesis that tissue factor dependent thrombin generation requires the continuous infusion of this cofactor, with plasma tissue factor playing an important role in hemostasis. The requirement of tissue factor for both the initiation and propagation phase has been suggested by Taubman et al[21]. In contrast, data from our laboratory and others were consistent with the notion that there is little or no tissue factor related activity in the blood of healthy humans or mice. We approached the question of the need for additional tissue factor to invoke a healthy clot response using a combination of numerical, synthetic proteome and whole blood experiments. In these experiments, repetitive additions of reactants to an ongoing clotting process were conducted to simulate the resupply condition which might occur with blood exiting a vessel perforation. These data were consistent with the notion that the reaction system once initiated by subvascular tissue factor no longer requires tissue factor to maintain thrombin generation in the growing thrombus [15]. This conclusion however can be applied only to blood from healthy individuals. A growing body of evidence suggests that active tissue factor exists in the blood of humans associated with inflammatory syndromes and thrombotic pathologies [22]. This circulating tissue factor, probably borne on microparticles, may play an important role in intravascular coagulation associated with thrombosis.
Controversy also exists with respect to the blood cell sources from which tissue factor is derived. It is generally accepted that monocytes are involved, while red cells are not. Tissue factor is present in monocytes and surface expressed following activation of these inflammatory cells by cytokines [23]. Some investigators have reported that both platelets and neutrophils can synthesize and express tissue factor [24, 25]; others have challenged these assertions [26]. Data from our laboratory have not confirmed platelet expression of tissue factor antigen or activity [26]. Osterud has challenged the observation of neutrophil tissue factor.[23]
A central controversy swirls about the control of tissue factor activity presentation. One school of thought represented by our laboratory [11] and that of Rao’s [27] is that tissue factor activity presented on inflammatory cells requires the presentation of accessories i.e. receptors and/or phospholipids which enhance the functional activity observed for cellular tissue factor presentation. Others have maintained that tissue factor is “encrypted” in an inactive form and activated by oxidation to form the disulfide bond closest to the membrane binding site of the protein (Cys186–Cys209) [28]. The encryption process derives from a molecular strain hypothesis forwarded by Hogg and colleagues which maintains that bond strain induces subsequent conformational changes in the molecule leading to factor VIIa binding and function [29]. Support for this concept evolves from three observations: 1) r-tissue factor with elimination of Cys186 of the pair shows decreased activity in factor X activation [30]; 2) a growing body of evidence suggests that protein disulfide isomerase may play a significant role in the blood coagulation process [31]; 3) treatment of cells with HgCl2 [28] (presumed to act as an oxidizing agent) increases tissue factor activity expression. However the role of the Cys186–Cys209 disulfide bond in tissue factor function has been the subject of controversy and challenged by the data of several studies[32, 33].
Two major breakthroughs in the studies of tissue factor occurred almost 30 years ago when the scarce natural protein was produced at significant levels using recombinant techniques. These proteins were produced either in E.coli or in Sf-9 cells as alluded to previously. Our laboratory has principally made use of natural tissue factor isolated from human placenta. This natural protein has roughly five times the activity of the recombinant tissue factor and this increase in activity is lost and becomes equivalent to E.coli produced tissue factor r1-243 when the carbohydrate on the protein is removed suggesting that recombinant proteins are less active as a consequence of alterations in or the absence of carbohydrate [9].
Summary
The concept of a tissue factor has been around for over a century. At present, the research community has access to the purified protein from natural and recombinant sources, methods to study the cellular expression of tissue factor, transgenic mice expressing low tissue factor levels and the structure of the extrinsic factor Xase at atomic levels. Thus, all the tools essential to understand tissue factor expression, activation and function appear to be at our collective disposal to resolve the issue of how, when and where tissue factor function is expressed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owen CAJ. A History of Blood Coagulation. Rochester, MN: Mayo Foundation for Medical Education and Research; 2001. [Google Scholar]
- 2.Bach R, Nemerson Y, Konigsberg W. Purification and characterization of bovine tissue factor. J Biol Chem. 1981;256:8324–31. [PubMed] [Google Scholar]
- 3.Spicer EK, Horton R, Bloem L, Bach R, Williams KR, Guha A, et al. Isolation of cDNA clones coding for human tissue factor: primary structure of the protein and cDNA. Proc Natl Acad Sci U S A. 1987;84:5148–52. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackman N, Morrissey JH, Fowler B, Edgington TS. Complete sequence of the human tissue factor gene, a highly regulated cellular receptor that initiates the coagulation protease cascade. Biochemistry. 1989;28:1755–62. doi: 10.1021/bi00430a050. [DOI] [PubMed] [Google Scholar]
- 5.Paborsky LR, Caras IW, Fisher KL, Gorman CM. Lipid association, but not the transmembrane domain, is required for tissue factor activity. Substitution of the transmembrane domain with a phosphatidylinositol anchor. J Biol Chem. 1991;266:21911–6. [PubMed] [Google Scholar]
- 6.Banner DW, D'Arcy A, Chene C, Winkler FK, Guha A, Konigsberg WH, et al. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–6. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 7.Paborsky LR, Harris RJ. Post-translational modifications of recombinant human tissue factor. Thromb Res. 1990;60:367–76. doi: 10.1016/0049-3848(90)90219-3. [DOI] [PubMed] [Google Scholar]
- 8.Bach R, Konigsberg WH, Nemerson Y. Human tissue factor contains thioester-linked palmitate and stearate on the cytoplasmic half-cystine. Biochemistry. 1988;27:4227–31. doi: 10.1021/bi00412a004. [DOI] [PubMed] [Google Scholar]
- 9.Krudysz-Amblo J, Jennings ME, 2nd, Mann KG, Butenas S. Carbohydrates and activity of natural and recombinant tissue factor. J Biol Chem. 2010;285:3371–82. doi: 10.1074/jbc.M109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krudysz-Amblo J, Jennings ME, 2nd, Matthews DE, Mann KG, Butenas S. Differences in the fractional abundances of carbohydrates of natural and recombinant human tissue factor. Biochim Biophys Acta. 2011;1810:398–405. doi: 10.1016/j.bbagen.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–96. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osterud B, Rapaport SI. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977;74:5260–4. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson JH, Mann KG. Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. J Biol Chem. 1991;266:11317–27. [PubMed] [Google Scholar]
- 14.Baugh RJ, Broze GJ, Jr, Krishnaswamy S. Regulation of extrinsic pathway factor Xa formation by tissue factor pathway inhibitor. J Biol Chem. 1998;273:4378–86. doi: 10.1074/jbc.273.8.4378. [DOI] [PubMed] [Google Scholar]
- 15.Orfeo T, Butenas S, Brummel-Ziedins KE, Mann KG. The tissue factor requirement in blood coagulation. J Biol Chem. 2005;280:42887–96. doi: 10.1074/jbc.M505506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedner U, Glazer S, Pingel K, Alberts KA, Blomback M, Schulman S, et al. Successful use of recombinant factor VIIa in patient with severe haemophilia A during synovectomy. Lancet. 1988;2:1193. doi: 10.1016/s0140-6736(88)90259-0. [DOI] [PubMed] [Google Scholar]
- 17.van't Veer C, Mann KG. The regulation of the factor VII-dependent coagulation pathway: rationale for the effectiveness of recombinant factor VIIa in refractory bleeding disorders. Semin Thromb Hemost. 2000;26:367–72. doi: 10.1055/s-2000-8454. [DOI] [PubMed] [Google Scholar]
- 18.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–45. [PubMed] [Google Scholar]
- 19.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–62. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 20.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–97. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taubman MB, Giesen PL, Schecter AD, Nemerson Y. Regulation of the procoagulant response to arterial injury. Thromb Haemost. 1999;82:801–5. [PubMed] [Google Scholar]
- 22.Boles J, Mackman N. Role of tissue factor in thrombosis in antiphospholipid antibody syndrome. Lupus. 2010;19:370–8. doi: 10.1177/0961203309360810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osterud B. Tissue factor expression in blood cells. Thromb Res. 2010;125 (Suppl 1):S31–4. doi: 10.1016/j.thromres.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–50. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 25.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 26.Bouchard BA, Mann KG, Butenas S. No evidence for tissue factor on platelets. Blood. 2010;116:854–5. doi: 10.1182/blood-2010-05-285627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pendurthi UR, Rao LV. Role of tissue factor disulfides and lipid rafts in signaling. Thromb Res. 2008;122 (Suppl 1):S14–8. doi: 10.1016/S0049-3848(08)70012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–8. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 29.Chen VM, Hogg PJ. Allosteric disulfide bonds in thrombosis and thrombolysis. J Thromb Haemost. 2006;4:2533–41. doi: 10.1111/j.1538-7836.2006.02236.x. [DOI] [PubMed] [Google Scholar]
- 30.Rehemtulla A, Ruf W, Edgington TS. The integrity of the cysteine 186-cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–9. [PubMed] [Google Scholar]
- 31.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–8. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–83. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]