Abstract
Sequential processing of amyloid precursor protein (APP) by β- and γ-secretase leads to the generation of amyloid-β (Aβ) peptides, which plays a central role in Alzheimer's disease pathogenesis. APP is capable of forming a homodimer through its extracellular domain as well as transmembrane GXXXG motifs. A number of reports have shown that dimerization of APP modulates Aβ production. On the other hand, we have previously reported that N-cadherin-based synaptic contact is tightly linked to Aβ production. In the present report, we investigated the effect of N-cadherin expression on APP dimerization and metabolism. Here, we demonstrate that N-cadherin expression facilitates cis-dimerization of APP. Moreover, N-cadherin expression led to increased production of Aβ as well as soluble APPβ, indicating that β-secretase-mediated cleavage of APP is enhanced. Interestingly, N-cadherin expression affected neither dimerization of C99 nor Aβ production from C99, suggesting that the effect of N-cadherin on APP metabolism is mediated through APP extracellular domain. We confirmed that N-cadherin enhances APP dimerization by a novel luciferase-complementation assay, which could be a platform for drug screening on a high-throughput basis. Taken together, our results suggest that modulation of APP dimerization state could be one of mechanisms, which links synaptic contact and Aβ production.
Keywords: Alzheimer's disease, amyloid precursor protein, amyloid β, N-cadherin, synapse
Amyloid precursor protein (APP) is an integral membrane protein with a single transmembrane (TM) domain that is expressed in wide number of different cell types, including neurons (Reinhard et al. 2005). Sequential processing of APP by β- and γ-secretase leads to the generation of amyloid-β (Aβ) peptides with varying lengths, whereas α-secretase-mediated APP cleavage, which occurs in the middle of the Aβ sequence, precludes generation of Aβ (De Strooper and Annaert 2000; Selkoe 2001). According to the widely accepted ‘amyloid cascade hypothesis’ (Hardy and Selkoe 2002), Aβ production plays a critical role in Alzheimer's disease (AD). Thus, identifying factors involved in the regulation of APP metabolism is crucially important for the understanding of AD pathogenesis.
It has been previously shown that APP and many other substrates of γ-secretase form homodimers (Beher et al. 1996; Ferguson et al. 2000; Scheuermann et al. 2001; Marambaud et al. 2002). As for APP, it has been reported that several different domains of APP could mediate its homodimerization. For example, heparin can induce bridging of two adjacent extracellular E1 domains to form APP homodimer (Dahms et al. 2010). E1 domain is composed of growth factor like domain (GFLD) (Rossjohn et al. 1999) and copper binding domain (CuBD) (Barnham et al. 2003; Kong et al. 2007). Moreover, a recent study showed that the loop region located between the GFLD and CuBD domain is critical for inducing APP homodimerization (Kaden et al. 2008). Furthermore, it is also suggested that extracellular E2 domain can support cis- and/or trans- dimerization of APP (Wang and Ha 2004). Lastly, three GXXXG motifs within the juxtamembrane and TM regions of APP are also known to mediate APP dimerization as well as Aβ dimerization (Munter et al. 2007, 2010; Kienlen-Campard et al. 2008). Importantly, dimerization of APP is suggested to impact Aβ production. For instance, addition of the loop peptide to disrupt APP dimerization via E1 domain leads to reduction of Aβ production (Kaden et al. 2008), whereas disruption of transmembrane GXXXG motif affects γ-secretase-mediated cleavage of APP, resulting in altered Aβ42/40 ratio (Munter et al. 2007, 2010). However, the cellular mechanism, which regulates APP dimerization, remains largely unknown. Interestingly, APP is implicated in maintenance of synaptic contact by forming trans-dimers, suggesting the possibility that N-cadherin and APP are functionally linked (Soba et al. 2005), although there are few reports connecting synaptic contact with APP cis-dimerization.
On the other hand, several lines of evidence provided that Aβ production is linked to the synaptic activity (Kamenetz et al. 2003; Lesné et al. 2005; Cirrito et al. 2008), which prompted us to investigate the role of synaptic contact in APP metabolism. N-cadherin, another known substrate for csecretase (Marambaud et al. 2003), is a representative cell adhesion molecule, which is involved in synaptic contact formation, especially in hippocampal excitatory neurons (Benson and Tanaka 1998). N-cadherin not only maintains synaptic structure, but also actively regulates synaptic plasticity (Tang et al. 1998; Togashi et al. 2002). Importantly, N-cadherin interacts with presenilin1 (PS1), a causative gene of familial AD, and a catalytic core of the γ-secretase, at the synaptic sites (Marambaud et al. 2003). Moreover, N-cadherin-based synaptic contact is neuroprotective by enhancing phosphoinositide 3-kinase (PI3K)/Akt (Baki et al. 2004) and suppressing p38MAPK (Ando et al. 2011) signal transduction. In addition, we have previously reported that N-cadherin modulates the PS1 function by changing its subcellular localization, thus affecting Aβ production in two ways: (i) by increasing extracellular Aβ release, and (ii) by reducing the Aβ42/40 ratio (Uemura et al. 2009a), indicating that N-cadherin-mediated synaptic contact is profoundly linked to APP metabolism. Thus, we asked whether N-cadherin-based cell adhesion alters the state of APP dimerization, and how it affects APP metabolism.
Here, we demonstrate by immunoprecipitation and lucif-erase-based protein complementation assay that N-cadherin expression facilitates dimerization of full-length APP through its extracellular domains. We propose that dimerization of APP is mediated by a direct interaction between APP and N-cadherin, based on the findings that were confirmed in the mouse brain at the endogenous expression level. In addition, we found that dimerization of APP increases the production of soluble APP (sAPP) β as well as extracellular Aβ release. These results indicate that N-cadherin-based synaptic contact affects the state of full length-APP dimerization, thereby modulating Aβ production. We thus propose that this may represent a mechanism by which synaptic activity is linked to Aβ production.
Materials and methods
Plasmids
APP770-myc and APP770-V5 constructs, expressing the full-length human APP770 tagged with myc or V5 in C-terminus were described elsewhere (Kinoshita et al. 2003). The expressing construct of full-length human N-cadhrein (NcadHA) was reported previously (Uemura et al. 2006). SP-C99-Flag encoding signal peptide sequence and C99 of APP were described elsewhere (Uemura et al. 2010). APPluc1 and APPluc2 constructs were generated by subcloning full-length human APP695 into pcDNA3/1 vector containing N-terminal and C-terminal half of humanized Gaussia luciferase respectively, using the primers TTTTTGGCGGGCCGCGATGCTGCCCGGTTTGGCACTGCTC and TTTTTATCGATGTTCTGCATCTGCTCAAAGAACTTGTA. (pcDNA-hGLuc1 and pcDNA-hGLuc2 were kindly provided by Dr. S. Michnick, University of Montreal, Canada). Negative control plasmids for split luciferase assay, luc1 and luc2 were generated by PCR that used APPluc1 and APPluc2 as templates. Luc1 was generated by PCR using the primers GCGCTAGCGCCACCATGAAGCCCACCGAGAACAACGAAGAC and GCAAGCTTTTAGCCTATGCCGCCCTGTGCG. Luc2 was generated by PCR using the primers GCGCTAGCGCCACCATGGAGGCGATCGTCGACATTCTT and GCAAGCTTTTAGTCACCACCGGCCCCCTTGAT. The PCR products were cloned as a NheI-HindIII fragment into the pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). The precise reading frame of the construct was verified by sequencing.
Cells and transient transfection
Human Embryonic Kidney (HEK) 293 cells were maintained in Dulbecco's modified Eagle's medium Dulbecco's modified Eagle's medium (Nacalai tesque, Kyoto, Japan) containing 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin at 37°C in 5% CO2 incubator. Primary neurons were obtained from the cerebral cortices of fetal mice (14 day's gestation) and cultured in Neurobasal medium supplemented with B-27 (Invitrogen). For transient expression, HEK293 cells were plated at the density of 105 cells/cm2, 24 h before transfection (8 μg DNA/6 cm dish) with Transfectin™, according to the manufacturer's instruction (Bio-Rad Laboratories, Hercules, CA, USA).
Antibodies and reagents
The polyclonal anti-HA-tag antibody, monoclonal anti-β-actin antibody, monoclonal anti-V5-tag antibody, monoclonal anti-Flag-tag antibody and polyclonal anti-APP C-terminal antibody were purchased from Sigma (St Louis, MO, USA). The control normal mouse IgG was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The monoclonal anti-PS1 C-terminal antibody was from Chemicon (Temecula, CA, USA). The monoclonal anti-N-cadherin C-terminal antibody was from BD Biosciences (Franklin Lakes, NJ, USA). The polyclonal anti-BACE1 C-terminal antibody was from Calbiochem, San Diego, CA, USA. The polyclonal anti-ADAM10 (a disintegrin and metalloproteinase 10) C-terminal antibody was from Millipore. Secondary antibodies were from GE Healthcare Japan (Tokyo, Japan). Polyclonal anti-gluc antibody was purchased from New England Biolab (Ipswich, MA, USA). Luciferase assays were performed using a Luciferase kit according to the manufacturer's instructions (BioLabs). Briefly, the cell extract transiently transfected with the luciferase construct was mixed with the luciferase assay reagent, and light emission was measured in a luminometer.
Immunoprecipitation and western blots
Amyloid precursor protein homodimerization and APP-N-cadherin interaction were analyzed by immunoprecipitation. HEK293 cells were co-transfected with APP770-myc and APP770-V5 in the presence or absence of N-cadherin construct. Cells were lysed in TNE buffer [10 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1% NonidetP-40 (Roche Applied Science, Indianapolis, IN, USA) containing a protease inhibitor mix] 48 h after co-transfection, followed by the immunoprecipitation, using the monoclonal anti-V5 tag antibody (Sigma). Samples were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane and blotted with anti-myc-tag antibody (MBL, Nagoya, Japan). For the analysis of C99 homodimerization, cell lysates were precipitated with the monoclonal anti-Flag antibody (Sigma), followed by the blotting with the polyclonal anti-myc-tag (MBL). Endogenous murine Ncad-APP interaction was also identified by immunoprecipitation. The wild type C57BL/6 mice brains of 6 months of age brain was lysed in ristocetin-induced platelet agglutination buffer (20 mM Tris–HCl pH7.4, 150 mM NaCl, 0.1% SDS, 1% TritonX-100, 1% Sodium Deoxycholate, 5 mM EDTA containing a protease inhibitor mix). Mouse brain lysate was immunoprecipitated by anti-N-cadherin antibody or the anti-APP antibody. The immunoprecipitates were washed five times with ristocetin-induced platelet agglutination buffer (20 mM Tris–HCl pH7.4, 150 mM NaCl, 0.1% SDS, 1% TritonX-100, 1% Sodium Deoxycholate, 5 mM EDTA containing a protease inhibitor mix), followed by the boiling in two times SDS sample buffer (124 mM Tris–HCl pH6.8, 4% SDS, 10% glycerol, 0.02% bromophenol blue and 4% 2-mercapto ethanol) and the western blot.
Measurement of extracellular Aβ and sAPP
HEK293 cells transiently expressing APP and N-cadherin were plated at density of 1.5 × 106 cells/12 well dish. The medium was exchanged 24 h after transfection, followed by incubation for another 24 h. The aliquot of the conditioned medium was collected for analysis. The Aβ40 and Aβ42 peptides were measured by using Human β Amyloid (1–40) or (1–42) ELISA kit (WAKO, Osaka, Japan). The sAPPα and sAPPβ were measured by using Human sAPPα Assay Kit and Human sAPPβ Assay Kit (IBL, Gumma, Japan), according to the manufacturer's instruction.
Inhibition of cell–cell contact by N-cadherin antagonist
The cyclic pentapeptide contained the cell adhesion recognition motif [His-Ala-Val (HAV), ADH-1, was kindly provided by Adherex Technologies (Durham, NC, USA) and characterized previously (Ando et al. 2011). HEK293 cells transiently expressing APP and N-cadherin were treated with 1 mg/mL of ADH-1 for 24 h.
Statistical analysis
Signals on films were quantified with NIH Image software (National Institutes of Health). Comparison was performed using a Student's t-test. For comparison of multiparametric analysis, one-way anova, followed by the post hoc analysis by Fisher's protected least significant difference (PLSD) was used. Data were expressed as means ± SD, and statistical significance was assessed at p < 0.05.
Results
N-cadherin expression enhances APP dimerization
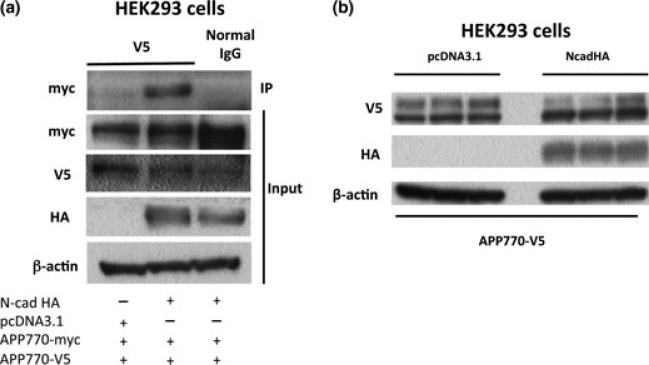
To investigate the effect of the N-cadherin expression on APP dimerization, HEK293 cells were co-transfected with myc- and V5-tagged APP770 constructs (APP-myc and APP-V5) in the presence or absence of HA-tagged N-cadherin (NcadHA). Cells were harvested 48 h after the transfection, and cell lysates were subjected to immunoprecipitation using anti-V5 antibody, followed by immunoblotting with anti-myc antibody. More APP-V5 was associated with APP-myc upon NcadHA expression, indicating that N-cadherin expression enhanced APP dimerization (Fig. 1a). The expression levels of APP were not significantly different between HEK293 cells with or without N-cadherin transfection (Fig. 1b, see also Figure S1), suggesting that the increase in the level of APP dimers is not attributable to the increase in total cellular APP levels.
Fig. 1.
N-cadherin expression enhances full-length APP dimerization. (a) HEK293 cells were co-transfected with APP770-myc and APP770-V5 in the presence or absence of NcadHA and analyzed by immunoprecipitation assay, using anti-V5 antibody. (b) HEK293 cells were transiently co-transfected with APP770-V5 in the presence or absence of NcadHA (n = 3). The expression levels of APP were analyzed by western blot.
Next, we asked whether the cis-dimerization of APP (dimerization of the APP molecules inserted within the same cell plasma membrane) or the trans-dimerization of APP (dimerization of the APP molecules inserted in the opposing cells plasma membrane) is enhanced by the N-cadherin expression. For this, APP-V5 and APP-myc were separately transfected into HEK293 cells cultured in different dishes (Figure S2a), followed by co-culture of the transfected cells. The immunoprecipitation assay showed that trans-dimerization of APP was observed by the co-expression of N-cadherin. However, the level of APP trans-dimer was significantly lower, compared to the dimer observed after cotransfection of APP-V5 and APP-myc, indicating that most of the APP-dimer observed in our experimental system represents APP cis-dimer (Figure S2b).
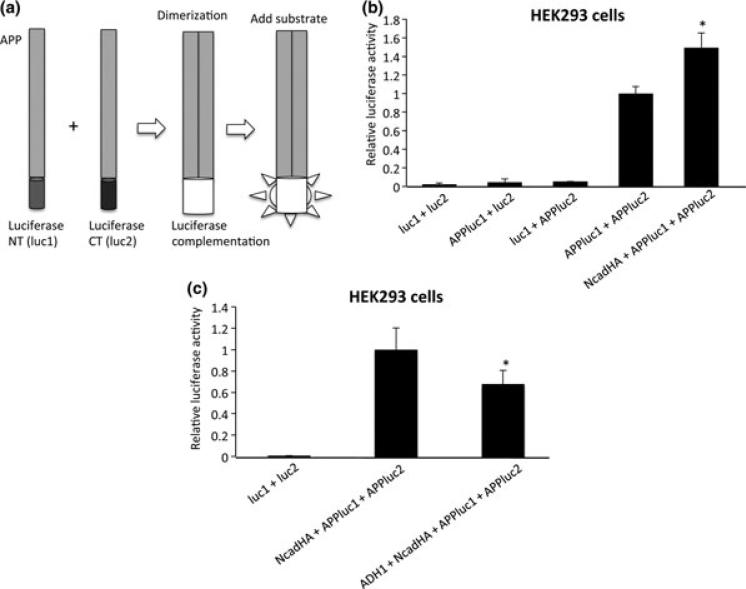
As an alternative and quantitative approach for the assessment of APP cis-dimerization, we performed a split luciferase assay. For this, APP was fused with either N-terminal (luc1) or C-terminal (luc2) half of humanized Gaussia luciferase. As APP molecules form dimers, luc1 and luc2 reconstitutes into active luciferase, whose activity can be visualized by the addition of a substrate (Remy 2006, Fig. 2a). HEK293 cells were co-transfected with APPluc1 and APPluc2 in the presence or absence of N-cadherin constructs. Co-transfection of cells with luc1 + luc2, APP-luc1 + luc2 or luc1 + APPluc2 did not show significant increase in luciferase activity, indicating that non-specific association and folding of luc1 and luc2 did not occur. On the other hand, co-expression of APPluc1 and APPluc2 showed a significant increase in the luciferase activity, demonstrating that APP dimerization accelerated the folding of luc1 and luc2 into mature luciferase. Moreover, luciferase activity in NcadHA + APPluc1 + APPluc2-transfected cells was approximately 1.5-fold higher compared to that in APPluc1 + APPluc2-transfected cells (Fig. 2b, see also Fig. S3), further validating an increase in the APP cis-dimerization in the presence of N-cadherin.
Fig. 2.
Split luciferase assay for quantitative analysis of APP cisdimerization. (a) APP was fused with either N-terminal (luc1) or C-terminal (luc2) half of humanized Gaussia luciferase. As they form dimers, they fold into active luciferase, whose activity can be visualized by the addition of luciferase substrate. (b) HEK293 cells were cotransfected with APPluc1 and APPluc2 in the presence or absence of NcadHA. Luciferase activity in NcadHA + APPluc1 + APPluc2-transfected cells showed an approximately 1.5-fold higher activity than that in APPluc1 + APPluc2-transfected cells (*p < 0.01). Conversely, luc1 + luc2, APPluc1 + luc2 and luc1 + APPluc2 did not show significant increase in luciferase activity. (luc1 + luc2 n = 6, APPluc1 + luc2 n = 6, luc1 + APPluc2 n = 3, APPluc1 + APPluc2 n = 9, NcadHA + APPluc1 + APPluc2 n = 6). (c) ADH-1 treatment inhibit APP dimerization. HEK293 cells were co-transfected with APPluc1, APP-luc2 and NcadHA. After transfection, cells were treated with 1 mg/mL of ADH-1 for 24 h. Luciferase activity was significantly decreased in cells treated with ADH-1 (*p < 0.01). (luc1 + luc2 n = 6, Ncad + APPluc1 + APPluc2 n = 6, ADH-1 + Ncad + APPluc1 + APPluc2 n = 6).
N-cadherin forms complex with APP
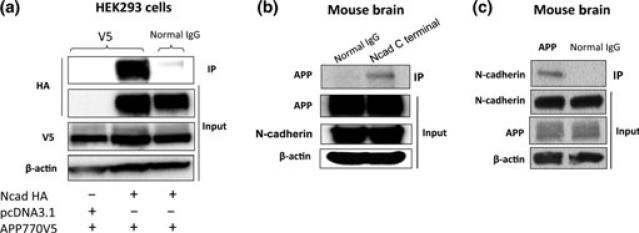
Next, we analyzed whether N-cadherin could physically interact with APP. For this, NcadHA and APP-V5 were co-transfected into HEK293 cells, and the cell lysate was subjected to immunoprecipitation assay using anti-V5 antibody. NcadHA was shown to be associated with APP-V5 (Fig. 3a). To determine whether N-cadherin associates with APP in the endogenous level, we analyzed the interaction between N-cadherin and APP in wild type mouse brains. The whole brain lysate from 6-month-old male C57BL/6 mice was immunoprecipitated by anti-N-cadherin antibody, followed by the blotting with anti-APP-antibody (Fig. 3b). Another immunoprecipitation experiment, using anti-APP antibody for pull-down and anti-N-cadherin for detection again showed the interaction between N-cadherin and APP (Fig. 3c). The result confirmed the physical interaction between N-cadherin and APP in the mouse brain.
Fig. 3.
N-cadherin forms complex with APP in wild-type mouse brain. (a) HEK293 cells were transfected with APP770-V5 in the presence or absence of NcadHA. Cell lysates were pulled down with anti-V5 antibody, followed by the blotting with anti-HA antibody. (b) The whole brain lysate of 6-month-old male C57BL/6 mice was immunoprecipitated by anti-N-cadherin antibody, followed by the blotting with anti-APP-antibody. (c) The same whole brain lysate was immunoprecipitated by anti-APP antibody, followed by the blotting with anti-N-cadherin-antibody.
N-cadherin interacts with, but does not facilitate the dimerization of C99
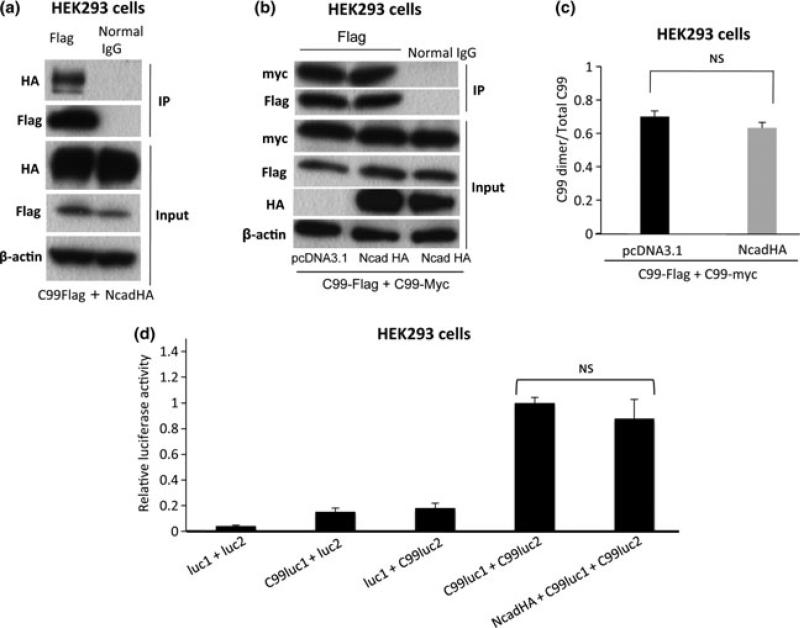
Previous reports have demonstrated that extracellular domain of APP plays a significant role in APP cis-dimerization as well as in Aβ production (Rossjohn et al. 1999; Barnham et al. 2003; Kong et al. 2007; Kaden et al. 2008). To determine whether APP extracellular domain is necessary for the N-cadherin-induced APP dimerization, we analyzed the effect of N-cadherin in dimerization of C99, which corresponds to the β-secretase-cleaved C-terminal fragment of APP, and lacks the extracellular E1 and E2 domains. First, we asked whether N-cadherin interacts with C99. HEK293 cells were transfected with C99-Flag together with NcadHA. The cell lysates were subjected to immunoprecipitation using anti-Flag antibody. Immunoblotting with anti-HA antibody revealed that C99 interacts with the N-cadherin (Fig. 4a). This indicates that the domain of APP, which mediates the interaction with N-cadherin, is located within either trans-membrane or cytoplasmic region. Next, to test whether N-cadherin expression could facilitate the dimerization of C99 (i.e. dimerization through the intramembrane GXXXG motif), HEK293 cells were co-transfected with both Flag and myc-tagged C99 (C99-Flag and C99-myc) together with N-cadherin or empty vector constructs, followed by immunoprecipitation using anti-Flag antibody. As shown in Fig. 4(b), N-cadherin expression did not significantly affect C99 dimerization (see also Fig. 4c for quantification analysis). These results indicate that APP interacts with N-cadherin via its C-terminal fragment, whereas N-cadherin-induced APP dimerization is mediated by APP extracellular domain. We further validated these findings by split luciferase assay. For this, C99 fused with luc1 (C99luc1) was co-expressed with C99 fused with luc2 (C99luc2) in the presence or absence of N-cadherin in HEK293 cells. The co-expression of NcadHA did not change the luciferase activity, indicating that N-cadherin expression have no effect on the dimerization state of C99 (Fig. 4d).
Fig. 4.
Interaction of N-cadherin with C99 and the effect of N-cadherin expression on C99 dimerization. (a) NcadHA and C99-Flag were co-expressed in HEK293 cells. Cell lysates were pulled down with anti-Flag antibody, followed by the blotting with anti-HA antibody. (b) HEK293 cells were co-transfected C99-Flag and C99-myc in the presence or absence of NcadHA. Cell lysates were pulled down with anti-Flag antibody, followed by the blotting with anti-myc antibody. (c) The ratio of C99 dimer/Total C99 was compared between the cells transfected with control vector and NcadHA. (n.s.: p = 0.4858, n = 3). (d) HEK293 cells were co-transfected with C99luc1 and C99luc2 in the presence or absence of NcadHA. Luciferase activity in NcadHA + C99luc1 + C99luc2-transfected cells was not significantly different from that in C99luc1 + C99luc2-transfected cells. (luc1 + luc2 n = 6, C99luc1 + luc2 n = 6, luc1 + C99luc2 n = 6, C99luc1 + C99luc2 n = 6, NcadHA + C99luc1 + C99luc2 n = 6).
N-cadherin expression increases the β-secretase mediated cleavage of APP
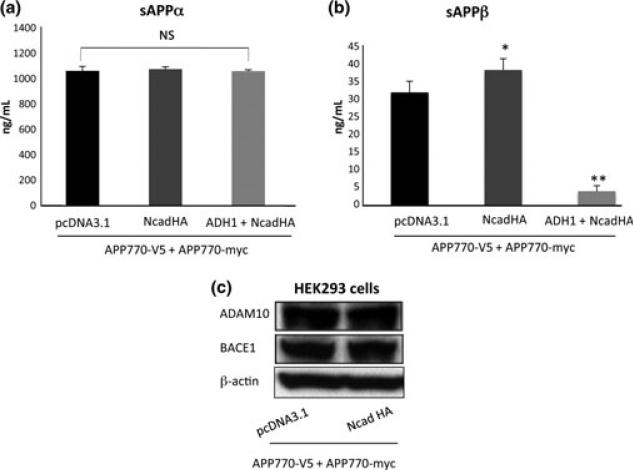
Amyloid precursor protein is processed by α-secretase, resulting in the shedding of a sAPPα, whereas sAPPα is produced by the β-secretase cleavage. Thus, sAPPα and sAPPβ can be surrogate markers of α- and β-secretase activity, respectively. Since we found that N-cadherin strengthens dimerization of the full length APP presumably via its extracellular domains, we next investigated the effect of N-cadherin expression on the APP extracellular cleavages by β- and α-secretases. HEK293 cells were co-transfected with APP-myc and APP-V5 together with N-cadherin construct. The culture media was subjected to ELISA assay for detection of sAPPα and sAPPβ. The level of sAPPα released in the media was not significantly different between the control cells and cells over-expressing N-cadherin (Fig. 5a). Interestingly, the level of sAPPβ was significantly elevated by the expression of NcadHA (Fig. 5b), indicating that β-secretase-mediated cleavage of APP is increased by the N-cadherin expression. The N-terminal extracellular domain of N-cadherin harbors the homophilic cell adhesion recognition sequence, HAV. It has been established that ADH-1, which mimics the natural HAV sequence of N-cadherin, can specifically disrupt N-cadherin-mediated cell adhesion (Ando et al. 2011). Treatment of the cells with N-cadherin antagonist, ADH-1, significantly reduced sAPPβ (Fig. 5b), but had no effect on sAPPα production, indicating that N-cadherin expression primarily affects β-secretase (BACE1)-mediated cleavage of APP (Fig. 5a). We examined whether N-cadherin expression affects the protein levels of BACE1 (i.e. β-secretase) and ADAM10 (i.e. α-secretase) by western blot, both of which did not change after the N-cadherin expression (Fig. 5c). Finally, we tested the effect of ADH-1 treatment on APP cis-dimerization by split luciferase assay. Treatment of HEK293 cells expressing APPluc1, APPluc2 and NcadHA significantly reduced luciferase activity, suggesting that the effect of ADH-1 on the metabolism of APP was, at least partially, mediated by a disruption of APP cis-dimerization (Fig. 2c).
Fig. 5.
Effects of N-cadherin expression for alpha and beta-secretase cleavage. (a) The level of sAPPα released in the media was compared between the cells expressing pcDNA3.1 and NcadHA by ELISA (n.s.: p = 0.3519, n = 5). ADH-1 treatment of the NcadHA expressing cells did not affect the level of sAPPα (n.s.: p = 0.9045, n = 5). (b) The level of sAPPβ was significantly increased by the expression of NcadHA (*p < 0.001, n = 8). Treatment of cells with ADH-1 reduced sAPPb (**p < 0.0001, n = 8). (c) Analysis of ADAM10 and BACE1 expression by western blotting in cells expressing APP770 or co-expressing APP770 and N-cadherin.
N-cadherin expression enhances the extracellular release of Aβ
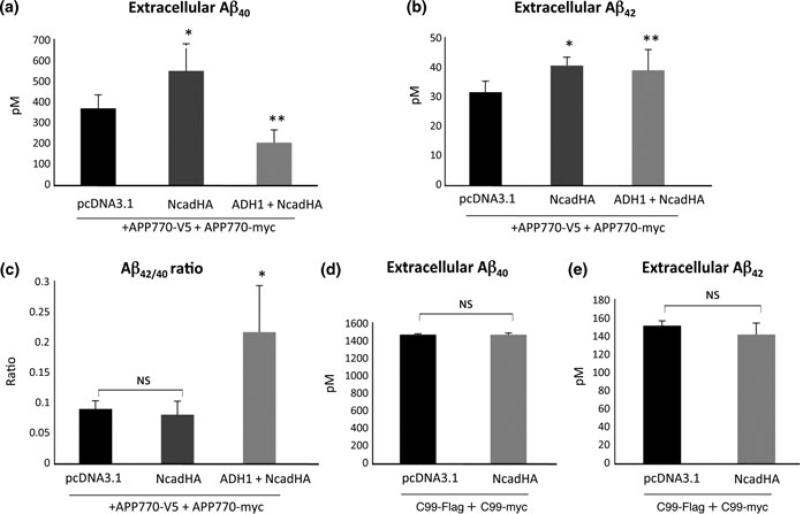
Given that N-cadherin expression induces cis-dimerization of APP and enhances the production of the sAPPβ, we assumed that N-cadherin expression could modulate Aβ production via controlling full length APP dimerization. HEK293 cells were co-transfected with APP770-myc and APP770-V5 in the presence or absence of NcadHA, and culture media was subjected to ELISA assay for detection of Aβ40 and Aβ42 48 h after the transfection. We observed a significant increase of both Aβ40 and Aβ42 under the expression of N-cadherin, compared to that in cells transfected with an empty vector (Fig. 6a and b). Interestingly, application of N-cadherin antagonist (ADH-1) to N-cadherin-expressing cells drastically reduced Aβ40 production, whereas production of Aβ42 remained unaffected (Fig. 6a and b). As a result, the Aβ42/40 ratio was significantly higher in the cells treated with ADH-1 (Fig. 6c).
Fig. 6.
Effects of N-cadherin expression on Aβ production. (a,b) Both Aβ40 and Aβ42 are increased under the expression of full-length N-cadherin, compared with the expression of empty vector (pcDNA3.1) (a,b: *p < 0.01, n = 6). Treatment of ADH-1 to N-cadherin expressing cells significantly reduced Aβ40 production (a: **p < 0.01, n = 6), whereas production of Aβ42 was increased compared to control (b: **p < 0.05, n = 6). (c) The Ab42/40 ratio was significantly higher in the cells treated with ADH-1 (*p < 0.001, n = 6). (d,e) HEK293 cells were co-transfected with C99-Flag and C99-myc with or without NcadHA. The production of Aβ40 as well as Aβ42 was almost identical between each condition.
To determine whether N-cadherin expression could enhance extracellular Aβ production from C99, HEK293 cells were co-transfected with C99-Flag and C99-myc, with or without N-cadherin constructs. We found that N-cadherin expression failed to enhance extracellular Aβ production from C99. The amount of Aβ40 and Aβ42 was almost identical in each condition (Fig. 6d and e). Since C99 dimerization is not facilitated by N-cadherin (Fig. 4b), these data suggest that dimerization of full-length APP, but not the dimerization of C99, is correlated to the enhancement of extracellular Aβ release.
Discussion
Increasing numbers of evidence supports the idea that the amount of released Aβ is tightly linked to the synaptic activity. For example, studies using the slice cultures and cultured cortical neurons show an increase in Aβ secretion concomitant with the neuronal activation, and suggest that neuronal activation shifts cleavage of APP to favor β-secretase over α-secretase (Kamenetz et al. 2003; Lesné et al. 2005). In addition, in vivo experiments demonstrate that Aβ levels released in mouse brain are dynamically regulated by the synaptic activity (Cirrito et al. 2008). On the other hand, N-cadherin has been shown to not only provide a structural backbone of the synaptic contact, but also to be functionally involved in synaptic plasticity (Tang et al. 1998; Togashi et al. 2002). Moreover, APP and its paralogs amyloid precursor-like proteins (APLP1 and 2) are implicated in synaptic contact via trans-dimerization (Soba et al. 2005). Thus, interaction between N-cadherin and APP, followed by APP dimerization could constitute one of the mechanistic links between the neuronal activation and an increase in the Aβ production.
Recent studies demonstrate that dimerization of APP is tightly linked to the mode of Aβ production. However, many of the previous studies are based on mutagenesis around the ectodomain (Scheuermann et al. 2001; Munter et al. 2007) or the GXXXG motifs within the transmembrane (or juxtamembrane) domain (Munter et al. 2007). Other studies are based on induced dimerization with the C-terminal tagging (Eggert et al. 2009) or on cell-free in vitro assay with synthesized peptides (Gorman et al. 2008). Thus, the cellular mechanism affecting dimerization state of the wild-type APP has not been clarified.
In the present study, we demonstrated by immunoprecipitation and luciferase-based complementation assay that N-cadherin expression facilitates APP dimerization. Actually, in our immunoprecipitation assay, we could not rule out the possibility that APP forms oligomers, instead of ‘dimers’, after N-cadherin expression. However, since our luciferase-based assay utilizes conformational changes of the luc1 and luc2 (both of which are fused to the C-terminus of APP) into mature Gaussia luciferase, the detected luciferase activity most likely represents cis- (not trans-) dimerization of APP. Moreover, N-cadherin-driven APP cis-dimerization is most likely mediated through the APP extracellular (E1 and/or E2) domain, since although N-cadherin did interact with the C99, it failed to enhance the dimerization of C99, which lacks the E1 as well as E2 domains.
As we previously reported (Uemura et al. 2009a), the expression of N-cadherin enhanced extracellular release of Aβ peptides, which was significantly reversed by the addition of N-cadherin antagonist (ADH-1). Indeed, the level of Aβ production after ADH-1 treatment was less than that of the non-treated cells (Fig. 6a), most likely because of suppression of the endogenous N-cadherin in HEK293 cells. Interestingly, N-cadherin expression failed to enhance the extracellular Aβ release from the cells expressing C99 (Fig 6d and e). Concomitantly, N-cadherin expression did not facilitate the dimerization of C99 (Fig. 4b and c). Thus, the increased extracellular release of Aβ from full-length APP after N-cadherin expression might be attributable to enhanced APP cis-dimerization. Accordingly, ADH-1 treatment of N-cadherin expressing cells reduced extracellular Aβ release as well as APP dimerization (Fig. 2c and 6a). Moreover, when we analyzed the levels of sAPPα and sAPPβ as surrogates for alpha- and beta-secretase activities, we observed an increase in sAPPβ production without the change of the BACE1 level (Fig. 5). These findings are in line with the recent report by Kaden et al. (Kaden et al. 2008), showing that disruption of APP dimers with a peptide targeting loop domain located between GFLD and CuBD leads to decreased Aβ production, accompanied by a decrease in the sAPPb. In addition, another report demonstrated that stabilization of the APP dimers by introducing cysteine mutation into the juxtamembrane domain led to a significant increase in the Aβ (Scheuermann et al. 2001). Interestingly, it has been proposed that BACE1 could also form dimers (Schmechel et al. 2004), and that APP homodimers might be substrates for the BACE1 oligomers (Multhaup 2006). Moreover, BACE1 activity can be significantly altered according to the subcellular membrane compartment change, such as lipid raft (Cordy et al. 2003). Taken together, these results suggest that dimerization of APP at the extracellular domain could induce a structural change and/or a change in the subcellular distribution of APP, which makes dimers more favorable substrates for β-secretase (BACE1), compared to APP monomers.
On the other hand, there have been a number of reports indicating that APP dimerization mediated by the TM GXXXG motifs could modulate the Aβ42/40 ratio, rather than the total amount of Aβ produced (Munter et al. 2007, 2010). Since most of these findings are based on mutagenesis within the Aβ sequence, which could interfere with APP processing as well as with APP dimerization, the impact of dimerization via TM GXXXG motifs on APP processing is still a matter of debate (Gorman et al. 2008; Eggert et al. 2009). Our immunoprecipitation data suggest that N-cadherin-based cell–cell adhesion may not have a direct impact on APP dimerization mediated by GXXXG motifs, since it does not promote the C99 dimerization. Therefore, we presume that there might be a different domain, which controls full length APP dimerization by N-cadherin expression. In addition, we presume an alternative mechanism through which N-cadherin expression modulates Aβ42/40 ratio. Specifically, we have previously found that N-cadherin interaction with PS1 induces a conformational change (i.e. ‘open’ conformation, in which PS1 N-terminus and C-terminus are located further apart) of PS1 to favor production of Aβ40 over Aβ42 (Uemura et al. 2009a,b). It has been repeatedly shown that conformational change of PS1 is tightly linked to the length of the Aβ peptide produced (Lleó et al. 2004; Berezovska et al. 2005; Uemura et al. 2009a). Thus, it is plausible that alteration of the Aβ42/40 ratio after ADH-1 treatment (Fig. 6c) was caused by a direct effect on PS1 conformation, rather than on APP dimerization mediated by the GXXXG motifs.
Finally, since experimental data from the present study predict enhanced Aβ production upon APP dimerization, drugs targeting APP dimerization could be beneficial for preventing amyloidosis associated with AD treatment. The luciferase-based assay system we developed for monitoring APP dimerization allows fast and semi-quantitative assessment of the amount of APP cis-dimers, and would provide a promising platform for drug screening on a high-throughput basis.
Supplementary Material
Acknowledgement
This work was supported by KANAE Foundation for the promotion of medical science (KU), NIH AG15379 (OB) and by Ministry of Education, Culture, Sports, Science and Technology (20300124, AK). We are grateful to Dr. S. Michnick, University of Montreal, Canada, who kindly provided pcDNA-hGLuc1 and pcDNA-hGLuc2 plasmid and Adherex Technologies (Durham, NC) for providing ADH-1.
Abbreviations used
- Aβ
amyloid-β
- AD
Alzheimer's disease
- ADAM10
a disintegrin and metalloproteinase 10
- APP
amyloid precursor protein
- CuBD
copper binding domain
- GFLD
growth factor like domain
- HAV
His-Ala-Val
- HEK
Human Embryonic Kidney
- luc1
humanized Gaussia luciferase N-terminus
- luc2
humanized Gaussia luciferase C-terminus
- NcadHA
HA-tagged N-cadherin
- PS1
presenilin 1
- sAPP
soluble APP
- SDS
sodium dodecyl sulfate
- TM
transmembrane
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article:
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Ando K, Uemura K, Kuzuya A, et al. N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease. J. Biol. Chem. 2011;286:7619–7628. doi: 10.1074/jbc.M110.158477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, McKinstry WJ, Multhaup G, et al. Structure of the Alzheimer's disease amyloid precursor protein copper binding domain. A regulator of neuronal copper homeostasis. J. Biol. Chem. 2003;278:17401–17407. doi: 10.1074/jbc.M300629200. [DOI] [PubMed] [Google Scholar]
- Beher D, Hesse L, Masters CL, Multhaup G. Regulation of amyloid protein precursor (APP) binding to collagen and mapping of the binding sites on APP and collagen type I. J. Biol. Chem. 1996;271:1613–1620. doi: 10.1074/jbc.271.3.1613. [DOI] [PubMed] [Google Scholar]
- Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J. Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezovska O, Lleo A, Herl LD, Frosch MP, Stern EA, Bacskai BJ, Hyman BT. Familial Alzheimer's disease presenilin 1 mutations cause alterations in the conformation of presenilin and interactions with amyloid precursor protein. J. Neurosci. 2005;25:3009–3017. doi: 10.1523/JNEUROSCI.0364-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc. Natl Acad. Sci. USA. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms SO, Hoefgen S, Roeser D, Schlott B, Gührs KH, Than ME. Structure and biochemical analysis of the heparin-induced E1 dimer of the amyloid precursor protein. Proc. Natl Acad. Sci. USA. 2010;107:5381–5386. doi: 10.1073/pnas.0911326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- Eggert S, Midthune B, Cottrell B, Koo EH. Induced dimerization of the amyloid precursor protein leads to decreased amyloid-beta protein production. J. Biol. Chem. 2009;284:28943–28952. doi: 10.1074/jbc.M109.038646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KM, Darling PJ, Mohan MJ, Macatee TL, Lemmon MA. Extracellular domains drive homo- but not hetero-dimerization of erbB receptors. EMBO J. 2000;19:4632–4643. doi: 10.1093/emboj/19.17.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman PM, Kim S, Guo M, Melnyk RA, McLaurin J, Fraser PE, Bowie JU, Chakrabartty A. Dimerization of the transmembrane domain of amyloid precursor proteins and familial Alzheimer's disease mutants. BMC Neurosci. 2008;9:17. doi: 10.1186/1471-2202-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Kaden D, Munter LM, Joshi M, et al. Homophilic interactions of the amyloid precursor protein (APP) ectodomain are regulated by the loop region and affect beta-secretase cleavage of APP. J. Biol. Chem. 2008;283:7271–7279. doi: 10.1074/jbc.M708046200. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kienlen-Campard P, Tasiaux B, Van Hees J, et al. Amyloidogenic processing but not amyloid precursor protein (APP) intracellular C-terminal domain production requires a precisely oriented APP dimer assembled by transmembrane GXXXG motifs. J. Biol. Chem. 2008;283:7733–7744. doi: 10.1074/jbc.M707142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J. Cell Sci. 2003;116:3339–3346. doi: 10.1242/jcs.00643. [DOI] [PubMed] [Google Scholar]
- Kong GK, Adams JJ, Harris HH, et al. Structural studies of the Alzheimer's amyloid precursor protein copper-binding domain reveal how it binds copper ions. J. Mol. Biol. 2007;367:148–161. doi: 10.1016/j.jmb.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Lesné S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J. Neurosci. 2005;25:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleó A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, Frosch MP, Irizarry M, Hyman BT. Nonsteroidal anti-inflammatory drugs lower Abeta42 and change presenilin 1 conformation. Nat. Med. 2004;10:1065–1066. doi: 10.1038/nm1112. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Multhaup G. Amyloid precursor protein and BACE function as oligomers. Neurodegener. Dis. 2006;3:270–274. doi: 10.1159/000095266. [DOI] [PubMed] [Google Scholar]
- Munter LM, Voigt P, Harmeier A, Kaden D, Gottschalk KE, Weise C, Pipkorn R, Schaefer M, Langosch D, Multhaup G. GxxxG motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of Abeta42. EMBO J. 2007;26:1702–1712. doi: 10.1038/sj.emboj.7601616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munter LM, Botev A, Richter L, Hildebrand PW, Althoff V, Weise C, Kaden D, Multhaup G. Aberrant amyloid precursor protein (APP) processing in hereditary forms of Alzheimer disease caused by APP familial Alzheimer disease mutations can be rescued by mutations in the APP GxxxG motif. J. Biol. Chem. 2010;285:21636–21643. doi: 10.1074/jbc.M109.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Hébert SS, De Strooper B. The amyloid-beta precursor protein: integrating structure with biological function. EMBO J. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy I, Michnick SW. A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat. Methods. 2006;3:977–979. doi: 10.1038/nmeth979. [DOI] [PubMed] [Google Scholar]
- Rossjohn J, Cappai R, Feil SC, et al. Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat. Struct. Biol. 1999;6:327–331. doi: 10.1038/7562. [DOI] [PubMed] [Google Scholar]
- Scheuermann S, Hambsch B, Hesse L, Stumm J, Schmidt C, Beher D, Bayer TA, Beyreuther K, Multhaup G. Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer's disease. J. Biol. Chem. 2001;276:33923–33929. doi: 10.1074/jbc.M105410200. [DOI] [PubMed] [Google Scholar]
- Schmechel A, Strauss M, Schlicksupp A, Pipkorn R, Haass C, Bayer TA, Multhaup G. Human BACE forms dimers and colocalizes with APP. J. Biol. Chem. 2004;279:39710–39717. doi: 10.1074/jbc.M402785200. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Soba P, Eggert S, Wagner K, et al. Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J. 2005;24:3624–3634. doi: 10.1038/sj.emboj.7600824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Uemura K, Kihara T, Kuzuya A, Okawa K, Nishimoto T, Ninomiya H, Sugimoto H, Kinoshita A, Shimohama S. Characterization of sequential N-cadherin cleavage by ADAM10 and PS1. Neurosci. Lett. 2006;402:278–283. doi: 10.1016/j.neulet.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Uemura K, Lill CM, Banks M, et al. N-cadherin-based adhesion enhances Abeta release and decreases Abeta42/40 ratio. J. Neurochem. 2009a;108:350–360. doi: 10.1111/j.1471-4159.2008.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Lill CM, Li X, Peters JA, Ivanov A, Fan Z, DeStrooper B, Bacskai BJ, Hyman BT, Berezovska O. Allosteric modulation of PS1/gamma-secretase conformation correlates with amyloid beta(42/40) ratio. PLoS ONE. 2009b;4:e7893. doi: 10.1371/journal.pone.0007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Farner KC, Hashimoto T, Nasser-Ghodsi N, Wolfe MS, Koo EH, Hyman BT, Berezovska O. Substrate docking to γ-secretase allows access of γ-secretase modulators to an allosteric site. Nat. Commun. 2010;1:130. doi: 10.1038/ncomms1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ha Y. The X-ray structure of an antiparallel dimer of the human amyloid precursor protein E2 domain. Mol. Cell. 2004;15:343–353. doi: 10.1016/j.molcel.2004.06.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.