Abstract
The p57Kip2 cyclin-dependent kinase inhibitor (CDKi) has been implicated in embryogenesis, stem-cell senescence and pathologies, but little is known of its role in cell cycle control. Here, we show that p57Kip2 is targeted by the p38 stress-activated protein kinase (SAPK). Phosphorylation of p57Kip2 at T143 by p38 enhances its association with and inhibition of Cdk2, which results in cell-cycle delay upon stress. Genetic inactivation of the SAPK or the CDKi abolishes cell-cycle delay upon osmostress and results in decreased cell viability. Oxidative stress and ionomycin also induce p38-mediated phosphorylation of p57 and cells lacking p38 or p57 display reduced viability to these stresses. Therefore, cell survival to various stresses depends on p57 phosphorylation by p38 that inhibits CDK activity. Together, these findings provide a novel molecular mechanism by which cells can delay cell cycle progression to maximize cell survival upon stress.
Keywords: cell cycle, cell stress, cell survival, p38 SAPK, p57 CDKi
Introduction
Mammalian cell cycle progression throughout the G1 phase is controlled by signalling pathways that regulate the activities of G1 cyclin-dependent kinases (CDKs) Cdk4/6-CyclinD and Cdk2-CyclinE/A, which are responsible for modulating the expression, activity and stability of many cell-cycle regulatory proteins (Malumbres and Barbacid, 2005). CDK activity is regulated by two unrelated families, INK and Cip/Kip, of CDK inhibitors (CDKis) (Vidal and Koff, 2000; Besson et al, 2008). The Cip/Kip family includes p21Cip1, p27Kip1 and the p57Kip2 proteins (Vidal and Koff, 2000). Although all Cip/Kip family members share a high homology in the N-terminal CDKi domain and the C-term region, p57 harbours a large central domain enriched in proline residues, which may confer unique functions not shared by p21 or p27 (Lee et al, 1995; Pateras et al, 2009). Notably, p57 is the only CDKi which play an essential role in mouse embryogenesis and p57−/− mice display several developmental defects and a phenotype that resembles the Beckwith–Wiedeman syndrome (Yan et al, 1997; Zhang et al, 1997). Loss of p57 contributes to the occurrence of soft tissue carcinomas, Wilm’s tumours and, in certain cells, a decrease in its expression has been related to increased invasiveness and metastasis, which suggests a role of p57 as a putative tumour suppressor (Matsuoka et al, 1995; Orlow et al, 1996; Pateras et al, 2009; Borriello et al, 2011). In addition, it has been shown that p57 mediates cell-cycle progression through diverse mechanisms such as the inhibition of G1 CDKs, particularly Cdk2 (Hashimoto et al, 1998). Remarkably, p57 has recently been shown to maintain haematopoietic stem cells (HSCs) quiescence by retaining CyclinD into the cytoplasm (Matsumoto et al, 2011; Zou et al, 2011). However, the regulation of p57 as well as its biological role in cell-cycle control is not well defined yet, possibly due to its essentiality and the lack of proper tools for its detection and study.
Stress-activated protein kinases (SAPKs) play a key role in controlling different cell-cycle checkpoints (Ambrosino and Nebreda, 2001; Bulavin and Fornace, 2004). Mammalian p38 SAPK has been implicated in cell cycle arrest induced by several stimuli at both G2/M and G1/S phases, at least in part, through the stabilization of p21Cip1 mRNA or p27Kip1 protein (Bulavin et al, 2001; Dmitrieva et al, 2002; Bulavin and Fornace, 2004; Pedraza-Alva et al, 2006; Reinhardt et al, 2007; Cuadrado et al, 2009; Lafarga et al, 2009). In budding yeast, the p38-related SAPK Hog1 controls cell cycle at different phases such as S, G2/M (Clotet et al, 2006; Yaakov et al, 2009) and G1 (Escoté et al, 2004; Adrover et al, 2011). In G1, Hog1 directly phosphorylates and controls the activity of the CDKi Sic1, which is related to the members of the mammalian Cip/Kip family, and prevents entry into S phase until proper cellular adaptation to osmostress is achieved (Escoté et al, 2004).
The functional and structural conservation of Hog1 and p38 (Galcheva-Gargova et al, 1994; Han et al, 1994; de Nadal et al, 2002) prompted us to test whether p38 was able to phosphorylate and regulate the activity of the mammalian Cip/Kip family of CDKis. Here, we report that stress-activated p38 phosphorylates and regulates the activity of the p57 CDKi. Phosphorylated p57 delays cell cycle and this delay is critical for cell survival in response to stress. This defines a novel role for the p57 CDKi as an integrator of stress signals to regulate cell-cycle progression.
Results
p38 SAPK phosphorylates the p57Kip2 CDKi in vitro
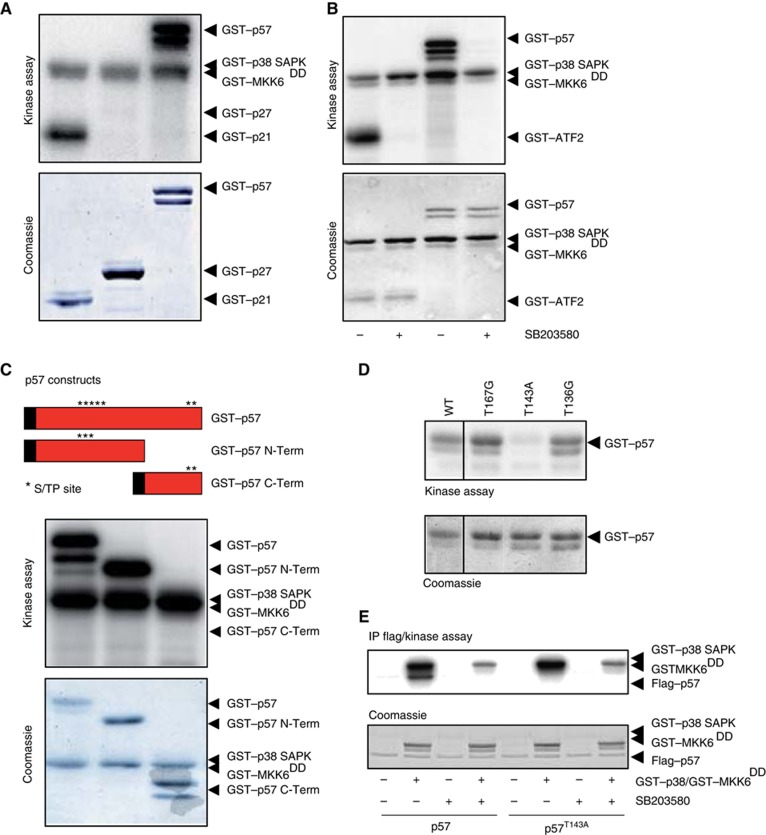
To analyse whether p38 SAPK was able to regulate some members of the Cip/Kip family of CDKis, we initially expressed in bacteria GST-fused p21Cip1, p27Kip1 and the p57Kip2 proteins. Purified proteins were subjected to an in vitro phosphorylation assay with activated p38. In-vitro activated p38 SAPK was able to phosphorylate the CDKis p21Cip1 and p57Kip2 but not p27Kip1 (Figure 1A). Since p21 was already known to be a p38 target (Kim et al, 2002; Todd et al, 2004) we focussed our efforts to further characterize p57 as a novel putative substrate for the p38 SAPK.
Figure 1.
p38 SAPK phosphorylates the CDKi p57 at T143 in vitro. (A) All proteins were purified from E. coli. GST–p57 was expressed as a doublet protein being the shorter a cleavage fragment of full-length GST–p57. GST–p38 SAPK phosphorylates human GST–p21 and mouse GST–p57 but not human GST–p27. (B) GST–ATF2 and GST–p57 phosphorylation is prevented by the p38 SAPK inhibitor SB203580. (C) The mouse p57 protein harbours five putative phosphorylation sites following the minimum SAPK S/TP motif clustered in two regions. A GST–p57 N-term, containing three putative sites, and a GST–p57 C-term, containing two putative sites, mutants were made and assayed in vitro in the presence of GST–p38 SAPK. The GST–p57 N-term variant is phosphorylated as well as wild-type GST–p57 whereas the GST–p57 C-term variant is not phosphorylated by the p38 SAPK. (D) The three putative p38 SAPK sites T167, T143 and T139 were mutated to either glycine or alanine and assayed in vitro. The GST–p57 T143A mutant is not phosphorylated by GST–p38 SAPK in vitro. (E) HeLa cells were transfected with Flag-tagged wild-type p57 and p57T143A. Forty-eight hours post transfections, cells were lysed and immunoprecipitated with anti-Flag agarose beads. Immunoprecipitates were assayed in vitro with GST–p38 SAPK in the presence or the absence of the p38 inhibitor SB203580. Only wild-type Flag–p57 was phosphorylated by p38 SAPK. Representative kinase assays and coomassie blue stained gels are shown.
The phosphorylation of p57 in vitro by p38 was fully prevented by the p38 inhibitor SB203580. ATF2, a known p38 substrate, was used as positive control (Figure 1B). The p57 protein contains five putative S/TP MAPK consensus sites. Thus, we generated two p57 truncated variants; the N-term containing three S/TP sites and the C-term containing two S/TP sites. In vitro kinase assays showed that the N-terminal p57 fragment was phosphorylated to the same extent as the full-length protein whereas the C-term fragment was not phosphorylated at all (Figure 1C). The three S/TP sites found at the p57 N-term fragment were then mutated in full-length p57 to either glycine or alanine and assayed in vitro. Mutation at T143 completely abolished in-vitro phosphorylation of p57 by p38 whereas mutation of p57 at T139 or T167 did not alter phosphorylation of p57 by p38 (Figure 1D).
To further confirm that p57 was a direct substrate for p38, we expressed Flag-tagged wild-type p57 and mutant p57T143A in HeLa cells. Flag immunoprecipitates were assayed in vitro with active p38 SAPK in the absence or the presence of SB203580. Wild-type p57 but not p57T143A was specifically phosphorylated by active p38 (Figure 1E). Therefore, p38 directly phosphorylates p57 at T143 in vitro.
p38 SAPK interacts with the p57Kip2 CDKi
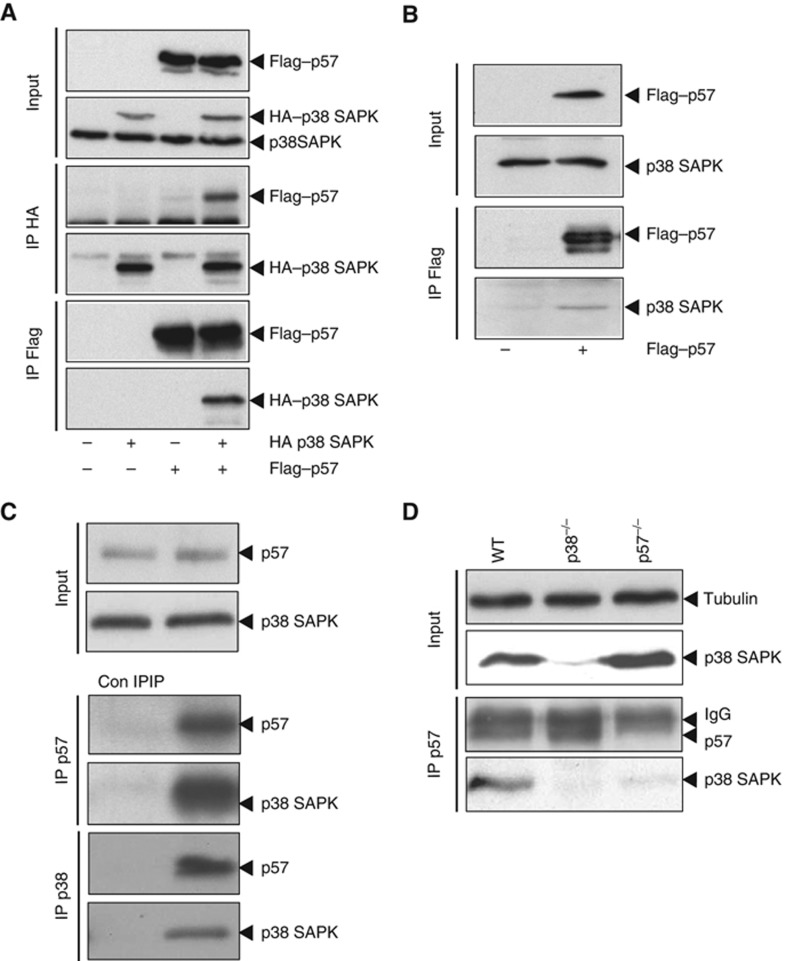
Most SAPKs interact with their corresponding substrates in cells. Thus, we tested whether p38 was able to interact with p57 by performing immunoprecipitation experiments in extracts from HeLa cells expressing Flag-tagged p57 and HA-tagged p38. Binding of HA–p38 was observed when Flag–p57 was precipitated from cell extracts (Figure 2A). Correspondingly, Flag–p57 was also able to co-immunoprecipitate when HA–p38 was precipitated using anti-HA antibodies (Figure 2A). Notably, we were also able to co-immunoprecipitate Flag-tagged p57 with endogenous p38 SAPK (Figure 2B). By using specific antibodies against endogenous p57 and p38 proteins, we were able to confirm the interaction of the two proteins in HeLa cells (Figure 2C). This interaction was also confirmed in wild-type MEF cells and it was abolished in p38−/− or p57−/− cells (Figure 2D). These results show that the CDKi p57 and the p38 SAPK do interact in vivo and form a stable complex.
Figure 2.
p38 SAPK and p57Kip2 form a stable complex in vivo. (A) HA–p38 and Flag–p57 were transfected into HeLa cells for 48 h. Cell lysates were then immunoprecipitated with either anti-Flag agarose beads or anti-HA coupled sepharose beads and analysed by western blot with anti-HA and anti-Flag antibodies. (B) Flag–p57 was transfected into HeLa cells for 48 h. Cell lysates were then immunoprecipitated with anti-Flag agarose beads and analysed by western blot with anti-p38 and anti-Flag antibodies. (C) HeLa cell extracts were immunoprecipitated with a control IgG (Con IP), anti-p57 or anti-p38 coupled sepharose beads and analysed by western blot with anti-p38 and anti-p57 antibodies. (D) Wild-type, p38−/− and p57−/− MEF cell lysates were immunoprecipiated with mouse anti-p57 coupled sepharose beads and analysed by western blot with anti-p38 and rabbit anti-p57 antibodies. Tubulin was used to monitor the input protein levels. Representative western blots are shown.
The p38 SAPK phosphorylates the p57Kip2 CDKi in vivo
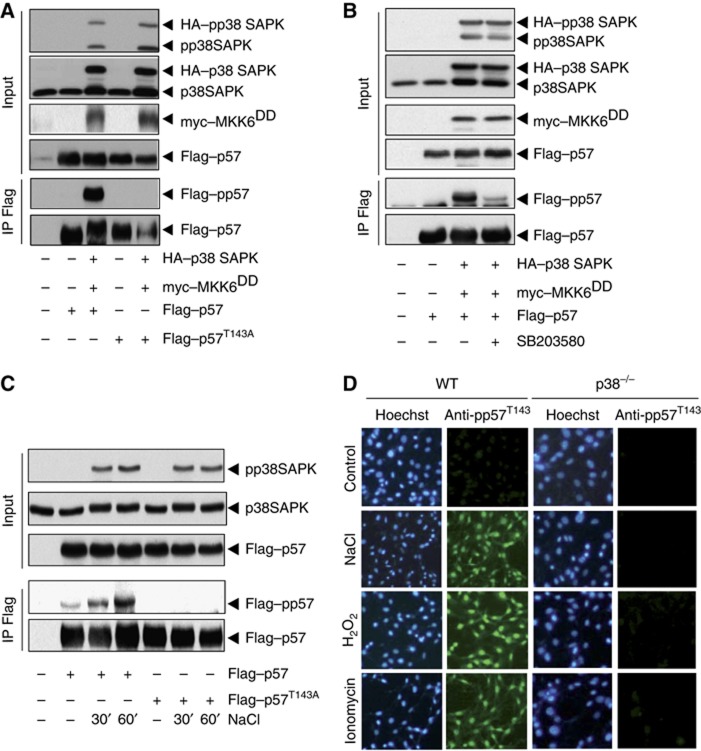
Due to the fact that T143 is a novel p38 target site not described to date, to detect p38 SAPK-mediated p57 phosphorylation in vivo, we took advantage of a generic anti-phospho S/T antibody that was able to specifically recognize p57 phosphorylation at T143. Thus, E. coli purified GST–p57 and GST–p57T143A proteins were incubated in vitro with cold ATP in the absence or presence of activated p38 and analysed by western blot. Only wild type p57, but not p57T143A was recognized by the anti-phospho S/T antibody (Supplementary Figure S1A). We next transfected HeLa cells with wild-type Flag-tagged p57 or Flag-tagged p57T143A in the presence of HA-tagged p38 and myc-tagged MKK6DD (a constitutively active form of the MKK6 MAPKK). The analysis of Flag immunoprecipitates revealed that wild-type p57 was strongly phosphorylated when p38 SAPK was activated by MKK6DD. In contrast, the p57T143A mutant was not phosphorylated by p38 (Figure 3A). Importantly, incubation of the cells with the p38 SAPK inhibitor SB203580 precluded p57 phosphorylation indicating that in vivo p57 phosphorylation required p38 activation (Figure 3B). To rule out that p57 phosphorylation was due to p38 and MKK6DD overexpression, we then assessed p57 phosphorylation upon osmostress. HeLa cells expressing Flag–p57 or Flag–p57T143A were subjected to osmostress and we found that only p57 but not p57T143A was phosphorylated (Figure 3C). The importance of finding a novel in-vivo p38 substrate prompted us to generate specific antibodies targeting phosphorylated p57 at T143. Thus, a phosphopeptide surrounding the p57 T143 site was used to immunize rabbits and the collected anti-sera was affinity purified. The antibody specifically recognized the phosphopeptide but not the non-phosphorylated peptide. Next, we phosphorylated in vitro purified wild-type GST–p57 and GST–p57T143A in the presence of p38 and MKK6DD with cold ATP. The purified anti-pp57 antibody was able to specifically recognize p57 phosphorylation at T143A (Supplementary Figure S1B). Then, we expressed wild-type Flag-tagged p57 in HeLa cells in the absence or the presence of the p38 SAPK inhibitor Birb 0796. Cells were osmostressed and analysed by western blot. The anti-pp57 antibody was able to specifically recognize p57 phosphorylation in vivo upon p38 SAPK activation (Supplementary Figure S1C). Correspondingly, phosphorylation of Flag–p57 upon osmostress was also abolished in p38−/− cells (Supplementary Figure S1D). We next assessed in vivo phosphorylation p57 by immunofluorescence using the specific phospho-p57 antibody. Wild-type and p38−/− MEFs were subjected to osmostress and found that whereas no phosphorylation of p57 was detected in the absence of stress, strong nuclear fluorescence was detected upon osmostress. The increase on p57 phosphorylation upon osmostress was not observed in p38−/− cells (Figure 3D). Altogether, these results show that p57 is phosphorylated at T143 in vivo by the p38 SAPK.
Figure 3.
The CDKi p57 is phosphorylated at T143 in vivo by stress-activated p38 SAPK. (A) HeLa cells were transfected with wild-type Flag-p57 and Flag–p57T143A in the presence or absence of HA–p38 SAPK and myc-MKK6DD for 48 h. Cell lysates were immunoprecipitated with anti-Flag agarose beads and analysed by western blot with anti-pp38, anti-p38, anti-myc, anti-phospoS/T and anti-Flag antibodies. (B) HeLa cells were transfected with Flag–p57 in the presence or absence of HA–p38 SAPK and myc-MKK6DD for 48 h. The p38 SAPK inhibitor SB203580 was added to a final concentration of 10 μM 24 h prior harvesting the cells. Cell lysates were analysed as in (A). (C) HeLa cells were transfected with Flag–p57. Forty-eight hours post transfection, cells were treated with 100 mM NaCl for the indicated times. Cell lysates were analysed as in (A). Representative western blots are shown. (D) Wild-type and p38−/− MEF cells were grown on glass covers and treated with 100 mM NaCl, 600 μM H2O2 or 7.5 mM ionomycin for 60 min prior to fixation. p57 phosphorylation at T143 was detected by indirect immunofluorescence. Nuclear DNA was stained with Hoeschst 33342. Pictures were taken using an inverted Olympus CKX 41 microscope and the Olympus CellˆD imaging software. Representative pictures are shown.
p57 phosphorylation at T143 by p38 regulates p57 activity towards Cdk2 in vitro
We then analysed whether p57 phosphorylation by p38 was modulating p57 activity. It has been shown that protein phosphorylation can alter the stability or localization of Cip/Kip CDKis (Tsvetkov et al, 1999; Ishida et al, 2002; Kim et al, 2002; Liang et al, 2002; Shin et al, 2002; Kotake et al, 2005; Kossatz et al, 2006). Thus, we initially monitored endogenous p57 half live in HeLa cells treated with NaCl or anisomycin (a known activator of p38). Protein synthesis was stopped by the addition of cycloheximide 30 min prior to stressing the cells. p57 protein levels were followed over time by western blot. Neither osmostress nor anisomycin altered p57 half live (Supplementary Figure S2A), albeit this was under the control of the proteosome as previously reported (Supplementary Figure S2B; Kamura et al, 2003). To further confirm that p57 protein half life was not affected by cell stress, we expressed wild type Flag-tagged p57 in HeLa cells. As observed with endogenous p57, Flag-tagged p57 protein half life was neither affected by osmostress nor anisomycin (Supplementary Figure S2C). We then monitored whether p57 localization was altered upon osmostress by following the localization of a p57-DsRed construct. p57 was found to be localized mainly in the nucleus and it did not change its localization upon stress (Supplementary Figure S3B). Similar results were obtained when endogenous p57 was followed in cell fractionation (Supplementary Figure S3A). Of note, CyclinD was also mainly nuclear (Supplementary Figure S3A). The localization of p38 did not change significantly under the conditions tested but there was a significant amount of active p38 present in the nuclei of the cells (Supplementary Figure S3A and B). Altogether, phosphorylation of p57 by p38 neither affects its stability nor its localization.
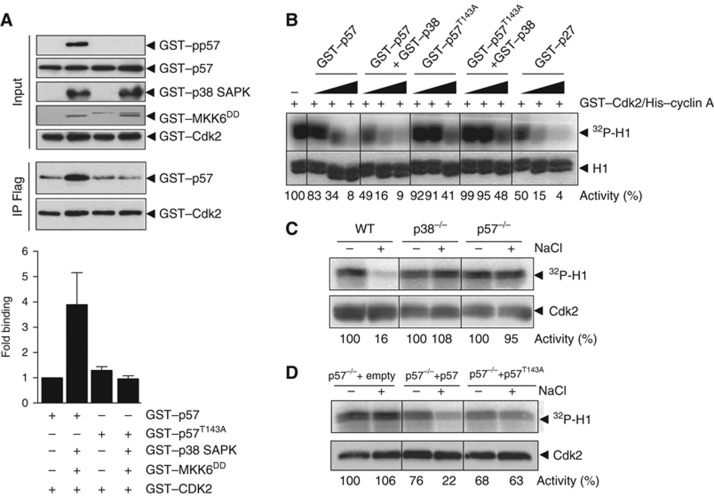
p57 preferentially binds to Cdk2 (Hashimoto et al, 1998) and thus, we asked whether T143 phosphorylation altered the ability of p57 to interact with and inhibit Cdk2. Wild type GST–p57 and mutant GST–p57T143A purified from bacteria were phosphorylated in vitro by activated p38 SAPK and binding to Cdk2 was assessed. Binding of p57 to Cdk2 increased almost four-fold when phosphorylated by p38. Remarkably, binding of the p57T143A mutant to Cdk2 was not affected after incubation with p38 (Figure 4A). Therefore, phosphorylation of p57 by p38 increased the association of p57 with Cdk2.
Figure 4.
p57 phosphorylation at T143 regulates Cdk2 activity. (A) All proteins were purified from E. coli. Wild-type GST–p57 and GST–p57T143A was phosphorylated in vitro with activated GST–p38 SAPK using cold ATP. Phosphorylated proteins were then incubated with purified GST–CDK2/His–CyclinA complexes. GST–CDK2 was immunoprecipitated with anti-CDK2 antibodies and the presence of GST–p57 was assessed by western blot with anti-p57 antibodies. Inputs were analysed by western blot with the shown antibodies. The graph represents the average and s.e.m. of three independent experiments. (B) Increasing amounts of the CDK inhibitors GST–p57, GST–p57T143A and GST–p27 were phosphorylated or not with activated GST–p38 SAPK using cold ATP. The phosphorylated proteins were then incubated with purified GST–CDK2/His–CyclinA complexes. CDK2 activity was assayed in the presence of radiolabelled 32P-γ-ATP and Histone H1 as substrates and analysed by phosphoimaging. (C) Wild-type, p38−/− and p57−/− MEFs were incubated with 100 mM NaCl for 4 h. Cell lysates were then immunoprecipitated with anti-CDK2 antibodies. Endogenous CDK2 activity was assayed as in (B). (D) p57−/− MEFs were infected with lentiviruses carrying an empty vector, a wild-type p57 or p57T143A. Infected MEFs were then treated and assayed as in (C). Representative kinase assays and western blots are shown. The average CDK2 activity of two independent experiments is shown in (B–D).
An increase in the affinity of p57 towards CDK2 could result in a decrease on Cdk2 activity. Thus, we tested whether phosphorylation of p57 could inhibit more efficiently Cdk2 activity. We incubated increasing amounts of purified GST–p57 (wild-type and the p57T143A mutant) previously incubated or not with active p38 and then, analysed Cdk2/CyclinA activity in vitro. Increasing amounts of GST–p57 inhibited gradually the activity of Cdk2 as it was observed by incubation of Cdk2 with p27. Remarkably, the inhibition of Cdk2 activity was more pronounced when p57 was phosphorylated by p38 (Figure 4B). Correspondingly, the ability of p57T143A to inhibit Cdk2 did not increase by preincubation with p38 (Figure 4B). Thus, phosphorylated p57 at T143 inhibits more efficiently Cdk2–CyclinA activity than non-phosphorylated p57.
Osmostress regulates Cdk2 activity in vivo through p57 phosphorylation at T143
If phosphorylation of p57 by p38 increases its ability to inhibit Cdk2, then osmostress should be expected to cause a decrease in Cdk2 activity in vivo. To assess Cdk2 activity, wild-type, p38−/− and p57−/− MEFs were challenged with 100 mM NaCl for 4 h and cell lysates were then immunoprecipitated with anti-Cdk2 antibodies. Endogenous Cdk2 activity was assayed in the presence of radiolabelled 32P-γ-ATP and Histone H1 as substrate. Cdk2 activity from wild-type MEFs was reduced to <20% upon osmostress (Figure 4C). This effect was dependent on p38 SAPK and p57 since it was not observed in p38−/− or p57−/− MEF cells (Figure 4C). Therefore, Cdk2 activity was inhibited in response to osmostress depending on p38 and p57.
To analyse whether phosphorylation of p57 by p38 was promoting the decrease in Cdk2 activity, we infected p57−/− MEF cells with lentiviruses expressing wild-type or the p57T143A mutant. Both proteins were expressed to a similar extend in p57−/− MEF cells (Supplementary Figure S4). Osmostress provoked a reduction on endogenous Cdk2 activity in cells carrying wild type p57 but not in cells carrying the p57T143A mutant (Figure 4D). Therefore, p57 phosphorylation at T143 is essential to regulate Cdk2 activity in vivo.
Osmostress regulates G1 progression in a p38- and p57-dependent manner
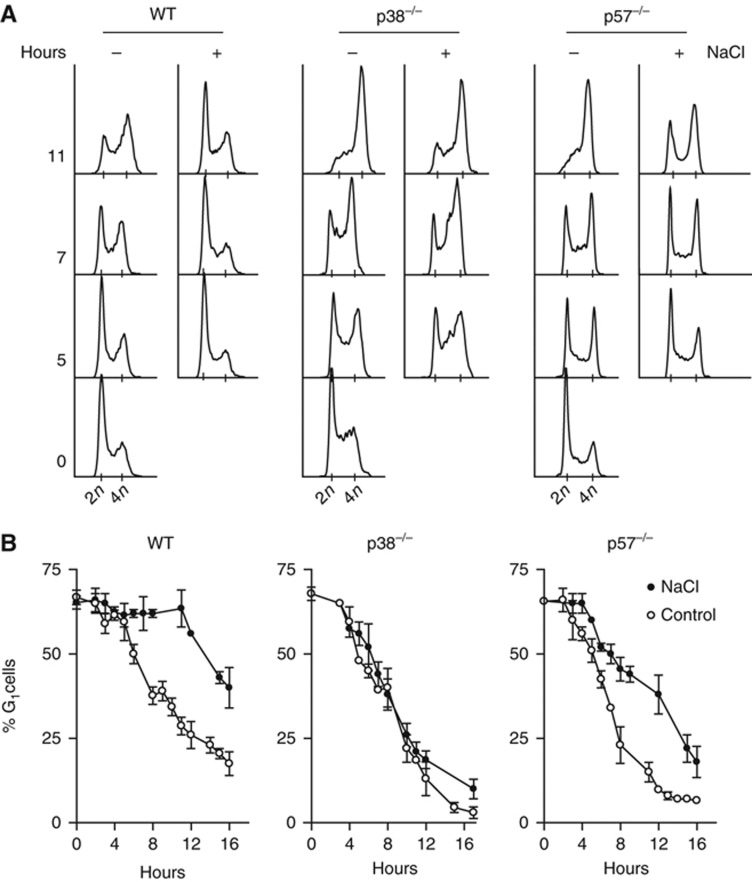
Since phosphorylation of p57 by p38 induced a reduction on Cdk2 activity, we analysed whether p57 was important to mediate a cell-cycle delay in G1 upon osmostress. Wild-type, p38−/− and p57−/− knockout MEFs cultures were subjected to osmostress (100 mM NaCl and 200 mM) and cell-cycle progression was followed by FACS. Nocodazole was added 1 h after osmostress to trap the cells at G2/M. In response to osmostress, wild-type cells clearly delayed the transition to G2/M (Figure 5A and B). In contrast, p38−/− and p57−/− cells were not able to delay cell-cycle progression to the same extent upon osmostress (Figure 5A and B; Supplementary Figure S9). Both cells express p18, p21 and p27 CDKis and it is worth noting that p21 is upregulated in the absence of p57 (Supplementary Figure S5A). Of note, p21 protein levels in p57−/− cells were not affected upon osmostress and were downregulated upon the reintroduction of p57 (Supplementary Figure S5B and C). Taken together, our data indicate that both p38 and p57 are required for cell-cycle delay in response to osmostress.
Figure 5.
Osmostress mediates a G1 cell-cycle delay in a p38 SAPK- and p57-dependent manner. (A) Wild-type, p38−/− and p57−/− MEFs were stressed with 100 mM NaCl. One hour later, nocodazole was added to trap the cells at the G2/M transition. Cell-cycle progression was monitored by FACS by collecting samples every 2 h. Representative DNA profiles are shown. (B) The percentage of wild-type, p38−/− and p57−/− MEFs in G1 from three independent experiments is shown. Solid circles represent osmostressed MEF cells. Open circles are non-stressed control MEF cells.
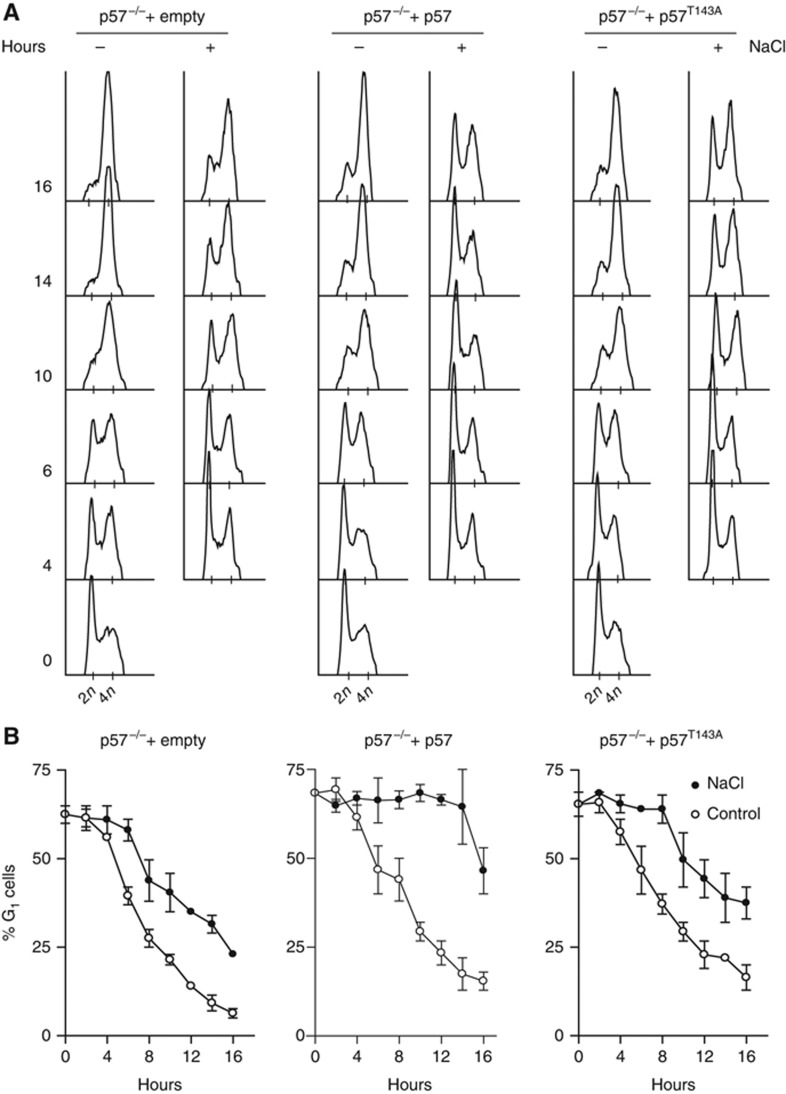
To address the involvement of p57 phosphorylation at T143 on cell-cycle progression, we then infected p57−/− MEFs with viruses expressing wild-type p57 or p57T143A and subjected them to stress (100 mM NaCl). p57−/− cells expressing wild-type p57 arrested at G1 upon osmostress as well as wild-type cells (Figure 6A and B versus 5A and B, ). In contrast, albeit cells arrested a little longer than p57−/− cells, expression of p57T143A was not able to proper delay cell-cycle progression upon stress (Figure 6A and B). Therefore, phosphorylation of p57 at T143 by p38 is critical to impose a G1 delay upon osmostress.
Figure 6.
The osmostress-induced G1 delay is rescued in p57−/− MEFs infected with wild-type p57 but not with p57T143A. (A) p57−/− MEFs infected with lentiviruses carrying an empty vector, a wild-type p57 or p57T143A were stressed with 100 mM NaCl. One hour later, nocodazole was added to trap the cells at the G2/M transition. Cell-cycle progression was monitored by FACS by collecting samples every 2 h. Representative DNA profiles are shown (B). The percentage of p57−/− infected with lentiviruses carrying an empty vector, a wild-type p57 or p57T143A in G1 from three independent experiments is shown. Solid circles represent osmostressed MEF cells. Open circles are non-stressed control MEF cells.
The p38 SAPK and p57 CDKi promote cell survival upon osmostress
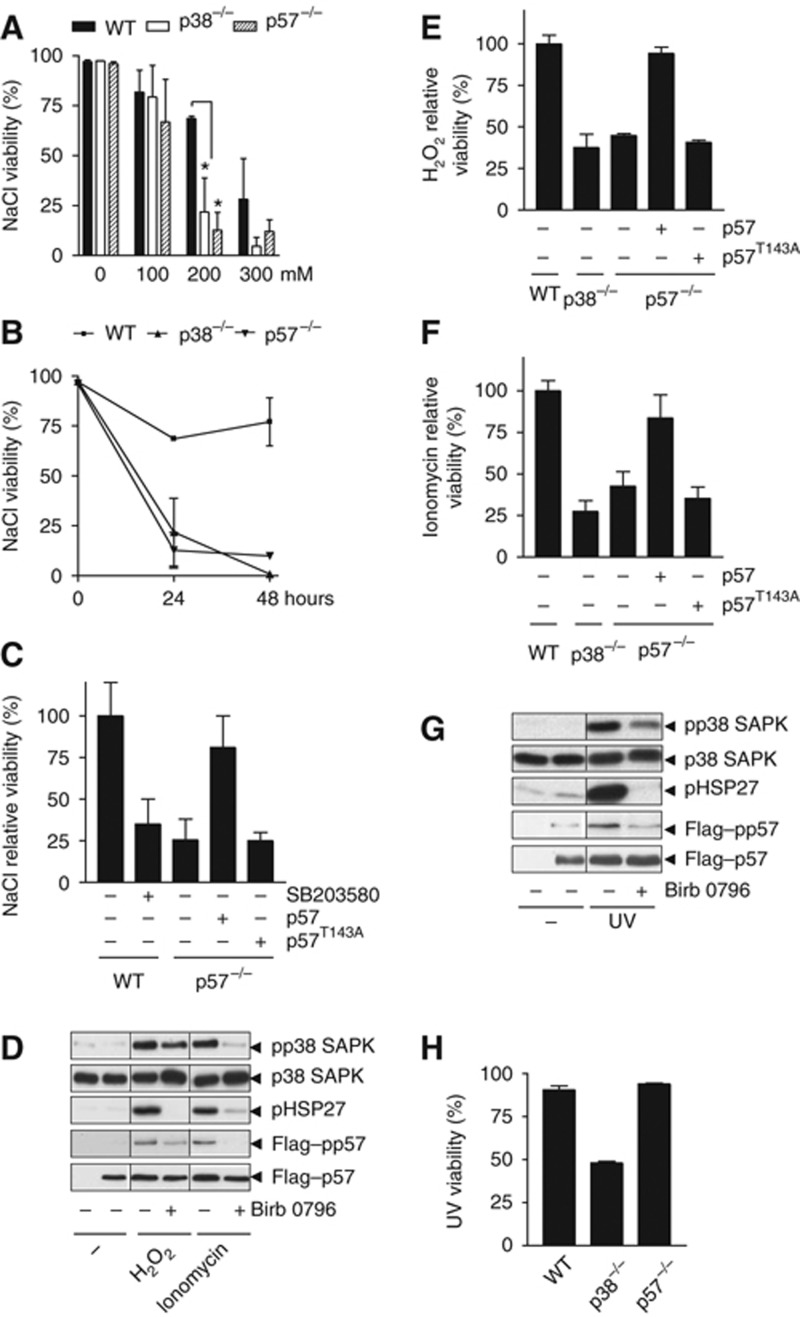
We reasoned that delaying cell-cycle progression in G1 could be necessary to guarantee cell adaptation and survival to osmostress. To assess the biological relevance of this cell-cycle delay induced by p57 phosphorylation, we monitored cell viability by propidium iodide (PI) staining by FACS in response to osmostress. We subjected wild-type, p38−/− and p57−/− cells to increasing amounts of NaCl and found that cell viability was compromised at increasing NaCl concentrations. Notably, both p38−/− and p57−/− cells displayed a strong reduction on their ability to survive to osmostress when compared with wild type (Figure 7A and B). Similar results were obtained when cell viability was assessed using an MTT assay (Supplementary Figure S6). MK2 and Cdt1 have been defined as targets for p38 SAPK involved in cell cycle (Manke et al, 2005; Reinhardt et al, 2007; Chandrasekaran et al, 2011). To assess the relevance of those proteins in response to osmostress, MK2 and Cdt1 were downregulated by the use of a chemical inhibitor (MK2 inhibitor III) or an siRNA against Cdt1. The downregulation of either MK2 or Cdt1 did not alter significantly cell survival in response to osmostress in wild-type or p57−/− and p38−/− cells (Supplementary Figures S10 and S11). Therefore, both p38 and p57 are critical for cell survival in response to osmostress.
Figure 7.
p38 SAPK and p57 promote cell survival upon stress. (A) Cell viability was assessed by FACS in wild-type, p38−/− and p57−/− MEFs 24 h after the addition of the indicated amounts of NaCl. Statistical significance was determined by one-way ANOVA followed by a Dunnett’s multiple comparison test. A value of P<0.05 was considered statistically significant and represented by (*) in the bar graph. (B) Cell viability was assessed on wild-type, p38−/− and p57−/− MEF cells 24 and 48 h after the addition of 200 mM NaCl. (C) Cell viability upon 200 mM NaCl treatment is compromised when p38 SAPK is inhibited by SB203580 in wild-type MEFs. Reintroduction of wild-type p57 but not p57T143A into p57−/− rescued cell viability 24 h after the addition of NaCl. (D) HeLa cells were transfected with Flag–p57. Forty-eight hours post transfection, cells were incubated in the presence or the absence of the specific p38 SAPK inhibitor Birb 0796. Birb 0796 was added to a final concentration of 0.5 μM 2 h prior to the treatment with 600 μM H2O2 and 7.5 mM ionomycin for 60 min. Cell lysates were analysed by western blot with anti-pp38, anti-p38, anti-pHSP27, anti-pp57 and anti-Flag antibodies. (E, F) Cell viability is compromised in p38−/− and p57−/− MEFs after oxidative and ionomycin stress. Reintroduction of wild-type p57 but not p57T143A into p57−/− MEF cell rescued cell viability 24 h after oxidative and ionomycin stress. (G) HeLa cells were transfected with Flag–p57. Forty-eight hours post transfection, cells were incubated in the presence or the absence of the specific p38 SAPK inhibitor Birb 0796. Birb 0796 was added to a final concentration of 0.5 μM 2 h prior to the treatment with 5 mJ of UV for 60 min. Cell lysates were analysed as in (D). (H) Cell viability is compromised in p38−/− MEFs but not in wild type and p57−/− 24 h after irradiating the cells with 5 mJ of UV. Data are represented as mean±s.e.m.
We then studied the relevance of p57 phosphorylation by p38 to promote cell survival upon osmostress and found that knockout cells expressing wild-type p57 were able to survive similarly to wild-type cells, whereas cells expressing p57T143A displayed a survival rate as low as the observed in p57−/− knockout cells (Figure 7C). Therefore, phosphorylation of p57 by p38 is essential to promote cell survival upon osmostress.
Survival to oxidative stress or ionomycin also depends on p38 and p57
In addition to osmostress, p38 is activated by other stimuli such as oxidative stress, ionomycin and UV (Huot et al, 1997; Bulavin et al, 2001; Elzi et al, 2001). Thus, we wondered whether p38 and p57 were also essential to promote cell survival upon these stresses. Initially, we assessed whether oxidative stress or ionomycin was able to induce p38-mediated p57 phosphorylation. Cells expressing Flag–p57 were subjected to H2O2 (600 μM H2O2) or ionomycin (7.5 μM) for 1 h and phosphorylation of p38 and p57 (at Thr143) was assessed by western blot by using specific antibodies. Phosphorylation of Hsp27 was assessed as a control of p38 activation. Exposure of cells to these stresses induced phosphorylation of p57 that was prevented by incubation with a p38 inhibitor (Birb 0796) (Figure 7D). Similar results were obtained when endogenous p57 phosphorylation was assessed by immunofluorescence using the phospho-p57 antibody. Moreover, p57 phosphorylation upon stress was abolished in p38−/− cells (Figure 3D). Remarkably, cell viability was strongly compromised in p38−/− and p57−/− MEFs in the presence of H2O2 and ionomycin (Figure 7E and F; Supplementary Figure S6). Of note, the decrease on cell viability is accompanied with an increase on apoptosis as assessed by DNA nuclear condensation (Supplementary Figure S7). Furthermore, whereas p57−/− cells expressing wild-type p57 were able to survive similarly to wild type upon stress, cells expressing p57T143A displayed a survival rate as low as the observed in p57−/− cells (Figure 7E and F; Supplementary Figure S6). p27 has been shown to act downstream of p38 in response to drug induced DNA damage (Cuadrado et al, 2009) and it could be redundant with p57 in HSCs (Matsumoto et al, 2011; Zou et al, 2011). Thus, we then addressed p27−/− sensitivity to different kind of stresses. In contrast to p57−/− cells, cells deficient in p27 displayed a survival rate similar to wild type pointing out towards the critical role of p57 in cell survival upon stress (Supplementary Figure S8).
H2O2 and ionomycin are known to arrest cells in G1 (Chua et al, 2009; Scotto et al, 1999), in contrast, exposure to UV promotes S and G2/M delays. UV induced phosphorylation of both p38 and p57 (Figure 7G); however, it only reduced cell viability and induced nuclei condensation in p38−/− cells but not in p57−/− cells (Figure 7H; Supplementary Figures S6 and S7). Taken together, these findings show that phosphorylation of p57 by p38 is critical for cell survival to different type of stresses that impact in G1.
Discussion
Integration of environmental cues to cell-cycle control is critical to understand how cells respond and adapt to stress. SAPKs mediate signal transduction to stress and control several aspects of cell physiology from transcription to cell-cycle regulation (de Nadal et al, 2011). Previous reports have implicated mammalian p38 SAPK in the control of cell-cycle progression (Ambrosino and Nebreda, 2001). However, little was known on the regulation of G1 by p38 and the possible relevance of this process in the response to stress. Here, we provide evidence that the p57 CDKi is a novel target of p38 SAPK that mediates cell-cycle control in response to extracellular insults. Our results indicate that phosphorylation of p57 on Thr143 by p38 SAPK mediates cell-cycle arrest at G1 in response to stress and promotes cell survival. Furthermore, we found that p57 has a critical role for cell survival to various types of stresses that also activate p38 at G1.
The role of p57 in cell-cycle control and its biological functions have been elusive, mainly for the difficulty to detect this protein in many cells and tissues as well as the fact that mice lacking p57 show strong developmental defects (Pateras et al, 2009). p57 have been involved in regulation of signalling by its association with the JNK kinase (Chang et al, 2003). In contrast, we have shown that cells deficient in p57 do not show altered p38 signalling. Recent data pointed out the relevance of p57 in bone marrow HSCs. p57 is critical to brake cycling HSCs, which is important for maintaining quiescence and permit self-renewal activity (Matsumoto et al, 2011; Tesio and Trumpp, 2011; Zou et al, 2011). This role seems to be shared with p27 since expression of p27 at the p57 locus partly bypasses p57 requirement (Matsumoto et al, 2011). In addition, both p27 and p57 seem to share the same mechanism of control, which involves interaction with Hsc70 and CyclinD in the cytoplasm to prevent entry of CyclinD at the nucleus (Zou et al, 2011). In response to stress, the regulation of cell-cycle progression by p57 seems to be completely different. First, p57 is mainly a nuclear protein whose nuclear localization does not change in response to stress. Similarly, cyclinD localization is mainly nuclear and does not change upon stress. Moreover, we have found that phosphorylation of p57 increases its ability to inhibit CDK2 activity. Both in vitro and in vivo analyses have shown that phosphorylation of p57 at Thr143 by p38 is a key factor for CDK2 inhibition. Therefore, in response to stress p57 imposes a cell-cycle delay differently from that observed to maintain HSC quiescence. Of note, it has been shown that p27 is upregulated in p57−/− HSC cells and that expression of p27 in the p57 locus can replace p57 function on HSC quiescence (Tesio and Trumpp, 2011; Zou et al, 2011). However that was not the case in p57−/− MEFs which expressed similar p27 protein levels as wild type MEFs. Furthermore, phosphorylation of p57 by p38 lays in a region that is conserved neither in p27 nor in p21. Taken together, it is likely that p57 has a specific function in cell-cycle regulation upon stress that is not shared by the other Cip/Kip inhibitors.
It has been shown that downregulation of p57, both transcriptionally or translationally, is frequent in many human cancers (Pateras et al, 2009), indicating that the levels of p57 might be important to control cell-cycle progression as it has been described by other inhibitors (Besson et al, 2008). For instance, p38 SAPK promotes the expression and stabilization, directly or indirectly, of p21 and p27 CDKis in response to different stimuli (Kim et al, 2002; Todd et al, 2004; Cuadrado et al, 2009; Lafarga et al, 2009; Swat et al, 2009). In contrast, we do not observe a stress-induced change on p57 protein levels, intracellular localization or stability but rather a change on the affinity of the CDKi towards Cdk2. We have shown that phosphorylated p57 binds more efficiently to Cdk2 which results in a more efficient inhibition of Cdk2 activity both in vitro and in vivo. Our results support a novel regulatory mechanism by which changes on the affinity of the CDKi towards the CDK, caused by specific phosphorylation, inhibits more efficiently CDK activity and resulting in delayed cell-cycle progression.
Cells deficient in p38 or p57 display a dramatic impairment on viability upon osmostress, suggesting that cell-cycle arrest in G1 play an important role in the survival of stressed cells. Remarkably, cells expressing a non-phosphorylatable mutant of p57 are as sensitive to osmostress as the p57−/− or p38−/− cells. Therefore, the role of p38 on promoting cell survival in response to stress in G1 is mainly mediated by p57 phosphorylation. Of note, p57−/− cells still express p21 and p27 CDKis further supporting a prevalent role of p57 in delaying cell-cycle progression in response to osmostress. Of note, p57−/− cells have increased p21 levels, independently of stress, suggesting that cells induce the expression of p21 to compensate for the loss of p57. Despite this fact, p21 upregulation was not sufficient to neither impose a G1 delay nor promote cell survival upon an osmotic shock.
In addition to osmostress, p38 is activated by other insults such as oxidative stress, ionomycin and UV. All those stimuli not only induce p38 activation but also p57 phosphorylation at Thr143. Correspondingly, activation of p38 by MKK6DD also results in p57 phosphorylation at Thr143. Both p38−/− and p57−/− deficient cells are also extremely sensitive to oxidative stress and ionomycin. Importantly, sensitivity of p57−/− cells to those stresses is suppressed by expression of wild-type p57 but not the p57T143A mutant, indicating that phosphorylation of p57 in response to unrelated stresses is a general mechanism to modulate cell-cycle progression and to maximize cell survival. On the other hand, we have found that albeit p57 is phosphorylated, it is not essential for cell survival in response to UV. It is known that UV induces DNA damage that is repaired later during the cell cycle (Sinha and Häder). For instance, upon UV damage, p38 contributes to G2/M cell-cycle delay via inhibition of CDC25B/C phosphatases which leads to CDK1/CyclinB inhibition (Bulavin et al, 1999, 2001; Manke et al, 2005). It is therefore possible that the relevance of p57 is restricted to stress-induced G1 arrest and that alternative p38-mediated targets control other phases of cell-cycle progression. This strongly suggests that p38 is controlling a network of cell-cycle components to maximize cell survival in response to external stimuli.
In summary, this study uncovers a novel function for p57 that integrates external signals transduced by p38 SAPK to control the cell-cycle machinery, establishing a checkpoint in G1 which is critical to delay cell-cycle progression and permit cellular adaptation to stress.
Materials and methods
Cells, transfection and infection
Human embryo kidney 293T (HEK 293T), HeLa, wild-type MEFs, p38α−/− (Ambrosino et al, 2003), p27−/− and p57−/− MEF cells were maintained in Dulbecco’s modified Eagle’s medium (Biological Industries) containing 10% fetal calf serum (Sigma) and supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 100 U/ml Penicillin and 100 μg/ml Streptomycin (Gibco) and cultured in a 5% CO2 humidified incubator at 37°C. Primary p57 null MEFs, a gift from Dr Manuel Serrano (CNIO, Madrid), were spontaneously immortalized following the classical 3T3 immortalization protocol described by Todaro and Green (1963). When indicated, cells were incubated with 10 μM SB203580 (Calbiochem) for 30 min, 0.5 μM BIRB 0796 (Axon Medchem) for 2 h, 150 μM MK-2 Inhibitor III (Calbiochem) for 30 min, Cyclohexamide (Sigma) for 20 min and 10 μM MG132 for 10 min (Sigma) prior to the stress. Cells were stressed with NaCl (ranging from 100 to 300 mM), 600 μM H2O2 (Sigma), 7.5 μM Ionomycin (Sigma) or UV (5 mJ). DNA transfections and siRNA transfections of HEK 293T, HeLa and MEF cells were performed using respectively FuGENE 6 (Roche Diagnostics) or Oligofectamine (Invitrogen) following manufacturer’s protocol. MEF cells were infected for up to 3 days with supernatants containing lentiviruses produced in transfected HEK 293T cells. Briefly, HEK 293T cells were co-transfected with the lentiviral vector pWPI along with the lentiviral packaging and envelope vectors pMDG2 and psPAX2 and left for 48 h before harvesting the media. After a brief centrifugation to remove cell debris, cleared supernatants were added directly to cell culture dishes growing MEF cells.
Plasmids constructs and siRNAs
pcDNA3-Flag was obtained from Dr Pura Muñoz-Canoves (UPF, Spain). Constructs to express GST–CDK2 (human), His–cyclinA (bovine) and GST–Cak1/Civ1 in E. coli have been previously described (Brown et al, 1995, 1999; Ferby et al, 1999). The lentiviral vectors pWPI, pMD2G and psPAX2 were obtained from Dr Didier Trono (EPFL, Lausanne). The mouse p57 cDNA was PCR out from the Cdkn1c/5930414J15 plasmid and cloned into the EcoRI and XhoI sites of pGEX4T1 (GE Healthcare) to generate a GST–p57 fusion protein using the oligonucleotides mp57.1 forward CAT GAA TTC ATG GAA CGC TTG GCC TCC and mp57.1 reverse CAT CTC GAG TCA TCT CAG AGC TTT GCG. The GST–p57 N-Term and C-term were obtained using the full-length GST–p57 as a template by PCR with the complementary oligonucleotides N-term mp57 reverse CAT CTC GAG GAC CTG TTC CTC GCC GTC and the C-term mp57 forward CAT GAA TTC GAC CCG ATC CCG GAC GCG and subcloned into the EcoRI and XhoI sites of the pGEX4T1 vector. The GST–p57 mutants were generated using the Quickchange XL site directed mutagenesis kit from Stratagene following manufacturers’ instructions with the following mutagenesis primer pairs: T139G (GTG GCG GAG CCC GGG CCA CCC GCG ACC and GGT CGC GGG TGG CCC GGG CTC CGC GAC), T143A (ACC CCA CCC GCG GCC CCG GCC CCG GCT and AGC CGG GGC CGG GGC CGC GGG TGG GGT), T167G (ACC TCC GAC CCG GGT CCG GAC CCG ATC and GAT CGG GTC CGG ACC CGG GTC GGA GGT). C-terminal Flag-tagged versions of full-length p57 and p57T143A were PCR out from the pGEX4T1 vectors and cloned into the BamHI and EcoRI restriction sites of a pCDNA3-Flag using the oligonucleotides mp57.2 forward CAT GGA TTC ATG GAA CGC TTG GCC TCC and mp57.2 reverse CAT GAA TTC CAG ACG TTT GCG CGG. Ds-Red p57 wt and Ds-Red p57T143A were generated by subcloning wild-type p57 and p57 T143A into the pDs-Red-Express-N1 (Clontech). Lentiviral expressing Flag-tagged p57 was obtained from the pCDNA3-Flag vectors by PCR using the oligonucleotides: 5p57PacI forward CAT TTAATTAA ATG GAA CGC TTG GCC TCC and 3p57PacI reverse CAT TTAATTAA TCA TTT ATC GTC ATC GTC CTT GTA ATC TCT CAG ACG TTT GCC CGG and cloned into the pWPI PacI restriction site. The underlined sequences indicate restriction enzymes used. All the constructs and site-directed mutations were checked by sequencing using the ABI-Prism kit from Applied Biosystems. Cdt1 protein levels were knocked down using the EMU044861 Mission esiRNA (Sigma).
Bacterial expression and purification of recombinant proteins
E. coli were grown at 37°C until they reached an OD600 of 0.5 absorbance units. At this point, GST- or His-tagged proteins were induced for 4 h by adding 1 mM IPTG and switching the culture temperature to 25°C. After induction, cells were collected by centrifugation and resuspended in 1/50th volume of STET 1 × buffer (100 mM NaCl, 10 mM Tris–HCl pH 8.0, 10 mM EDTA pH 8.0, 5% Triton X-100 supplemented with 2 mM DTT and the 1 mM PMSF, 1 mM Benzamidine, 200 μg/ml Leupeptine and 200 μg/ml Pepstatine). Cells were lysed by ice-cold brief sonication and cleared by high speed centrifugation. GST-fused proteins were pulled down from supernatants with 300 μl of gluthatione-sepharose beads (GE Healthcare, 50% slurry in equilibrated with STET) by mixing 45 min at 4°C. The gluthatione-sepharose beads were collected by brief centrifugation and washed four times in STET 1 × buffer and two times in 50 mM Tris–HCl pH 8.0 buffer supplemented with 2 mM DTT. The GST-fused proteins were then eluted in 200 μl of 50 mM Tris–HCl pH 8.0 buffer supplemented with 2 mM DTT and 10 mM reduced gluthatione (Sigma) by mixing for 45 min at 4°C and stored at −80°C. His-tagged cyclinA was purified from E. coli as described but incubating the sonicated lysate supernatant with Talon metal affinity beads (Clontech) for 90 min at 4°C. The beads were washed with 60 mM Imidazole, 500 mM NaCl and 20 mM Tris–HCl (pH 8.0). His–cyclinA was eluted in the same buffer supplemented with 1 M Imidazole. Fractions containing the purified recombinant proteins were dialysed overnight against 50 mM Tris (pH 8.0), 50 mM NaCl, 0.1 mM EDTA, 0.5 mM DTT, and 5% glycerol and stored in aliquots at −80°C.
Western blot and immunoprecipitation assays
Transfected cells were washed with ice-cold PBS and scraped into 500 μl of IP/lysis buffer (10 mM Tris–HCl pH 7.5, 1% NP-40, 2 mM EDTA, 50 mM NaF, 50 mM β-glycerolphosphate, 1 mM Sodium Vanadate, 1 mM PMSF, 1 mM Benzamidine, 200 μg/ml Leupeptine and 200 μg/ml Pepstatine). The lysates were cleared by microcentrifugation and 10% retained as input. The remainders were subjected to immunoprecipitation with either 5 μl of agarose-conjugated anti-Flag (Sigma) or 50 μl sepharose-protein A beads (GE Healthcare, 50% slurry equilibrated in IP buffer) coupled to specific antibodies by mixing overnight at 4°C. Immune complexes were collected by brief centrifugation and washed rapidly three times with IP buffer. Immunoprecipitates and the input samples were subjected to PAGE–SDS and western blotting. Commercially available antibodies used were as follows: mouse monoclonal anti-α-Tubulin (Sigma, S9026), mouse monoclonal anti-Flag (Sigma, S2220), rabbit polyclonal anti-Cdk2 (Santa Cruz, sc-163), rabbit polyclonal anti-cyclinD (Santa Cruz, sc-717), rabbit polyclonal anti-p57(Santa Cruz, sc-8298), mouse monoclonal anti-p57 (Santa Cruz, sc-56341), rabbit polyclonal anti-p21 (Abcam, ab7960), rabbit polyclonal anti-p27 (Santa Cruz, sc-528), rabbit polyclonal anti-p38α SAPK (Santa Cruz, sc-535), rabbit monoclonal anti-pp38 SAPK (Cell Signaling, clone 3D7), rabbit polyclonal anti IκBα (Santa Cruz, sc-371), rabbit polyclonal anti-Histone 3 (Abcam, ab-1791), rabbit polyclonal anti-Cdt1 (Santa Cruz, sc-28262), rabbit polyclonal anti-HSP27 (Stressgen, #SPA-523) and Mouse anti-phospho-serine/threonine (BD Transduction Laboratories). Mouse monoclonal anti-HA and mouse monoclonal anti-myc were house made from the 12CA5 and 9E10 hybridomas, respectively. Rabbit polyclonal antibodies specifically targeting p57 phosphorylation at T143 were generated by Genscript Corporation. Horse Radish Peroxidase conjugated anti-rabbit and anti-mouse antibodies and the Enhanced Chemiluminiscence kit were from GE Healthcare.
Immunocytochemistry
Cells were grown in chamber slides and fixed in 95% ethanol, 5% acetic acid for 10 min and permeabilized with 1% formaldehyde, 0.25% Triton X-100 in TBS for 5 min. Fixed cells were blocked with 3% BSA/TBS for 30 min. The primary antibodies were incubated at 1/50 dilution in blocking buffer overnight. The secondary anti-rabbit IgG-FITC antibody (Sigma, F0382) was used at 1/100 dilution in blocking buffer for 1 h.
Cytoplasm and nuclear fractionation
Cells were rinsed and scrapped off with cold PBS. Cell pellets were then resuspended and incubated for 10 min in cold hypotonic buffer (10 mM HEPES-KOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 1 mM PMSF). After brief vortexing and pelleting, the supernatant was kept as a cytosolic fraction. The pellet nuclear fraction was then resuspended and incubated for 20 min in cold high salt buffer (20 mM HEPES-KOH pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 1 mM PMSF). After spinning, the supernatant was kept as the nuclear fraction.
In-vitro p38 SAPK and Cdk2 kinase assay
GST–p38α SAPK was activated in vitro in a small volume (15 μl/assay) by mixing with GST–MKK6DD in 1 × kinase assay buffer (50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 2 mM DTT) in the presence of 100 μM cold ATP plus/minus 10 μM SB203580 (Calbiochem) for 20 min at 30°C. In all, 15 μl of the activated GST–p38 SAPK was used to phosphorylate in vitro either eluted GST-fused proteins or immunoprecipitates from mammalian expressed proteins. The reactions were carried in 1 × kinase assay buffer in the presence of 1 μCi/assay of radiolabelled 32P-γ-ATP (3000 Ci/mmol from Perkin-Elmer) in a final volume of 40 μl/assay for 20 min at 30°C. Cdk2 immunoprecipitates from MEF cell extracts were incubated in 1 × kinase assay buffer buffer in the presence of 50 μM cold ATP, 1 μCi/assay of radiolabelled 32P-γ-ATP (3000 Ci/mmol from Perkin-Elmer) and 4 μg of histone H1 (Roche Diagnostics) in a final volume of 40 μl/assay for 20 min at 30°C. Reactions were stopped by adding SB5X (250 mM Tris–HCl pH 6.8, 0.5 M DTT, 10% SDS, 20% glycerol, 0.5% Bromophenol Blue) and boiling at 100°C for 5 min. Phosphorylated proteins were subjected to PAGE–SDS and coomassie blue stained or transfer blotted onto a PVDF membrane and exposed to BIOMAX XAR films (KODAK). The incorporated radioactivity was quantified by phosphoimaging using a Typhoon 8600 apparatus and the ImageQuant software from Molecular Dynamics.
Activation of recombinant Cdk2 and inhibition of Cdk2-cyclinA by p57
Purified bacterially expressed GST–Cdk2 (200 ng/assay) was activated by incubation with GST–Cak1/Civ1 (100 ng/assay) in the presence of 100 μM cold ATP and followed by the addition of His–cyclinA (200 ng/assay) in 1 × kinase buffer. Active Cdk2–cyclinA complexes were then incubated with 10, 20 and 50 ng of either non-phosphorylated or in-vitro phosphorylated GST–p57, GST–p57T143A or GST–p27kip1 proteins for 1 h at 4°C. The recombinant complexes were then assayed for 15 min at 30°C in a final volume of 10 μl of kinase buffer (50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 2 mM DTT, 150 μM cold ATP) containing 2 μCi/assay of radiolabelled 32P-γ-ATP (3000 Ci/mmol) and 4 μg of histone H1. The reactions were stopped with sample buffer and analysed by SDS–PAGE, autoradiography and phosphoimaging.
Cell cycle, cell viability and nuclear condensation
Exponentially growing wild-type MEFs, p38α knockout MEFs and p57 knockout MEFs were stressed with 100 mM NaCl. One hour later, nocodozale (Sigma) was added to a final concentration of 100 ng/ml to trap the cells at the G2/M phase. DNA was labelled in vivo by incubating MEF cells with 8 μM of Hoechst 33342 (Sigma) for 1 h before being trypzinized and collected for cell-cycle FACS analysis. Cell viability upon osmostress was assessed by labelling living cells with 1 μg/ml PI (Sigma) for 10 min followed by FACS analysis. The stained cells were acquired on an LSR flow cytometer (Becton Dickinson) using the CellQuest software. Cell-cycle profiles and viability were then analysed with the WinMDI software. The MTT viability assay was assessed using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (Sigma). Cells were incubated with MTT (0.2 mg/ml) for 60 min at 37°C. The blue formazan derivative was solubilized in DMSO and the dual wavelength was measured at 560 and 620 nm in a BioRad plate reader. DNA nuclear condensation was evaluated by labelling the cells with 8 μM Hoechst 33342 for 60 min at 37°C. Nuclei was visualized in an Olympus CKX 41 fluorescent microscope using an excitation/emission wavelength of 350/460 nm. Cells with condensed and/or fragmented chromatin were quantified.
Supplementary Material
Acknowledgments
We thank Dr M Serrano (CNIO) for plasmids, cells and helpful discussions. L Subirana and S Obejas for technical support. I Ferreiro and EJ Ruiz were supported by PhD fellowships (FPI) from the MICINN (Spanish Government), and A Gubern by the ‘Juan de la Cierva’ program. This work was supported by the MICINN grant BFU2007-66503 and BFU2010-15839 to M Joaquin and BIO2009-07762, Consolider Ingenio 2010 programme (grant CSD2007-0015) and through contract No. ERAS-CT-2003-980409 of the European Commission, DG Research, FP6 as part of an EURYI scheme award and the Fundación Marcelino Botín to F Posas. F Posas and E de Nadal are recipient of an ICREA Acadèmia (Generalitat de Catalunya).
Author contributions: MJ, AG, DGN, IF and EJR performed the experiments presented in the manuscript. MJ, AG, EN, AR and FP have (1) designed the experiments, (2) contributed to the discussion and interpretation of the data and (3) written the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adrover M, Zi Z, Duch A, Schaber J, González-Novo A, Jimenez J, Nadal-Ribelles M, Clotet J, Klipp E, Posas F (2011) Time-dependent quantitative multicomponent control of the G1-S network by the stress-activated protein kinase Hog1 upon osmostress. Sci Signal 4: ra63. [DOI] [PubMed] [Google Scholar]
- Ambrosino C, Mace G, Galban S, Fritsch C, Vintersten K, Black E, Gorospe M, Nebreda AR (2003) Negative feedback regulation of MKK6 mRNA stability by p38alpha mitogen-activated protein kinase. Mol Cell Biol 23: 370–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino C, Nebreda AR (2001) Cell cycle regulation by p38 MAP kinases. Biol Cell 93: 47–51 [DOI] [PubMed] [Google Scholar]
- Besson A, Dowdy S, Roberts J (2008) CDK inhibitors: cell cycle regulators and beyond. Dev Cell 14: 159–169 [DOI] [PubMed] [Google Scholar]
- Borriello A, Caldarelli I, Bencivenga D, Criscuolo M, Cucciolla V, Tramontano A, Oliva A, Perrotta S, Della Ragione F (2011) p57(Kip2) and cancer: time for a critical appraisal. Mol Cancer Res 9: 1269–1284 [DOI] [PubMed] [Google Scholar]
- Brown N, Noble M, Endicott J, Garman E, Wakatsuki S, Mitchell E, Rasmussen B, Hunt T, Johnson L (1995) The crystal structure of cyclin A. Structure 3: 1235–1247 [DOI] [PubMed] [Google Scholar]
- Brown N, Noble M, Endicott J, Johnson L (1999) The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat Cell Biol 1: 438–443 [DOI] [PubMed] [Google Scholar]
- Bulavin D, Higashimoto Y, Popoff I, Gaarde W, Basrur V, Potapova O, Appella E, Fornace AJ (2001) Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411: 102–107 [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Fornace AJ (2004) p38 MAP kinase's emerging role as a tumor suppressor. Adv Cancer Res 92: 95–118 [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ (1999) Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18: 6845–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran S, Tan TX, Hall JR, Cook JG (2011) Stress-stimulated mitogen-activated protein kinases control the stability and activity of the Cdt1 DNA replication licensing factor. Mol Cell Biol 31: 4405–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TS, Kim MJ, Ryoo K, Park J, Eom SJ, Shim J, Nakayama KI, Nakayama K, Tomita M, Takahashi K, Lee MJ, Choi EJ (2003) p57KIP2 modulates stress-activated signaling by inhibiting c-Jun NH2-terminal kinase/stress-activated protein kinase. J Biol Chem 278: 48092–48098 [DOI] [PubMed] [Google Scholar]
- Chua PJ, Yip GW, Bay BH (2009) Cell cycle arrest induced by hydrogen peroxide is associated with modulation of oxidative stress related genes in breast cancer cells. Exp Biol Med (Maywood) 234: 1086–1094 [DOI] [PubMed] [Google Scholar]
- Clotet J, Escoté X, Adrover M, Yaakov G, Garí E, Aldea M, de Nadal E, Posas F (2006) Phosphorylation of Hsl1 by Hog1 leads to a G2 arrest essential for cell survival at high osmolarity. EMBO J 25: 2338–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado M, Gutierrez-Martinez P, Swat A, Nebreda A, Fernandez-Capetillo O (2009) p27Kip1 stabilization is essential for the maintenance of cell cycle arrest in response to DNA damage. Cancer Res 69: 8726–8732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E, Alepuz PM, Posas F (2002) Dealing with osmostress through MAP kinase activation. EMBO Rep 3: 735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E, Ammerer G, Posas F (2011) Controlling gene expression in response to stress. Nat Rev Genet 12: 833–845 [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, Bulavin D, Fornace AJ, Burg M (2002) Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. Proc Natl Acad Sci USA 99: 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzi DJ, Bjornsen AJ, MacKenzie T, Wyman TH, Silliman CC (2001) Ionomycin causes activation of p38 and p42/44 mitogen-activated protein kinases in human neutrophils. Am J Physiol Cell Physiol 281: C350–C360 [DOI] [PubMed] [Google Scholar]
- Escoté X, Zapater M, Clotet J, Posas F (2004) Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1. Nat Cell Biol 6: 997–1002 [DOI] [PubMed] [Google Scholar]
- Ferby I, Blazquez M, Palmer A, Eritja R, Nebreda A (1999) A novel p34(cdc2)-binding and activating protein that is necessary and sufficient to trigger G(2)/M progression in Xenopus oocytes. Genes Dev 13: 2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Dérijard B, Wu IH, Davis RJ (1994) An osmosensing signal transduction pathway in mammalian cells. Science 265: 806–808 [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808–811 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kohri K, Kaneko Y, Morisaki H, Kato T, Ikeda K, Nakanishi M (1998) Critical role for the 310 helix region of p57(Kip2) in cyclin-dependent kinase 2 inhibition and growth suppression. J Biol Chem 273: 16544–16550 [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Marceau F, Landry J (1997) Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res 80: 383–392 [DOI] [PubMed] [Google Scholar]
- Ishida N, Hara T, Kamura T, Yoshida M, Nakayama K, Nakayama K (2002) Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J Biol Chem 277: 14355–14358 [DOI] [PubMed] [Google Scholar]
- Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama K (2003) Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci USA 100: 10231–10236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Mercer S, Ewton D, Yan Z, Jin K, Friedman E (2002) The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem 277: 29792–29802 [DOI] [PubMed] [Google Scholar]
- Kossatz U, Vervoorts J, Nickeleit I, Sundberg H, Arthur J, Manns M, Malek N (2006) C-terminal phosphorylation controls the stability and function of p27kip1. EMBO J 25: 5159–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y, Nakayama K, Ishida N, Nakayama K (2005) Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation. J Biol Chem 280: 1095–1102 [DOI] [PubMed] [Google Scholar]
- Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda A (2009) p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol 29: 4341–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Reynisdóttir I, Massagué J (1995) Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev 9: 639–649 [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor M, Han K, Lee J, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland J (2002) PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8: 1153–1160 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30: 630–641 [DOI] [PubMed] [Google Scholar]
- Manke I, Nguyen A, Lim D, Stewart M, Elia A, Yaffe M (2005) MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell 17: 37–48 [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y, Nakayama K, Nakayama KI (2011) p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell 9: 262–271 [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ (1995) p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev 9: 650–662 [DOI] [PubMed] [Google Scholar]
- Orlow I, Iavarone A, Crider-Miller SJ, Bonilla F, Latres E, Lee MH, Gerald WL, Massagué J, Weissman BE, Cordón-Cardó C (1996) Cyclin-dependent kinase inhibitor p57KIP2 in soft tissue sarcomas and Wilms'tumors. Cancer Res 56: 1219–1221 [PubMed] [Google Scholar]
- Pateras IS, Apostolopoulou K, Niforou K, Kotsinas A, Gorgoulis VG (2009) p57KIP2: "Kip"ing the cell under control. Mol Cancer Res 7: 1902–1919 [DOI] [PubMed] [Google Scholar]
- Pedraza-Alva G, Koulnis M, Charland C, Thornton T, Clements J, Schlissel M, Rincón M (2006) Activation of p38 MAP kinase by DNA double-strand breaks in V(D)J recombination induces a G2/M cell cycle checkpoint. EMBO J 25: 763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt H, Aslanian A, Lees J, Yaffe M (2007) p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 11: 175–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto C, Delphin C, Deloulme JC, Baudier J (1999) Concerted regulation of wild-type p53 nuclear accumulation and activation by S100B and calcium-dependent protein kinase C. Mol Cell Biol 19: 7168–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin I, Yakes F, Rojo F, Shin N, Bakin A, Baselga J, Arteaga C (2002) PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med 8: 1145–1152 [DOI] [PubMed] [Google Scholar]
- Sinha RP, Häder DP (2002) UV-induced DNA damage and repair: a review. Photochem Photobiol Sci 1: 225–236 [DOI] [PubMed] [Google Scholar]
- Swat A, Dolado I, Rojas JM, Nebreda AR (2009) Cell density-dependent inhibition of epidermal growth factor receptor signaling by p38alpha mitogen-activated protein kinase via Sprouty2 downregulation. Mol Cell Biol 29: 3332–3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesio M, Trumpp A (2011) Breaking the cell cycle of HSCs by p57 and friends. Cell Stem Cell 9: 187–192 [DOI] [PubMed] [Google Scholar]
- Todaro G, Green H (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17: 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DE, Densham RM, Molton SA, Balmanno K, Newson C, Weston CR, Garner AP, Scott L, Cook SJ (2004) ERK1/2 and p38 cooperate to induce a p21CIP1-dependent G1 cell cycle arrest. Oncogene 23: 3284–3295 [DOI] [PubMed] [Google Scholar]
- Tsvetkov L, Yeh K, Lee S, Sun H, Zhang H (1999) p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 9: 661–664 [DOI] [PubMed] [Google Scholar]
- Vidal A, Koff A (2000) Cell-cycle inhibitors: three families united by a common cause. Gene 247: 1–15 [DOI] [PubMed] [Google Scholar]
- Yaakov G, Duch A, García-Rubio M, Clotet J, Jimenez J, Aguilera A, Posas F (2009) The stress-activated protein kinase Hog1 mediates S phase delay in response to osmostress. Mol Biol Cell 20: 3572–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Frisén J, Lee MH, Massagué J, Barbacid M (1997) Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev 11: 973–983 [DOI] [PubMed] [Google Scholar]
- Zhang P, Liégeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ (1997) Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387: 151–158 [DOI] [PubMed] [Google Scholar]
- Zou P, Yoshihara H, Hosokawa K, Tai I, Shinmyozu K, Tsukahara F, Maru Y, Nakayama K, Nakayama KI, Suda T (2011) p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell 9: 247–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.