Abstract
Staphylococcus aureus small colony variants (SCVs), which are characterized by slow growth and a range of morphological and metabolic changes including altered antibiotic resistance profiles, have been studied for several decades. This Closeup highlights findings described in this issue of EMBO Molecular Medicine by Tuchscherr et al (2011), who present strong evidence that SCVs can arise in chronic infection models when S. aureus is internalized in non-professional phagocytes and survives intracellularly. As the intracellular residency time increases, the proportion of SCVs grows and the host cell inflammatory response diminishes. The study suggests that this mode of phenotype switching is an essential feature of the S. aureus infection process and can explain an underlying cause of chronic and relapsing infections.
See related article in EMBO Mol Med (Tuchscherr et al (2011) EMBO Mol Med 3: 129-141)
Keywords: infection, intracellular, phenotype switch, SCV, Staphylococcus
Since the mid-1940s, the encounter of Staphylococcus aureus with antibiotics has been known to evoke sub-populations of persister cells, which help the overall population to survive. Persister cells represent a metabolically dormant state that is not induced by a heritable genetic change. Indeed, following removal of the antibiotic and sub-cultivation of the persister cells, they demonstrate antibiotic susceptibility indicating that they are clearly a phenotypic population variant. This survival strategy, widespread in bacteria, helps to account for chronic relapsing infections since complete therapeutic eradication is precluded (see Lewis, 2007, for review).
The study reported by Tuchscherr et al (2011) in this issue now elegantly illustrates a related S. aureus survival strategy: the induction of reversible phenotype switching that can occur following bacterial internalization and facilitates intracellular survival. Instead of classic persister cells induced by antibiotic encounter, a type of metabolically dormant cell, termed SCV, can arise during prolonged residence inside host cells. Although some recovered SCVs harbour heritable auxotrophies for biosynthetic defects in electron transport chain co-factors or thymidine biosynthesis, a large proportion of recovered SCVs are unstable and can revert to the wild-type phenotype—a feature remarkably similar to persister cells. The capacity to interconvert by this mode of phenotype switching permits S. aureus to alter its virulence potential rapidly (Fig 1A). Importantly, the study emphasizes how intracellular survival and phenotypic switching defines a powerful immune escape mechanism and an alternative route to explain chronic infection.
Figure 1. Intracellular phenotype switching to SCV and immune evasion of S. aureus.
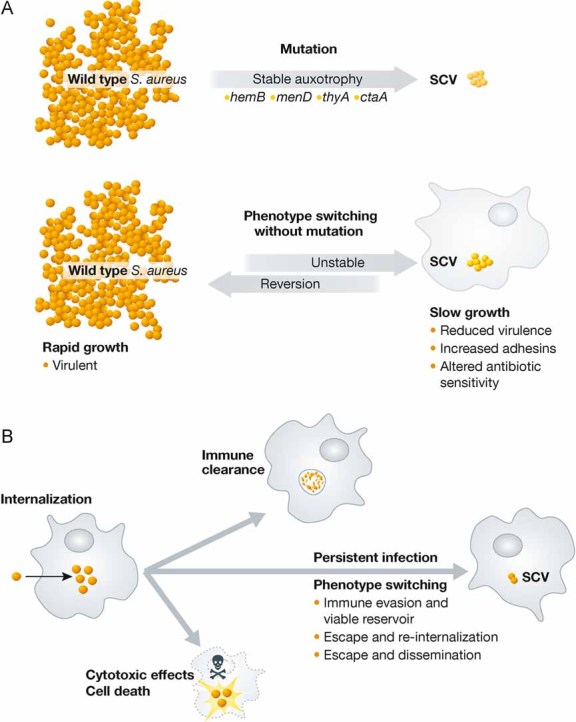
- Different routes to SCV formation. Stable SCVs are caused by a variety of mutations in certain bacterial genes leading to auxotrophy (upper panel). Phenotype switching without mutations predominantly results in unstable SCVs during intracellular growth as shown in the Tuchscherr study. SCVs display reduced virulence and altered antibiotic sensitivity; unstable SCVs arising from phenotype switching can rapidly revert to the wild type form with increased virulence and rapid growth (lower panel).
- Possible outcomes after intracellular S. aureus infection. After internalization into the host cell, not all bacteria undergo phenotype switching. S. aureus can be cleared, it can cause cell death or it can undergo a phenotype switch and cause a persistent infection. The phenotype switch to SCVs allows S. aureus to evade the immune response and leads to the formation of a viable reservoir of bacteria from which the bacteria can escape and disseminate or re-infect the host.
The Tuchscherr study builds upon two main avenues of S. aureus basic research: considerable prior knowledge of SCV genetics and physiology and the mounting evidence that S. aureus can invade and survive for several weeks within a wide range of cell types that are not specialized professional phagocytes.
SCVs are broadly defined as slow growing colonies with diameters roughly 1/10 the parental strain when cultivated on agar plates. In S. aureus, SCVs have been shown to have enhanced surface expression of adhesins, particularly fibronectin binding proteins (FnBPs) which aid internalization and decreased expression of exotoxins, which diminishes cytotoxicity (see Sendi et al, 2009, for review). Of particular importance are their decreased susceptibility to antibiotics and the absence of routine testing in clinical samples to detect their presence. These characteristics of SCVs, coupled with their capacity to revert to more rapidly growing forms, renders them ideal candidates to provoke persistent human infections. Several decades of research now attest to their likely involvement in disease pathology (Proctor et al, 2006).
S. aureus has not been traditionally thought of as an intracellular pathogen, although considerable evidence now exists that demonstrates its ability to enter a wide range of cell types (Garzoni et al, 2009). Host cell invasion is primarily dependent upon surface expression of S. aureus FnBPs (encoded by fnbA and fnbB), which engage host cell fibronectin and integrins. Uptake is mediated by an endocytic process mediated by F-actin. Once inside cells, the fate of internalized S. aureus can vary from rapid clearance to persistence from several days-to-weeks (Fig 1B). The presence of cytotoxic effects and the nature of the sub-cellular localization of internalized S. aureus is highly strain dependent, difficult to predict and renders comparison of published studies difficult.
S. aureus already is endowed with many features (e.g. the ability to promote asymptomatic nasal colonization of a large fraction of the world's population, resistance to lysozyme and innate immune defence strategies, toxin production and multiple drug resistance to name a few) that help define it as a formidable human pathogen causing relatively mild skin infections to life-threatening, invasive disease. A central question raised in the Tuchscherr study is whether the generation of SCVs is also an intrinsic feature of S. aureus biology and, therefore, an important key to understanding persistent and relapsing infection.
To address this question, the study employed both in vitro (cell culture using both cell lines and primary cells) and in vivo (tissue homogenates from murine haematogenous bacteraemia and human tissue samples from patients with chronic S. aureus infection) experimental systems. Viable bacteria could be recovered from A549 human lung adenocarcinoma cells for at least 4 weeks, whereas, viable bacteria could be recovered from primary cells such as osteoblasts or human umbilical vein endothelial cells (HUVEC), for up to 1 week. By monitoring the fraction of SCVs recovered as well as simultaneously measuring acute inflammatory cytokines (CCR5/RANTES or CXCL11/I-TAC) or ICAM-1/CD54 levels as a cytokine-driven inflammatory marker, a striking temporal snapshot of the infection process emerged. First, the proportion of SCVs recovered increased with increasing intracellular residence time. Secondly, as markers of an acute inflammatory response diminished to pre-infection control levels, viable intracellular bacteria still persisted. The authors concluded from this correlation that phenotype switching occurred to attenuate activation of the innate immune response.
. . .a striking temporal snapshot of the infection process emerged.
One of the strengths of the study was using quantitative RNA analysis over the same infection time course to examine the expression of a handful of S. aureus virulence factor genes (hla, encoding α-toxin, and fnbA) and the master regulator operon, agr (accessory gene regulator)—a sensory system in S. aureus that responds to population density and controls a large number of virulence factor genes. For review see Novick, 2003. The results clearly showed diminished hla and agrA levels over time suggesting reduced virulence, whereas, fnbA levels steadily increased, indicating the likely acquisition of an enhanced capacity for invasion/re-uptake in the event intracellular bacteria escaped.
Building on the knowledge of the transcription signature profiles derived from cell culture models of infection, the authors next examined RNA expression in tissue samples—firstly, a mouse haematogenous bacteraemia model of infection and secondly, human tissue samples derived from patients with chronic S. aureus infections. In both cases, the temporal RNA expression profiles obtained during the course of infection were strikingly similar to those obtained in cell culture models. It is important to stress that recovery of viable S. aureus from tissue homogenates by no means constitutes a proof of the existence of intracellular S. aureus in these in vivo experiments, but nevertheless it is striking that the RNA profile obtained from cell culture model systems, where it is relatively easy to demonstrate intracellular S. aureus, is concordant with profiles obtained from infected tissue samples. Assays for inflammatory cytokines in the mouse model provided additional concordant evidence that by the time the recovered SCV fraction was highest, acute inflammatory markers had returned to pre-infection control levels.
Taken together, these results demonstrate that S. aureus dynamically alters its agr and virulence factor expression to bypass innate immune defences and establish conditions favouring prolonged intracellular persistence. The phenotypic switching of S. aureus in these experiments constitutes strong evidence that the formation of SCVs is indeed an essential feature of the S. aureus life cycle and that formation of SCVs in an intracellular environment is an underlying cause of chronic infection and prolonged asymptomatic intervals.
According to ancient legend, Odysseus' clever ruse to hide armed men within a wooden horse in order to gain access to a strongly defended fortification ultimately culminated in the fall of Troy and brought victory for the Greeks. If we accept that S. aureus can hide intracellularly and easily change its phenotype to evade immune surveillance, then S. aureus has clearly learned from the Trojan horse. By military analogy, adequate intelligence of enemy plans can, in principle, help to construct appropriate countermeasures. What can be done to thwart the devious plans of this insidious pathogen knowing that it can hide intracellularly?
There are few intracellular antibiotics effective against S. aureus, with the exception of rifampicin, to which bacteria can acquire rapid resistance by point mutation if used in monotherapy. If the proportion of SCVs increases with intracellular residence time as this study shows, their decreased susceptibility to a range of antibiotics presents an additional problem. For the future, the authors advocate new pharmacotherapy research aiming to block phenotype switching which clearly has the projected benefits of reducing the formation of SCVs and perhaps concomitantly eradicating the formation of an intracellular reservoir.
Raising the awareness that S. aureus can provoke persistent infection by residing intracellularly, even transiently, requires consideration of those patients at risk for this type of infection—for example those with chronic osteomyelitis or endocarditis. Obtaining knowledge of conditions, which trigger phenotype switching and favour reactivation/egress of internalized bacteria to seed subsequent infection rounds, should be vigorously pursued. Definitive proof that intracellular S. aureus can cause human disease is at the limits of the technically feasible and hotly debated. The present study has taken a giant step to convince us of this possibility, and unlike King Priam of Troy, we would be wise to heed counsel of those who suspected a plot. The alternative is to suffer the consequences.
Acknowledgments
The authors declare that they have no conflict of interest.
References
- Garzoni C, et al. Trends Microbiol. 2009;17:59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Lewis K. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- Novick RP. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- Proctor RA, et al. Nat Rev Microbiol. 2006;4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- Sendi P, et al. Trends Microbiol. 2009;17:54–58. doi: 10.1016/j.tim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Tuchscherr L, et al. EMBO Mol Med. 2011 doi: 10.1002/emmm.201000115. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]


