Abstract
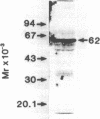
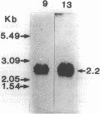
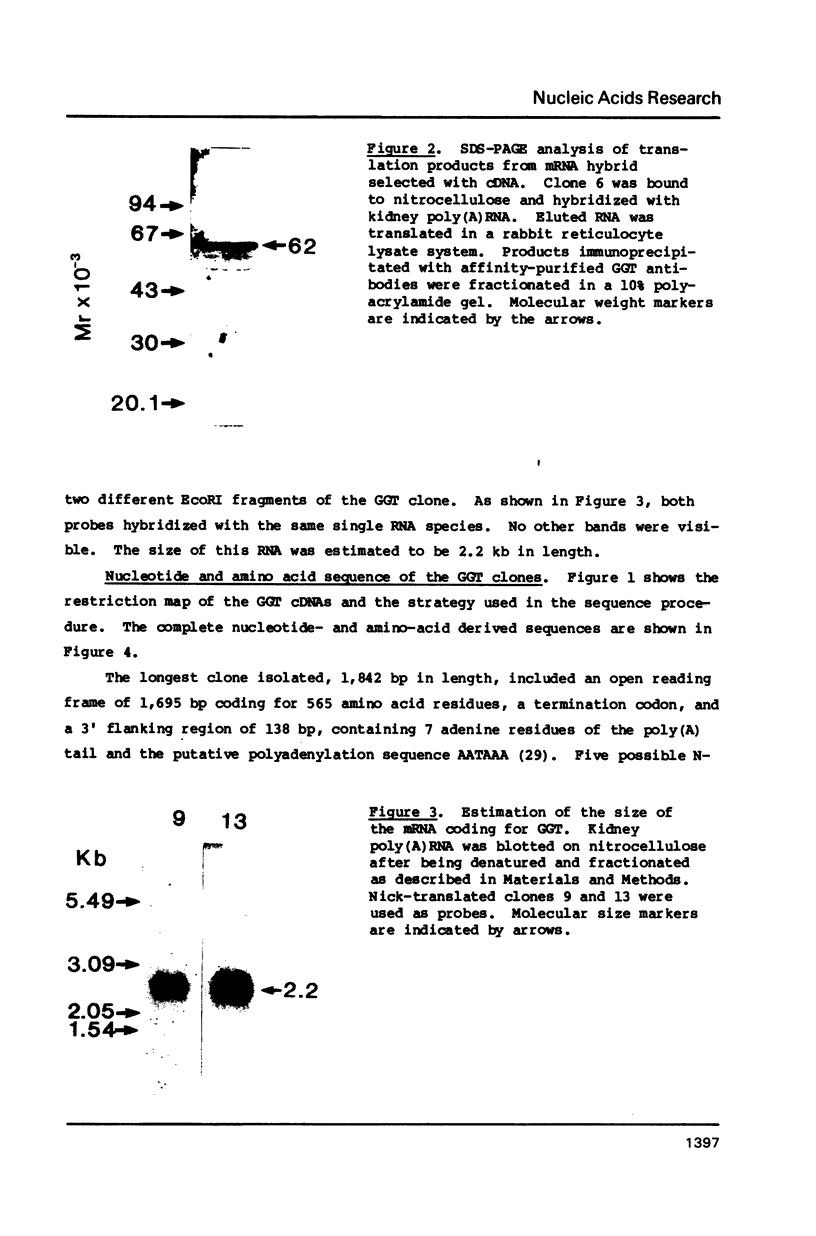
We have isolated several cDNA's complementary to gamma-glutamyltranspeptidase (GGT) mRNA by screening a rat kidney library constructed in lambda gtll with antibodies specifically reactive to the enzyme protein. The clone selected an mRNA that was translated into a 62 Kd peptide, corresponding to the GGT precursor. The longest clone isolated was 1842 bp long with an open reading frame coding for 565 amino acids. The length of the mRNA coding for GGT was estimated to be 2.2 kb long. The amino acid sequence derived from the nucleotide sequence matched the short sequences determined by us as well as by other authors.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. D., Meister A. Evidence that transpeptidation is a significant function of gamma-glutamyl transpeptidase. J Biol Chem. 1981 Mar 25;256(6):2988–2992. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Capraro M. A., Hughey R. P. Processing of the propeptide form of rat renal gamma-glutamyltranspeptidase. FEBS Lett. 1983 Jun 27;157(1):139–143. doi: 10.1016/0014-5793(83)81132-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P. Role of gamma-glutamyltranspeptidase in the renal metabolism of glutathione. Miner Electrolyte Metab. 1983;9(4-6):236–245. [PubMed] [Google Scholar]
- Das N. D., Shichi H. Tissue difference in gamma-glutamyl transpeptidase attributed to sialic acid content. Life Sci. 1979 Nov 19;25(21):1821–1827. doi: 10.1016/0024-3205(79)90429-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hanigan M. H., Pitot H. C. Gamma-glutamyl transpeptidase--its role in hepatocarcinogenesis. Carcinogenesis. 1985 Feb;6(2):165–172. doi: 10.1093/carcin/6.2.165. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Brew K., Grant G. A., Bradshaw R. A., Lennarz W. J. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. Studies with natural and synthetic peptides. J Biol Chem. 1979 Oct 10;254(19):9747–9753. [PubMed] [Google Scholar]
- Huseby N. E. Multiple forms of serum gamma-glutamyltransferase. Association of the enzyme with lipoproteins. Clin Chim Acta. 1982 Sep 1;124(1):103–112. doi: 10.1016/0009-8981(82)90324-2. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. I. Correlation between sialylation and isozymic forms. J Biochem. 1980 Apr;87(4):1243–1248. [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. III. Evidence that the amino terminus of the heavy subunit is the membrane binding segment. J Biochem. 1983 May;93(5):1427–1433. doi: 10.1093/oxfordjournals.jbchem.a134278. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. III. Evidence that the amino terminus of the heavy subunit is the membrane binding segment. J Biochem. 1983 May;93(5):1427–1433. doi: 10.1093/oxfordjournals.jbchem.a134278. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Kuno T., Katunuma N. Biosynthesis and degradation of gamma-glutamyltranspeptidase of rat kidney. J Biochem. 1983 Sep;94(3):755–765. doi: 10.1093/oxfordjournals.jbchem.a134416. [DOI] [PubMed] [Google Scholar]
- Mueckler M. M., Himeno M., Pitot H. C. In vitro synthesis and processing of a precursor to ornithine aminotransferase. J Biol Chem. 1982 Jun 25;257(12):7178–7180. [PubMed] [Google Scholar]
- Nakamura Y., Azuma T., Fukuyama H., Suzuki F., Nagata Y. Active site of intestinal gamma-glutamyltransferase faces luminal side of brush border membrane. J Nutr Sci Vitaminol (Tokyo) 1985 Apr;31(2):179–187. doi: 10.3177/jnsv.31.179. [DOI] [PubMed] [Google Scholar]
- Nash B., Tate S. S. Biosynthesis of rat renal gamma-glutamyl transpeptidase. Evidence for a common precursor of the two subunits. J Biol Chem. 1982 Jan 25;257(2):585–588. [PubMed] [Google Scholar]
- Nash B., Tate S. S. In vitro translation and processing of rat kidney gamma-glutamyl transpeptidase. J Biol Chem. 1984 Jan 10;259(1):678–685. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Reyes E., Barela T. D. Isolation and purification of multiple forms of gamma-glutamyl transpeptidase from rat brain. Neurochem Res. 1980 Feb;5(2):159–170. doi: 10.1007/BF00964329. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Matsuda Y., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. II. Location of the segment anchoring gamma-glutamyltranspeptidase to the membrane. J Biochem. 1980 Jun;87(6):1567–1571. doi: 10.1093/oxfordjournals.jbchem.a132898. [DOI] [PubMed] [Google Scholar]
- Vanderlaan M., Phares W. gamma-Glutamyltranspeptidase: a tumour cell marker with a pharmacological function. Histochem J. 1981 Sep;13(5):865–877. doi: 10.1007/BF01003295. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Mizumoto K., Kaziro Y. Limited tryptic digestion of messenger RNA capping enzyme from Artemia salina. Isolation of domains for guanylyltransferase and RNA 5'-triphosphatase. J Biol Chem. 1984 Apr 25;259(8):4695–4698. [PubMed] [Google Scholar]
- Yamashita K., Hitoi A., Tateishi N., Higashi T., Sakamoto Y., Kobata A. Organ-specific difference in the sugar chains of gamma-glutamyltranspeptidase. Arch Biochem Biophys. 1983 Sep;225(2):993–996. doi: 10.1016/0003-9861(83)90116-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]