Abstract
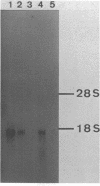
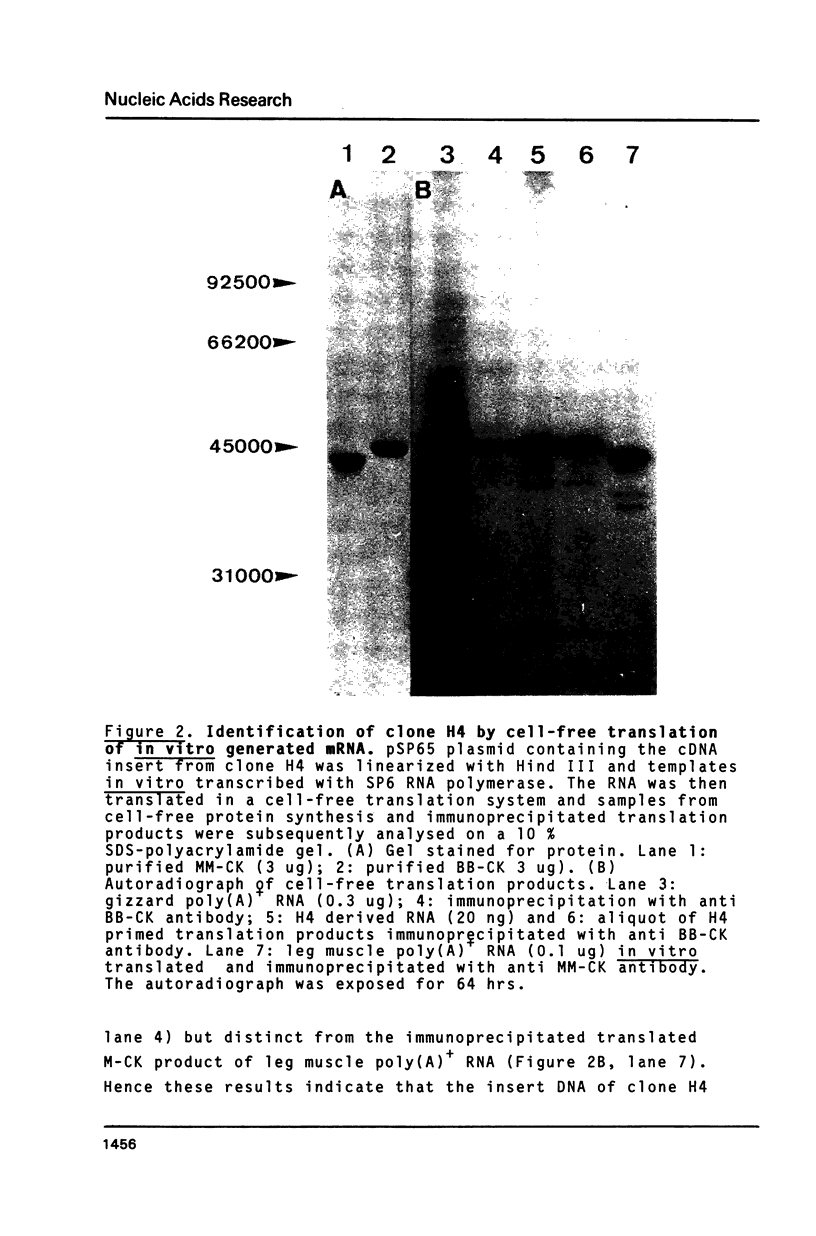
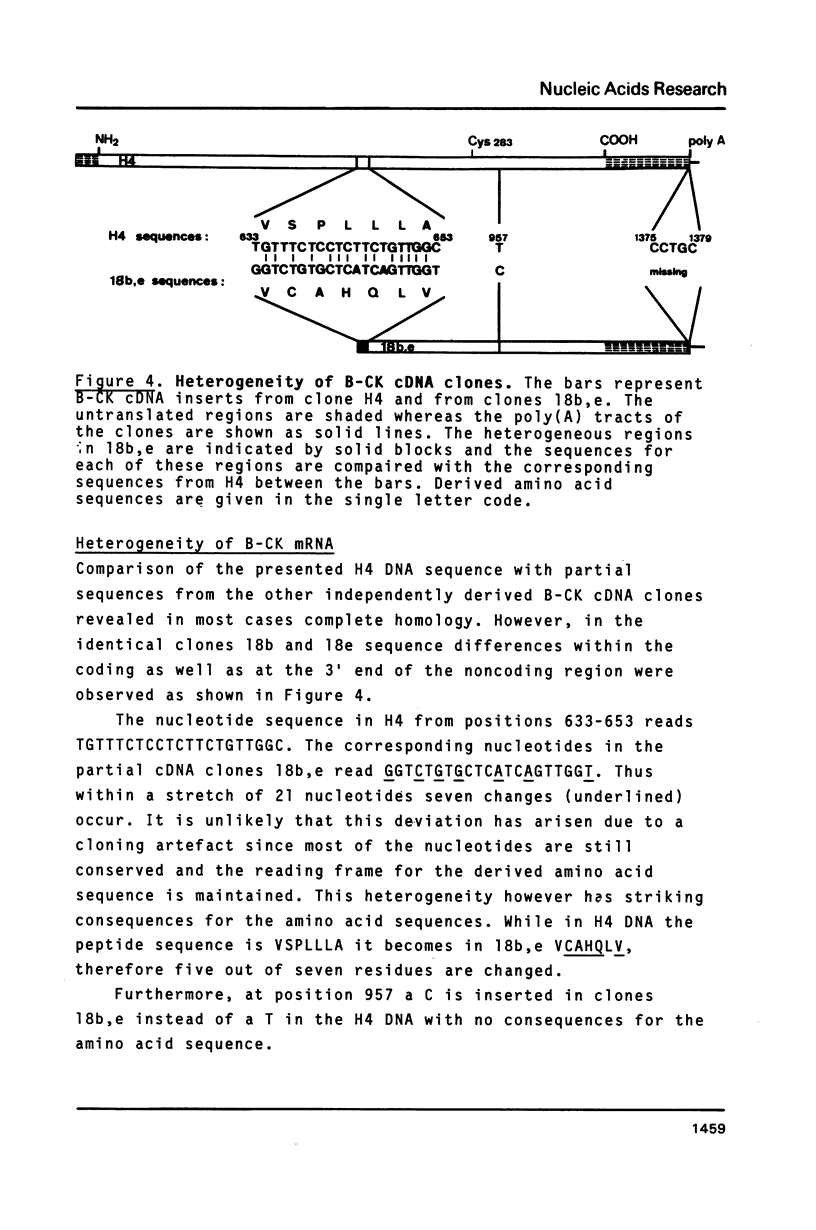
cDNA clones for chicken B-CK were isolated by immunoscreening from a gizzard cDNA library constructed in the expression vector lambda gtll. The entire coding portion in addition to the complete 3' untranslated region and 42 bp of the 5' noncoding part are represented in the clone H4. On RNA blots H4 insert DNA hybridized to a 1600 bp poly(A)+ RNA from gizzard, brain and heart but not to breast or skeletal muscle RNA. In vitro generated sense strand transcripts of H4 insert DNA were translated in vitro into a protein indistinguishable from isolated, authentic B-CK. The distinct nucleotide sequences of H4 insert DNA and M-CK cDNA were translated into 82% homologous amino acid sequences. Sequence heterogeneity among the B-CK cDNA clones within both the 3' noncoding and even in the coding region indicates the existence of multiple B-CK mRNA species.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravatti M., Perriard J. C., Eppenberger H. M. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Biosynthesis of creatine kinase subunits M and B. J Biol Chem. 1979 Feb 25;254(4):1388–1394. [PubMed] [Google Scholar]
- Dawson D. M., Eppenberger H. M., Kaplan N. O. The comparative enzymology of creatine kinases. II. Physical and chemical properties. J Biol Chem. 1967 Jan 25;242(2):210–217. [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Sefton B. M., Hunter T., Walter G., Singer S. J. Immunofluorescent localization of the transforming protein of Rous sarcoma virus with antibodies against a synthetic src peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5322–5326. doi: 10.1073/pnas.79.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordahl C. P., Evans G. L., Cooper T. A., Kunz G., Perriard J. C. Complete cDNA-derived amino acid sequence of chick muscle creatine kinase. J Biol Chem. 1984 Dec 25;259(24):15224–15227. [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Levels of mRNA for creatine kinase subunits M and B. J Biol Chem. 1979 Aug 10;254(15):7036–7041. [PubMed] [Google Scholar]
- Perriard J. C., Perriard E. R., Eppenberger H. M. Detection and relative quantitation of mRNA for creatine kinase isoenzymes in mRNA from myogenic cell cultures and embryonic chicken tissues. J Biol Chem. 1978 Sep 25;253(18):6529–6535. [PubMed] [Google Scholar]
- Pickering L., Pang H., Biemann K., Munro H., Schimmel P. Two tissue-specific isozymes of creatine kinase have closely matched amino acid sequences. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2310–2314. doi: 10.1073/pnas.82.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg U. B., Eppenberger H. M., Perriard J. C. Occurrence of heterogenous forms of the subunits of creatine kinase in various muscle and nonmuscle tissues and their behaviour during myogenesis. Eur J Biochem. 1981 May;116(1):87–92. doi: 10.1111/j.1432-1033.1981.tb05304.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Kunz G., Frischauf A., Lehrach H., Mähr R., Eppenberger H. M., Perriard J. C. Molecular cloning and expression during myogenesis of sequences coding for M-creatine kinase. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6589–6592. doi: 10.1073/pnas.79.21.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- THOMSON A. R., EVELEIGH J. W., MILES B. J. AMINO-ACID SEQUENCE AROUND THE REACTIVE THIOL GROUPS OF ADENOSINE TRIPHOSPHATE--CREATINE PHOSPHOTRANSFERASE. Nature. 1964 Jul 18;203:267–269. doi: 10.1038/203267a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Metabolite channeling: a phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell. 1985 May;41(1):325–334. doi: 10.1016/0092-8674(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Gmür R., Lebherz H. G., Siegrist M., Wallimann T., Eppenberger H. M. Differentiation in cultures derived from embryonic chicken muscle. II. Phosphorylase histochemistry and fluorescent antibody staining for creatin kinase and aldolase. Dev Biol. 1976 Feb;48(2):284–307. doi: 10.1016/0012-1606(76)90091-9. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Maier V., Eppenberger H. M. Creatine kinase and aldolase isoenzyme transitions in cultures of chick skeletal muscle cells. Dev Biol. 1974 Mar;37(1):63–89. doi: 10.1016/0012-1606(74)90170-5. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Eppenberger H. M. Localization and function of M-line-bound creatine kinase. M-band model and creatine phosphate shuttle. Cell Muscle Motil. 1985;6:239–285. doi: 10.1007/978-1-4757-4723-2_8. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]