Abstract
Microbial translocation has been linked to systemic immune activation in HIV-1 disease, yet mechanisms by which microbes may contribute to HIV-associated intestinal pathogenesis are poorly understood. Importantly, our understanding of the impact of translocating commensal intestinal bacteria on mucosal-associated T cell responses in the context of ongoing viral replication that occurs early in HIV-1 infection is limited. We previously identified commensal Escherichia coli-reactive T helper (Th)1 and Th17 cells in normal human intestinal lamina propria (LP). Here, we established an ex vivo assay to investigate the interactions between Th cell subsets in primary human LP mononuclear cells (LPMC), commensal E. coli and CCR5-tropic HIV-1Bal. Addition of heat-killed E. coli to HIV-1-exposed LPMC resulted in increases in HIV-1 replication, CD4 T cell activation and infection, and IL-17 and IFN-γ production. Conversely, purified LPS derived from commensal E. coli did not enhance CD4 T cell infection. E. coli exposure induced greater proliferation of LPMC Th17 than Th1 cells. Th17 cells were more permissive to infection than Th1 cells in HIV-1-exposed LPMC cultures, and Th17 cell infection frequencies significantly increased in the presence of E. coli. The E. coli-associated enhancement of infection was dependent on the presence of CD11c+ LP dendritic cells and, in part, dependent on MHC Class II-restricted antigen presentation. These results highlight a potential role for translocating microbes in impacting mucosal HIV-1 pathogenesis during early infection by increasing HIV-1 replication and infection of intestinal Th1 and Th17 cells.
INTRODUCTION
One of the hallmarks of progressive HIV-1 disease is a gradual depletion of peripheral blood CD4 T cells. However, HIV infection is also characterized by a significant assault to the gastrointestinal (GI) tract resulting in a myriad of structural and immunological complications. The impact of HIV on the GI tract has been observed since the early days of research into the epidemic when HIV infection was associated with GI abnormalities such as diarrhea, weight loss, malnutrition, malabsorption and villous atrophy (1). Recent studies have highlighted the central role the intestinal mucosal immune system plays in HIV-1 pathogenesis. Studies using a pathogenic SIV infection model demonstrated that significant and rapid depletion of intestinal CD4 T cells occurred as early as 7 days post infection (2–4) and was associated with early epithelial barrier dysfunction and high levels of viral replication (2, 4). Early in vitro studies demonstrated that human lamina propria (LP) CD4 T cells were also naturally permissive to HIV-1 infection and, unlike peripheral blood CD4 T cells, required no prior stimulation for productive infection (5). Indeed, greater than 50% of human intestinal CD4 T cells are depleted during acute and early HIV infection, and significant depletion of these cells has been noted throughout all stages of HIV-1 disease (6–9). The susceptibility of LP T cells to HIV-1 or SIV infection at steady state likely relates to their increased activation status and expression of HIV/SIV co-receptors such as CCR5 and α4β7 (10–14). Depletion occurs directly through lysis of infected cells (4, 15, 16) and indirectly through apoptosis of infected and uninfected CD4 T cells (15). With respect to HIV infection, higher HIV viral DNA and RNA levels were observed within GI tract CD4 T cells compared to peripheral blood CD4 T cells from subjects during acute and early infection, with infection detected in both activated and non-activated mucosal CD4 T cells (9). In a recent study, d’Ettorre et al demonstrated that in chronically HIV-infected, untreated donors, HIV DNA load was greater in the gut mucosa than in peripheral blood (17).
Human T helper (Th) 17 cells are a subset of CD4 T cells that have been shown to produce IL-17A, IL-17F, IL-22 and IL-26 (18) and to play an important role in both mucosal defense against extracellular bacterial and fungal pathogens and in epithelial barrier maintenance and regeneration (19). Thus, the loss of these cells would be expected to have an impact on both intestinal homeostasis and immunity. A number of recent studies have highlighted that the specific depletion of Th17 cells is associated with disease progression both in SIV and HIV infections (20–24). Indeed, the preservation of Th17 cells during chronic SIV infection of sooty mangabeys has been associated with the non-pathogenic phenotype of this natural host of SIV (20).
HIV/SIV-associated epithelial barrier dysfunction and CD4 T cell depletion, in particular Th17 cell depletion, may contribute to reduced protection from microbial products translocating from the lumen into the LP and into the systemic circulation. Using a model of intestinal inoculation of Salmonella typhimurium during acute SIV-infection of rhesus macaques, Raffatellu et al observed depletion of Th17 cells, a decrease in epithelial barrier integrity and the dissemination of S. typhimurium (25). In a recent study, Estes et al demonstrated the presence of not only microbial products such as LPS, but also Escherichia coli, in colonic LP and in lymph nodes of chronically infected rhesus macaques providing more direct evidence for the occurrence of gut-associated microbial translocation in SIV infection (26). Indirect evidence for this phenomenon occurring in HIV-1 infection has also been demonstrated. Increased levels of microbial products were observed in the circulation of HIV-1-infected individuals and were found to be associated with T cell activation (27, 28).
Epithelial barrier dysfunction is known to occur early in SIV and HIV disease (2, 29–31) in association with microbial translocation (26). However, the mechanisms by which translocated microbes induce mucosal immune dysfunction and impact viral replication remain poorly understood. We have previously identified significant frequencies of human LP effector CD4 T cells that produced IFN-γ and IL-17 in response to commensal bacteria in normal human intestinal tissue (32). The in vitro expansion of these bacteria reactive T cells was dependent upon the presence of a subset of LP dendritic cells. We hypothesized that in the setting of HIV infection and a ‘leaky’ gut barrier, LP mononuclear cells (LPMC) would have increased exposure to bacteria and bacterial products. This, in turn, would lead to the activation of bacteria-reactive T cells, increasing their permissiveness to HIV infection and replication. Using an in vitro assay that mimics the early interactions between HIV-1, commensal bacteria and primary LPMC, we present evidence to suggest that IL-17-producing intestinal CD4 T cells are not only preferentially infected, but that productive infection is further enhanced in the presence of commensal E. coli. These results provide a mechanism for how microbial translocation may contribute to increased viral replication and CD4 T cell depletion in mucosal tissue.
MATERIALS AND METHODS
Tissue samples and preparation of LPMC
Human intestinal tissue samples (n=10 jejunums, n=11 colons) were obtained from patients undergoing elective abdominal surgery, represent otherwise discarded tissue and were considered macroscopically normal as previously described (32, 33). All patients undergoing surgery signed a release to allow the unrestricted use of discarded tissues for research purposes, and all protected patient information was de-identified to the laboratory investigators. This research was reviewed by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Anschutz Medical Campus and was granted exempt research status. LPMC were isolated from tissue samples and released LPMC were cryopreserved and stored in liquid nitrogen as detailed elsewhere (32, 33). Within all LPMC samples (n=21), the median percentage of total viable cells was 75.5% (range 30.6–96.6%) and the median percentage of viable, CD45+ LPMC was 86.6% (range 54.5–97.7%), as assessed using flow cytometry staining protocols. Percentages of T cells as a fraction of viable, CD45+ LPMC were evaluated in the majority of samples: CD3+ T cells (76.4%, 53.7–95.2%; n=20), CD4+ T cells (61.0%, 43.4–75.2%; n=18) and CD8+ T cells (12.2%, 4.8–41.4%).
Preparation of PBMC
Peripheral blood samples were collected from healthy adults, self-identifying as HIV-1 negative, who voluntarily gave written informed consent to participate. Collection of blood samples was approved by the Colorado Institutional Review Board (COMIRB) at the University of Colorado Anschutz Medical Campus. PB mononuclear cells (PBMC) were isolated from heparinized blood by standard Ficoll-Hypaque (Amersham Biosciences, Pacataway, NJ) density gradient centrifugation, as described previously (34) and were cryopreserved and stored in liquid nitrogen as detailed elsewhere (32, 33).
Surface and Intracellular Flow Cytometry Staining (IFC) assays
Standard flow-cytometry staining protocols for surface markers and for intracellular cytokine or HIV-1 p24 expression are detailed elsewhere (32, 33, 35). CD45 (PerCp-Cy5.5; eBioscience, San Diego, CA), CD3 (FITC, BD Biosciences, San Jose, CA; PE-Texas Red, ECD, Beckman Coulter, Fullerton, CA), CD4 (APC, APC-Cy7, AF700, all BD Biosciences) and CD8 (APC, FITC, both BD Biosciences; AF405, Invitrogen, Carlsbad, CA). PE-Cy5 CD38 and a matched isotype control (BD Biosciences) were used to evaluate T cell activation. Intracellular cytokines were detected using the following: V450 IL-17 and AF700 IFN-γ with V450 mouse IgG1 and AF700 mouse IgG1 used as isotype controls (all BD Biosciences). Intracellular HIV-1 p24 was detected using PE (RD1) KC57 (specific for the 55, 39, 33 and 24kD proteins of the core antigens of HIV-1) and PE (RD1) mouse IgG1 as a matched isotype control (both Beckman Coulter). To evaluate CD11c and CD19 percentages in total LPMC and in CD11c/CD19-depleted LPMC, the following antibodies were used: PerCp-Cy5.5 CD45, PE-Cy5 CD11c and APC-H7 CD19 (both from BD Biosciences). In all staining protocols, a Live/Dead Fixable Dead Cell Stain (Invitrogen) was included. At the completion of the staining procedure, LPMC were resuspended in 1% paraformaldehyde (Sigma-Aldrich, St Louis, MO) prior to acquisition on a LSRII Flow Cytometer (BD Biosciences).
Commensal heat-killed E. coli and B. fragilis stocks and CCR5-tropic HIV-1Bal stock
E. coli stocks (#25922, ATCC, Manassass, VA) were expanded overnight in RPMI 1640 + 10% FBS at 37°C, 5% CO2 or were plated on Brain Heart Infusion agar (BD Diagnostics, Sparks, MD) and incubated at 37°C for 2 days. B. fragilis stocks (#25285, ATCC) were expanded by culturing on Brucella plates (BD Diagnostics) and incubated at 37°C for 2 days in anaerobic conditions using a BD GasPack EZ Anaerobe Pouch System (BD Diagnostics). After expansion, bacteria were heat-killed (HK) at 56°C for 2hrs, washed and resuspended at 3×109bacteria/ml in DPBS and stored in single use aliquots at −20°C.
To prepare HIV-1 viral stocks for use in the in vitro assays, PBMCs were resuspended at 2×106cells/ml in RPMI 1640 + 1% penicillin/streptomycin/L-glutamine (CM) + 10% human AB serum and stimulated with 5μg/ml phytohemagglutinin (PHA; Sigma-Aldrich) for 3 days at 37°C, 5% CO2. PHA-blasted PBMCs were collected and resuspended at 1×106cells/ml in CM + 10% human AB serum + 10U/ml recombinant IL-2 (rIL-2; Roche, Indianapolis, IN) and cultured with CCR5-tropic HIV-1Bal (Catalog Number 510, NIH AIDS Research & Reference Reagent Program, Germantown, MD) for 7 days at 37°C, 5% CO2. Fresh PHA-blasted PBMCs, resuspended at 1×106cells/ml in CM + 10% human AB serum + 10U/ml rIL-2 were added at Day 7 as additional ‘feeder cells’. At Day 14 after initial viral inoculation, supernatants were collected and centrifuged at 1400rpm for 10mins to remove cells and cellular debris. CCR5-tropic HIV-1Bal supernatants were frozen in single-use aliquots at −80°C. HIV-p24 content of these viral stocks was determined using the HIV-1 p24 Elisa (PerkinElmer, Waltham, MA) and viral stock concentrations ranged from 0.11–0.26μg/ml.
Mitogenic stimulation of LPMC
LPMC were resuspended at 1×106 cells/ml in RPMI 1640 (Invitrogen) + 1% penicillin/streptomycin/L-glutamine (Sigma-Aldrich) + 10% human AB serum (Gemini-Bio-Products, West Sacramento, CA) + 500μg/ml piperacillin/tazobactam (Wyeth, Madison, NY) ± 250μg/ml Amphotericin B (Invitrogen) (complete medium; CM) and stimulated with 100ng/ml PMA (Sigma-Aldrich) and 1 μg/ml ionomycin (Sigma-Aldrich) in the presence of 1μg/ml Brefeldin A (Golgi Plug; BD Biosciences) for 5hrs at 37°C, 5% CO2. LPMC were collected and intracellular percentages of IFN-γ and IL-17-producing cells determined by IFC assay.
In vitro infection assays
LPMC were resuspended at 1×106 cells/ml in CM and cultured for 15–20hrs with 0.08–0.2μg p24/ml CCR5-tropic HIV-1Bal. LPMC were washed in warm DPBS and resuspended at 1×106 cells/ml in CM and cultured with or without HK bacteria at 5 bacteria:1 LPMC in 48 well plates for 2–3 days at 37°C, 5% CO2. In some assays, ultrapure LPS derived from commensal E. coli strain K12 (10μg/ml; InvivoGen, San Diego) was added to HIV-1Bal-exposed LPMC in 96 well plates. To block MHC Class II presentation, LEAF purified anti-human HLA-DR (Clone L243; 10–20μg/ml; BioLegend, San Diego, CA) was added to LPMC in 96 well plates 30 mins prior to the addition of E. coli and again up to 24hrs later. LEAF purified mouse IgG2a isotype (Biolegend) was used as the control antibody.
At the completion of the culture period, culture supernatants were collected and frozen at −20°C for subsequent assessment of secreted IFN-γ, IL-17 and HIV-1 p24. LPMC were collected and either immediately assessed for surface expression of CD38 and intracellular expression of HIV-1 p24 by flow cytometry or underwent mitogenic stimulation, and intracellular HIV-1 p24 percentages within cytokine-producing cells determined by IFC assay.
T cell proliferation assays
LPMC were pre-labeled with 5μM 5,6-carboxyl-fluorescein diacetate succinimidyl ester (CFSE; Invitrogen) in HBSS for 15mins at 37°C and washed with CM. CFSE-labeled LPMC were resuspended at 1×106 cells/ml in CM and cultured with or without HK E. coli (5 E. coli:1 LPMC) for 7 days at 37°C, 5% CO2. Phytohaemagglutinin (PHA, 5μg/ml; Remmel, Lenexa, KS) was used as a positive control and all samples had PHA-induced CD4+ T cell proliferation greater than 50% (range: 52.7–98.8%; data not shown). To determine proliferation of cytokine-producing cells, total LPMC were then collected after 7 days of in vitro culture, underwent mitogenic stimulation and assessed for intracellular cytokine expression by IFC assay.
Identification of cytokine-producing bacteria-reactive LP CD4 T cells
LPMC were resuspended at 1×106 cells/ml in CM and cultured for 4hrs with E. coli or B. fragilis (5 bacteria:1 LPMC) prior to the addition of 1μg/ml Brefeldin A. After an additional 14–16hrs, LPMC were collected and frequencies of CD4+ T cells producing IFN-γ or IL-17 determined by IFC assay.
Depletion of CD11c+ and CD19+ LP cells
LPMC were stained with biotinylated CD11c (Miltenyi Biotec, Auburn, CA) ± biotinylated CD19 (eBioscience). Control LPMC were resuspended in buffer only. In all cases, FcR blocking reagent (Miltenyi Biotec) was added. Biotinylated antibody-bound cells were incubated with 32×105 Biotin Binder Dynabeads (Invitrogen) per 1×106 LPMC for 30mins at 4°C under constant rotation. Dynabead-bound LPMC were magnetically removed following the manufacturers recommended protocol. CD11c+ LPMC were depleted by a median 80.3%, (range 71.4–93.8%) and CD19+ LPMC were depleted by 91.4% (82.3–95.6%) compared to total viable, CD45+ LPMC (n=7). Following depletion of CD11c+ and CD19+ LPMC, the percentage of CD3+ T cells increased from 78.7% (range 60.9–88.8%) to 85.2% (69.6–93.9%) of viable, CD45+ LPMC. For LPMC samples depleted of CD11c only (n=3), CD11c+ LPMC were depleted by 85.5% (82.6–95%).
LPMC were either labeled with CFSE and cultured with or without HK E. coli (5 E. coli:1 LPMC), as described above, for 5 days or were pre-exposed to HIV-1Bal (0.08μg p24/ml) for 19–20hrs, washed and cultured with or without HK E. coli (5 E. coli:1 LPMC) for an additional 3 days, as described above.
Flow cytometric acquisition and analysis
For all in vitro assays, a lymphocyte gate was established using Forward Scatter-Area and Side Scatter-Area properties within viable LPMC. Doublets were then excluded using a Forward Scatter-Height versus Forward Scatter-Width dot plot. A total CD3 gate was established from within this population of total LPMC. In all assays, except for the in vitro infection assays, CD4 T cells were then gated from within the CD3 gate. In our initial in vitro infection assays, we observed significant down-regulation of CD4 on CD3+ LP T cells in infected LPMC cultures (data not shown) therefore, a CD8− gate was established to include all CD4 T cells for these assays.
To evaluate expression of CD38, intracellular p24 and intracellular cytokines, matched isotype antibodies were used to establish background staining and specific expression then determined by removing the background staining. Specific proliferation in response to E. coli was determined by establishing a CFSElo gate based on the unstimulated condition.
All flow cytometry data was acquired on an LSRII Flow Cytometer (BD Biosciences). To control for the accuracy and precision of measurements taken over the course of the study, routine quality control using the Cytometer Setup & Tracking feature within the BD FACSDiva software version 6.1.2 (BD Biosciences) was performed daily as previously detailed (35).
IFN-γ, IL-17 and HIV-1 p24 ELISAs
The recommended manufacturer’s protocols were followed for IFN-γ (BD Biosciences), IL-17 (eBioscience) and HIV-1 p24 (PerkinElmer) ELISAs. The lower detection limits were 4.7pg/ml for IFN-γ, 4pg/ml for IL-17 and 12.5pg/ml for HIV-1 p24.
Statistical Analysis
Non-parametric statistics were used. Wilcoxon Matched-pairs Signed Rank test was used to evaluate paired data. The Friedman test was used for matched-paired comparisons across multiple groups with a Multiple Dunn’s Comparison test performed when the overall p value was <0.05. When non-parametric tests failed due to very small sample sizes, parametric tests were used. Paired t test was used to evaluate paired data. The Repeated Measures ANOVA was used for matched-paired comparisons across multiple groups with a Bonferroni’s Muiltiple Comparison test performed when the overall p value was <0.05. All statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Exposure to commensal E. coli increases HIV-1 replication and infection of LP T cells in vitro
In agreement with previous studies (5), we observed productive infection of LPMC exposed to HIV-1Bal in the absence of exogenous stimulation (Fig. 1). LPMC pre-exposed to HIV-1Bal + E. coli significantly produced higher levels of HIV-1 p24 compared to those with virus only (Fig. 1A). To determine if increased viral replication and infection in the presence of commensal E. coli was specific to intestinal T cells, we obtained PBMC from unmatched donors and exposed them to HIV-1Bal and E. coli using the same in vitro infection protocol (n=9). Evaluation of HIV-p24 levels within culture supernatants failed to demonstrate an increase in HIV-p24 levels when PBMC were cultured with HIV-1Bal + E. coli versus HIV-1Bal alone, and rather a decrease in HIV-p24 was noted (Fig. 1B). Thus, in contrast to HIV-1Bal + E. coli exposed LPMC, we did not observed a similar enhancing effect of bacteria on HIV replication in HIV-exposed PBMC, demonstrating the gut-specificity of this process.
FIGURE 1.
Exposure to commensal E. coli increases HIV-1 replication and infection of LP T cells in vitro. LPMC or PBMC were pre-exposed to CCR5-tropic HIV-1Bal and cultured with or without heat-killed E. coli for 2–3 additional days. A–B, p24 levels within (A) LPMC (n=7 jejunums, closed circles; n= 5 colons, open circles) and (B) PBMC culture supernatants (n=9). C, Gating strategy used to identify intracellular HIV-1 p24+ LP CD4 T cells. An initial viable lymphocyte gate was established and doublets excluded using a Forward Scatter Height versus Forward Scatter Width gate (profiles not shown). To account for the down-regulation of CD4 after exposure to HIV-1Bal in vitro, LP CD4 T cells were identified as CD3+CD8− (left plot). The net expression of HIV-1 p24 within LP CD3+CD8− T cells exposed to HIV-1Bal only (upper panel) or to HIV-1Bal and E. coli (lower panel) was established by using an isotype control (Mouse IgG1). C, Cumulative net percentages of intracellular HIV-1 p24+CD3+CD8− T cells within LPMC cultures (n=7 jejunums, closed circles; n=3 colons open circles). Statistical significance was determined using the Wilcoxon matched pairs signed rank test.
To enumerate the percentage of productively infected LP CD4 T cells, we assessed intracellular p24 expression using flow cytometry. We observed significant down-regulation of CD4 on CD3+ LP T cells in infected LPMC cultures (data not shown), therefore we assessed expression of intracellular HIV-1 p24 in CD3+ CD8− T cells (Fig. 1C). Addition of E. coli to HIV-1Bal-exposed LPMC also resulted in significantly higher percentages of CD8− T cells expressing intracellular HIV-1 p24 than in infected cultures without bacteria (Fig. 1D).
Exposure to HIV-1Bal and commensal E. coli increases T cell activation and cytokine production in vitro
T cell activation state is closely linked to the efficiency of HIV-1 infection and replication, therefore we also assessed CD38 expression, an indicator of T cell activation (14), on T cells in LPMC cultures stimulated in vitro, as described above, using flow cytometry. The addition of E. coli to HIV-1-exposed LPMC significantly increased CD38 expression on CD8− LP T cells above that observed in HIV-1Bal only cultures (Fig. 2A). CD38 expression on CD8+ T cells exposed to HIV-1Bal and E. coli (median MFI: 547, range 404–1736) was also greater than that on CD8+ T cells exposed to HIV-1Bal only (577, 317–1101) although this difference failed to reach statistical significance (p=0.07). The absolute levels of CD38 expression on CD8− T cells (886, 745–3011) were higher in response to HIV-1Bal + E. coli, than levels observed on CD8+ T cells (547, 404–1736; p=0.004).
FIGURE 2.
Exposure to HIV-1Bal and commensal E. coli increases T cell activation and cytokine production in vitro. LPMC were pre-exposed to CCR5-tropic HIV-1Bal and cultured with or without heat-killed E. coli for 2–3 additional days. A, Cumulative data showing mean fluorescence intensity (MFI) of CD38 expression on CD3+CD8− T cells minus isotype control values (net MFI) (n=6 jejunums, closed circles; n=3 colons, open circles). B, IFN-γ and C, IL-17 within culture supernatants (n=7 jejunums, closed circles; n=5 colons, open circles). Statistical significance was determined using the Wilcoxon matched pairs signed rank test.
Low levels of IFN-γ and IL-17 were detected in culture supernatants from LPMC exposed to HIV-1Bal only (Fig. 2B,C). However, both IFN-γ and IL-17 significantly increased in the presence of E. coli (Fig. 2B,C). In a subset of experiments, LPMC were pre-exposed to HIV-1Bal and cultured with a range of doses of E. coli for 2–3 days. As the ratio of E. coli to LPMC decreased from 5:1 to 0.2:1, a trend towards decreasing levels of CD38 expression on CD8− T cells and decreasing IFN-γ and IL-17 production was observed (data not shown), showing bacterial dose-dependent effects on CD8− T cell activation.
Statistically higher levels of IFN-γ and IL-17, and a trend towards higher levels of p24, were detected in culture supernatants from jejunal LPMC compared to colon LPMC stimulated with E. coli after pre-exposure to HIV-1Bal (Supplementary Table 1). However, irrespective of anatomical location, the amount of cytokines produced and p24 levels were always greater following combined HIV-1Bal and bacterial exposure than those observed in HIV-1Bal only.
Purified LPS from commensal E. coli does not increase HIV-1 infection within LPMC
Increased levels of LPS in the blood of HIV-infected individuals has been associated with systemic immune activation(27), and increased LPS levels were observed in the colon of SIV-infected rhesus macaques during the late acute stage of SIV infection (26). Therefore, we wished to determine whether exposure of LPMC to purified commensal E. coli-derived LPS would lead to increased infection of LP CD4 T cells as was observed with whole E. coli. As in previous assays, the addition of whole E. coli increased HIV-1 infection and replication within CD8− T cells and production of IFN-γ and IL-17 within total LPMC (Fig. 3A–D). However, the addition of purified E. coli-derived LPS did not increase levels of HIV-1 p24 (Fig. 3A) or frequencies of HIV-1 p24+ CD8− T cells (Fig. 3B) above those observed in cultures exposed only to HIV-1Bal. This was likely related to the lack of T cell activation and expansion in response to LPS as indicated by minimal induction of IFN-γ (Fig. 3C) or IL-17 (Fig. 3D).
FIGURE 3.
Exposure to HIV-1Bal and commensal E. coli-derived LPS does not increase HIV-1 replication and infection of LP T cells or IFN-γ and IL-17 cytokine production in vitro. LPMC were pre-exposed to CCR5-tropic HIV-1Bal and cultured with or without heat-killed E. coli or purified LPS derived from commensal E. coli (Strain K12) for 2–3 additional days. A–D, Cumulative data showing (A) p24 levels within culture supernatants, (B) net percentages of intracellular HIV-1 p24+CD3+CD8− T cells within LPMC cultures, (C) IFN-γ and (D) IL-17 levels within culture supernatants. Each symbol represents an individual sample (n=4 jejunums). Statistical significance was determined using the Repeated Measures ANOVA (overall P value) with a Bonferroni’s Multiple Comparison test. **P<0.01, ***P<0.001.
Expansion of IFN-γ and IL-17-producing LP CD4+ T cells in response to E. coli stimulation in vitro
High percentages of CD4+ T cells capable of producing IFN-γ were measured in primary LPMC (median: 65.2% of CD4+ T cells, range 33.6–73.2%) after a 5 hour stimulation with PMA/ionomycin, with lower frequencies found of CD4+ T cells capable of producing either IL-17 alone (1.9%, 1.4–10.6%) or co-producing both IL-17 and IFN-γ (1.0%, 0.1–5.6%) (Supplementary Fig. 1).
To determine the proliferative capacity of the cytokine+ CD4+ T cell subsets in response to bacteria, LPMC were initially labeled with CFSE, infected with HIV-1, then cultured with bacteria. E. coli-induced proliferation was detectable by flow cytometry after 5–7 days, but viability of HIV-exposed LPMC substantially decreased after 5 days in culture. Thus, the proliferative capacity of specific cytokine+ CD4+ T cell subsets in response to E. coli was subsequently determined after 7 days of stimulation with E. coli in the absence of HIV-1Bal. In agreement with our previous study (32), we observed a significant increase in the percentage of CFSElo, proliferating, CD4+ T cells in LPMC cultured in the presence of E. coli compared to unstimulated cultures (Fig. 4A). An example of the gating strategy used to evaluate proliferation of the cytokine+ CD4+ T cell subsets is shown in Fig. 4B. A hierarchy of proliferation within the specific cytokine populations in response to E. coli was observed (Fig. 4C). The highest fraction of proliferating cells was observed among the IL-17+IFN-γ+ co-producing CD4+ T cells, with lower percentages in the IL-17+IFN-γ− CD4+ T cell population and the lowest fraction occurring within IFN-γ+IL-17−CD4+ T cells (Fig. 4C). A trend towards greater proliferation of all IL-17-producing CD4 T cells than in total IFN-γ-producing T cells was noted (p=0.06; data not shown).
FIGURE 4.
E. coli induces the expansion of LP Th17 cells. CFSE-labeled LPMC were cultured for 7 days with or without heat-killed E. coli followed by a 5hr mitogenic stimulation. A, Percentages of CFSElo cells within CD3+CD4+ T cells in unstimulated cultures and within cultures stimulated with E. coli (n= 3 jejunums, closed circles; n=3 colons, open circles). Statistical significance was determined using the Wilcoxon matched pairs signed rank test. B, Gating strategy used to determine the percentages of proliferating cells within each cytokine+ T cell population in response to E. coli. CD3+CD4+ T cells were gated from within a viable lymphocyte gate with a doublet discrimination gate applied (profiles not shown). IFN-γ and IL-17 expressing-CD4+ T cells were determined using isotypes. The CFSElo gate within IL-17+IFN-γ+, IL-17+IFN-γ−, and IFN-γ+IL-17− CD4+ T cells was established from unstimulated cultures. C, Cumulative results from 6 samples (n= 3 jejunums, closed circles; n=3 colons, open circles). Statistical significance determined using the Friedman test with a Multiple Dunn’s Comparison test. **P<0.01.
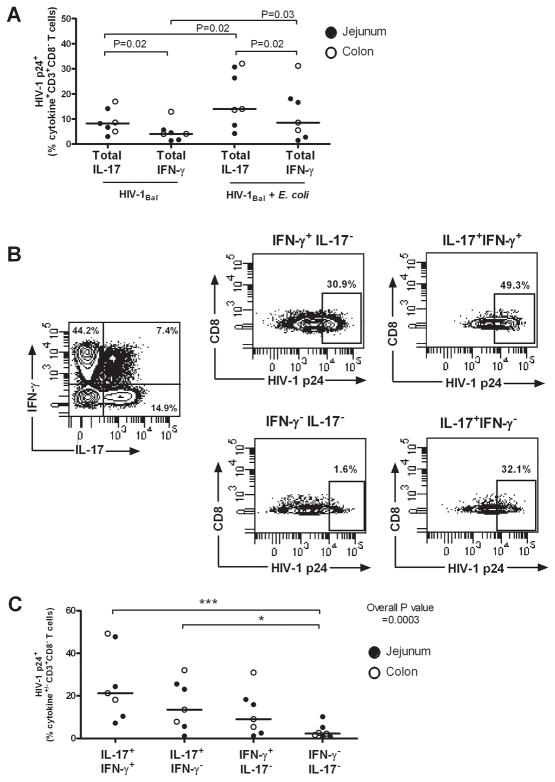
Infection frequencies of IFN-γ- and IL-17-producing T cell subsets increase in response to exposure to HIV-1Bal and commensal E. coli in vitro
We next assessed whether expansion of IL-17+ CD4+ T cells in response to E. coli (Fig. 4) corresponded to greater levels of productive infection within this population of cytokine-producing T cells. Although intracellular HIV-1 p24 was detected in both IFN-γ and IL-17-producing CD8− T cells following HIV-1Bal infection, a significantly higher percentage IL-17-producing T cells expressed p24, indicating an inherent preference of HIV-1Bal for LP Th17 cells (Fig. 5A). The percentage of infected IL-17+ and IFN-γ+CD8− T cells increased after infection with HIV-1Bal in the presence of E. coli, with the fraction of infected IL-17+ CD8− T cells remaining statistically higher than IFN-γ+ CD8− T cells (Fig. 5A). We next evaluated intracellular HIV-1 p24 expression within each cytokine-producing T cell subset in response to HIV-1Bal + E. coli using the gating shown in Fig. 5B. The highest median percentage of HIV-1 p24+ cells was observed in the IL-17+IFN-γ+ CD8− T cell population and the next highest percentage observed within CD8− T cells producing IL-17 only (Fig. 5C). Elevated percentages of HIV-1 p24+ cells were observed in IFN-γ+ IL-17− CD8− T cells compared to non-cytokine producing T cells, but these were lower than those noted for either of the IL-17-producing populations (Fig. 5C).
FIGURE 5.
Greater productive HIV-1 infection of LP Th17 cells co-producing IFN-γ. LPMC (n=4 jejunums, closed circles; n=3 colons, open circles) were pre-exposed to CCR5-tropic HIV-1Bal and cultured with or without heat-killed E. coli for 3 additional days followed by a short-term mitogenic stimulation. A, Net percentages of IL-17+CD3+CD8− (Total IL-17) or IFN-γ+CD3+CD8− (Total IFN-γ) T cells expressing HIV-1 p24 within LPMC cultures in response to HIV-1Bal only and to HIV-1Bal + E. coli. Statistical significance was determined using the Wilcoxon matched pairs signed rank test. B, Gating strategy to determine net HIV-1 p24 expression within CD3+CD8− T cell cytokine± subsets (IL-17+IFN-γ+, IL-17+IFN-γ−, IFN-γ+IL-17− and IFN-γ−IL-17−) in response to HIV-1Bal + E. coli. C, Cumulative percentages of net CD3+CD8−cytokine+/− HIV-1 p24+ T cells within LPMC cultures in response to HIV-1Bal + E. coli. Statistical significance was determined using the Friedman test with a Multiple Dunn’s Comparison test for each of the cytokine+ T cell populations relative to IFN-γ−IL-17−CD3+CD8− T cells. *P<0.05, ***P<0.001.
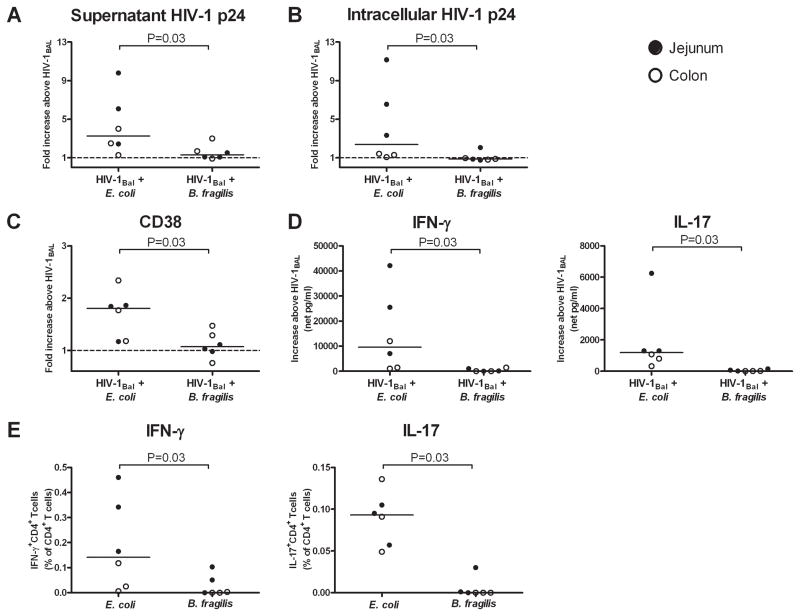
The ability of specific bacteria to enhance HIV-1 infection is related to frequencies of bacteria-reactive CD4 T cells in LPMC
To determine whether enhancement of HIV-1 replication in LPMC was unique to E. coli or was generalizable to other enteric bacteria, we compared T cell infection frequencies and cytokine responses to E. coli versus those to Bacteroides fragilis, another gram negative commensal organism. The Bacteroides group has been shown to be an abundant taxa in intestinal mucosal biopsies (36) and, therefore, might be likely to translocate into the intestinal LP during epithelial barrier breakdown. However, when LPMC were pre-exposed to HIV-1Bal and then cultured with either E. coli or B. fragilis, B. fragilis failed to induce the increases in HIV-1 replication, T cell activation and infection, and cytokine production that were observed following E. coli exposure (Fig. 6A–D). To determine whether these differential responses were potentially related to differences in frequencies of resident bacteria-reactive LP CD4+ T cells, the frequencies of IFN-γ- and IL-17-producing cells responding to each bacterial species was measured in normal LPMC using intracellular cytokine staining, as described (32). LPMC contained significantly higher frequencies of IFN-γ- and IL-17-producing CD4+ T cells that recognized E. coli than recognized B. fragilis (Fig. 6E).
FIGURE 6.
E. coli induces greater productive infection and activation of LP T cells compared to B. fragilis. LPMC (n=3 jejunums, closed circles; n=3 colons, open circles) were pre-exposed to CCR5-tropic HIV-1Bal and cultured with or without heat-killed E. coli or B. fragilis for 3 additional days. Fold increases in A, HIV-1 p24 levels within culture supernatants, B, percentages of intracellular HIV-1 p24+CD3+CD8− T cells and C, CD38 expression by CD3+CD8− T cells within LPMC cultures exposed to HIV-1Bal and either E. coli or B. fragilis above that observed in LPMC cultures exposed to HIV-1Bal only. D, IFN-γ and IL-17 levels detected in culture supernatants within LPMC cultures exposed to HIV-1Bal and either E. coli or B. fragilis above that observed in LPMC cultures exposed to HIV-1Bal only (net). E, Frequencies of CD4+ T cells expressing IFN-γ or IL-17 after 18–20hrs exposure to either E. coli or B. fragilis. Statistical significance was determined using the Wilcoxon matched pairs signed rank test.
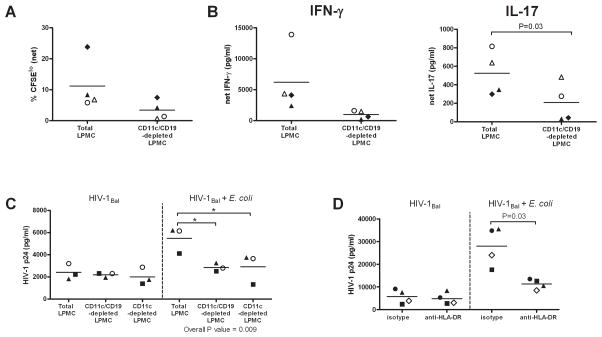
E. coli-associated proliferation, cytokine production and enhancement of infection of LP CD4+ T cells requires the presence of CD11c+ LP cells and is, in part, MHC-Class II restricted
We previously showed that bacteria-specific LP T cell proliferation was dependent on the presence of a subset of LP dendritic cells (32). We wished to determine if the E. coli-associated increase in HIV-1 infection of LP T cells observed in our current in vitro assays was also dependent on the presence of APCs or rather represented a direct effect of E. coli on T cells. To enrich for T cells, LPMC were depleted of CD11c+ (DCs) and CD19+ (B cells) cells using magnetic cell separation techniques. Depletion of DCs and B cells trended towards reduced proliferation of E. coli-reactive LP T cells relative to that observed in total LPMC cultures (P=0.07; Fig. 7A), with an overall mean decrease of 71.5% (SEM: 8.5%; n=4). In tandem with the decreased proliferation in response to E. coli, lower levels of IFN-γ and IL-17 were also observed within the T cell-enriched LPMC cultures compared to levels detected within total LPMC cultures following E. coli stimulation (Fig. 7B) although this did not reach statistical significance for IFN-γ. This observed decrease in cytokine production equated to a mean decrease of 82.5% (SEM: 5.6%) in IFN-γ and 66.8% (SEM: 15.1%) in IL-17 levels. Thus, the E. coli induced expansion and cytokine production was not occurring primarily through direct stimulation of T cells and implicated a role for CD19 and/or CD11c+ LP cells.
FIGURE 7.
CD11c+ DCs are required for E. coli-induced expansion and enhancement of infection of LP T cells. LPMC were enriched for CD3+ T cells by depletion of CD11c (DC) and CD19 (B cells) LP cells (CD11c/CD19-depleted LPMC). CFSE-labeled LPMC were cultured for 5 days with or without heat-killed E. coli (5 E. coli:1 LPMC). A, E. coli-induced proliferation of CD3+ CD4+ T cells was evaluated by assessing the percentage of CFSElo cells within CD3+CD4+ T cells in cultures stimulated with E. coli and are shown with background CD3+CD4+ T cell proliferation detected within unstimulated LPMC cultures removed (net; n=1 colon, 3 jejunums). B. Culture supernatants were collected and assayed for IFN-γ and IL-17 with values expressed as the net pg/ml produced in response to E. coli stimulation after removing IFN-γ or IL-17 amounts detected in unstimulated cultures. Each symbol represents one sample (n=1 colon, n=3 jejunums). Statistical significance for Fig. A, B was determined using the Paired t test. C, Levels of HIV-1 p24 detected within culture supernatants from total LPMC, CD11c/CD19-depleted LPMC or CD11c-depleted LPMC pre-exposed to CCR5-tropic HIV-1Bal and cultured with or without heat-killed E. coli (5 E. coli:1 LPMC) for an additional 3 days. Each individual symbol represents one sample (n=3 jejunums). Statistical significance was determined using the Repeated Measures ANOVA (overall P value) with a Bonferroni’s Multiple Comparison test. *P<0.05. D, Levels of HIV-1 p24 detected within culture supernatants from total LPMC pre-exposed to CCR5-tropic HIV-1Bal and cultured with or without E. coli (5 E. coli:1 LPMC) in the presence of purified mouse IgG2a (isotype) or purified anti-HLA-DR for an additional 3 days. Each individual symbol represents one sample (n=4 jejunums). Statistical significance was determined using the Paired t test.
We next investigated whether the requirement for CD19+ and CD11c+ LP cells in the expansion of E coli-reactive T cells also impacted bacteria-associated enhancement of HIV-1 infection of LP T cells. To further define the role of DCs, we also depleted LPMC of CD11c+ DCs only. Minimal changes in the levels of HIV-1 p24 from HIV-1-exposed LPMC cultured in the absence of E. coli were detected when CD11c and CD19 LP cells were depleted (Fig. 7C). However, we observed a decrease in the bacterial enhancement of infection in CD11c/CD19-depleted cultures (Fig. 7C), with levels of supernatant HIV-1 p24 decreasing by 46.9% (SEM: 4.5%). Comparison with CD11c-depleted cultures showed similar reductions in infection levels in the presence of E. coli (Fig. 7C), with HIV-1 p24 levels decreasing by 49.4% (SEM: 9.3%) suggesting that depletion of DCs rather than B cells was responsible for the majority of the effects observed. Notably, mean levels of HIV-1 p24 detected in CD11c/CD19- and CD11c-depleted cultures stimulated with E. coli were similar (CD11c/CD19-depleted: 2858pg/ml; CD11c-depleted: 2908 pg/ml) to levels observed within culture supernatants from total LPMC exposed only to HIV-1Bal (2417pg/ml), further highlighting the role for LP DCs in driving the E. coli-associated enhancement of HIV-1 infection of LP T cells.
We have previously shown that expansion of E. coli-specific LP T cells was partially dependent on HLA-DR molecules (32). To further delineate potential mechanisms behind the current observations that LP DCs were required for the E. coli-associated enhancement of LP T cell infection, we evaluated levels of infection in the presence of an HLA-DR blocking antibody. Minimal effect of HLA-DR blocking on the levels of HIV-1 infection was observed in the absence of E. coli (Fig. 7D). However, significantly reduced levels of HIV-1 p24 were detected in culture supernatants after stimulation of HIV-1Bal-exposed LPMC to E. coli in the presence of the HLA-DR blocking antibody (Fig. 7D), with levels in the presence of anti-HLA-DR antibody decreasing by 56.2% (SEM: 9.5%) relative to levels detected in the presence of the isotype control. Taken together, these results show that the bacterial enhancement of HIV replication and infection within LP CD4 T cells does not occur through direct stimulation of T cells, but rather requires the presence of CD11c+ DC and is partially MHC Class II-restricted.
DISCUSSION
In recent years, Th17 cell dynamics during HIV infection have been extensively studied, and it is well established that significant depletion of these cells occurs both in peripheral blood (20, 37–40) and in the intestinal mucosa (20, 22, 24, 41). Decreases in intestinal Th17 cells during HIV infection may be a factor contributing to the translocation of bacteria and bacterial products into the LP (26) and into the systemic circulation of HIV-1 infected individuals (27, 28). Indeed, microbial translocation occurs throughout the course of HIV disease and has been associated with systemic immune activation, a predictor of disease progression (27, 28, 42, 43). A link between microbial translocation and poor clinical outcomes such as cardiovascular disease, dementia and mortality has also been suggested (44–46).
The mechanisms underlying the depletion of Th17 cells are being actively investigated. HIV infection of peripheral blood Th17 cells has previously been demonstrated both in vivo (20) and in vitro (37, 39, 47). To evaluate if Th17 cells were preferentially infected in vivo, Brenchley et al stimulated PBMC from HIV-1 infected donors with anti-CD3 and observed no preferential infection of peripheral blood Th1 or Th17 cells (20). In chronically SIV-infected rhesus macaques, spleen IFN-γ+ and IL-17+ T cells had similar levels of SIV DNA (21). In contrast, Gosselin et al identified IL-17-producing T cells by surface expression of CCR6 and observed higher levels of integrated DNA within CCR6+ memory T cells relative to CCR6-memory T cells isolated from untreated, viremic HIV-infected individuals (37), suggesting that preferential infection of Th17 cells in vivo may in fact occur. Further, in vitro infection studies have suggested that peripheral blood Th17 cells were more permissive to HIV infection than Th1 cells (37, 47). However, studies to address the mechanisms responsible for HIV-associated depletion of intestinal Th17 cells have been hampered by the small numbers of LPMC typically obtained from clinical gut biopsies.
In the present study, we evaluated how HIV-1 replication and infection of resident LP T cells were influenced by exposure of LPMC to commensal bacteria in order to better understand the mechanisms by which microbial translocation might contribute to HIV pathogenesis at the mucosal level, particularly during early infection where epithelial barrier breakdown, increased translocation of intestinal microbes and increased viral replication is known to occur. Using an in vitro system modeling these early interactions between LPMC, HIV-1 and translocated whole bacteria we demonstrate that exposure to commensal E. coli increases the activation and expansion of resident E. coli-reactive T cells resulting in increased HIV-1 replication and infection of IFN-γ and IL-17-producing CD4 T cells. A hierarchy of infection was noted with the greatest fraction of HIV-1 p24-expressing cells observed within the IL-17+IFN-γ+ population, followed by cells producing only IL-17, and finally those that produced IFN-γ only. This infectivity profile paralleled the proliferation profile of Th1 and Th17 subsets in response to E. coli. Furthermore, the ability of E. coli to enhance HIV-1 replication in LPMCs was linked to the frequency of resident LP CD4 T cells able to recognize that specific bacterial species, as HIV-1 replication was not enhanced in response to B. fragilis, a commensal organism recognized by few LP T cells.
It is well established that activated/memory T cells, including HIV-1-specific CD4 T cells, are preferentially infected by HIV-1 (14, 48–51). Studies have also implicated pathogenic bacteria in enhancing HIV-1 replication. Bacterial vaginosis-associated microflora was shown to enhance HIV replication within a promonocytic cell line chronically infected with HIV-1 provirus (52), and higher levels of HIV gag DNA were observed in Mycobacterium tuberculosis-specific peripheral CD4 T cells than in total memory CD4 T cells, in HIV-1-infected subjects with active tuberculosis (53). In addition, this group demonstrated higher HIV infection rates within M. tuberculosis-specific CD4 T cells following in vitro mitogenic stimulation (53). Within the intestine, the activated phenotype of LP T cells has been linked to their natural susceptibility to HIV infection and to their rapid subsequent depletion (5, 14). The activated state of intestinal T cells may be partially explained by the presence of commensal bacteria-reactive T cells (32). Our current study expands on these previous findings by addressing the potential impact of translocating commensal bacteria on the local immune response within the intestinal LP during HIV-1 infection. Notably, our study suggests that the translocation of certain enteric bacteria across a leaky epithelial barrier into the intestinal LP could increase the pool of activated resident LP CD4 T cells, particularly Th17 cells, resulting in increased infection rates. These in vitro findings provide a physiologic basis by which translocating microbes might contribute to mucosal Th17 cell depletion, but this theoretical process remains to be verified as a true mechanism of HIV-1 pathogenesis in vivo.
In studies to address the potential mechanisms behind the bacteria-associated enhancement of HIV-1 infection and replication within LP T cells, we demonstrated that exposure of infected LPMC to purified LPS, a known TLR4 agonist (54), did not result in the increases in HIV-1 replication that were observed using whole bacteria. We previously identified MHC class II-restricted, Th1 and Th17 bacteria-reactive T cells within the LP of normal human intestinal tissue (32) and showed that their proliferation was DC-dependent. We now show that the ability of bacteria to enhance HIV-1 replication in LP CD4 T cells is also dependent on the presence of LP DCs, and is in part, MHC Class II restricted. Taken together, these findings suggest that innate stimulation of single bacterial TLR alone is insufficient to result in T cell activation and increased infection and that antigen presentation by DCs is likely critical to the expansion of resident bacteria-reactive CD4 T cells necessary for increased HIV-1 replication. However, other factors, such as cytokines, are also likely involved in the enhancement of HIV-1 replication as MHC Class II blockade only partially inhibited this process. The bacterial antigens, either alone or in combination, that are responsible for the observed enhancement of infection and replication, as well as the DC-derived cytokines or chemokines involved, remain to be determined.
One of the more intriguing observations made in our study is the bacteria-induced selective expansion and infection of Th17 cells that co-produce IFN-γ. Although not extensively studied, this Th17/Th1 subset has been described in other investigations addressing human peripheral blood Th17 responses in the setting of HIV-1 infection. IL-17 and IFN-γ co-producing T cells expressed CCR5 and were productively infected after exposure to CCR5-tropic HIV-1 in vitro, (37, 47) and in one study, the highest levels of in vitro infection and depletion were noted in IL-17+IFN-γ+ CD4 T cells (47). During early HIV infection, higher frequencies of HIV-specific IL-17+IFN-γ+ blood CD4 T cells have also been noted in vivo (55). To the best of our knowledge, this current study is the first to show that commensal E. coli induced expansion of human LP IL-17+IFN-γ+ CD4 T cells, increasing the frequency of productively infected cells when concurrently exposed to CCR5-tropic HIV-1 in vitro.
In a recent study tracking IL-17-producing cells in an in vivo mouse model, Hirota et al demonstrated that IL-23 was necessary not only for the induction of full effector function of Th17 cells, but also for induction of the Th1-associated transcription factor Tbet and the subsequent switch of these cells to a IL-17+IFN-γ+ T cell phenotype (56). Importantly, this plasticity of IL-17-producing cells appeared only during EAE, a chronic inflammatory disease, and not after infection of the skin with Candida albicans which resulted in acute inflammation, rapid clearance of the fungus and a relatively anti-inflammatory environment. A pro-inflammatory environment as a requirement for ‘switching’ of Th17 cells to IL-17- and IFN-γ-co-producing T cells has also been observed in a mouse model of ocular inflammation (57). Thus, in the context of our HIV-1-infection assay, we speculate that the expansion of these cells and their increased infection in the presence of HIV-1 and E. coli in vitro may result in part from the pro-inflammatory milieu generated. However, further detailed analysis of LP Th1/Th17 cells, including their expression of Th1 and Th17 transcription factors and dependence on specific cytokines induced by enteric bacteria, is necessary to define a possible role for them in HIV-1-associated mucosal immune dysfunction.
Why do IL-17-producing LP CD4 T cells, and in particular those that co-produce IL-17 and IFN-γ, constitute the greatest fraction of infected cells after exposure to HIV-1 and E. coli? Several studies have shown that intestinal Th17 cells are highly infectable, which may be in part attributable to the high level of baseline activation of LP CD4 T cells (14) as well as the expression of HIV co-receptors. LP CD4+ T cells express high levels of CCR5, (5, 58) and CCR5-expressing LP Th17 cells were shown to be preferentially depleted in HIV-infected subjects (20). In addition, peripheral blood IL-17+IFN-γ+ CD4 T cells were shown to highly express CCR5 (37, 47). Recently, HIV and SIV have been shown to bind the gut-homing receptor α4β7 on peripheral blood CD4+ T cells in vitro and in vivo, (10, 11) and the α4+β7hi T cells contained a substantial proportion of IL-17-producing cells (11). Intestinal α4+β7hi CD4 T cells were also preferentially infected in the early stages of SIV infection (59). Thus, expression of α4β7, as well as CCR5, may explain the greater infection frequency of intestinal Th17 cells in our in vitro infection assay. In addition, peripheral blood Th17 cells were shown to have a reduced ability to produce MIP-1α and MIP-1β, CCR5 ligands associated with blocking of HIV replication, relative to IFN-γ-producing T cells, thus providing another potential mechanism for a greater infectivity rate of IL-17- versus IFN-γ-producing T cells (47).
Our data suggest that the intrinsic targeting of IL-17-producing cells by HIV-1 can be further enhanced upon exposure to E. coli, a process that appears to be dependent upon the baseline frequency of bacteria reactive cells and their capacity to proliferate upon bacterial exposure. In particular, the selective activation and expansion of IL-17/IFN-γ co-producing cells in response to bacteria likely contributes to their selective targeting by HIV-1 in our in vitro model system. Given the pivotal role that Th17 cells play in mucosal homeostasis and epithelial barrier function (19), the infection and depletion of a relatively small number of Th17 cells could have a significant impact of mucosal immunity and the clinical outcome of HIV infection. Additional in vivo work is necessary to determine whether commensal bacteria enhance the targeting of Th17 subsets in the setting of HIV-1 infection.
Although depletion of LP CD4 T cells during HIV infection is likely multi-factorial, our study provides one potential mechanism by which translocation of selected enteric bacteria from the intestinal lumen into the LP could enhance the infection and depletion of LP CD4 T cells, especially those producing IL-17 or co-producing IL-17 and IFN-γ. Based on our results, we propose that during early HIV infection, as HIV replicates in the intestinal LP resulting in inflammation and damage to the integrity of the epithelial barrier, increased exposure of LP CD4 T cells to translocating commensal bacteria would lead to the activation, expansion, and increased infection and depletion of bacteria-reactive Th17 and Th17/Th1 LP cells. In this study, an in vitro model was used to address the dynamic interactions between HIV-1, commensal bacteria and LPMC that would be harder to capture in ‘snapshots’ using clinical samples. However, clinical studies are necessary to confirm that these in vitro findings are relevant to mucosal pathogenesis in either early or chronic HIV-1 infection. An understanding of the role of microbial translocation in mucosal pathogenesis and its attendant mechanisms will be important in facilitating the development of therapeutic approaches aimed at blocking this process at the mucosal level.
Supplementary Material
Acknowledgments
We would like to thank the surgical teams of the Department of Surgery, University of Colorado Hospital for their assistance in obtaining tissue samples. We acknowledge Dave Shugarts, ACTG Clinical Trials Unit, University of Colorado Anschutz Medical Campus, and Nancy Madinger and the staff of the Clinical Microbiology Laboratory, University of Colorado Anschutz Medical Campus, for technical assistance.
Footnotes
This work was supported by the National Institute of Health grants 1RO1 DK088663 and 5K24 AI074343.
References
- 1.Kotler DP, Gaetz HP, Lange M, Klein EB, Holt PR. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;101:421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- 2.Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, Stahl-Hennig C, Kaup FJ, Stallmach A, Zeitz M. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–1123. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 3.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 5.Lapenta C, Boirivant M, Marini M, Santini SM, Logozzi M, Viora M, Belardelli F, Fais S. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur J Immunol. 1999;29:1202–1208. doi: 10.1002/(SICI)1521-4141(199904)29:04<1202::AID-IMMU1202>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 11.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, Mattapallil JJ. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poles MA, Elliott J, Taing P, Anton PA, Chen IS. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol. 2001;75:8390–8399. doi: 10.1128/JVI.75.18.8390-8399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, Lackner AA. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veazey RS, I, Tham C, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 16.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 17.d’Ettorre G, Paiardini M, Zaffiri L, Andreotti M, Ceccarelli G, Rizza C, Indinnimeo M, Vella S, Mastroianni CM, Silvestri G, Vullo V. HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr HIV Res. 2011;9:148–153. doi: 10.2174/157016211795945296. [DOI] [PubMed] [Google Scholar]
- 18.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 19.Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, Zaffiri L, Tryniszewska E, Tsai WP, Vaccari M, Parks RW, Venzon D, Douek DC, O’Shea JJ, Franchini G. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chege D, Sheth PM, Kain T, Kim CJ, Kovacs C, Loutfy M, Halpenny R, Kandel G, Chun TW, Ostrowski M, Kaul R. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. Aids. 2011;25:741–749. doi: 10.1097/QAD.0b013e328344cefb. [DOI] [PubMed] [Google Scholar]
- 23.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 25.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, Hirsch VM, Silvestri G, Douek DC, Miller CJ, Haase AT, Lifson J, Brenchley JM. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010:6. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 28.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epple HJ, Allers K, Troger H, Kuhl A, Erben U, Fromm M, Zeitz M, Loddenkemper C, Schulzke JD, Schneider T. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology. 2010;139:1289–1300. doi: 10.1053/j.gastro.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Estes JD, Duan L, Jessurun J, Pambuccian S, Forster C, Wietgrefe S, Zupancic M, Schacker T, Reilly C, Carlis JV, Haase AT. Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J Infect Dis. 2008;197:420–429. doi: 10.1086/525046. [DOI] [PubMed] [Google Scholar]
- 31.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, Dandekar S. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe R, Dillon S, Rogers L, McCarter M, Kelly C, Gonzalez R, Madinger N, Wilson CC. Evidence for dendritic cell-dependent CD4(+) T helper-1 type responses to commensal bacteria in normal human intestinal lamina propria. Clin Immunol. 2009;131:317–332. doi: 10.1016/j.clim.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dillon SM, Rogers LM, Howe R, Hostetler LA, Buhrman J, McCarter MD, Wilson CC. Human intestinal lamina propria CD1c+ dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J Immunol. 2010;184:6612–6621. doi: 10.4049/jimmunol.1000041. [DOI] [PubMed] [Google Scholar]
- 34.Dillon SM, Robertson KB, Pan SC, Mawhinney S, Meditz AL, Folkvord JM, Connick E, McCarter MD, Wilson CC. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dillon SM, Friedlander LJ, Rogers LM, Meditz AL, Folkvord JM, Connick E, McCarter MD, Wilson CC. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. J Virol. 2011;85:397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillevet P, Sikaroodi M, Keshavarzian A, Mutlu EA. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem Biodivers. 2010;7:1065–1075. doi: 10.1002/cbdv.200900322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, Fonseca S, Wacleche V, El-Far M, Boulassel MR, Routy JP, Sekaly RP, Ancuta P. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol. 2010;184:1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ndhlovu LC, Chapman JM, Jha AR, Snyder-Cappione JE, Pagan M, Leal FE, Boland BS, Norris PJ, Rosenberg MG, Nixon DF. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. Aids. 2008;22:990–992. doi: 10.1097/QAD.0b013e3282ff884e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, Goulder P, Klenerman P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 40.Salgado M, Rallon NI, Rodes B, Lopez M, Soriano V, Benito JM. Long-term non-progressors display a greater number of Th17 cells than HIV-infected typical progressors. Clin Immunol. 2011;139:110–114. doi: 10.1016/j.clim.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, Alfano M, Poli G, Rossouw T. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 43.Wallet MA, Rodriguez CA, Yin L, Saporta S, Chinratanapisit S, Hou W, Sleasman JW, Goodenow MM. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. Aids. 2010;24:1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B, Brenchley JM, Douek DC, Lederman MM. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Hed A, Khaitan A, Kozhaya L, Manel N, Daskalakis D, Borkowsky W, Valentine F, Littman DR, Unutmaz D. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis. 2010;201:843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 49.Gowda SD, Stein BS, Mohagheghpour N, Benike CJ, Engleman EG. Evidence that T cell activation is required for HIV-1 entry in CD4+ lymphocytes. J Immunol. 1989;142:773–780. [PubMed] [Google Scholar]
- 50.Spina CA, Prince HE, Richman DD. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 52.Al-Harthi L, Roebuck KA, Olinger GG, Landay A, Sha BE, Hashemi FB, Spear GT. Bacterial vaginosis-associated microflora isolated from the female genital tract activates HIV-1 expression. J Acquir Immune Defic Syndr. 1999;21:194–202. doi: 10.1097/00126334-199907010-00003. [DOI] [PubMed] [Google Scholar]
- 53.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, Ambrozak DR, Louder M, Ampofo W, Pollakis G, Hill B, Sanga E, Saathoff E, Maboko L, Roederer M, Paxton WA, Hoelscher M, Koup RA. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beutler B. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr Opin Microbiol. 2000;3:23–28. doi: 10.1016/s1369-5274(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 55.Yue FY, Merchant A, Kovacs CM, Loutfy M, Persad D, Ostrowski MA. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:6767–6771. doi: 10.1128/JVI.02550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, Grovit-Ferbas K, Mackay CR, Chen ISY, Giorgi JV. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. Aids. 2000;14:1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 59.Kader M, Bixler S, Roederer M, Veazey R, Mattapallil JJ. CD4 T cell subsets in the mucosa are CD28+Ki-67-HLA-DR-CD69+ but show differential infection based on alpha4beta7 receptor expression during acute SIV infection. J Med Primatol. 2009;38(Suppl 1):24–31. doi: 10.1111/j.1600-0684.2009.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.