Abstract
Objective. To determine the prevalence and the genotypes of Enterocytozoon bieneusi in stool specimens from HIV patients. Methods. This cross-sectional study was carried out in Kinshasa hospitals between 2009 and 2012. Detection of microsporidia including E. bieneusi and E. intestinalis was performed in 242 HIV-infected patients. Typing was based on DNA polymorphism of the ribosomal DNA ITS region of E. bieneusi. PCRRFLP generated with two restriction enzymes (Nla III and Fnu 4HI) in PCR-amplified ITS products for classifying strains into different lineages. The diagnosis performance of the indirect immune-fluorescence-monoclonal antibody (IFI-AcM) was defined in comparison with real-time PCR as the gold standard. Results. Out of 242 HIV-infected patients, using the real-time PCR, the prevalence of E. bieneusi was 7.9% (n = 19) among the 19 E. bieneusi, one was coinfected with E. intestinalis. In 19 E. bieneusi persons using PCR-RFLP method, 5 type I strains of E. bieneusi (26.3%) and 5 type IV strains of E. bieneusi (26.3%) were identified. The sensitivity of IFI-AcM was poor as estimated 42.1%. Conclusion. Despite different PCR methods, there is possible association between HIVinfection, geographic location (France, Cameroun, Democratic Republic of Congo), and the concurrence of type I and type IV strains.
1. Introduction
It is established that Enterocytozoon bieneusi (E. bieneusi) is the most commonly characterized microsporidia species among human beings. Microsporidia, obligate intracellular parasites, lack eukaryotic ribosomal features and peroxisomes [1]. Their spores do penetrate and infect eukaryotic cells in various invertebrate and vertebrate organisms. The literature reports epidemiology, causes, diagnosis, and digestive disorders related to microsporidiosis among HIVpatients [2–7].
In Kinshasa region, The capital city of The Democratic Republic of Congo (DRC), we detected E. bieneusi infection in HIV patients using only light microscopy and Fungi Fluor [8] as well as conventional polymerase chain reaction (PCR) method [9]. We could confirm the sensitivity of the diagnosis of E. bieneusi infection by a real-time PCR assay in comparison with traditional methods [10, 11].
E. bieneusi genotypes were also identified by PCR-restriction fragment length polymorphism (RFLP) analysis [12, 13].
Therefore, the objective of this study was to determine the prevalence and the genotypes of E. bieneusi in stool specimens among HIV patients by developing a rapid and efficient real-time PCR and PCR-RFLP approach.
2. Materials and Methods
2.1. Study Design
This study was designed as a descriptive cross-sectional approach between December 2009 and January 2012.
2.2. Ethical Considerations
The institutional review boards and the Committee of Ethics of the University of Kinshasa Faculty of Medicine approved the protocol of the study which was conducted in compliance with the principles of Helsinki Declaration. The procedures of the study were explained, and an informed consent sheet was signed by each participant or a designated literate substitute when necessary.
2.3. Study Setting
In the Kinshasa community, Democratic Republic of Congo, the Cliniques Universitaires de Kinshasa (CUK) as the teaching hospital at the south-western part of Kinshasa city, the general referral hospital of Kinshasa (HGRK) in the center of Kinshasa city, the general referral hospital of Kintambo (HGRKint) at the Northeastern Kinshasa city, and military referral hospital of Camp Kokolo (HMRK) at the western part of Kinshasa city were randomly selected.
2.4. Patients and Clinical Specimens
We included 242 consecutive HIV-infected patients. The clinical signs characteristic of HIV disease were collected among all participants.
2.5. Diagnosis of E. bieneusi Infection
We collected 242 fresh stool samples in pH 7.2 buffer stored at + 4°C before analysis. The stool specimens from all 242 patients were diluted at PBS solution for microscopic examination.
Microscopic examination and specific staining were done both in Kinshasa University Parasitology laboratories (CUK) and in the Pitié Salpêtrière Hospital (PSL) Parasitology Mycology Laboratory, Paris, France. Stool samples (one for each patient) were studied using optical microscopy (direct examination and trichrome specific staining as modified by Weber) for microsporidia detection [14].
The indirect immunofluorescence-monoclonal antibody (IFI-AcM) techniques were used for the identification of E. bieneusi and E. intestinalis [15, 16].
2.6. Genomic DNA Extraction
DNA extraction was performed by using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the supplier's protocol.
2.7. Real-Time PCR
We carried out a real-time PCR for all samples at the Saint Louis Hospital Parasitology Mycology service in Paris, France, using a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) for all three species identification (E. bieneusi and E. intestinalis).
For E. bieneusi, the real-time PCR assay amplified a 102bp fragment of the small subunit ribosomal RNA gene, with FEB1 (5′-CGCTGTAGTTCCTGCAGTAAACTATGCC-3′) and REB1 (5′-CTTGCGAGCGTACTATCCCCAGAG-3′) primers and a fluorescent TaqMan probe (5′-ACGTGGGCGGGAGAAATCTTAGTGTTCGGG-3′), as previously described [10]. For E. intestinalis, the real-time PCR assay was performed by using FEI1 (5'-GCAAGGGAGGAATGGAACAGAACAG-3′) and REI1 (5′-CACGTTCAGAAGCCCATTACACAGC-3′)-primers, with the following fluorescent TaqMan probe: 5′-FAM-CGGGCGGCACGCGCACTACGATA-TAMRA-3′, as previously described [10, 11].
2.8. PCR-RFLP for E. bieneusi Genotype Identification
The PCR-RFLP assay was performed on a 9700 PCR system (Applied Biosystems) as previously described [12, 13]. The RFLP analysis was performed on a 2% agarose gel by comparing the number and the length of the obtained PCR undigested and digested fragments by using Fnu4HI and NlaIII restriction enzymes.
2.9. Statistical Analysis
Data were expressed as proportions (%) for categorical variables and means with standard deviations for continuous variables. Differences were compared by the chi-square test for proportions and by the Student's t-test for continuous variables with results considered statistically significant for P value <0.05. All analyses were performed by use of STATA (version 11) software package.
3. Results
3.1. Clinical Profile of Patients
Of 242 HIV/AIDS patients, 35.9% (n = 87) were males and 64.1% (n = 155) were females: sex ratio of 2 women: 1 man. The mean age of the participants was 39.2 ± 11.8 years (range: 15–73).
Table 1 presents the clinical signs of the study population. Asthenia and diarrhea were the most frequent signs among the participants.
Table 1.
Clinical signs of our HIV patients.
| Clinical signs | N/242 | % |
|---|---|---|
| Asthenia | 88 | 36,3 |
| Diarrhea | 83 | 34,3 |
| Pulmonary signs | 52 | 21,4 |
| Cutaneous signs | 42 | 17,3 |
| Anorexia | 28 | 11,5 |
| Fever | 25 | 10,3 |
| Emaciation | 14 | 5,7 |
| Anemia | 5 | 2 |
3.2. Molecular Evaluation and Prevalence
Out of 242 HIV-infected patients, using the real-time PCR, the prevalence of E. bieneusi was 7.9% (n = 19). Among the 19 E. bieneusi, one was coinfected with E. intestinalis.
Table 2 presents the findings from IFI-AcM, real-time PCR, and genotypes. The diagnosis efficiency of IFI-AcM was defined with comparison with the real-time PCR as follows: sensitivity of 42.1%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 95%.
Table 2.
Microsporidia (E. bieneusi, E. intestinalis, and genotypes).
| N/19 | IFI-AcM Eb,Ei | PCR RT Eb,Ei | Genotypes par RFLP |
|---|---|---|---|
| 07 | Eb | Eb | Type 4 |
| 08 | No | Eb | ND |
| 10 | Eb | Eb | Type 4 |
| 12 | No | Eb | ND |
| 30 | No | Eb | Type 1 |
| 34 | Eb | Eb | Type 4 |
| 36 | Eb | Eb | ND |
| 37 | Eb | Eb | ND |
| 39 | Eb | Eb | ND |
| 40 | Eb | Eb | ND |
| 44 | No | Eb | Type 1 |
| 49 | No | Eb | Type 1 |
| 63 | Eb | Eb, Ei | Type 4 |
| 89 | No | Eb | ND |
| 93 | No | Eb | ND |
| 105 | No | Eb | Type 4 |
| 134 | No | Eb | ND |
| 183 | No | Eb | Type 1 |
| 220 | No | Eb | Type 1 |
Figure 1 shows the function of the relative fluorescent signal (Delta Rn) according to the cycle number.
Figure 1.
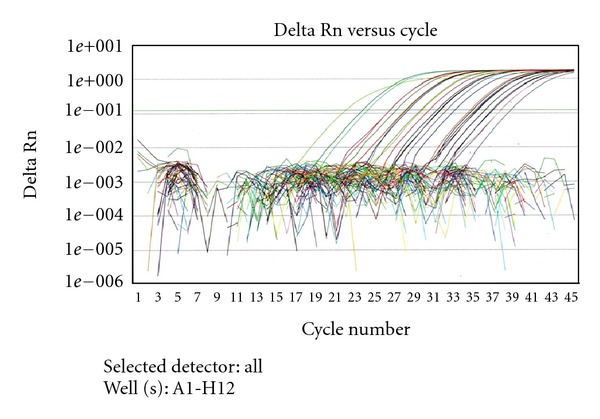
Amplification curves obtained with the E. bieneusi-specific real-time PCR assay.
The sensitivity and reproducibility of real-time PCR was assessed by repeated testing of serial dilutions (Figure 2). The relation between Ct value and the decimal logarithm of E. bieneusi small subunit rRNA gene copy number per μl was as follows: slope = −3.397 and intercept = 41.747.
Figure 2.
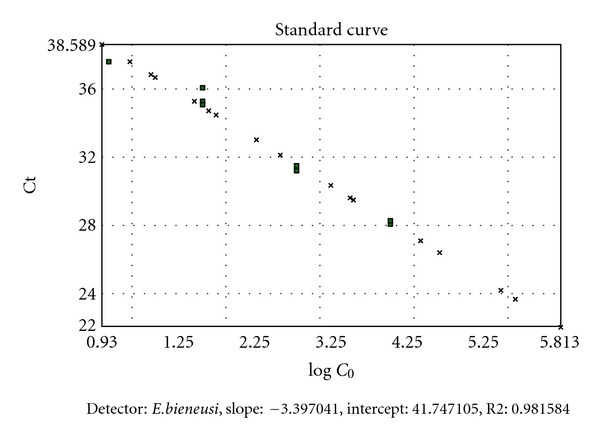
Standard curve representing the threshold cycle (Ct) values as a function of the decimal logarithms of E. bieneusi small subunit rRNA gene copy number per μl.
PCR-RFLP analysis of the amplification products of the ITS region was then performed on the 19 E. bieneusi stool isolates (Figure 3). We found two genetically unrelated lineages: type I strains without digestion of amplicons with Fnu 4HI, and type IV strains with digestion of amplicons with NlaIII and Fnu4HI.
Figure 3.
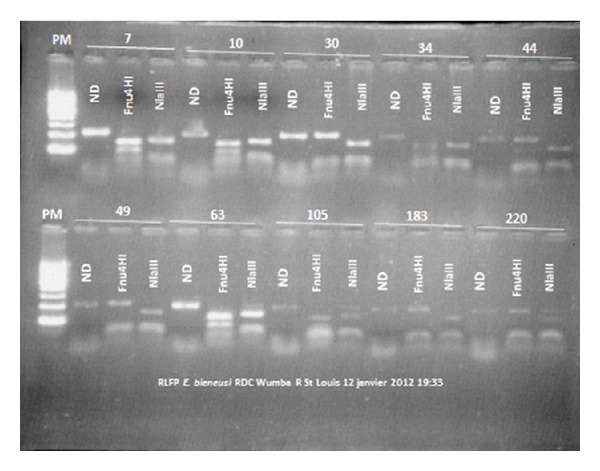
RFLP analysis of E. bieneusi PCR products after digestion with Fnu4HI and NlaIII enzymes. ND: not digested, PM: molecular weight marker.
4. Discussion
In the present study, we have used two real-time PCR assays and a PCR-RFLP assay for the quantitative detection of E. bieneusi DNA and strain genotyping from stool specimens.
Clinical features from the HIV-infected participants were similar to the frequency of diarrhea reported among other African patients [2–7].
The prevalence of E. bieneusi identified by PCR in these HIV Congolese patients was estimated at 8.2% (7.9% of E. bieneusi), which was higher than the prevalence of microsporidia found using similar PCR techniques in other African countries (less than 5%) [4, 17–25]. These low rates of microsporidiosis could be related to the location and availability of antiretroviral therapy (ART). Indeed, the prevalence of microsporidia including E. bieneusi in HIV-infected people has dramatically decreased in countries where ART is widely available [26, 27]. However, in most African countries including our Congolese study, few patients have access to ART [1, 8, 9], which could explain the higher prevalence found in our study and in some other African studies among HIV-infected individuals [8, 9, 28].
In this study, we used a rapid and efficient qPCR method combined with PCR-RFLP genotyping and IIF-MAb for determining intestinal microsporidiosis from stool specimens among HIV-infected patients. Thus, we confirmed the best diagnostic of E. bieneusi using more sensitive and specific real-time PCR than the diagnosis of E. intestinalis [10–13].
The literature reports that E. bieneusi is a relatively homogeneous entity with PCR-RFLP-based putative polymorphism of the ITS region of E. bieneusi [5]. This putative polymorphism of the ITS region of E. bieneusi had a genetic diversity of E. bieneusi [5].
Among the 19 E. bieneusi cases we studied, we identified 5 type I strains of E. bieneusi (26.3%) and 5 type IV strains. By contrast, HIV-infected patients in France were in majority infected with type I strains [12, 13]. Interestingly, type IV strains were also encountered in a previous study in Cameroon [18]. Furthermore, Tumwine et al. found a majority of genotype K strains, which correspond to type IV in our classification, in children from Uganda [29].
4.1. Findings and Current Understanding in the Field within the Field
The present work and the work by Liguory et al. [12, 13] were performed using the same PCR-RFLP developed by Liguory team. Our typing was based on DNA polymorphism of the ribosomal DNA internal transcribed spacer (ITS) region of E. bieneusi. PCR-RFLP generated with two restriction enzymes (Nla III and Fnu4HI) in PCR-amplified ITS products at classifying type I, type II, type III, and type IV [12, 13].
Santín et al. [30] were among the leaders to reduce confusion associated with the identification of genotypes within E. bieneusi after the meeting during IWOP-10. According to the consensus [30], previously, the correspondence for the nomenclature was as follows: genotype B belongs to type I, genotype C belongs to type II, genotype, undetermined genotype does not belong to type III, and genotype K belongs to genotype IV [13, 30, 31].
Despite the standard methods for determining the genotypes of E. bieneusi based on the DNA sequence of the internal transcribed spacer (ITS) region, the r-RNA gene in the publication of Santín et al. [30], the present work in Kinshasa (DRC), and the previous works in France [12, 13] and in Cameroun [31] showed a significant association between HIV-infection and genotypes I and IV E. bieneusi. Genotype IV E. bieneusi was only present among HIV-patients from Nigeria [32], Uganda [29], Gabon [31], and Portugal [33]. The genotypes II and III E. bieneusi were not identified in the present study from Kinshasa (DRC) as they are not yet reported from Africa. However, genotypes II and III E. bieneusi are more frequent among HIV-negative people from Europe [12, 13]. Genotype I in HIV-patients is commoner and more frequent than genotype IV in Europe [12, 13, 34] than in HIV-patients from Central Africa including Democratic Republic of Congo with the present study and Cameroun [31].
In this study, the genotype I–genotype IV E. bieneusi ratio was 1 in HIVpatients and emerging: genotype I E. bieneusi in 5 cases of HIV/AIDS versus genotype IV E. bieneusi in 5 cases of HIV/AIDS. Possible rapid travels between France and francophone Central Africa may be a factor contributory to the emerging genotype I E. bieneusi.
4.2. Implications for Public Health
The significant diagnosis efficiency of PCR methods for E. bieneusi will have implications on management of HIV-related microsporidia.
The accurate identification and differentiation of microsporidian species by real-time PCR techniques will improve therapy, clinical manifestations, and prognosis [35–37].
Modes of transmission and sources of human infection by E. bieneusi or HIV and molecular analyses developed by real-time PCR and RFLP should be useful for epidemiological studies [1, 5, 8, 9, 35–39].
5. Conclusion
The prevalence of E. bieneusi is emerging. We used a sensitive, specific, rapid, and efficient approach for typing E. bieneusi obtained from stool specimens by real-time PCR and PCR-RFLP assays. Genotype I E. bieneusi is more prevalent among HIV-patients from Europe than the genotype I–genotype IV E. bieneusi estimated 1 in HIV-infected patients from the present study in Kinshasa, Democratic Republic of Congo.
Conflict of Interests
The authors have not received any funding or benefits from industry, agency of financing, or elsewhere to conduct this study.
Acknowledgments
The authors thank Professor Francis Derouin of the Hospital of Saint Louis, Paris, France, and the students of the University the Protestant of Congo (UPC), Kinshasa, DRC, for sample collection, Mrs. Annie Claude Guillo-Olczyk, Isabelle Jolly, Isabelle Meyer, and Liliane Ciceron for excellent technical assistance. We also thank Mr. Alain Gaulier and Mrs. Mireille Gaulier, Garenne Colombes, France, for excellent social assistance. they acknowledge with thanks also the Physician Directors and Physicians of The hospitals for permission to carry out this study.
References
- 1.Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clinical Microbiology Reviews. 1994;7(4):426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asmuth DM, DeGirolami PC, Federman M, et al. Clinical features of microsporidiosis in patients with AIDS. Clinical Infectious Diseases. 1994;18(5):819–825. doi: 10.1093/clinids/18.5.819. [DOI] [PubMed] [Google Scholar]
- 3.Kotler DP, Orenstein JM. Clinical syndromes associated with microsporidiosis. In: Wittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. Washington, DC, USA: ASM Press; 1999. pp. 258–292. [Google Scholar]
- 4.Maiga I, Doumbo O, Dembele M, et al. Human intestinal microsporidiosis in Bamako (Mali): the presence of Enterocytozoon bieneusi in HIV seropositive patients. Cahiers Santé. 1997;7(4):257–262. [PubMed] [Google Scholar]
- 5.Liguory O, David F, Sarfati C, et al. Diagnosis of infections caused by Enterocytozoon bieneusi and Encephalitozoon intestinalis using polymerase chain reaction in stool specimens. AIDS. 1997;11(6):723–726. doi: 10.1097/00002030-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. Comparison of three staining methods for detecting microsporidia in fluids. Journal of Clinical Microbiology. 1995;33(12):3138–3145. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotler DP, Orenstein JM. Prevalence of intestinal microsporidiosis in HIV-infected individuals referred for gastroenterological evaluation. American Journal of Gastroenterology. 1994;89(11):1998–2002. [PubMed] [Google Scholar]
- 8.Wumba R, Enache-Angoulvant A, Develoux M, et al. Prévalence des infections opportunistes digestives parasitaires à kinshasa (république démocratique du congo), résultats d’une enquête préliminaire chez 50 patients au stade sida. Revue Médecine Tropicale. 2007;67(2):145–148. [PubMed] [Google Scholar]
- 9.Wumba R, Longo-Mbenza B, Mandina M, et al. Intestinal parasites infections in hospitalized aids patients in kinshasa, democratic Republic Of Congo. Parasite. 2010;17(4):321–328. doi: 10.1051/parasite/2010174321. [DOI] [PubMed] [Google Scholar]
- 10.Menotti J, Cassinat B, Porcher R, Sarfati C, Derouin F, Molina JM. Development of a real-time polymerase-chain-reaction assay for quantitative detection of Enterocytozoon bieneusi DNA in stool specimens from immunocompromised patients with intestinal microsporidiosis. Journal of Infectious Diseases. 2003;187(9):1469–1474. doi: 10.1086/374620. [DOI] [PubMed] [Google Scholar]
- 11.Menotti J, Cassinat B, Sarfati C, Liguory O, Derouin F, Molina JM. Development of a real-time PCR assay for quantitative detection of Encephalitozoon intestinalis DNA. Journal of Clinical Microbiology. 2003;41(4):1410–1413. doi: 10.1128/JCM.41.4.1410-1413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liguory O, David F, Sarfati C, Derouin F, Molina JM. Determination of types of Enterocytozoon bieneusi strains isolated from patients with intestinal microsporidiosis. Journal of Clinical Microbiology. 1998;36(7):1882–1885. doi: 10.1128/jcm.36.7.1882-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liguory O, Sarfati C, Derouin F, Molina JM. Evidence of different Enterocytozoon bieneusi genotypes in patients with and without human immunodeficiency virus infection. Journal of Clinical Microbiology. 2001;39(7):2672–2674. doi: 10.1128/JCM.39.7.2672-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The New England Journal of Medicine. 1991;326(3):161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 15.Cisse OA, Ouattara A, Thellier M, et al. Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in bamako (Mali) Journal of Clinical Microbiology. 2002;40(5):1715–1718. doi: 10.1128/JCM.40.5.1715-1718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Accoceberry I, Thellier M, Desportes-Livage I, et al. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi . Journal of Clinical Microbiology. 1999;37(12):4107–4112. doi: 10.1128/jcm.37.12.4107-4112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarfati C, Bourgeois A, Menotti J, et al. Prevalence of intestinal parasites including microsporidia in human immunodeficiency virus-infected adults in Cameroon: a cross-sectional study. The American Journal of Tropical Medicine and Hygiene. 2006;74(1):162–164. [PubMed] [Google Scholar]
- 18.Kadende P, Nkurunziza T, Floch JJ, et al. Infectious diarrhoea conconmitant with African AIDS. Review of 100 cases observed in Bujumbura (Burundi) Medecine Tropicale. 1989;49(2):129–213. [PubMed] [Google Scholar]
- 19.Drobniewski F, Kelly P, Carew A, et al. Human microsporidiosis in African AIDS patients with chronic diarrhea. Journal of Infectious Diseases. 1995;171(2):515–516. doi: 10.1093/infdis/171.2.515. [DOI] [PubMed] [Google Scholar]
- 20.Kelly P, Davies SE, Mandanda B, et al. Enteropathy in Zambians with HIV related diarrhoea: regression modelling of potential determinants of mucosal damage. Gut. 1997;41(6):811–816. doi: 10.1136/gut.41.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumbo T, Sarbah S, Gangaidzo IT, et al. Intestinal parasites in patients with diarrhea and human immunodeficiency virus infection in Zimbabwe. AIDS. 1999;13(7):819–821. doi: 10.1097/00002030-199905070-00011. [DOI] [PubMed] [Google Scholar]
- 22.van Gool T, Luderhoff E, Nathoo KJ, Kiire CF, Dankert J, Mason PR. High prevalence of Enterocytozoon bieneusi infections among HIV-positive individuals with persistent diarrhoea in Harare, Zimbabwe. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1995;89(5):478–480. doi: 10.1016/0035-9203(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 23.Dieng Y, Dieng TH, Diouf G, Coll-Seck AM, Diallo S. Screening for microsporidian spores in AIDS patients at the fann University Hospital, Dakar (Senegal): preliminary results. Medecine et Maladies Infectieuses. 1998;28(3):265–267. [Google Scholar]
- 24.Lebbad M, Norrgren H, Nauclér A, Dias F, Andersson S, Linder E. Intestinal parasites in HIV-2 associated AIDS cases with chronic diarrhoea in Guinea-Bissau. Acta Tropica. 2001;80(1):45–49. doi: 10.1016/s0001-706x(01)00142-5. [DOI] [PubMed] [Google Scholar]
- 25.Samé-Ekobo A, Lohoué J, Mbassi A. Clinical and biological study of parasitic and fungal diarrhea in HIV patients in the urban and suburban areas of Yaounde. Cahiers Sante. 1997;7(6):349–354. [PubMed] [Google Scholar]
- 26.Le Moing V, Bissuel F, Costagliola D, et al. Decreased prevalence of intestinal cryptosporidiosis in HIV-infected patients concomitant to the widespread use of protease inhibitors. AIDS. 1998;12(11):1395–1397. doi: 10.1097/00002030-199811000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. The Lancet. 1998;351(9098):256–261. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 28.Mwachari C, Batchelor BIF, Paul J, Waiyaki PG, Gilks CF. Chronic diarrhoea among HIV-infected adult patients in Nairobi, Kenya. Journal of Infection. 1998;37(1):48–53. doi: 10.1016/S0163-4453(98)90561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Buckholt MA, Tzipori S. Enterocytozoon bieneusi among children with diarrhea attending Mulago hospital in Uganda. The American Journal of Tropical Medicine and Hygiene. 2002;67(3):299–303. doi: 10.4269/ajtmh.2002.67.299. [DOI] [PubMed] [Google Scholar]
- 30.Santín M, Fayer R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. Journal of Eukaryotic Microbiology. 2009;56(1):34–38. doi: 10.1111/j.1550-7408.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 31.Breton J, Bart-Delabesse E, Biligui S, et al. New highly divergent rRNA sequence among biodiverse genotypes of Enterocytozoon bieneusi strains isolated from humans in Gabon and Cameroon. Journal of Clinical Microbiology. 2007;45(8):2580–2589. doi: 10.1128/JCM.02554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinbo FO, Okaka CE, Omoregie R, Dearen T, Leon ET, Xiao L. Molecular characterization of Cryptosporidium spp. in HIV-infected persons in Benin City, Edo State, Nigeria. The The American Journal of Tropical Medicine and Hygiene. 2012;86(3):441–445. doi: 10.4269/ajtmh.2012.11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobo ML, Xiao L, Antunes F, Matos O. Microsporidia as emerging pathogens and the implication for public health: a 10-year study on HIV-positive and -negative patients. International Journal for Parasitology. 2012;42:197–205. doi: 10.1016/j.ijpara.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Rinder H, Katzwinkel-Wladarsch S, Löscher T. Evidence for the existence of genetically distinct strains of Enterocytozoon bieneusi . Parasitology Research. 1997;83(7):670–672. doi: 10.1007/s004360050317. [DOI] [PubMed] [Google Scholar]
- 35.Hartskeerl RA, Schuitema ARJ, Van Gool T, Terpstra WJ. Genetic evidence for the occurrence of extra-intestinal Enterocytozoon bieneusi infections. Nucleic Acids Research. 1993;21(17, article 4150) doi: 10.1093/nar/21.17.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina JM, Goguel J, Sarfati C, et al. Potential efficacy of fumagillin in intestinal microsporidiosis due to Enterocytozoon bieneusi in patients with HIV infection: results of a drug screening study. AIDS. 1997;11(13):1603–1610. doi: 10.1097/00002030-199713000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Pol S, Romana CA, Richard S, et al. Microsporidia infection in patients with the human immunodeficiency virus and unexplained cholangitis. The New England Journal of Medicine. 1992;328(2):95–99. doi: 10.1056/NEJM199301143280204. [DOI] [PubMed] [Google Scholar]
- 38.Hutin Y, Sombardier MN, Sarfati C, Decazes JM, Modai J, Molina JM. Risk factors for intestinal microsporidiosis in patients infected with human immunodeficiency virus (HIV). Proceedings of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 1997; American Society for Microbiology; pp. 1–150. Abstracts. [Google Scholar]
- 39.Swaminathan B, Barrett TJ. Amplification methods for epidemiologic investigations of infectious diseases. Journal of Microbiological Methods. 1995;23(1):129–139. [Google Scholar]


