In this issue, Kalichman et al(1) report the results of a cross-sectional study on anal intercourse(AI) of heterosexual men and women in South Africa(SA)(1). This study is a welcome addition to the literature since there are relatively few studies quantifying rates of AI among heterosexual populations, especially in SA. Overall, 14% men and 10% women reported AI in the previous three months. Among those, 56% did in at least 50% of all sex acts and reported using condoms as often for AI as for vaginal intercourse(VI). Despite this, the authors concluded that the rate of AI was relatively low among heterosexuals and that, “even among those who do engage in AI, most do so at significantly lower rates than VI,” (total VI=9.90, total AI=8.47) based on reported mean frequencies of all (protected and unprotected) VI and AI (Table 3). However, we believe that the observed differences in unprotected VI and AI are not sufficient to assert this with confidence, especially for men (mean frequency of unprotected AI and VI acts: 3.61 and 4.56 respectively for females, 3.11 and 3.04 for males). They further argued that, although the role of AI remains unclear, the HIV epidemic in SA cannot be primarily attributed to AI and that AI “should neither be the focus of nor ignored by HIV prevention interventions in SA”. They also suggest that, relative to VI, AI is unlikely to account for a large fraction of new HIV infections in Africa, but its role should not be underestimated given its higher transmission efficiency.
As stated by the authors, an important limitation of these results is that the AI rate may be underestimated, given the sensitivity of subject matter and the interviewing method used (self-administered questionnaire with minimal assistance). Studies have shown that collection methods for sensitive data can lead to substantial under-reporting which varies across methods, settings and populations(2), making comparisons difficult. For example, in a study among married men from the general population in Cotonou-Benin, 3.5% reported ever having AI with a woman in face-to-face interviews compared to 17.5% when using pooling booth surveys(3). Given the difficulty in evaluating the magnitude of the under-reporting, we must be cautious in concluding that AI is practiced at relatively low rates and then drawing further conclusions on its relative importance for HIV transmission within a population.
The reported AI rates were similar to those reported by younger populations in the US(4–5), but were higher than reported by other African(6–7) or European(8) studies. However, the paucity of data, differences between populations and methods, and the lack of information on frequency of sex acts makes comparison across studies difficult(9–11). Additional information related to AI practices that may play an important role in HIV transmission include lifetime AI, who is having unprotected AI with whom (e.g. casual or stable partner), at what age, when (at the start or later in a relationship) and the motivation for doing so.
Nevertheless, even in the face of uncertainties in the estimates, an important question is: Does 10–15% of the population engaging in AI constitute too small a fraction to influence the course of HIV epidemics, despite the high risk associated with AI?
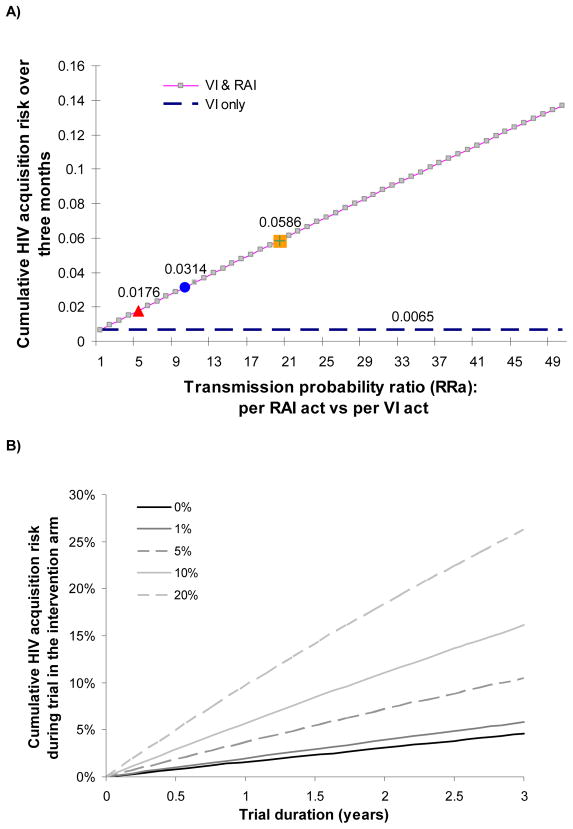
A recent meta-analysis suggested per-act HIV transmission probabilities of 1.7%(95%CI 0.3–8.9) for receptive AI and 0.08%(95%CI 0.06–0.11) for male-to-female VI from studies in developed countries, which roughly translates to a 20-fold increase in HIV risk per receptive AI act compared to receptive VI(12). However, detailed data on the transmission probability of insertive AI are lacking(13). Assuming that women engaging in AI have 8.2 unprotected sex acts over 3 months (with a HIV positive partner), 43% of which are receptive AI (as reported by the current study(1)), then increases in per-act HIV transmission probability of AI compared to VI of 5-, 10- and 20-fold translate into 3-, 5- and 9-fold increases respectively in the three-month cumulative risk of HIV acquisition, compared to the situation where all acts are VI (cumulative risk=0.0065)(Figure A).
To further appreciate the potential role of AI, it also helps to think about other risk factors for HIV transmission. Increased risk due to AI is as large as the best estimates of the relative increase in infectivity during the acute phase of HIV, which has been estimated to range between 9.2-fold (95%CI 4 · 5–18 · 8)(12) and 26-fold (95%CI 12–47)(14); the acute phase is much shorter than the period of time during which AI may be practiced. Given that 22% of the participants in the current study reporting AI tested HIV positive (compared to 9% amongst those who did not), the potential for transmission may not be negligible, especially if the rate of partnership turnover is fast. In the current study(1), those engaging in AI reported 2.3 partners in the past 3 months compared to 1.4 for those who did not.
AI (and VI) may be more risky in SA than other developed countries due to the presence of other risk factors that increase HIV susceptibility and infectivity. For VI, a meta-analysis reported higher HIV transmission probability for developing (0.3%) than developed countries (0.08%)(12). However, it was not possible to know if the difference was due to under-reporting of unprotected sex acts, AI, or extramarital partners or due to higher prevalence of key cofactors for HIV transmission. In all cases, AI may have a greater role than first appears due to under-reporting and/or because of interacting risk factors.
Figure B highlights another reason why the role of AI should not be underestimated as it may also jeopardise development of new vaginal microbicides by reducing their estimated effectiveness in clinical trials and also reducing their effectiveness in the field upon roll-out. Even 5% AI practice can have a marked effect on the cumulative HIV risk. The effectiveness of a microbicide with 80% efficacy per VI act reduces from 79% and 78% effectiveness for 1 and 3 year trials respectively, if there is no AI practised, to 60% and 58% if used vaginally but not anally with 5% of AI(13). This may lead to a drastic reduction in the statistical power in microbicide trials(15).
Given these considerations, it seems premature to conclude that AI is not or was not important in HIV transmission. Acknowledging that AI may be important does not mean that VI is not important but it gives us the mandate to improve understanding of AI’s role. The next two steps should be to use transmission dynamic modeling to understand under which conditions AI can have a significant impact and to collect data to validate the resulting hypotheses. This latter calls for more studies similar to Kalichman et al(1), with a strong emphasis on using validated methods which reduce social desirability and recall biases.
Figure 1.
A) Cumulative risk of HIV acquisition for females over three months (assuming 100% of sex acts with a HIV positive partner) as a function of the relative increase in per-act HIV transmission probability per receptive anal intercourse (AI) compared to receptive vaginal intercourse (VI). The figure shows that AI, practiced at a rate similar to what was reported in this study(1), can rapidly increase the cumulative risk of HIV infection within a relationship with a HIV positive partner. The cumulative risk is derived by CumRisk = 1 ((1 − pv)nact* fv · (1 − RRa · pv)nact* fa where pv= per-act male-to-female vaginal transmission probability; nact = total number of unprotected sex acts in three months; fv and fa (fv=1 − fa) are the fractions of sex acts which are vaginal and anal respectively and RRa is the increase in risk per-act for AI compared to VI. RRa = pa/pv where pa= per-act receptive AI transmission probability. We assumed nact=8.17 unprotected sex acts over three months of which 43% are AI (fv=56%, fa=43%) (as reported by Kalichman et al for women reporting AI (1)); the assumed male-to-female per-act HIV transmission probability for VI is set to the developed country estimate of pv=0.08%(12).
B) Relationship between cumulative risk of HIV acquisition for females in the intervention arm during a hypothetical microbicide trial of different durations (x-scale) where 0%, 1%, 5%, 10% and 20% of sex acts are receptive anal intercourse (AI), when the microbicide has 80% efficacy when used vaginally and adherence is 100% for vaginal intercourse but 0% for AI. We assumed that 2.0 acts/week are not protected by condoms and that 25% of all acts are with HIV-infected partners. Male-to-female VI transmission probability is assumed to be 0.3%, as for developing countries and 1.7% for AI(12). The dark solid line represents the risk in absence of AI (0%) which when compared to the trial arm without microbicide (not shown) produces an effectiveness of 79% and 78% in a trial of 1 and 3 years respectively. The cumulative risk is calculated using a similar method to that used for Figure A, allowing for reduced per-act transmission probability according to efficacy of microbicide (further details in (13)).
Key messages.
It is premature to conclude that heterosexual AI is not important to overall HIV transmission in South Africa based solely on the results of Kalichman et al [1].
Given the high infectivity associated with receptive anal intercourse (AI), heterosexual AI can significantly increase the individual risk of HIV infection
Additional studies quantifying the prevalence and frequency of episodes of unprotected AI among heterosexuals and the contribution of AI to the HIV epidemic overall, especially in sub Saharan Africa are important.
Quantifying AI in clinical trials is also important because it has significant implications for the evaluation of interventions (such as microbicides) designed to control HIV spread
Acknowledgments
Funding
This work was supported by the Wellcome Trust (GR082623MA to RFB); BM was supported by the National Institutes of Health (grant number 5 U01 AI068615-03). The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Wellcome Trust or NIH.
Footnotes
Author’s contribution
MCB wrote the first draft and produced some of the results and figures. RFB also produced results and some figures and revised the different drafts of the manuscript. BM significantly contributed to the first draft of the manuscript, verified results and edited subsequent drafts.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Sexually Transmitted Infections and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence.
References
- 1.Kalichman SC, Simbayi L, Cain D, Jooste S. Heterosexual anal intercourse among community and clinical settings. Cape Town, South Africa: STI; 2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips A, Gomez G, Boily MC, Garnett GP. Abstract ISSTDR. London: Jun, 2009. A systematic review and meta-analysis of interviewing tools to investigate HIV risk behaviour in developing countries. [Google Scholar]
- 3.Minani I, Alary M, Lowndes CM, et al. Abstract OS1.8.03 ISSTDR. London: Jun, 2009. Higher levels of HIV-related risky behaviour reported in Polling Booth Surveys (PBS) compared to Face-to-Face Interviews in a General Population Survey (GPS) in Cotonou-BENIN (West Africa) [Google Scholar]
- 4.Tian LH, Peterman TA, Tao G, Brooks LC, Metcalf C, Malotte CK, Paul SM, Douglas JM, Jr RESPECT-2 Study Group. Heterosexual anal sex activity in the year after an STD clinic visit. Sex Transm Dis. 2008 Nov;35(11):905–9. doi: 10.1097/OLQ.0b013e318181294b. [DOI] [PubMed] [Google Scholar]
- 5.Houston AM, Fang J, Husman C, Peralta L. More than just vaginal intercourse: anal intercourse and condom use patterns in the context of “main” and “casual” sexual relationships among urban minority adolescent females. J Pediatr Adolesc Gynecol. 2007 Oct;20(5):299–304. doi: 10.1016/j.jpag.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Lane T, Pettifor A, Pascoe S, Fiamma A, Rees H. Heterosexual anal intercourse increases risk of HIV infection among young South African men. AIDS. 2006;2;20(1):123–125. doi: 10.1097/01.aids.0000198083.55078.02. [DOI] [PubMed] [Google Scholar]
- 7.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 8.Nicolosi A, Correa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology. 1994 Nov;5(6):570–5. doi: 10.1097/00001648-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Karim SS, Ramjee G. Anal sex and HIV transmission in women. Am J Public Health. 1998 Aug;88(8):1265–6. doi: 10.2105/ajph.88.8.1265-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallah ED, Grunitzky-Bekele M, Bassabi K, Dodzro K, Sadzo A, Balogou AK, et al. Sexual behavior, knowledge and attitudes to AIDS and sexually transmitted diseases of students at the University of Benin (Togo) 2. Vol. 9. Sante (Montrouge, France): 1999. Mar-Apr. pp. 101–9. [PubMed] [Google Scholar]
- 11.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomized controlled trial. Lancet. 2002;360:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 12.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009 Feb;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. doi: 10.1093/ije/dyq057. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008 Sep 1;198(5):687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 15.McGowan I. Microbicide Trials Network. Alliance for Microbicide Development Annual Meeting; Key Bridge Marriot, Arlington, VA, USA. 24 April 2008; [accessed 20 May 2009]. (slide #18 available at: http://microbicide.org/galleries/amd/McGowan.MTNTrialLearning.AMDMeeting11.FINAL.24Apr08.pdf . [Google Scholar]