Abstract
Introduction
Sensorineural hearing loss affects a high percentage of the population. Ototoxicity is a serious and pervasive problem in patients treated with cisplatin. Strategies to ameliorate ototoxicity without compromising on antitumor activity of treatments are urgently needed. Similar problems occur with aminoglycoside antibiotic therapy for infections. Noise-induced hearing loss affects a large number of people. The use of ear protection is not always possible or effective. The prevention of hearing loss with drug therapy would have a huge impact in reducing the number of persons with hearing loss from these major causes.
Areas covered
This review discusses significant research findings dealing with the use of protective agents against hearing loss caused by cisplatin, aminoglycoside antibiotics and noise trauma. The efficacy in animal studies and the application of these protective agents in clinical trials that are ongoing are presented.
Expert opinion
The reader will gain new insights into current and projected future strategies to prevent sensorineural hearing loss from cisplatin chemotherapy, aminoglycoside antibiotic therapy and noise exposure. The future appears to offer numerous agents to prevent hearing loss caused by cisplatin, aminoglycoside antibiotics and noise. Novel delivery systems will provide ways to guide these protective agents to the desired target areas in the inner ear and will circumvent problems with therapeutic interference of anti-tumor and antibiotics agents and will minimize undesired side effects.
Keywords: aminoglycoside antibiotics, anti-inflammatory agents, antioxidants, cisplatin, cochlea, drug delivery, nanotechnology, noise-induced hearing loss, ototoxicity, reactive oxygen species, sensorineural hearing loss, siRNA, stem cell therapy
1. Introduction
Sensorineural hearing loss is caused by damage to the inner ear (cochlea) or to the neural pathways from the inner ear (retrocochlear) to the brain. This sensory deficit may be congenital or acquired. There are no known cures (medical or surgical) for this kind of hearing loss because it is permanent. Some causes are: birth injury, drugs toxic to the auditory system, noise exposure, head trauma, aging, tumors, and genetic syndromes, [1]. In this review, we will address SNHL that is drug induced (platinum drugs and aminoglycosides), noise-induced, and age induced (briefly), drugs in clinical trials, a brief overview of the experimental drugs most likely to succeed and effective routes of delivery. A broad and generalized molecular mechanism for cochlear insult and injury is provided further below in section 4.
2. Drug induced hearing loss
There are two main classes of drugs that can cause permanent hearing loss: Aminoglycoside antibiotics and platinum-based chemotherapeutic agents.
2.1 Cisplatin induced hearing loss
Cisplatin is used for treatment of solid tumors like ovarian, testicular, cervical, lung, head and neck and bladder cancers. Side effects of cisplatin that can limit dosing include nephrotoxicity, neurotoxicity and ototoxicity. Nephrotoxicity can be reduced using saline hydration with or without mannitol diuresis; there are no clinically proven protective modalities for cisplatin ototoxicity. Elevations of audiometric thresholds have been reported in some studies in 75–100% of patients treated with cisplatin [2]. Hearing loss is usually bilateral and irreversible, and is particularly severe in young children with neuroblastoma, CNS malignancies, and in adults with head and neck cancers, in which the base of the skull or brain may be irradiated [3]. Hearing in young children impairs speech, cognitive and social development. There is an imperative need for treatments to prevent cisplatin ototoxicity. This goal could be accomplished by inhibition of uptake transporters, ROS generating systems.
2.2 Aminoglycoside ototoxicity
Aminoglycoside antibiotics are used in the treatment of gram-negative bacterial infections like tuberculosis; tularemia and other hospital acquired serious infections. Dose-limiting side effects include cochlear and/or vestibular toxicity and nephrotoxicity. Cochlear toxicity is primarily due to death of outer hair cells in the organ of Corti [4]. As with cisplatin ototoxicity, aminoglycoside ototoxicity has been linked to ROS and RNS generation, wherein antioxidant therapy has been shown to be otoprotective [5–7].
3. Noise induced hearing loss
Exposure to high levels of noise is the most common cause of hearing loss in adults. According to the latest NIDCD reports more than 30 million people in the US alone are exposed to hazardous levels of noise regularly (1). Among the causes of NIHL are: death or damage of the organ of Corti [8–13], ischemia of the inner ear [14–20], and increased metabolic activity leading to excessive ROS generation and lipid peroxidation [21–27]. Our laboratory as well as others has shown that noise exposure induces reactive oxygen species (ROS) generation in the cochlea as early as 1 hr post exposure to a secondary peak seen several hours post exposure [28,29], which persist for several days after noise trauma noise exposure. This leads to hair cell damage and death that continues for days after noise exposure [30]. Control of ROS generation in NIHL by administration of single or multiple antioxidants may provide an effective therapeutic strategy.
4. Age related hearing loss or Presbycusis
Age related hearing loss (ARHL or AHL) is the gradual loss of hearing with age. According to National Institute of deafness and other communication disorders (NIDCD), about 30–35 percent of adults between the ages of 65 and 75 years have a hearing loss. It is estimated that 40–50 percent of people 75 and older have a hearing loss. Presbycusis is one of the most common conditions affecting the elderly. It is estimated that by 2025, approximately 24.5 million Americans will be affected (Heman-Ackah S et. al, US Census Bureau, State Interim Population Projections by Age and Sex: 2004–2030 [31]. Literature review suggests that (not taking genetic predisposition or any other health condition like high blood pressure, diabetes etc.) accumulation of oxidative damage to the inner ear may be the most fundamental cause of AHL.
Riva et al., [32] showed in cd/1 mice that the combination of “HIF-ROS” induced multiple reactions within the cochlea, including a strong inflammatory response demonstrated by increased expression of TNF-alpha, and inhibition of neuronal protection mechanisms, including repression of IGF-1.
Caloric restriction suppresses apoptotic cell death in the mouse cochlea and prevents late onset of presbycusis. Calorie restricted (CR) mice were shown to retain normal hearing without any cochlear degeneration. CR mice demonstrated a significant decrease in the number of TUNEL-positive cells and cleaved caspase-3-positive cells relative to middle-age control mice. Microarray analysis revealed that CR down-regulated the expression of 24 apoptotic genes, including Bak and Bim. These findings suggest that apoptosis of key cells is an important mechanism of presbycusis in mammals, and that CR can retard this process by suppressing apoptosis in the inner ear [33].
ARHL in C57BL/6J mice is mediated by mitochondrial apoptosis via a Bak-dependent mechanism. Bak knockout mice were protected against outer hair cell loss and the death of spiral ganglion neurons in the cochlea and prevented ARHL. A mitochondrially targeted catalase transgene was found to suppress Bak expression in the cochlea and prevented loss of outer hair cells and spiral ganglion neurons and ARHL. Oral alpha-lipoic acid and coenzyme Q10 suppressed Bak expression in the cochlea, reducing cell death and preventing ARHL [34]. These findings suggest that antioxidants may reduce oxidative stress in the cochlea and long-term therapy with these agents may retard the onset of presbycusis in humans.
An inverse association between total n-3 Polyunsaturated Fatty Acid (PUFA) intake and prevalent hearing loss in a survey of participants aged 50 years of age or greater in a population-based survey of age-related hearing loss (1997–1999 to 2002–2004). The authors used a semi-quantitative food-frequency questionnaire and calculated PUFA and fish intakes. Participants who had 2 or more servings of fish per week compared with participants who had less than 1 serving of fish per week had a significantly reduced risk of developing presbycusis at audiometric follow-up. These findings further support a role of oxidative stress in ARHL. Dietary intervention with n-3 PUFAs could delay the development of ARHL [35].
Antioxidants may have a protective effect against AHRL. C57BL/6 mice were fed with a combination agent containing six antioxidant agents targeting four sites within the oxidative pathway: L-cysteine-glutathione mixed disulfide, ribose-cysteine, NW-nitro-L-arginine methyl ester, vitamin B12, folate, and ascorbic acid. Auditory brainstem response (ABR) thresholds were recorded at baseline and every three months following initiation of treatment. Combination antioxidant therapy effectively decreased ABR threshold shifts. Antioxidant therapy may provide a useful method for reducing the severity of presbycusis.[36].
5. Mechanism of Cochlear injury and targets/approaches in prevention
A very general mechanism that is common to all the insulting agents in addition to their specific pathways is the chronic generation of reactive oxygen species (ROS) in all the three subregions of the organ of Corti: stria vascularis, spiral ligament and spiral ganglionic cells. This ROS overload leads to the depletion of cochlear antioxidant enzyme system (e.g. superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione reductase (GSH-R) etc.), that scavenge and neutralize the superoxides generated. Thus, increase in ROS generation leads to increase in proinflammatory cytokines-leading to inflammation, superoxide formation- eventually forming peroxinitryls and 4-hydroxynonenol (4HNE) and activation and cleavage of pro-apoptotic enzymes such as caspases, among other pathways. Drug targets in clinical trials as well as experimental animal models have traditionally been anti-inflammatory, anti-oxidants and ROS scavengers. Recently, innovations such as antibodies (TNF-α inhibitor), siRNA (NOX3, TRPV1 in cisplatin ototoxicity), caspase inhibitors, JNK pathway inhibitors (for noise exposure) and inhibitors of copper transporters (for cisplatin ototoxicity) have come to play in prevention of cochlear injury and hearing loss. Figure. 1 provides a broad overview of the mechanism of cochlear insult and the potential inhibitors.
Figure 1. A general overview of cochlear injury and targets or approaches in prevention.
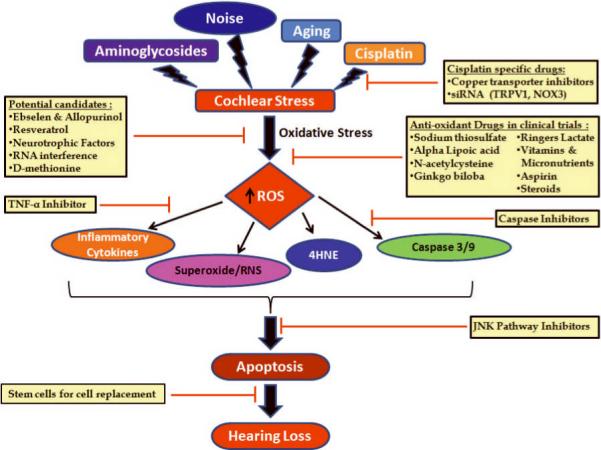
Acquired sensorineural hearing loss has been chiefly subdivided into 4 main categories hearing loss due to aminoglycosides, aging, noise exposure and cisplatin treatment. While each of these ototoxic agents and aging have their own specific molecular pathways causing hearing loss, they all produce oxidative stress, leading to increased reactive oxygen species (ROS) generation. Most of the drugs in clinical trials as well as the potential candidate drugs target increased ROS generation. Some of the latest drugs or approaches in prevention of sensorineural hearing loss target the downstream effector molecules like TNF-α, caspases, stress pathways and partial or complete replacement of dead and damaged sensory hair cells.
6. Initial Impression
The current trend of thought suggests control of ROS generation in acquired sensorineural hearing loss (SNHL) by single or multiple antioxidants approach will alleviate hearing loss. This is confirmed by the drugs that are being investigated/tested in clinical trials for sudden sensorineural hearing loss, noise induced and cisplatin induced hearing loss.
A comprehensive review of the literature on acquired SNHL suggests that the first line of defense seems to inhibit the surge of free radicals that result due to various insults to the cochleae either by antioxidant therapy or by inhibition of inflammation and pro-inflammatory cytokines, specific ROS generating system or by blockage of ROS/stress induced apoptotic pathways. This approach is reflected in the drugs/drug targets being tested in vivo in the laboratories and in clinical trials in the US. We have tried to focus on the latest discoveries (experimental) and short listed molecules/drugs/targets that are likely to succeed in the clinical trials as well as the routes of administration. Thus we have listed several categories the up and coming drug candidates that may help in the amelioration of acquired SNHL.
7. Drugs in clinical trials
The majority of drugs on clinical trials for prevention of hearing loss are antioxidants [37], or anti-inflammatory drugs like steroids. This is consistent with the global scientific view that damage due to increased ROS generation whether acute or chronic leads to eventual cochlear hair cell damage and death. Given below is a comprehensive summary of the drug interventions in clinical trials. Though some of these trials have been completed, no outcomes have been posted as yet. However, we believe that some of these drugs may enter the general market in the next few years. By definition “Clinical trials (also called medical research and research studies) are used to determine whether new drugs or treatments are both safe and effective.
7.1 Antioxidants and ROS scavengers
7.1.1 Sodium thiosulfate
The high affinity of sulfur for platinum groups forms the basis for their use against cisplatin toxicity. Such molecules may chelate cisplatin to inactivate it. Included are some of the animal studies that formed the basis of ongoing clinical trials: administration of sodium thiosulfate (STS) along with cisplatin protected against cisplatin-induced hearing loss as assessed by auditory brainstem responses (ABR) [38, 39]. Two clinical trials are currently in phase III and are recruiting participants. The details of these trials are given below:
7.1.1.1 Clinical Trial I: (NCT00716976)
Sodium Thiosulfate in Preventing Hearing Loss in Young Patients Receiving Cisplatin for Newly Diagnosed Germ Cell Tumor, Hepatoblastoma, Medulloblastoma, Neuroblastoma, Osteosarcoma, or Other Malignancy, is currently recruiting participants. In this study, the experimental group of patients will receive sodium thiosulfate administered intravenously over 15 minutes, 6 hours after the completion of each cisplatin infusion. Treatment with sodium thiosulfate continues until the completion of cisplatin therapy. Periodic audiological assessments will be made. The control group of patients will not receive any interventions.
7.1.1.2 Clinical Trial II: (NCT00652132) Cisplatin With or Without Sodium Thiosulfate in Treating Young Patients With Stage I, Stage II, or Stage III Childhood Liver Cancer. This study is recruiting participants
The control group of patients will receive cisplatin IV over 6 hours on day 1. Treatment will be repeated every 2 weeks for 4 courses. Patients with progressive disease after course 4 will be taken off study. Patients without evidence of disease progression will proceed to surgery. Beginning within 3 weeks after surgery, patients will receive cisplatin IV over 6 hours on day 1. Treatment will be repeated every 2 weeks for 2 courses in the absence of disease progression or unacceptable toxicity. In the experimental group of patients the same treatment plan will be followed with the additional administration of sodium thiosulfate intravenously over 15 minutes (beginning 6 hours after completion of cisplatin). Blood and tumor biopsies will be collected periodically for biological and pharmacological studies consisting of biomarker analysis, gene expression profiling, IHC, proteomic analysis, and gene rearrangement analysis. Patients will also undergo auditory evaluations at baseline and at the completion of study treatment or at an age of at least 3.5 years to measure ototoxicity and hearing impairment. Additionally, after completion of study treatment, patients will be followed periodically for at least 5 years.
7.1.1.3 Clinical Study (SIOPEL 6)
Recently, SIOPEL (including the German GPOH, the Japanese Study Group for Pediatric Liver Tumors, and several US centers) began the first large randomized trial of STS (SIOPEL 6) to reduce ototoxicity in children with hepatoblastoma. This phase 3 trial plans to enroll 250 children with standard-risk hepatoblastoma for preoperative treatment with single-agent cisplatin or cisplatin and STS. STS will be given 6 hours after cisplatin infusion to rescue cisplatin ototoxicity without compromising antitumor activity. A randomized phase 3 clinical trial examining the efficacy of STS for preventing hearing loss from cisplatin in newly diagnosed children with hepatoblastoma, germ cell tumors, medulloblastoma, and osteosarcoma has been recently activated by the Children's Oncology Group [40].
7.1.1.4 Comments
STS forms a complex with cisplatin that leads to its inactivation and excretion by kidneys, thus interfering with the tumoricidal activity of cisplatin. Thus, even though STS is being administered 6 hr post cisplatin, there is a strong possibility of it inactivating cisplatin as both the drugs are administered intravenously. Cisplatin acts by accumulation in the cell and DNA adduct formation and this typically starts at 24hr post administration. Interference with anti-tumor efficacy is likely to occur through decrease in the area under the curve (AUC) of cisplatin and/or its monohydrated complex [41, 42]. Furthermore, in the presence of STS, the anti-cancer effect of cisplatin may be diminished by the accelerated disappearance of platinum [43]. Therefore there is concern that systemic administration of STS at this early time point may interact with cisplatin and inactivate it. Thus, an alternative route of administration like trans-tympanic injection might result in a better outcome. In this case, a ventilation tube in the tympanic membrane may be a better alternative and STS could be administered as ear drops over the entire period of cisplatin therapy.
7.1.2 Alpha Lipoic Acid
Antioxidant compounds like lipoic acid have also showed protection from age [44], noise [45, 46] and cisplatin induced ototoxicity in rats [47, 48]. There are no known inhibitory effects of lipoic acid on cisplatin chemotherapy.
7.1.2.1 Clinical Trial 1: (NCT00477607) Alpha-Lipoic Acid in Preventing Hearing Loss in Cancer Patients Undergoing Treatment with Cisplatin
This study is currently recruiting participants and is in Phase II and III. In this study the patients will receive either oral alpha-lipoic acid supplement or a placebo once a day beginning 1 week before the start of cisplatin treatment and continuing for up to 1 month after the completion of cisplatin. During cisplatin treatment, patients will discontinue supplement 1 day prior to the cisplatin treatment and resume daily supplements 2 days post treatment. Hearing will be assessed at baseline, on each day of chemotherapy, and at 1 and 3 months post chemotherapy. Patients will be followed for 3 months after completion of treatment with cisplatin.
7.1.2.2 Comments
This compound has shown several beneficial effects and thus we expect it to ameliorate cisplatin ototoxicity. Systemic lipoic acid has been shown to reduce cisplatin nephrotoxicity. On the other hand, since lipoic acid chelates metals, it has the potential to interfere with the chemotherapeutic activity of cisplatin. This concern will need to be addressed in clinical trials.
7.1.3 N-acetylcysteine (NAC)
NAC has been shown to be otoprotective in both noise induced, aminoglycoside as well as cisplatin ototoxicity in the animals in several studies. Some examples of the studies: Feldman et al. [49] showed otoprotection by NAC against gentamicin induced ototoxicity in hemodialysis patients. NAC's protective action in noise induced hearing loss in chinchilla was shown by Kopke et al., [50], and in guinea pigs by Fetoni et al., [51]. In guinea pigs, transtympanic administration of 2% NAC and Ringer's lactate via antero-superior quadrant myringotomies, prior to cisplatin injection resulted in the preservation of the distortion product otoacoustic emissions (DPOAE) [52].
7.1.3.1 Clinical Trial 1: (NCT01138137) N-acetylcysteine given IV with Cisplatin and Paclitaxel in Patients with Ovarian Cancer
This study is in phase I and is recruiting participants. The primary aim of this study is to determine the Maximum Tolerated Dose (MTD) and assess the toxicity of IV NAC in conjunction with IP cisplatin and IV/IP paclitaxel in subjects with stage 3 or 4 epithelial ovarian cancer that has been surgically debulked. In this study, the patients will undergo chemotherapy for epithelial ovarian cancer with paclitaxel IV, 135 mg/m2 (Day 1) and IP cisplatin 100 mg/m2 (Day2), followed by Taxol IP, 60 mg/m 2 (Day 8) every 3 weeks for 6 courses. Sixty minutes prior to each course of IP cisplatin, IV NAC (starting at 150 mg/kg) will be infused over 30 minutes. A dose escalation scheme will be followed.
7.1.3.2 Clinical Trial II: (NCT00552786) Antioxidation Medication for Noise-induced Hearing Loss. This study has been completed
In this study, 53 noise-exposed workers from steel industries in Taiwan were recruited. The duration of medication (N-acetylcysteine (NAC), 600mg twice daily, or placebo) was 2 weeks, followed by washout for 2 weeks, and then a crossover of NAC and placebo was maintained for further 2 weeks. The results published for this study indicate that NAC prevented temporary daily threshold shifts at high frequencies but did not seem to affect the temporary threshold shift at low frequencies. Interestingly, when the participants were grouped by GST M1/T1 genotypes, the NAC effect was only significant among workers with null genotypes in both GSTM1 and GSTT1 (p = 0.004). NAC may prevent noise-induced TTS among occupationally noise-exposed men. The protective effect of NAC was more prominent in subjects with both GSTM1-null and GSTT1-null genotypes [53]
7.1.3.3: Comments
The participants in this study were exposed to industrial noise for ~16.3 yrs, prior to participation in this clinical trial. The duration of the treatments were short (14days of NAC or placebo, then two weeks washout and then two weeks of crossover with placebo or NAC). Temporary threshold shifts at the end of 2 weeks show protection with NAC at high frequencies (3000, 4000 and 6000 Hz) and was even more pronounced when there was a mutation in the GST gene. In our opinion NAC seems to be otoprotective in the industrial noise setting. We believe that depending on the outcome of a larger phase III trial NAC may not only be found to be otoprotective, but also be one of latest drugs that works better on a certain gene pool.
7.1.4 Ginkgo Biloba
Ginkgo biloba is a potent antioxidant and ROS scavenger that has been shown to be a very effective otoprotectant in sudden hearing loss and cisplatin ototoxicity in the rat [54]
7.1.4.1 Clinical Trials: (NCT01139281) The Protective Effect of Ginkgo Biloba Extract (GBE761) on Cisplatin-induced Ototoxicity in Humans
This compound was in phase II clinical trials and has recently been completed.
The subjects were randomized and allocated in two groups: Control Group (CG) and Study Group (SG). The study group received GBE761(120mg twice a day) plus cisplatin and was guided to ingest GBE761 just before initial cisplatin dosage. The maximum cumulative cisplatin dosage was 300mg/m2. They were followed up for ninety days. Comparisons were made between baseline distortion-product otoacoustic emissions measurements and those DPOAE records after maximum cumulative cisplatin dosage.
7.1.4.2: Comments
Based on the potent antioxidant nature of this compound added to the fact that ginkgo is not known to interfere with cisplatin efficacy [55], we expect this study to have positive outcomes. Toxicity studies show that Ginkgo biloba extract is relatively safe. However, side effects have been reported, including, bleeding, gastrointestinal disturbances, headaches, dizziness, and allergic skin reactions.
7.1.5. Ringer's Lactate
Ringer's lactate solution is isotonic with blood and has historically been used for fluid resuscitation and counteracts acidosis due to acute fluid loss or renal failure. Trans-tympanic lactate administration in guinea pigs has been shown to offer significant partial protection against cisplatin-induced ototoxicity, only at a mid-frequency (2 KHz) [56], and when applied by anterosuperior quadrant myringotomies, prior to cisplatin injection resulted in the preservation of the distortion product otoacoustic emissions (DPOAE) [52]. However, another recent study using the intratympanic application of Ringer's lactate solution through a tympanostomy ventilation tube did not provide protection against cisplatin-induced ototoxicity in chinchillas [57]
7.1.5.1 Clinical Trial 1: (NCT00584155) Transtympanic Ringer's Lactate for the Prevention of Cisplatin Ototoxicity
This clinical trial has been completed. Treatment plan: Lactated Ringer's will be placed in one ear and an inactive saline solution will be placed in the other ear. The active drug is Lactated Ringer's with 0.03% ofloxacin solution. Lactated Ringer's with 0.03% ofloxacin, the patient will be instructed to place one dropper full of solution in the external auditory canal. The bottle will be marked right or left ear. The drops will be administered at the start time, 30 minutes after chemotherapy starts and hourly for 4 hours after infusion. As a placebo comparator each patient will also receive a bottle containing normal saline and 0.03% ofloxacin. The patients will use these drops in the contra lateral ear. According to the treatment plan the patients will use drops in their ears; Lactated Ringer's will be placed in one ear and the placebo comparator solution will be placed in the other. Each participant will receive a hearing evaluation before each dose of Cisplatin and another evaluation 2 to 4 weeks after the final treatment. The primary outcome measure is a comparison of the pre-treatment audiogram with the post-treatment audiogram. No results have been posted for this study as yet.
7.1.5.2 Clinical Trial 2: (NCT01108601) Transtympanic Ringer's Lactate for the Prevention of Cisplatin Ototoxicity
This study is currently recruiting participants and is in Phase I and II of clinical trials. In this study to ensure adequate delivery of the solution to the middle ear, a small pressure equalizing tube will be inserted under local anesthesia before commencement of chemotherapy treatment in the patients. For each patient, only one ear will receive the Ringer's Lactate solution (Ringer's Lactate (0.03% Ciprofloxacin)). The other ear will act as a control. The patient will be instructed to administer four drops of RL solution to the experimental ear twice a day during their chemotherapy treatment. Pre-, mid-(if available) and post-chemotherapy treatment audiograms will be compared to determine changes in hearing from baseline and between ears. Hearing will also be assessed every six months after chemotherapy treatment for up to four years to determine possible long-term effects.
7.1.5.3 Comments
Ringer's lactate administration via ventilation tube may show favorable results, as the lactate is known to act as a buffer for the prevention of acidosis, provide an alternate source of bicarbonate and may also provide an alkalizing effect in the cochlea. However, a chinchilla study failed to demonstrate protection against cisplatin ototoxicity [57]. Thus, human clinical trials may not demonstrate efficacy of this agent against cisplatin ototoxicity.
7.1.6 Dietary supplements: Vitamins and minerals
Cochlear free radical generation (ROS/RNS) is a key contributor in the development of noise induced hearing loss (NIHL) and it starts from 2hrs post exposure and lasts for up to 10 days. Antioxidant therapy has been shown to be effective and as such animal studies have shown that vitamins that act as ROS scavengers like vitamins A, C, and E act in synergy with minerals like magnesium (Mg) to effectively prevent noise-induced damage to the inner ear [58]. These animal studies have culminated in the testing of this combination of vitamins and minerals Aquaquell® in a clinical trial, details of which are given below:
7.1.6.1 Clinical trial: (NCT00808470) Micronutrients to Prevent Noise-induced Hearing Loss
This study is in phase II clinical trial and is open for recruitment. In the long term study in Spain, subjects who are assigned to treatment group in either Airbase or Factory populations will be given dietary supplement (18 mg beta-carotene 500 mg vitamin C (delivered as 500 mg ascorbic acid) 270 mg vitamin E (delivered as 305 mg alpha-tocopherol acetate) 315 mg magnesium (delivered as 1949 mg magnesium citrate)), orally in capsule form. The total daily dose will be divided into two equal half-doses, and the half doses will be consumed for two consecutive days (cross-over studies) or daily, for two years. Placebo comparators will be used. This is a two year study.
In the short-term studies in Sweden and the United States; all subjects will be treated with active dietary supplement in one arm of the study. All subjects will also participate in placebo arm, and order of treatments is masked. The total daily dose will be divided into two equal half-doses, and the half doses will be consumed for two consecutive days (cross-over studies) or daily, for two years.
7.1.6.2 Comments
The scope of success for this study is good. However, side effects may be troublesome. Magnesium citrate has been used as a laxative and could cause diarrhea. This could compromise compliance with this treatment regimen if patients develop diarrhea and stop taking this combination.
7.1.7 Summary
While anti-oxidant therapy seems to be the therapy of choice, it is not without potential side effects of its own. It is imperative to maintain a delicate balance between oxidants-antioxidant system of the body, as ROS is an important signalling molecule in several cellular processes including immune response and in development.
7.2. Anti-inflammatory drugs
7.2.1 Salicylate/Aspirin
Salicylates have been shown to be otoprotective in both cisplatin as well as noise induced ototoxicity. Animal studies have shown that salicylate is effective against cisplatin-induced outer hair cell damage. In a study by [59], subcutaneous administration of sodium salicylate, 90 min prior to cisplatin infusion, attenuated the loss of outer hair cells seen with cisplatin. An additional advantage of salicylate administration was no apparent effect on the antitumor activity of cisplatin [60].
Mice receiving a single injection of salicylic acid (an antioxidant drug which also reversibly depresses the motor protein prestin of the cochlear amplifier in the outer hair cells) just before, and in other mice, just after, 3.5 h of 113-dB SPL broadband noise exposure. The permanent threshold shift (PTS) in mice pretreated with salicylic acid just before the noise exposure was significantly smaller than that in mice exposed to the same noise without salicylic acid. The PTS in the latter was not significantly different from that in mice who received the drug just after the noise. Thus treatment with salicylates, just before noise exposure, may protect the ear from a noise-induced hearing loss [61].
7.2.1.1 Clinical trials: (NCT00578760) Does Aspirin Have a Protective Role against Chemotherapeutically Induced Ototoxicity?
This study has not started recruiting participants as yet. The participants will ingest 325mg aspirin orally during the entire course of chemotherapy. Hearing loss will be evaluated.
7.2.1.2 Comments
The scope of success for this study is good. Though the concerns remain, that oral administration of aspirin with cisplatin chemotherapy may increase nausea and upset the gastric balance and cause gastric bleeding. GI bleeding was a problem in the clinical study performed in China with aspirin and gentamicin. The risks of bleeding will be increased in patients treated with cisplatin because of its propensity to cause thrombocytopenia.
7.2.1.3 Clinical study: Use of Aspirin to alleviate aminoglycoside ototoxicity
A prospective, randomized, double-blind placebo-controlled clinical trial of aspirin administration to patients receiving gentamicin at two hospitals in China was carried out. Gentamicin-induced hearing loss was mostly moderate. The incidence of hearing loss in the placebo group was 13 percent but was significantly lower in the aspirin group 3 percent. Gentamicin efficacy was not affected by aspirin. However, gastric symptoms occurred more frequently in the aspirin-treated group, and three patients had to be discontinued from the study because of gastric bleeding [62].
7.2.2 Steroids
The use of corticosteroids to combat cisplatin and aminoglycoside ototoxicity is based on the premise that attenuation of the ROS generated inflammatory response in the cochlea results in otoprotection. Corticosteroids studies in animals and in cochlear explants have been shown to attenuate the cisplatin and aminoglycoside induced generation of ROS in the cochlea, and thus prevent hearing loss [63–65].
7.2.3 TNF-α inhibitors
Cochlear insult causes inflammation that leads to auditory hair cell damage and death. Pro-inflammatory cytokines like TNF-α, IL-6, IL-1β have been shown to be released by the spiral limbus, spiral ligaments, OHC, and supporting cells of the organ of Corti on cisplatin exposure [66–68], aging [69] and SSHL [66]. The use of TNF-α neutralizing antibody infliximab or etanercept either by intraperitoneal or subcutaneous route of administration provided complete protection from cisplatin ototoxicity [67, 68]. TNF-α antibody Enbrel® (Amgen and Pfizer Inc) is FDA approved for autoimmune disorders including rheumatoid arthritis, thus will be closest to getting approved for another indication for use. Unfortunately, there are no clinical trials for the use of this drug as an otoprotectant planned as yet.
7.2.3.1 Comments
Enbrel® is immunosuppressive and that may be a concern for usage systemically, however, local delivery via transtympanic injections, micro-catheters or via ventilation tubes to the inner ear will provide the same amount of hearing preservation. This drug would be a success in the auditory field as an otoprotectant from multiple causes, if delivered locally.
8 Apoptosis inhibitors
Acoustic trauma as well as aminoglycosides induces stress leading to death and damage of the outer hair cells of the inner ear. Activation of apoptotic pathways like mitogen-activated protein kinase (MAPK)/c-Jun-N terminal kinase (JNK) signal cascade has been shown, thus inhibitors of these pathways such as D-JNKI-1 (AM-111) and SP600125 by transtympanic injections have been successful in conservation of hearing in animals [70–72]. AM-111 is in the clinical trials for acute sensorineural hearing loss.
8.1 Clinical trial 1: Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study
In this study patients with acute acoustic trauma after exposure to exposure to firecrackers in Berlin and Munich on New Year's Eve 2005/2006 were recruited. 11 patients were randomly selected from this group and treated with a single intratympanic admistration of AM-111 at a concentration of 0.4 mg/ml or 2 mg/ml within 24 h after noise exposure. Pure tone audiometry and otoacoustic emissions were assessed before treatment and on days 3 and 30 post treatment. Results of the study showed that AM-111 was therapeutically effective in noise induced hearing loss [73].
8.2 Comments
In this study 5 of the participants reported 13 adverse events, and as there were no control untreated patients, there is not enough data to show any protective effects of AM-111 post noise exposure. A larger study with more participants is required to test the efficacy of AM-111 in noise induced hearing loss. However, because of the high incidence of side effects, this drug may not prove tolerable in patients with noise trauma.
9 Potential Drug candidates from animal studies likely to succeed
9.1 Targeted drug delivery to the cochleae
The isolated and unique anatomical position of the cochlea makes it practically a closed system. It is precisely for this very reason that cochlear targeted drug delivery is extremely difficult. The challenge faced by systemic administration of drugs is to get the otoprotective drug to the cochleae at a sufficiently high concentration rapidly without overwhelming the system or possible interactions or competition with another drug like cisplatin or aminoglycoside antibiotic. Lately, several investigators are using the transtympanic injections that directly target the cochlea without seeming to interfere with systemic drugs administered. The potential drawback is the number of transtympanic injections that may be required and the potential fear of scar tissue formation or tympanic perforation after repeated injections, particularly if corticosteroids are injected. However insertion of a ventilation tube in the tympanic membrane could facilitate repeated drug administration by obviating the need for repeated penetrations of the tympanic membrane. Direct intracochlear application of otoprotective drugs by cochleostomy has been carried out primarily in animal experiments. This approach in humans runs the risk of further damage by entering the cochlea. Future refinements in technique to avoid further damage to the cochlea may make this approach feasible in the future.
9.2 Novel Drugs: RNA interference
“RNAi” refers to double stranded gene silencing RNA's 20–30 nucleotides in length and include microRNA (miRNA) and short inhibitory RNA (siRNA) [74–76]. siRNA silencing is gene specific and has been shown to be very effective in animals, with the drawback of a short life span as exo- and endonucleases in blood, serum degrade siRNA [77]. However, the anatomical isolation of the cochlea serves to circumvent this problem; in addition the presence of very few blood vessels decreases the problem of degradation of the RNAi or siRNA further. Thus the cochlea like the eye is a rather privileged organ specifically in the face of novel gene targeted treatment options. So far drug delivery to the cochlea had been problematic, but in the recent years our laboratory has optimized the route of transtympanic delivery of drugs in animals. Transtympanic injections in humans are done as an outpatient procedure, is relatively non invasive and limits the systemic exposure of the drug in the patient and is probably the best route of target organ drug delivery. The first gene to be silenced by siRNA (round window delivery) was the gap junction protein, beta-2 (GJB2) in the mouse [78].
Three recent research articles have shown that a single transtympanic injection of siRNA 48h prior to drug administration alleviates hearing loss in the rat. Silencing of the Transient Receptor Potential Vanilloid 1 (TRPV1) gene that was shown to be up-regulated in cisplatin treatment was reported after round window membrane delivery [79]. Transtympanic injections of the same siRNA showed comparable protection from cisplatin ototoxicity as with round window administration. The application of siRNA against NOX3, cochlear specific NADPH oxidase enzyme and showed dose dependant rescue from cisplatin induced hearing loss by a single transtympanic injection [80]. The third drug target was siRNA against signal transducers and activators of transcription 1 (STAT1), a transcription factor involved in inflammatory pathways, and showed inhibition of temporary threshold shift [81]. Because these molecular targets are gene specific and are administered transtympanically (less invasive and cochlear targeted delivery) they seem to be ideal drug candidates for otoprotection and prevention of hearing loss. Furthermore these siRNA molecules will not be likely to compromise the chemotherapeutic activity of cisplatin.
9.3 Nanotechnology and drug administration to the cochlea
Nanotechnology offers and exciting potential new method for drug delivery to the cochlea. These methods could provide an excellent way to deliver siRNAs to the cochlea to prevent hearing loss from ototoxic drugs or noise trauma. Lipid nanocapsules are potential vectors for drug delivery into the spiral ganglion cells, nerve fibers, hair cells, and spiral ligament [82]. The local application of rhodamine nanoparticles to the RWM was more effective in targeted delivery to the cochlea than systemic application [83].
The distribution of polylactic/glycolic acid-encapsulated iron oxide nanoparticles (PLGA-NPs) in chinchilla cochleae was studied after application on the round window membrane (RWM). Nanoparticles were found in cochleae with or without exposure to magnet forces appearing in the RWM, perilymph, endolymph, and multiple locations in the organ of Corti using transmission electron microscopy. Electron energy loss spectroscopy confirmed iron elements in nanoparticles. The nanoparticles were distributed throughout the inner ear after application on the chinchilla RWM, with and without magnetic forces [84]. Cy3-labeled silica nanoparticles were placed on the round window membrane of adult mice. Hearing thresholds were determined after nanoparticle delivery by auditory brainstem responses (ABRs). Fluorescent microscopy demonstrated Cy3-labeled nanoparticles signals in the sensory hair cells and the spiral ganglion neurons of both the treated and contralateral inner ears on histological examination. The investigators detected no hearing loss or inflammation was noted in the treated cochlea [85].
9.4 D-methionine
D-methionine, a sulfur-containing amino acid, has also been studied extensively in the prevention of cisplatin-induced hearing loss. Both the systemic (intraperitoneal or oral) and local administration of D-methionine effectively reduced cisplatin ototoxicity [86–88]. D-methionine also elevated the levels of antioxidant enzymes while reduced the levels of malondialdehyde (marker of lipid peroxidation) after cisplatin administration [89]. The concern remains whether systemic administration of D-methionine would potentially inhibit the anti-tumor efficacy of cisplatin. D-methionine has been shown to protect against permanent noise-induced hearing loss when administered both 48 h before and 48 h after noise exposure of chinchillas (6 h 105 dB SPL 4 kHz narrow band) [90].
9.5 Ebselen and allopurinol
The combined oral delivery of ebselen and allopurinol was found to be effective in reducing nephrotoxicity, myelotoxicity, hepatotoxicity and ototoxicity in rats bearing breast or ovarian cancer treated with a repeated cisplatin dosing schedule. Not only was the anti-tumor activity of cisplatin preserved, rather, the mortality, morbidity and outcome were improved in the ovarian cancer model. These findings make this combination of protective agents very attractive [91]. However, one of the rare but serious adverse effects of allopurinol is a hypersensitivity reaction that can be fatal. This should cause one to be very cautious about using this combination of drugs to reduce cisplatin ototoxicity.
Oral administration of ebselen in guinea pigs were exposed for 5 h to 125 dB sound pressure level octave band noise centered at 4 kHz was protective. No adverse systemic or auditory function effects were observed in unexposed controls administered 30 mg/kg ebselen. These observations suggest that ebselen attenuates noise-induced cochlear damage [92].
9.6 Resveratrol
Resveratrol is the active polyphenol found in the skin of red grapes and is thus abundant in red wine. Resveratrol ingestion (430/mcg/kg/day, oral gavage) for three weeks prior to noise exposure and continued post noise exposure period of four more weeks showed significant preservation of hearing [93] in rats.
9.7 Neurotrophic factors
T-817MA (1-{3-[2-(1-benzothiophen-5-yl) ethoxy] propyl}-3-azetidinol maleate) was synthesized as a candidate therapeutic agent for the treatment of Alzheimer's Dementia [94]. This factor was tested for its neuroprotective effects in noise induced hearing loss in guinea pigs [95]. In this study, oral T-817MA was administered in drinking water (0.2, 0.7 mg/ml), 10 days before noise exposure and through the testing period of 10days post exposure. ABR thresholds changes were significantly reduced and hair cells were preserved, suggesting T-817MA protects the cochlea functionally and morphologically.
9.8 Caspase Inhibitors
Caspase family of cysteine proteases plays a key role in apoptosis. In the auditory hair cells caspases 3 and 9 have been shown to participate in apoptotic pathway [96, 97]. Inhibitors of caspases 3 (z-DEVD-fmk) and caspases 9 (z-LEHD-fmk) when administered by intracochlear perfusion showed significant protection from cisplatin ototoxicity [98] in guinea pigs. A more detailed review on the mechanism of caspase action and other cell death pathways and recent patents for cochlear protection was recently published [99]. The use of cochlear perfusion to administer these agents has the potential to cause further damage in the cochlea because of the use of this invasive route of administration. It may not be safe to administer caspase inhibitors by the systemic route because of potential interference with the anti-tumor effects of cisplatin.
9.9 Copper transport Inhibitors
Copper transporters have been demonstrated in cochlear tissue and appear to control cisplatin entry into the cochlea. Inhibitors of copper transporters including cimetidine and copper sulphate have been found to protect against cisplatin ototoxicity in mice [100, 101]. However, copper sulphate is toxic and the therapeutic value is questionable. On the other hand, cimetidine is clinically used for H1 blockade in gastric disturbances and has the potential to be a safe protective agent against cisplatin ototoxicity. Cimetidine was shown not to interfere with the antitumor effect of cisplatin [101]. However, one must be cautious about blocking cisplatin uptake by copper transport inhibitors because of potential interference with uptake by tumor cells.
9.10 Stem cell transplantation
Stem cells are unspecialized somatic or embryonic cells with the ability to differentiate and develop into a cell or tissue population of choice. Research on cochlear regeneration has utilized a variety of stem cells introduced into the damaged cochlea to determine whether they could repopulate and integrate into the injured sensory epithelium. Parker et al., 2007 [102] showed that male neural stem cells transplanted into the scala tympani of noise damaged cochlea of female mice and guinea pigs, were found in the cochlear nerve, where they differentiated into satellite cells (a type of neural supporting cell) and neurons expressing markers characteristic of the normal cells found in the spiral ganglion. Stem cells that infiltrated the organ of Corti or the lateral wall differentiated into cochlear-specific cell types, including inner and outer hair cells, pillar cells, Deiter's cells, fibrocytes in the lateral wall and cells within the stria vascularis.
Exciting new research has shown that mouse embryonic stem and induced pluripotent stem cells were converted into otic progenitor cells. These cells were manipulated so that they aggregated into epithelial clusters demonstrating hair cell-like morphology with stereociliary bundles. Following mechanical stimulation, bundle-bearing cells in these clusters generated currents resembling transduction currents from immature hair cells [103].
Even though the use of stem cells to repair cochlear injury is a relatively new and promising field, it is still in its infancy. There is an urgent need for aggressive investigation and fine-tuning of stem cell transplantation techniques to be able to bring this novel technique from the laboratory to the bedside of deaf patients.
10 CONCLUSION
Recent studies have demonstrated the efficacy of a wide variety of protective agents against hearing loss and cochlear damage from noise and ototoxic injury from aminoglycosides and cisplatin. Most of these investigations were carried out in vitro or in rodent models. Several clinical trials have been initiated to study the effects of these agents in patients. Protective strategies have employed antioxidant compounds, anti-inflammatory agents or RNA silencing to achieve positive results. Cisplatin ototoxicity has been shown to be ameliorated by antioxidant compounds. Clinical trials using sodium thiosulfate, alpha-lipoic acid, N-acetylcysteine, and ginkgo biloba extract are ongoing. There is concern about the interference with the anti-neoplastic effect of cisplatin with antioxidants, especially with sodium thiosulfate. Animal studies using anti-inflammatory agents, like dexamethasone and etanercept look promising. The former drug has been used by trans-tympanic injection in mice. The administration of cimetidine appears promising for inhibition of a copper transporter in the cochlea. This drug could be given orally or by transtympanic injection, RNA silencing using trans-tympanic administration of siRNA against NOX3 or TRPV1 has been shown to protect against cisplatin ototoxicity in rats. Silencing of the copper transporter (CTR1) in vitro decreased the cytotoxicity of cisplatin. The implementation of nanotechnology to deliver siRNAs or other therapeutic agents may offer future strategies to protect the cochlea from drug or noise-induced hearing loss in the future. Clinically effective treatments for prevention of aminoglycoside antibiotic ototoxicity include aspirin, N-acetylcysteine. The problem with the use of aspirin is gastrointestinal toxicity that may limit or preclude the administration of this agent in patients receiving aminoglycoside or cisplatin therapy. Promising agents for protection against noise-induced hearing loss include antioxidants: D-methionine, ebselen, resveratrol, N-acetylcysteine, lipoic acid and micronutrients with antioxidant vitamins. These are being tested in clinical trials to determine efficacy in patients. The use of oral dosing makes these protective agents particularly attractive for this indication. There also appears to be a time window after noise exposure that will allow the delayed administration of a protective agent and still ameliorate the hearing loss that would otherwise be incurred.
11 Expert Opinion
The key findings in research done in this field to date are that a wide variety of potential protective agents have been reported against noise and otototoxic drugs in animals. These include antioxidants, inhibitors of cell death pathways, anti-inflammatory drugs and siRNAs. Weaknesses in research to date are that numerous studies have shown the efficacy of protective drugs in vitro. Fewer investigations have been carried out in vivo, and most of these reports have utilized rodent models (rat, guinea pig, gerbil, or mouse). There have been reports that a wide range of drugs appear to protect against hearing loss from ototoxic insults from cisplatin, aminoglycoside antibiotics and noise. However, most experimental studies have demonstrated only partial protection. Very few clinical trials have been performed. There are challenges in delivery of protective agents to the cochlea. Some drugs or genes have been delivered by intracochlear perfusion, which is too invasive for application to patients. Very few investigations have used the oral route of administration. Very little information on side effects has been reported, including the potential for interference with desired therapeutic effects of chemotherapy or antimicrobial therapy. There is great potential that single agents or combinations of drugs will be effective in protecting the cochlea against damage from noise or ototoxic drugs.
The ultimate goal in this field is effective protection against inner ear injury with no undesirable side effects and without interference with therapeutic action of ototoxic agents. Delivery should be noninvasive or only minimally invasive. Oral delivery would be ideal if the protective agent has the desired pharmacokinetic characteristics, however, intravenous or subcutaneous injection would also be acceptable.
The discovery of new compounds can be facilitated by testing potential agents for efficacy, toxicity and favorability of pharmacokinetic profile and rapidly excluding drugs that do not meet desired standards. In order to achieve this goal, a systematic approach with high throughput screening e.g., the zebrafish model or mammalian in vitro models (organotypic organ culture, hair cell precursor cultures, such as the UB-OC-1 or HEI-OC1 cell lines) followed by in vivo testing in mammalian animal models to provide proof of concept for efficacy, mechanisms of action and potential side effects. Ultimately clinical trials will be needed. The biggest challenge is to discover efficacious protective agents that can be given orally or by parenteral injection without significant side effects and without interference with therapeutic effects of cisplatin or aminoglycosides.
The field appears to be moving toward nanotechnology and gene silencing with siRNA. Novel developments of drug delivery in the future could provide exciting ways of providing protective agents in effective concentrations to the inner ear. The ability to deliver a variety of protective agents by trans-tympanic injection is of great interest at the present time. This allows the physician to deliver the protective compound to the inner ear in therapeutic concentrations and avoid systemic toxicity and interference with the pharmacokinetics and pharmacodynamics of the aminoglycoside antibiotics or cisplatin. Alternative technologies or approaches include gene therapy or RNA silencing using non-viral delivery methods, such as nanotechnology. These technologies provide potential alternative methods to supply protective molecules to counteract the toxic effects of noise or ototoxic drugs on the cochlea. The future application of stem cell therapy to the inner ear has the potential to replace damaged cells or to supply protective and/or trophic molecules to the cochlea. Currently, however, there are problems with effective delivery of stem cells that do not require invasive procedures.
Acknowledgments
Declaration of Interest LP Rybak is supported by a grant from the National Institutes of Health (RO1DC02396), while V Ramkumar is supported by a SIU School of Medicine Excellence in Academic Medicine Award and NIH grant (CA135494). D Mukherjea is additionally supported by a National Research Service Award (NRSA F32 award DC009950).
Abbreviations
- ABR
Auditory Brainstem Response
- CTR1
Copper Transporter 1
- DPOAE
Distortion Product Otoacoustic Emissions
- GST
Glutathione S Transferase
- HIF
Hypoxia Inducible Factor
- IL
Interleukin
- IP
Intra-peritonial
- JNK
c-Jun-N terminal kinase
- Mg
Magnesium
- MAPK
Mitogen-Activated Protein Kinase
- NAC
N-acetyl cysteine
- NIHL
Noise induced hearing loss
- NOX3
NADPH oxidase isoform 3
- PTS
Permanent Threshold Shift
- PLGA-NPs
Polylactic/Glycolic Acid-encapsulated iron oxide nanoparticles
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- RWM
Round Window Membrane
- SNHL
Sensorineural hearing loss
- siRNA
Short interfering RNA
- STAT1
Signal Transducers and Activators of Transcription 1
- STS
Sodium thiosulfate
- TRPV1
Transient Receptor Potential 1
- TNF-α
Tumor Necrosis Factor-α
References
- 1. www.asha.org.
- 2.McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug. Saf. 1995;13:228–244. doi: 10.2165/00002018-199513040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Chen WC, Jackson A, Budnick AS, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106:820–829. doi: 10.1002/cncr.21683. [DOI] [PubMed] [Google Scholar]
- 4.Warchol ME. Cellular mechanisms of aminoglycoside ototoxicity. Current Opinion in Otolaryngology & Head and Neck Surgery. 2010;18:454–458. doi: 10.1097/MOO.0b013e32833e05ec. [DOI] [PubMed] [Google Scholar]; ***This is an up-to-date review of the mechanisms of ototoxicity of aminoglycosides.
- 5.Kovacic P, Sacman A, Wu-Weis M. Nephrotoxins: Widespread role of oxidative stress and electron transfer. Curr Med Chem. 2009;9:823–847. doi: 10.2174/0929867024606803. [DOI] [PubMed] [Google Scholar]
- 6.Takumida M, Popa R, Anniko M. Free radicals in the guinea pig inner ear following gentamycin exposure. Orl J Otorhinolaryngol Relat Spec. 1999;61:63–70. doi: 10.1159/000027643. [DOI] [PubMed] [Google Scholar]
- 7.Evans P, Halliwell P. Free Radicals and hearing: cause, consequences, and criteria. Ann NY Acad Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- 8.Spoendlin H. Primary structural changes in the organ of corti after acoustic overstimulation. Acta Otolaryngol (Stockh) 1971;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins JE, Jr., Johnsson LG, Stebbins WC, et al. Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol (Stockh) 1976;81:337–343. doi: 10.3109/00016487609119971. [DOI] [PubMed] [Google Scholar]
- 10.Hamernik RP, Henderson D, Crossley JJ, et al. Interaction of continuous and impulse noise: Audiometric and histological effects. J Acoust Soc Am. 1974;55:117–121. doi: 10.1121/1.1928141. [DOI] [PubMed] [Google Scholar]
- 11.Hunter-Duvar IM, Bredberg G. Effects of intense auditory stimulation: Hearing losses and inner ear changes in the chinchilla. J Acoust Soc Am. 1974;55:795–801. doi: 10.1121/1.1914602. [DOI] [PubMed] [Google Scholar]
- 12.Hunter-Duvar IM, Elliott DN. Effects of intense auditory stimulation: Hearing losses and inner ear changes in the squirrel monkey. J Acoust Soc Am. 1973;54:1179–1183. doi: 10.1121/1.1914364. [DOI] [PubMed] [Google Scholar]
- 13.Mulroy MJ, Henry WR, McNeil PL. Noise-induced transient microlesions in the cell membranes of auditory hair cells. Hear Res. 1998;115:93–100. doi: 10.1016/s0378-5955(97)00181-0. [DOI] [PubMed] [Google Scholar]
- 14.Lipscomb DM, Roettger RL. Capillary constriction in cochlear and vestibular tissues during intense noise stimulation. Laryngoscope. 1973;83:259–263. doi: 10.1288/00005537-197302000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins JE, Jr, Johnsson LG, Preston RE. Cochlear microvasculature in normal and damaged ears. Laryngoscope. 1972;82:1091–1104. doi: 10.1288/00005537-197207000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson A, Dengerink H. The effects of noise on histological measures of the cochlear vasculature and red blood cells: A review. Hear Res. 1987;31:183–191. doi: 10.1016/0378-5955(87)90125-0. [DOI] [PubMed] [Google Scholar]
- 17.Axelsson A, Vertes D. Histological findings in cochlear vessels after noise. In: Hamernik R, Anderson D, Salvi R, editors. New perspectives in noise-induced hearing loss. Raven; New York: 1981. pp. 49–68. [Google Scholar]
- 18.Miller JM, Ren TY, Dengerink HA, et al. Cochlear blood flow changes with short sound stimulation. In: Axelsson A, Borchgrevink HM, Hamernik RP, Hellstrom PA, Henderson D, Salvi RJ, editors. Scientific basis of noise-induced hearing loss. Thieme Medical Publishers; New York: 1996. pp. 95–109. [Google Scholar]
- 19.Duvall AJ, 3rd, Robinson KS. Local vs systemic effects of acoustic trauma on cochlear structure and transport. Arch Otolaryngol Head Neck Surg. 1987;113:1066–1071. doi: 10.1001/archotol.1987.01860100044019. [DOI] [PubMed] [Google Scholar]
- 20.Santi PA, Duvall AJ., 3rd Stria vascularis pathology and recovery following noise exposure. Otolaryngology. 1978;86 doi: 10.1177/019459987808600229. [DOI] [PubMed] [Google Scholar]
- 21.Yamane H, Nakai Y, Takayama M, et al. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–508. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- 22.Ohlemiller KK, McFadden SL, Ding DL, et al. Targeted mutation of the gene for cellular glutathione peroxidase (gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- 24.McFadden SL, Ding DL, Ohlemiller KK, et al. The role of superoxide dismutase in age-related and noise-induced hearing loss: Clues from sod1 knockout mice. In: Willot JF, editor. Handbook of mouse auditory research; from behavior to molecular biology. CRC Press; New York: 2001. pp. 489–504. [Google Scholar]
- 25.Ohlemiller KK, McFadden SL, Ding DL, et al. Targeted deletion of the cytosolic cu/zn-superoxide dismutase gene (sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol. 1999;4:237–246. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- 26.Ohinata Y, Miller JM, Altschuler RA, et al. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–173. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- 27.Henderson D, Bielefeld EC, Harris KC, et al. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 28.Miller JM, Yamashita D, Minami S, et al. Mechanisms and prevention of noise-induced hearing loss. Otol Jpn. 2006;16:139–153. [Google Scholar]
- 29.Yamane H, Nakai Y, Takayama M, et al. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–508. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- 30.Bohne BA. Safe level for noise exposure? Ann Otol Rhinol Laryngol. 1976;85:711–724. doi: 10.1177/000348947608500602. [DOI] [PubMed] [Google Scholar]
- 31. http://www.census.gov/population/www/projections/projectionsagesex.html.
- 32.Riva C, Donadieu E, Magnan J, et al. Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp Gerontol. 2007;42(4):327–36. doi: 10.1016/j.exger.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Someya S, Yamasoba T, Weindruch R, et al. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging. 2007;28(10):1613–22. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Someya S, Xu J, Kondo K, et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106(46):19432–7. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopinath B, Flood VM, Rochtchina E, et al. Consumption of omega-3 fatty acids and fish and risk of age-related hearing loss. Am J Clin Nutr. 2010;92(2):416–21. doi: 10.3945/ajcn.2010.29370. [DOI] [PubMed] [Google Scholar]
- 36.Heman-Ackah SE, Juhn SK, Huang TC, et al. A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice. Otolaryngol Head Neck Surg. 2010;143:429–34. doi: 10.1016/j.otohns.2010.04.266. [DOI] [PubMed] [Google Scholar]
- 37. www.clinicaltrials.gov.
- 38.Wang J, Lloyd Faulconbridge RV, Fetoni A, et al. Local application of sodium thiosulfate prevents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology. 2003;45(3):380–93. doi: 10.1016/s0028-3908(03)00194-1. [DOI] [PubMed] [Google Scholar]
- 39.Otto WC, Brown RD, Gage-White L, et al. Effects of cisplatin and thiosulfate upon auditory brainstem responses of guinea pigs. Hear Res. 1988;35:79–85. doi: 10.1016/0378-5955(88)90042-1. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan MJ. Hepatoblastoma, cisplatin, and ototoxicity: good news on deaf ears. Cancer. 2009;115:5623–6. doi: 10.1002/cncr.24668. [DOI] [PubMed] [Google Scholar]
- 41.Goel R, Cleary SM, Horton C, et al. Effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J Natl Cancer Inst. 1989;81(20):1552–60. doi: 10.1093/jnci/81.20.1552. [DOI] [PubMed] [Google Scholar]
- 42.Videhult P, Laurell G, Wallin I, et al. Kinetics of Cisplatin and its monohydrated complex with sulfur-containing compounds designed for local otoprotective administration. Exp Biol Med (Maywood) 2006;231(10):1638–45. doi: 10.1177/153537020623101009. [DOI] [PubMed] [Google Scholar]
- 43.Tamura Y, Ikeda O, Nakasone Y, et al. Effect of sodium thiosulfate on cisplatin removal after intra-arterial embolization with a lipiodol-platinum suspension for hepatocellular carcinoma. Acta Radiol. 2010;51:383–8. doi: 10.3109/02841850903563429. [DOI] [PubMed] [Google Scholar]
- 44.Seidman MD, Khan MJ, Bai U, et al. Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol. 2000;2:161–7. doi: 10.1016/s0196-0709(00)80003-4. [DOI] [PubMed] [Google Scholar]
- 45.Pouyatos B, Gearhart C, Nelson-Miller A, et al. Lipoic acid and 6-formylpterin reduce potentiation of noise-induced hearing loss by carbon monoxide: preliminary investigation. J Rehabil Res Dev. 2008;45(7):1053–64. doi: 10.1682/jrrd.2007.12.0200. [DOI] [PubMed] [Google Scholar]
- 46.Diao MF, Liu HY, Zhang YM, et al. Changes in antioxidant capacity of the guinea pig exposed to noise and the protective effect of alpha-lipoic acid against acoustic trauma. Sheng Li Xue Bao. 2003;55(6):672–6. Chinese. [PubMed] [Google Scholar]
- 47.Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope. 1999(a);109(11):1740–1744. doi: 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Rybak LP, Husain K, Whitworth C, et al. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system. Toxicol Sci. 1999(b);47(2):195–202. doi: 10.1093/toxsci/47.2.195. [DOI] [PubMed] [Google Scholar]
- 49.Feldman L, Efrati S, Eviatar E, et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007;72(3):359–63. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]; ***This important clinical study demonstrated the efficacy of this protective agent against cisplatin ototoxicity in this group of highly vulnerable patients.
- 50.Kopke RD, Weisskopf PA, Boone JL, et al. Reduction of noise-induced hearing loss using LNAC and salicylate in the chinchilla. Hear Res. 2000;149(1–2):138–46. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 51.Fetoni AR, Ralli M, Sergi B, et al. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29(2):70–5. [PMC free article] [PubMed] [Google Scholar]
- 52.Choe WT, Chinosornvatana N, Chang KW. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol. 2004;25(6):910–915. doi: 10.1097/00129492-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Lin CY, Wu JL, Shih TS, et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269(1–2):42–7. doi: 10.1016/j.heares.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, Whitworth CA, Rybak LP. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol Neurotol. 2007;28(6):828–33. doi: 10.1097/mao.0b013e3180430163. [DOI] [PubMed] [Google Scholar]
- 55.Fukaya H, Kanno H. Experimental studies of the protective effect of ginkgo biloba extract (GBE) on cisplatin-induced toxicity in rats. Nippon Jibiinkoka Gakkai Kaiho. 1999;102(7):907–17. doi: 10.3950/jibiinkoka.102.907. [DOI] [PubMed] [Google Scholar]
- 56.Nadar SK, Lip GY. New insights into complement C3 and inflammation in hypertension. J Hum Hypertens. 2007;21(4):261–3. doi: 10.1038/sj.jhh.1002160. [DOI] [PubMed] [Google Scholar]
- 57.Munguia R, Sahmkow SI, Funnell WR, et al. Transtympanic Ringer's lactate application in the prevention of cisplatinum-induced ototoxicity in a chinchilla animal model. Otolaryngol Head Neck Surg. 2010;143:134–140. doi: 10.1016/j.otohns.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Le Prell C, Hughes L, Miller J. Free radical scavengers, vitamins A, C, and E, plus magnesium reduces noise trauma. Free Radic Biol Med. 2007;42(9):1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hyppolito MA, de Oliveira JA, Rossato M. Cisplatin ototoxicity and otoprotection with sodium salicylate. Eur Arch Otorhinolaryngol. 2006;263:798–803. doi: 10.1007/s00405-006-0070-6. [DOI] [PubMed] [Google Scholar]
- 60.Li G, Sha SH, Zotova E, et al. Salicylate protects hearing and kidney function from cisplatin toxicity without compromising its oncolytic action. Lab Invest. 2002;82:585–596. doi: 10.1038/labinvest.3780453. [DOI] [PubMed] [Google Scholar]
- 61.Adelman C, Freeman S, Paz Z, et al. Salicylic acid injection before noise exposure reduces permanent threshold shift. Audiol Neurootol. 2008;13(4):266–272. doi: 10.1159/000115436. [DOI] [PubMed] [Google Scholar]
- 62.Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354(17):1856–7. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]; ***This was a prospective, randomized, double-blind, placebo controlled trial of 195 patients receiving gentamicin for infection. They were randomly assigned to receive 14 days of supplementation either with aspirin or with placebo. Ototoxicity was assessed five to seven weeks after treatment.The incidence of hearing loss was significantly lower in the aspirin group compared with the placebo group. This well-designed study demonstrated the efficacy of aspirin as an antioxidant otoprotective agent.
- 63.Himeno C, Komeda M, Izumikawa M, et al. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;67:61–70. doi: 10.1016/s0378-5955(02)00345-3. [DOI] [PubMed] [Google Scholar]
- 64.Park SK, Choi D, Russell P, et al. Protective effect of corticosteroid against the cytotoxicity of aminoglycoside otic drops on isolated cochlear outer hair cells. Laryngoscope. 2004;114(4):768–71. doi: 10.1097/00005537-200404000-00033. [DOI] [PubMed] [Google Scholar]
- 65.Paksoy M, Ayduran E, Sanh A, et al. The protective effects of intratympanic dexamethasone and vitamin E on cisplatin-induced ototoxicity in rats. Med Oncol. 2010 doi: 10.1007/s12032-010-9477-4. epub 19 Mar; DOI 10.1007/s12032-010-9477-4. [DOI] [PubMed] [Google Scholar]
- 66.Van Wijk F, Staecker H, Keithley E, et al. Local perfusion of the tumor necrosis factor alpha blocker infliximab to the inner ear improves autoimmune neurosensory hearing loss. Audiol Neurootol. 2006;11(6):357–65. doi: 10.1159/000095897. [DOI] [PubMed] [Google Scholar]
- 67.So H, Kim H, Lee JH, et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol. 2007;8(3):338–55. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim MG, Yang HN, Kim HW, et al. IL-10 mediates rosiglitazone-induced kidney protection in cisplatin nephrotoxicity. J Korean Med Sci. 2010;25(4):557–63. doi: 10.3346/jkms.2010.25.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tadros SF, D'Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13(11):1303–21. doi: 10.1007/s10495-008-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pirvola U, Xing-Qun L, Virkkala J, et al. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20(1):43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Van De Water TR, Bonny C, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23(24):8596–607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dinh CT, Van De Water TR. Blocking pro-cell-death signal pathways to conserve hearing. Audiol Neurootol. 2009;14(6):383–92. doi: 10.1159/000241895. [DOI] [PubMed] [Google Scholar]
- 73.Suckfuell M, Canis M, Strieth S, et al. Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Otolaryngol. 2007;127(9):938–42. doi: 10.1080/00016480601110212. [DOI] [PubMed] [Google Scholar]
- 74.Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 75.Tabara H, Yasuda J. Physiological and technological aspects of RNAi. Tanpakushitsu Kakusan Koso. 2003;48(4 Suppl):469–79. [PubMed] [Google Scholar]
- 76.Mello CC. A conversation with Craig C Mello on the discovery of RNAi. Cell Death Differ. 2007;14(12):1981–4. doi: 10.1038/sj.cdd.4402249. [DOI] [PubMed] [Google Scholar]
- 77.Hoerter JHA, Walter NG. Chemical modification resolves the asymmetry of siRNA strand degradation in human blood serum. RNA. 2007;13(11):1887–1893. doi: 10.1261/rna.602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maeda Y, Fukushima K, Nishizaki K, et al. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Molec Genet. 2005;14:1641–1650. doi: 10.1093/hmg/ddi172. [DOI] [PubMed] [Google Scholar]
- 79.Mukherjea D, Jajoo S, Whitworth C, et al. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci. 2008;28:13056–13065. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mukherjea D, Jajoo S, Kaur T, et al. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13(5):589–98. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]; ***This study was the first to demonstrate the efficacy of siRNA directed against NOX3 NADPH oxidase in the cochlea by injection through the ear drum into the middle ear in protecting the inner ear from damage to outer hair cells, preventing oxidative stress and reducing hearing loss.
- 81.Mukherjea D, Jajoo S, Sheehan K, et al. NOX3 NADPH Oxidase Couples Transient Receptor Potential Vanilloid 1 to STAT1-Mediated Inflammation and Hearing Loss. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3497. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou J, Saulnier P, Perrier T, et al. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J Biomed Mater Res B Appl Biomater. 2008;87(1):10–8. doi: 10.1002/jbm.b.31058. [DOI] [PubMed] [Google Scholar]
- 83.Tamura T, Kita T, Nakagawa T, Endo Tet al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115(11):2000–5. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- 84.Ge X, Jackson RL, Liu J, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137(4):619–23. doi: 10.1016/j.otohns.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Praetorius M, Brunner C, Lehnert B, et al. Transsynaptic delivery of nanoparticles to the central auditory nervous system. Acta Otolaryngol. 2007;127(5):486–90. doi: 10.1080/00016480600895102. [DOI] [PubMed] [Google Scholar]; ***This interesting study used fluorescently-labeled nanoparticles placed on the round window membrane of adult mice. These nanoparticles were taken up by sensory cells and spiral ganglion cells in both the ipsilateral and contralateral inner ears as well as the central auditory pathways of the brainstem without causing hearing loss or inflammation in the cochlea. This shows a proof of concept that nanoparticles can be introduced into the inner ear by a relatively noninvasive method.
- 86.Campbell KCM, Meech RP, Klemens JJ, et al. Pevention of noise-and drug-induced hearing loss with D-methionine. Hear Res. 2007:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Giordano P, Lorito G, Ciorba A, et al. Protection against cisplatin ototoxicity in a Sprague-Dawley rat animal model. Acta Otorhinolaryngol Ital. 2006;26(4):198–207. [PMC free article] [PubMed] [Google Scholar]
- 88.Korver KD, Rybak LP, Whitworth C, et al. Round window application of D-methionine provides complete cisplatin otoprotection. Otolaryngol Head Neck Surg. 2002;126:683–689. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- 89.Campbell KC, Meech RP, Rybak LP, et al. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol. 2003;14(3):144–156. [PubMed] [Google Scholar]
- 90.Kopke RD, Coleman JK, Liu J, et al. Candidate's thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002;112(9):1515–32. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 91.Lynch ED, Gu R, Pierce, et al. Combined oral delivery of ebselen and allopurinol reduces multiple cisplatin toxicities in rat breast and ovarian cancer models while enhancing anti-tumor activity. Anti-Cancer Drugs. 2005;16:569–579. doi: 10.1097/00001813-200506000-00013. [DOI] [PubMed] [Google Scholar]
- 92.Pourbakht A, Yamasoba T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear Res. 2003;181(1–2):100–8. doi: 10.1016/s0378-5955(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 93.Seidman MD, Khan MJ, Bai U, et al. Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol. 2000;2:161–7. doi: 10.1016/s0196-0709(00)80003-4. [DOI] [PubMed] [Google Scholar]
- 94.Hirata K, Yamaguchi H, Takamura Y, et al. A novel neurotrophic agent, T-817MA [1-{3-[2-(1-benzothiophen-5-yl) ethoxy] propyl}-3-azetidinol maleate], attenuates amyloid-beta-induced neurotoxicity and promotes neurite outgrowth in rat cultured central nervous system neurons. J Pharmacol Exp Ther. 2005;314(1):252–9. doi: 10.1124/jpet.105.083543. [DOI] [PubMed] [Google Scholar]
- 95.Yamashita D, Shiotani A, Kanzaki S, et al. Neuroprotective effects of T-817MA against noise-induced hearing loss. Neurosci Res. 2008;61(1):38–42. doi: 10.1016/j.neures.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 96.Budihardjo I, Oliver H, Lutter M, et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 97.Van De Water TR, Lallemend F, Eshraghi AA, et al. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurotol. 2004;25(4):627–32. doi: 10.1097/00129492-200407000-00035. [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Ladrech S, Pujol R, et al. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64(24):9217–24. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- 99.Abi-Hachem RN, Zine A, Van De Water TR. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Pat CNS Drug Discov. 2010;5(2):147–63. doi: 10.2174/157488910791213121. [DOI] [PubMed] [Google Scholar]; ***This excellent and timely review demonstrates how the cochlea responds with inflammation and cell death toxins and how various approaches to protection of the inner ear have succeeded in counteracting these injury mechanisms.
- 100.More SS, Akil O, Ianculescu AG, et al. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010;30(28):9500–9. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ciarimboli G, Deuster D, Knief A, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176(3):1169–80. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]; ***This recent paper demonstrates how a clinically available agent, cimetidine, may reduce cisplatin ototoxicity and nephrotoxicity by inhibiting cisplatin uptake in the cochlea and kidney.
- 102.Parker MA, Corliss DA, Gray B, et al. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res. 2007;232(1–2):29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oshima K, Teo DT, Senn P, et al. LIF promotes neurogenesis and maintains neural precursors in cell populations derived from spiral ganglion stem cells. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]; ***This outstanding paper describes novel techniques to manipulate both embryonic and somatic stem cells in vitro to generate hair cell-like structures with stereocilia and mechanosensitive transduction currents reminiscent of those seen in immature hair cells.***Very Important Papers


