Abstract
Aims
We examined whether the relationship between anxiety and indicators of glucose metabolism in people without diabetes varies by race and gender.
Methods
Participants were 914 adults (777 white, 137 black) without diabetes in the MIDUS II study. Glucose metabolism was characterized by fasting glucose, insulin, HOMA-IR, and HbA1c. Hierarchical linear regressions stratified by race and gender examined whether anxiety was associated with glucose metabolism.
Results
After adjustment for potential confounders, positive relationships between anxiety and fasting glucose (p=.04), insulin (p=.01), and HOMA-IR (p=.02) but not HbA1c, were observed in black women only.
Conclusions
Our findings extend prior evidence about the links between psychosocial vulnerabilities and impaired glucose metabolism in black women, by documenting significant associations between anxiety and clinical indicators of glycemic control among black women without diabetes. Thus, anxiety might constitute an intervention target in black women, a subgroup disproportionately affected by type 2 diabetes, its complications, and premature mortality.
INTRODUCTION
Much research has documented the importance of mental health for diabetes care: people with diabetes have much higher rates of mental illness than people without diabetes, and having a mental illness such as depression is associated with poorer glycaemic control and increased mortality [1–5]. Compared to people without diabetes, those with diabetes had a 20% higher rate of lifetime diagnosis of anxiety [6], and anxiety disorders are associated with hyperglycemia among people with diabetes [7].
In addition to the research linking mental illness to glycaemic control in type 2 diabetes, mounting evidence demonstrates that psychosocial factors are also independently associated with glucose metabolism and type 2 diabetes risk among people without diabetes. Specifically, depressive symptoms, anger, and hostility are cross-sectionally and prospectively associated with nondiabetic glucose metabolism, particularly among women and black Americans [8–11]. Despite the well-recognized relationship between depression and diabetes and the comorbidity between depression and anxiety [12], the relationship between anxiety and glucose metabolism in people without diabetes remains unknown. Thus, we investigated whether trait anxiety is associated with glucose metabolism in people without diabetes, independently of depressive symptoms and other potential confounds, and whether any potential relationship is most pronounced among black women, compared to their male counterparts or white women.
RESEARCH DESIGN AND METHODS
Sample
Analyses are based on data from the biological subsample of the Midlife in the US (MIDUS) national study that included 1255 participants ages 34 to 84 (57% female) which includes an oversample of blacks living in Milwaukee, WI. Details on MIDUS participants and the biological subsample are available elsewhere [13, 14]. We excluded 341 participants for any of the following reasons: self-reported diabetes diagnosis, current use of anti-diabetic medications, HbA1c above 6.5%, fasting glucose above 126 mg/dl, or missing data on any variables in the analyses. Our analysis therefore includes complete data for 914 participants without diabetes.
Measures
Anxiety was measured using the Spielberger Trait Anxiety Inventory [15] and includes items such as “I wish I could be as happy as others seem to be”, “I take disappointments so keenly that I can’t put them out of my mind”, and “I worry too much”. Responses were based on a 4-point scale ranging from “almost never” to “almost always” (αcoefficient = 0.9). Fasting glucose, insulin, and HbA1c samples were obtained during an overnight stay in a general clinical research center. HOMA-IR was calculated using an established formula [16].
Statistical Methods
Glucose, insulin, and HOMA-IR were log-transformed to achieve normal distributions. All predictor variables included were mean-centered.
First, we evaluated relations between anxiety, glucose metabolism measures, and sociodemographic and health characteristics according to race and gender (black men and women, white men and women) by conducting factorial ANOVA and Tukey’s post-hoc tests. Second, hierarchical linear regression analyses stratified by race and gender examined the relationship between anxiety and nondiabetic glucose metabolism. Multivariate analyses controlled for age, body mass index (BMI), waist-to-hip ratio (WHR), total household income (unadjusted for family size), fasting triglycerides, HDL cholesterol, current depressive symptoms [17], lifetime depression diagnosis, current smoker, and engaging in exercise for 20 minutes 3 times a week. All covariates were added to the model simultaneously before trait anxiety.
RESULTS
Table 1 shows subject characteristics by gender and race. Bivariate analyses revealed significant differences between subgroups. Black women were younger, had lower incomes, and reported higher anxiety and current depressive symptoms than whites. Significant subgroup differences existed for all indicators of glucose metabolism.
Table 1.
Means (and SDs) or Proportions for All Measures Stratified by Race and Gender.
| African American | Caucasian | F statistic (df=3) | Significant Subgroup Differences | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Womena (n=86) | Menb (n=51) | Womenc (n=429) | Mend (n=348) | |||
| Primary Predictor | ||||||
| Anxiety | 39.3 (10.0) | 37.8 (10.2) | 33.9 (8.9) | 32.2 (8.2) | 17.87*** | ac, ad, bc, bd, cd |
| Outcomes | ||||||
| Glucose (mmol/L) | 5.3 (0.5) | 5.4 (0.5) | 5.2 (0.5) | 5.4 (0.5) | 12.85*** | bc, cd |
| Insulin (uIU/mL) | 15.7 (14.8) | 12.4 (12.5) | 10.4 (7.9) | 12.6 (9.6) | 8.70*** | ac, ad, cd, |
| HOMA-IR | 3.9 (4.0) | 3.1 (3.4) | 2.5 (2.1) | 3.1 (2.4) | 8.71*** | ac, cd |
| HbA1c (mmol/mol) | 41 (2.0) | 39 (3.1) | 40 (0.9) | 39 (2.0) | 5.36** | ad |
| HbA1c (%) | 5.9 (0.4) | 5.7 (0.5) | 5.8 (0.3) | 5.7 (0.4) | ||
| Covariates | ||||||
| Age (years) | 52.6 (10.5) | 50.5 (8.0) | 57.6 (11.4) | 57.6 (11.8) | 10.71*** | ac, ad, bc, bd, |
| Income (× 1000) | 37.1 (34.1) | 49.7 (49.9) | 74.8 (61.4) | 84.6 (59.9) | 18.39*** | ac, ad, bc, bd |
| BMI (kg/m2) | 32.6 (9.0) | 28.8 (6.1) | 28.1 (5.9) | 29.1 (4.6) | 14.21*** | ab, ac, ad |
| WHR | .86 (0.1) | .93 (0.1) | .83 (0.1) | .96 (.1) | 223.39*** | ab, ac, ad, bc, bd, cd |
| Triglycerides | 101.7 (70.8) | 117.9 (76.3) | 112.2 (64.4) | 140.6 (88.5) | 11.58*** | ad, cd |
| HDL cholesterol | 61.1(18.9) | 56.7 (19.3) | 63. (17.1) | 47.3 (14.6) | 60.25*** | ad, bd, cd |
| Current Depressive Symptoms | 13.5 (10.4) | 11.9 (9.3) | 7.9 (7.9) | 7.4 (7.4) | 16.65*** | ac, ad, bc, bd |
| Depression Diagnosis (1=Yes) | 0.30 | 0.14 | 0.28 | 0.17 | 5.70** | ad, cd |
| Currently Smoking (1=Yes) | 0.29 | 0.45 | 0.09 | 0.12 | 24.56*** | ab, ac, ad, bc, bd |
| Exercise 3 × week (1=Yes) | 0.57 | 0.82 | 0.81 | 0.81 | 9.70*** | ab, ac, ad |
Asterisks denote significance level of F-statistic, where
P < 0.01 and
P < 0.001.
Post-hoc comparisons were conducted using ANOVA; significant (P < 0.05) subgroup differences are denoted as ab: African American women vs. African American men; ac: African American women vs. Caucasian women; ad: African American women vs. Caucasian men; bc: African American men vs. Caucasian women; bd: African American men vs. Caucasian men; cd: Caucasian women vs. Caucasian men.
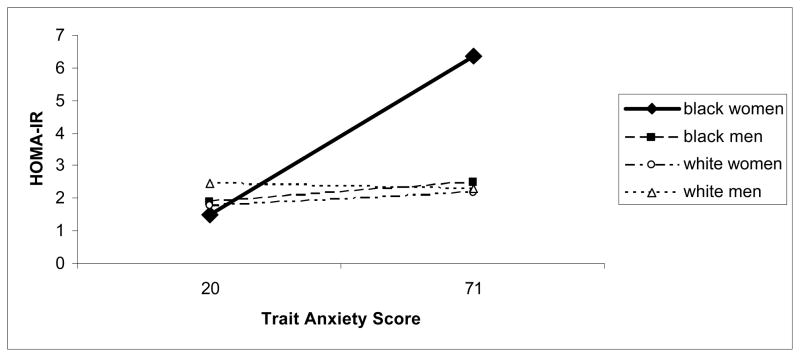
Hierarchical multiple regression models adjusted for all previously mentioned potential confounders and revealed that trait anxiety was associated with higher glucose (R2=.378, β=.407, p=.04), insulin (R2=.410, β=.475, p=.02), and HOMA-IR (R2=.429, β=.490, p=.01) only for African American women (see Figure 1). Such effects were not evident for HbA1c.
Figure 1. Trait Anxiety is Associated with Higher HOMA-IR among Black Women (p<.05).
Trait anxiety is associated with higher HOMA-IR (homeostasis model of assessment – insulin resistance) among black women (P < 0.05). Note: the relationship between anxiety and HOMA-IR is graphed using the full range of anxiety values.
DISCUSSION
Previous research has shown relationships between glucose metabolism and psychosocial risk factors such as depressive symptoms, anger, hostility, and acute stress [8–11, 18] for black women without diabetes. To the best of our knowledge, our study is the first to document an independent relationship between trait anxiety and indicators of glucose metabolism including glucose, insulin and HOMA-IR among black women without diabetes, a relationship evident despite the younger age of black compared to white women in this sample. These data are hypothesis generating and suggest that anxiety likely influences glycaemic control even before type 2 diabetes is fully developed.
Moreover, these results also suggest that black women who have almost double the risk for diabetes compared to their white counterparts (14) might be particularly vulnerable to the effects of anxiety on glucose metabolism. The underlying pathophysiologic mechanism relating anxiety to markers of glucose metabolism is uncertain. However, insight may come from an atherosclerosis study that demonstrated positive associations of trait anxiety with HOMA-IR and leptin/adiponectin ratio [19]. These authors posited that anxiety-related adiposity due to physical inactivity might influence upstream regulators of insulin resistance including heightened production of inflammatory substances. Indeed, although our study also does not address why black women are particularly vulnerable to the influence of anxiety on glucose metabolism, black women have a higher density of β-receptors in omental adipose tissue as compared with white women [20], thereby potentially increasing free fatty acids that negatively impact insulin sensitivity. Additionally, chronic psychological stress, a comorbid condition with anxiety, causes activation of the HPA axis and upregulation of the sympathetic nervous system which also results in impaired glucose handling [21] [22].
Our findings are consistent with a growing literature that links psychosocial vulnerability with dysregulated nondiabetic glucose metabolism in black women. Our results contribute to the existing literature by adding anxiety to the list of psychosocial vulnerabilities linked with glucose metabolism in black women without diabetes. Despite this, there is need for longitudinal population based studies comprised of larger numbers of black individuals than this study that will evaluate the role of anxiety in the preclinical development of diabetes. We also document that multiple indicators of glucose metabolism representing differing components involved in the pathophysiologic progression from no diabetes to diabetes are associated with anxiety. Taken together, the results suggest that anxiety might help facilitate progression to diabetes. From a public health perspective, our results additionally suggest that future studies are necessary to 1) prospectively confirm the role of anxiety in dysregulated glucose metabolism; 2) understand the impact of anxiety on health by race/ethnicity since blacks are substantially less likely to receive mental health specialist care [23, 24] and 3) to develop interventions that target anxiety along with established risk factors (e.g weight and physical activity) for diabetes risk reduction.
Acknowledgments
This research was supported by a grant from the National Institute on Aging (P01-AG020166; Carol D. Ryff, Principal Investigator) to conduct a longitudinal follow-up of the MIDUS (Midlife in the US) investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. We thank the staff of the Clinical Research Center at the University of Wisconsin-Madison, at the University of California—Los Angeles, and at Georgetown University for their support in conducting this study. Data collection was supported by the following grants M01- RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program, and 1UL1RR025011 (UW) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. The first author of this study was also supported, in part, by award number T32HD049302 from the Eunice Kennedy Shriver National Institute of Child Health And Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
Declaration of Competing Interests: Nothing to Declare.
References
- 1.Lustman PJ, Penckofer SM, Clouse RE. Recent advances in understanding depression in adults with diabetes. Curr Diab Rep. 2007;7:114–122. doi: 10.1007/s11892-007-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: findings from the National Health and Nutrition Examination Epidemiologic Follow-up Study, 1971–1992. Am J Epidemiol. 2003;158:416–423. doi: 10.1093/aje/kwg172. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd CE. Diabetes and mental health; the problem of co-morbidity. Diabet Med. 27:853–854. doi: 10.1111/j.1464-5491.2010.03067.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol. 2005;161:652–660. doi: 10.1093/aje/kwi089. [DOI] [PubMed] [Google Scholar]
- 5.Skinner TC, Carey ME, Cradock S, Dallosso HM, Daly H, Davies MJ, et al. Depressive symptoms in the first year from diagnosis of Type 2 diabetes: results from the DESMOND trial. Diabet Med. 27:965–967. doi: 10.1111/j.1464-5491.2010.03028.x. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Barker L, Ford ES, Zhang X, Strine TW, Mokdad AH. Diabetes and anxiety in US adults: findings from the 2006 Behavioral Risk Factor Surveillance System. Diabet Med. 2008;25:878–881. doi: 10.1111/j.1464-5491.2008.02477.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RJ, Grigsby AB, Freedland KE, de Groot M, McGill JB, Clouse RE, et al. Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med. 2002;32:235–247. doi: 10.2190/KLGD-4H8D-4RYL-TWQ8. [DOI] [PubMed] [Google Scholar]
- 8.Suarez EC. Sex differences in the relation of depressive symptoms, hostility, and anger expression to indices of glucose metabolism in nondiabetic adults. Health Psychol. 2006;25:484–492. doi: 10.1037/0278-6133.25.4.484. [DOI] [PubMed] [Google Scholar]
- 9.Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torrens JI, Kravitz HM, et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27:2856–2862. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- 10.Surwit RS, Williams RB, Siegler IC, Lane JD, Helms M, Applegate KL, et al. Hostility, race, and glucose metabolism in nondiabetic individuals. Diabetes Care. 2002;25:835–839. doi: 10.2337/diacare.25.5.835. [DOI] [PubMed] [Google Scholar]
- 11.Georgiades A, Lane JD, Boyle SH, Brummett BH, Barefoot JC, Kuhn CM, et al. Hostility and fasting glucose in African American women. Psychosom Med. 2009;71:642–645. doi: 10.1097/PSY.0b013e3181acee3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: Protocol, Measures, Sample, and Comparative Context. J Aging Health. 2010 doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radler BT, Ryff CD. Who Participates? Accounting for Longitudinal Retention in the MIDUS National Study of Health and Well-Being. J Aging Health. 22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spielberger CD. Manual for the state–trait anxiety inventory (Form Y) (“Self-evaluation questionnaire”) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Radloff L. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 18.Surwit RS, Williams RB, Lane JD, Feinglos MN, Kuhn CM, Georgiades A. Plasma epinephrine predicts fasting glucose in centrally obese African-American women. Obesity (Silver Spring) 2010;18:1683–1687. doi: 10.1038/oby.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narita K, Murata T, Hamada T, Kosaka H, Sudo S, Mizukami K, et al. Associations between trait anxiety, insulin resistance, and atherosclerosis in the elderly: a pilot cross-sectional study. Psychoneuroendocrinology. 2008;33:305–312. doi: 10.1016/j.psyneuen.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 20.McConnaughey MM, Sheets KA, Davis J, Privette J, Hickner R, Christian B, et al. Differences in beta-adrenergic receptor densities in omental and subcutaneous adipose tissue from obese African American and Caucasian women. Metabolism. 2004;53:247–251. doi: 10.1016/j.metabol.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Miller DB, O'Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51:5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- 22.Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 23.Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17:769–781. doi: 10.1097/JGP.0b013e3181ad4f5a. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]