Abstract
Clioquinol has been shown to have anticancer activity both in vitro and in vivo. The present study compared the cytotoxicity of clioquinol with six analogues, using human cancer cell lines. Of the analogues tested, 8-hydroxy-5-nitroquinoline (NQ) was the most toxic, with an IC50 that was 5-10 fold lower than other congeners. Its activity was enhanced by copper, but not zinc, and the use of a zinc-sensitive fluorophore showed that unlike clioquinol, NQ is not a zinc ionophore. NQ increased intracellular reactive oxygen species generation, an effect that was significantly enhanced by the addition of copper at levels that approximate those found in human plasma. NQ has been used in humans for the treatment of urinary infections. NQ is an 8-hydroxyquinoline derivative that is more potent than the halogenated 8-hydroxyquinolines, and may be less neurotoxic because it lacks zinc ionophore activity. NQ is another clinical used anti-microbial agent whose properties suggest that it may be useful in treating cancer.
Keywords: 5-amino-8-hydroxyquinoline, clioquinol, Raji, reactive oxygen species, cytotoxicity
1. Introduction
Clioquinol (5-chloro-7-iodo-8-hydoxyquinoline) is an antibiotic with metal-binding properties which has been shown to have anticancer activity in several experimental model systems [1; 2; 3; 4]. Clioquinol has been shown to operate via several different mechanisms of action. We have demonstrated that clioquinol targets zinc to lysosomes [5] and inhibits NF-kappa B activity [2; 6]. Clioquinol has shown by others to inhibit proteasome activity in various tumor lines [1; 4; 7]. Although clioquinol has a long history of use in humans, it was judged to be the cause of an epidemic of a rare neurological disease (subacute myeloptic neuropathy, SMON) in Japan and was banned in many countries. Since that time, others have pointed out that SMON was not seen in other countries where clioquinol was widely used, and have criticized the epidemiological data that led to its banning [4; 8; 9; 10]. Because of promising data in animal studies, clioquinol has been administered in clinical trials for Alzheimer's disease without recurrence of SMON [11; 12], prompting cautious re-evaluation of it use as a therapeutic agent.
A number of compounds that are structurally related to clioquinol are commercially available. These compounds include the parent compound (8-Hydroxyquinoline, 8HQ), as well as halogenated (5,7-Diiodo-8-hydroxyquinoline, IIQ; 5-Chloro-8-quinoline, CQ2; 5,7-Dichloro-8-quinolinol, CCQ) and non-halogenated derivatives (8-Hydroxy-5-nitroquinoline, NQ, and 5-Amino-8-quinolinol dihydrochloride, A8HQ). We have carried out a series of studies to compare the cytotoxicity of these compounds against human cancer cells. We report here that among the compounds tested, the non-halogenated 5-nitro derivative of 8-hydroxyquinoline (NQ) was the most cytotoxic, with an IC50 value 5-10 fold lower than clioquinol's.
2. Materials and Methods
2.1 Materials
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent was purchased from Promega (Madison, WI). Carboxy-H2DCFDA, a fluorescent probe for ROS detection, was from Invitrogen (Carlsbad, CA). Antibodies for Poly (ADP-ribose) polymerase (PARP) were from BIOMOL Research Laboratories, Inc. (Plymouth Meeting, PA), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from Promab Biotechnologies, Inc. (Richmond, CA). Clioquinol and its analogues, CuCl2, ZnCl2 and all other reagents are analytic grade and were purchased from Sigma Chemical Co. (St. Louis, MO).
2.2 Cell culture
Human tumor lines Raji (lymphoma), HL-60 (leukemia), and Panc-1 (pancreatic cancer) were from American Type Tissue Culture Collection (ATCC, Manassas, VA). A2780 (ovarian cancer) was a generous gift from Dr. Stephen Howell (University of California, San Diego). DHL-4 (lymphoma) was kindly provided by Dr. Linda Boxer (Stanford University). Cell lines were cultured in ATCC specified medium supplemented with 10% fetal bovine serum, 10,000 U/ml penicillin and 10,000μg/ml streptomycin under 5% CO2 at 37°C in a humid environment. All cells were routinely grown in 75-mm flasks and split twice a week.
2.3 Cell viability assays
The cytotoxicity of clioquinol and its six analogues were examined using the MTS reagent as previously described [13]. Briefly, Raji, DHL-4 and HL-60 cells were plated in 96-well plates at 10,000 cells/well, and A2780 and Panc-1 cells at 5,000/well. 24 hours after plating, cells were treated with various compounds at increasing concentrations for 72 hours in the presence or absence of 10 μM CuCl2 or 50 μM ZnCl2. 20 μL of the MTS reagent was added to each well and the plates were then incubated for another hour at 37°C. The optical density of each well was then read at 490 nm. Each experiment was performed in triplicate and repeated three times. Data are presented as percentages of values detected in control cells cultured under the same conditions. The drug potency was determined by calculating IC50 values of each compound using GraphPad Prism version 4.00 for Windows (San Diego, CA).
2.4 Detection of intracellular reactive oxygen species (ROS)
Intracellular reactive oxygen species (ROS) were analyzed by Flow Cytometry using a fluorescent probe, Carboxy-H2DCFDA, following the manufacturer's protocol. Raji cells were plated at 1×106 cells/well in a 6-well plate in RPMI 1640 medium supplemented with 10% fetal bovine serum, 10,000 U/ml penicillin and 10,000μg/ml streptomycin. 24 hours after plating, cells were treated with NQ and IIQ at indicated concentrations and durations in the presence or absence of 10 μM CuCl2. The medium was then replaced with serum free RPMI 1640 containing 10 μM Carboxy-H2DCFDA. After 60 min incubation, cells were washed with PBS and analyzed with FACScan Analytic Flow Cytometer (BD-Bioscience, San Jose, CA, excitation at 485 nm, emission at 538 nm). 100 μM tert-butyl hydroperoxide (TBHP, Sigma-Aldrich) was used as a positive control. Data was analyzed using the FlowJo6.7 software. The oxidation of Carboxy-H2DCFDA is detected by the increase of fluorescence that is proportional to the amount of total intracellular ROS generated. The value of ROS generation is presented as the mean of relative fluorescence units.
2.5 Detection of intracellular zinc
Intracellular free zinc ions were analyzed with the FluoZin-3 probes using fluorescence microscopy following the manufacturer's instructions (Invitrogen, Carlsbad, CA). A2780 cells were seeded in a 12-well plate with 3×105 cells per well. 24 hours after plating, the cells were treated with 10 μM of clioquinol, NQ, or IIQ in the presence or absence of 50 μM ZnCl2 for 30 minutes. The medium was then exchanged with fresh medium containing 1 μM FluoZin-3®. After incubating for 30 minutes, the medium was removed and the cells were washed three times with Hanks’ balanced salt solution (HBSS) and viewed on a Nikon Eclipse TE2000-U microscope. Zinc was detected as a green color (FluoZin-3®, excitation, 490/20 nm, emission, 528/38 nm),
2.7 Western Blot
Raji cells were plated at 1×106 cells/well in 100-mm diameter dish in RPMI 1640 and treated with 10 μM NQ, IIQ and CQ in the presence or absence of 10 μM CuCl2 for 24 hours. At the end of the incubation, cells were collected by centrifuge. Cells were lysed using the lysis buffer (50 mM Tris-HCl pH 7.8, 100 mM NaCl, 5 mM Na-EDTA, 0.1% SDS, 1mM PMSF, 1% Triton-X-100, and 2.5% Glycerol) and samples were sonicated on ice for 1 min (10 sec pulse on/off). Equal amounts of protein (20 μg per lane) were separated on 10% SDS PAGE gel and transferred onto a PVDF membrane. The membrane was blocked with 5% non-fat dry milk and then incubated with a 1:2000 dilution of the primary antibody against PARP or GAPDH overnight at 4 °C. The membrane was then washed three times with TBST and incubated with a secondary peroxidase-conjugated antibody for 1 hour. Signaling was detected using an enhanced chemiluminescence detection system (GE Healthcare Life Sciences, Piscataway, NJ). Each experiment was repeated three times to assess consistency of the results.
3. Results
3.1 Effects of clioquinol and its analogues on the viability of Raji cells
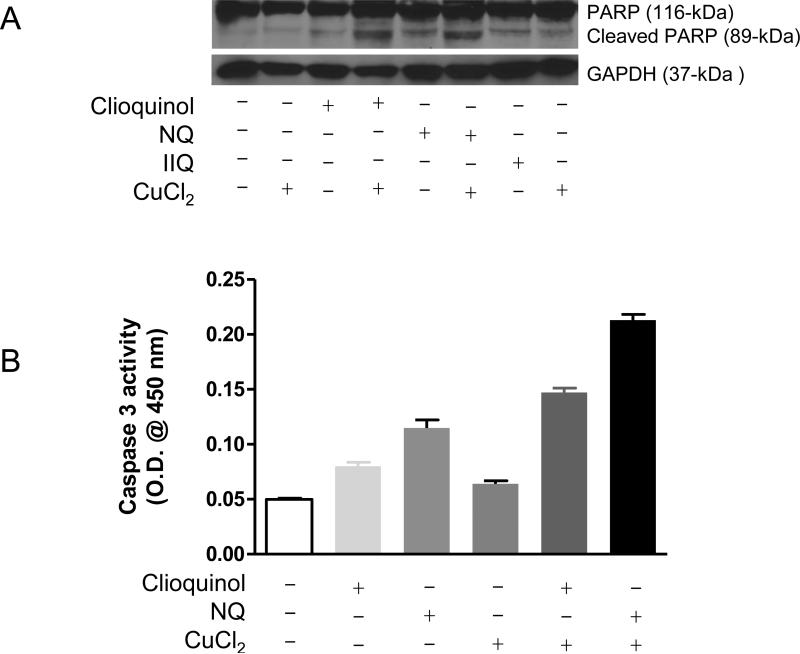
To compare the cytotoxicity of clioquinol and its analogues, Raji cells (a cell line derived from a human B cell lymphoma) were treated with increased concentrations of the compounds for three days in the presence or absence of 50 μM zinc and 10 μM copper. Cell viability was analyzed and IC50 values calculated (Table 1). As shown in Table 1, among the compounds tested, NQ was the most toxic with an IC50 of 438 nM, whereas IIQ was the least toxic with an IC50 close to 6 μM. The order of their efficacies in inhibiting cell viability was: NQ > CCQ > 8HQ > Clioquinol > A8HQ > CQ2 > IIQ. The cytotoxicity of each compound was enhanced by the addition of copper, regardless of the atoms in the C5 and C7 positions. However, in the presence of zinc, only the cytotoxicity of clioquinol and IIQ were augmented. To determine if the decreased viability was due to apoptosis, cell extracts were examined for PARP cleavage (Figure 1). Both clioquinol [2], and NQ induced PARP cleavage, which was enhanced by the addition of copper.
Table 1. Effects of clioquinol and analogues on the viability of Raji cells.
Cells were treated with increasing concentrations of the compounds in the presence or absence of 50 μM ZnCl2 or 10 μM CuCl2. Cell viability was analyzed using the MTS assay and IC50 values were calculated (mean ± SEM, from three independent experiments).
| Compounds | Structure | Drug alone IC50, μM | +ZnCl2 IC50, μM | +CuCl2 IC50, μM |
|---|---|---|---|---|
| Clioquinol |
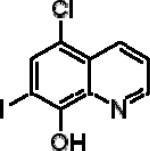
|
5.09±020 | 3.30±0.38 | 1.89±0.25 |
| 8-Hydroxy-5-nitroquinoline (NQ) |
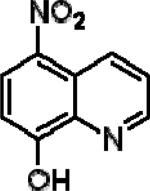
|
0.438±0.20 | 0.855±0.18 | 0.0003±0.0 |
| 5,7-Diiodo-8-hydroxyquinoline (IIQ) |
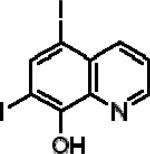
|
5.54±0.26 | 4.52±0.22 | 2.09±0.20 |
| 5-Chloro-8-quinoline (CQ2) |
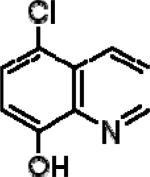
|
5.49±0.22 | 7.20±0.27 | 3.49±0.18 |
| 8-Hydroxyquinoline (8HQ) |
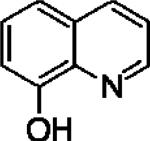
|
1.98±0.23 | 6.92±0.14 | 0.03±0.002 |
| 5,7-Dichloro-8-quinolinol (CCQ) |
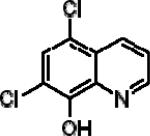
|
1.44±0.15 | 1.40±0.2 | 0.133±0.03 |
| 5-Amino-8-quinolinol dihydrochloride (A8HQ) |
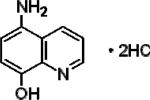
|
5.44±0.13 | 8.56±0.15 | 1.93±0.10 |
Figure 1. Effects of clioquinol, IIQ, and NQ on PARP cleavage in Raji cells.
Raji cells were treated with 10 μM of clioquinol, IIQ, and 5 μM NQ in the presence or absence of 10 μM CuCl2 for 4 hours. Total proteins were isolated and separated on 10% SDS PAGE gel, followed by Western blot analysis with specific antibodies against PPAR-1 and GAPDH. Shown are representatives of two experiments.
3.2 Effects of clioquinol, NQ and IIQ on the viability of HL60, DHL-4, Panc-1 and A2780 cells
To insure that the findings were not restricted to one cell type, we examined the cytotoxicity of NQ (the most toxic), clioquinol, and IIQ (the least toxic) on several additional types of human malignant cell lines, including HL60 (leukemia), DHL-4 (lymphoma), Panc-1 (pancreatic cancer), and A2780 (ovarian cancer). In all cases, NQ proved to be the most cytotoxic and IIQ the least, although the IC50 values differed among the cell lines (Table 2). This finding suggests that this class of compounds may be useful in treating a variety of types of cancer.
Table 2. Effects of clioquinol, IIQ, andNQ on the viability of HL60, DHL-4, A2780, and Panc-1 cells.
Cells were treated with increasing concentrations of the compounds in the presence or absence of 50 μM ZnCl2 or 10 μM CuCl2. Cell viability was analyzed using the MTS assay and IC50 values were calculated (mean ± SEM, from three independent experiments).
| Compounds | Cell lines (IC50, μM) | |||
|---|---|---|---|---|
| HL60 | DHL-4 | Panc-1 | A2780 | |
| Clioquinol | 12.90 ±0.16 | 2.71±0.18 | 5.89±0.28 | 13.00±2.1 |
| + ZnCl2 | 8.60±0.23 | 2.27±0.17 | 2.07±0.21 | |
| + CuCl2 | 7.51±0.22 | 0.69±0.13 | 0.76±0.19 | |
| NQ | 6.26±0.10 | 0.89±0.28 | 1.46±0.14 | 0.18±0.02 |
| + ZnCl2 | 5.22±0.62 | 1.15±0.24 | ||
| + CuCl2 | 0.15±0.12 | 0.07±0.15 | 0.19±0.02 | |
| IIQ | 93.00±10.00 | 24.7±0.17 | 72.00±7.00 | 13.60±1.9 |
| + ZnCl2 | 37.60±2.90 | 2.15±0.20 | ||
| + CuCl2 | 11.00±1.40 | 1.27±0.21 | 2.03±0.22 | |
3.3 Unlike clioquinol, NQ does not act as a zinc ionophore
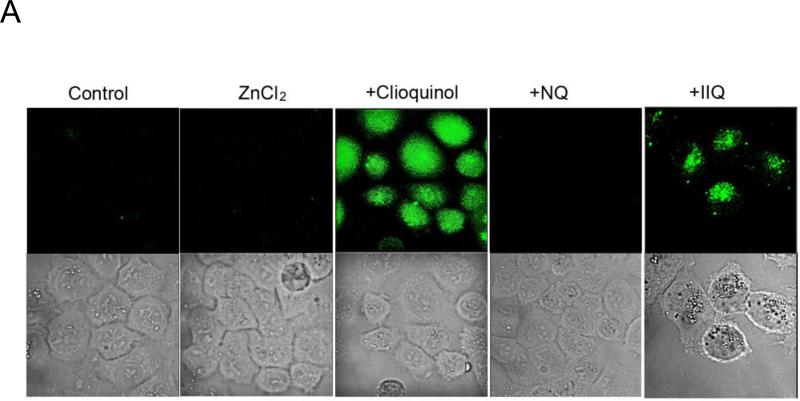
We previously reported that clioquinol acts as a zinc ionophore that targets zinc to lysosomes in cancer cells [5]. To understand whether NQ and IIQ also act as zinc ionophores, A2780 cells were treated with 10 μM of clioquinol, NQ, or IIQ for 30 min in the presence of 50 μM zinc. Using a zinc-sensitive fluorescent probe (FluoZin-3), we found that both clioquinol and IIQ increased intracellular zinc concentrations, while NQ did not (Figure 2). These findings are consistent with the cell viability assays, which showed enhancement of the cytotoxicity of clioquinol and IIQ, but not NQ, in the presence of added zinc. These observations suggest that NQ inhibits the viability of cancer cells through a mechanism that differs in part, from those responsible for the effects of halogenated 8-hydroxyquinoline derivatives.
Figure 2. NQ is not a zinc ionophore.
A2780 cells were loaded with 3 μM FluoZin-3 and treated with 50 μM ZnCl2 for 30 min in the presence of 10 μM NQ, IIQ or clioquinol. The images were captured with the Leica TCS NT Confocal Microscopy using 63x Plan APO 1.2 NA on living cells. Shown are representatives of three experiments.
3.4 Effects of NQ and IIQ on ROS generation in Raji cells
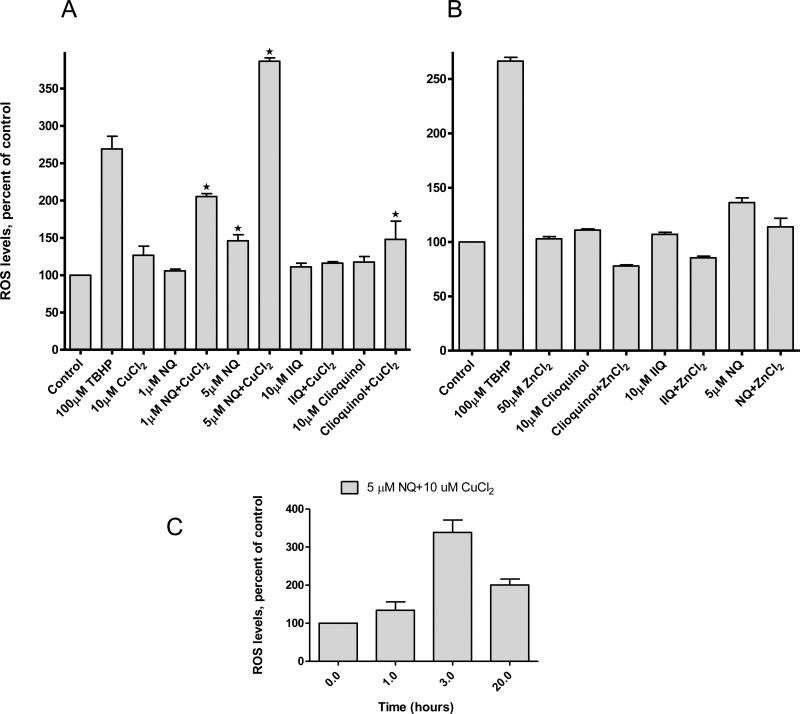
In considering why NQ was more potent than the other compounds tested, we considered how its nitro moiety might influence its activity. Some compounds containing nitro groups may generate nitro free radicals [14]. While these radicals are often not cytotoxic of themselves [15], they may initiate a series of redox reactions resulting in the formation of reactive oxygen species (ROS), which in turn may alter intracellular signaling [16]. To explore the possibility that NQ's cytotoxicity might involve a change in the cell's redox status, we utilized a fluorescent probe sensitive to ROS. Raji cells were treated with NQ or IIQ in the presence of 10 μM copper or 50 μM zinc and intracellular ROS levels determined using Carboxy-H2DCFDA. As shown in Figure 3, NQ itself increased ROS levels, an effect enhanced by additional copper. IIQ did not increase ROS levels whether used alone, or in combination with copper. Zinc, which is not considered to be a redox-active metal, did not enhance NQ's effects on ROS generation (data not shown).
Figure 3. Effects of NQ and IIQ on intracellular ROS generation in Raji cells.
Cells were treated with NQ or IIQ at indicated concentrations plus 10 μM CuCl2 for 3 hours. ROS levels were determined using the Carboxy-H2DCFDA probe. Florescence was detected as described in the Materials and Methods. Data are (mean ± SEM, n=3) expressed as percentages of untreated control cells.
4. Discussion
This is the first study to demonstrate that a nitro derivative of 8-hydroxyquinoline is more cytotoxic against human cancer cells than either the parent compound, or other congeners. (Others have generated Mannich bases of NQ which have enhanced cytotoxic activity when compared to clioquinol, but the activity of the parent compound (NQ) was not reported (16).) The cytotoxicity of NQ was enhanced by the addition of copper but not zinc and was associated with an increase in reactive oxygen species. Although the cytotoxicity of clioquinol was enhanced by zinc, NQ's cytotoxicity was not, suggesting that NQ did not act as a zinc ionophore. A zinc-sensitive fluorescent probe was used to confirm that NQ does not act to increase intracellular zinc concentrations. Zinc enhanced the cytotoxicity only of compounds that have iodine in the C7 position (clioquinol and IIQ), though the mechanism whereby iodine converts 8-hydroxyquinoline derivatives into zinc ionophores is uncertain. The increased anti-cancer efficacy of a nitro derivative due to enhanced ROS production has previously been documented in the case of a synthetic heteroarotinoid (17).
NQ has been studied in a limited manner in humans, mainly in patients with urinary tract infections [17]. As far as we can determine, there has not been any reports of neurological diseases noted in patients treated with NQ, unlike the case with halogenated derivatives [18]. The neurotoxicity of clioquinol has been attributed to its zinc-transporting activity [19; 20]. The fact that NQ lacks such activity provides an additional reason to consider it for clinical development.
After these studies were completed, a chemical library screen for inhibitors of methionine aminopeptidase identified NQ as such a compound [21]. The authors showed that NQ (nitroxoline) acts as an anti-angiogenic agent, in vitro and in vivo, and inhibited the growth of tumor xenografts in mice. We have previously found (unpublished observations) that clioquinol inhibits tube formation by the endothelial-derived cell line, EA.hy926, an effect that could explain part of its in vivo anti-tumor activity [2]. The data presented here suggest that the in vivo activity of NQ observed by Shim et al may be due to NQ's possessing both anti-tumor and anti-angiogenic activity and provide additional rationale for considering its use as an anti-cancer drug in humans.
Highlights.
We compared the cytotoxicity of clioquinol analogues in human cancer cells.
8-hydroxy-5-nitroquinoline (NQ) was the most toxic among 6 analogues tested.
Unlike clioquinol, NQ is not a zinc ionophore.
NQ increased generation of intracellular reactive oxygen species.
NQ is another anti-microbial agent that may be useful in treating cancer.
Acknowledgement
Financial support was obtained from the American Cancer Society (CNE-117557), the Susan G. Komen for the Cure Foundation (KG081083), the NIH OK-INBRE program (3P20RR016478-09S2), and the Oklahoma Center for the Advancement of Science and Technology (HR09-025).
Abbreviations used
- Clioquinol
5-chloro-7-iodo-8-quinolinol
- IIQ
5,7-Diiodo-8-hydroxyquinoline
- CQ2
5-Chloro-8-quinoline
- 8HQ
8-Hydroxyquinoline
- CCQ
5,7-Dichloro-8-quinolinol
- NQ
8-Hydroxy-5-nitroquinoline
- A8HQ
5-Amino-8-quinolinol dihydrochloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None Declared
References
- 1.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 3.Du T, Filiz G, Caragounis A, Crouch PJ, White AR. Clioquinol promotes cancer cell toxicity through tumor necrosis factor alpha release from macrophages. J Pharmacol Exp Ther. 2008;324:360–367. doi: 10.1124/jpet.107.130377. [DOI] [PubMed] [Google Scholar]
- 4.Mao X, Li X, Sprangers R, Wang X, Venugopal A, Wood T, Zhang Y, Kuntz DA, Coe E, Trudel S, Rose D, Batey RA, Kay LE, Schimmer AD. Clioquinol inhibits the proteasome and displays preclinical activity in leukemia and myeloma. Leukemia. 2009;23:585–590. doi: 10.1038/leu.2008.232. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Zhou Y, Lind SE, Ding WQ. Clioquinol targets zinc to lysosomes in human cancer cells. Biochem J. 2009;417:133–139. doi: 10.1042/BJ20081421. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Lou JR, Ding WQ. Clioquinol independently targets NF-kappaB and lysosome pathways in human cancer cells. Anticancer Res. 30:2087–2092. [PubMed] [Google Scholar]
- 7.Chen D, Cui QC, Yang H, Barrea RA, Sarkar FH, Sheng S, Yan B, Reddy GP, Dou QP. Clioquinol, a therapeutic agent for Alzheimer's disease, has proteasome-inhibitory, androgen receptor-suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts. Cancer Res. 2007;67:1636–1644. doi: 10.1158/0008-5472.CAN-06-3546. [DOI] [PubMed] [Google Scholar]
- 8.Cahoon L. The curious case of clioquinol. Nat Med. 2009;15:356–359. doi: 10.1038/nm0409-356. [DOI] [PubMed] [Google Scholar]
- 9.Tsubaki T, Honma Y, Hoshi M. Neurological syndrome associated with clioquinol. Lancet. 1971;1:696–697. doi: 10.1016/s0140-6736(71)92699-7. [DOI] [PubMed] [Google Scholar]
- 10.Meade TW. Subacute myelo-optic neuropathy and clioquinol. An epidemiological case-history for diagnosis. Br J Prev Soc Med. 1975;29:157–169. doi: 10.1136/jech.29.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regland B, Lehmann W, Abedini I, Blennow K, Jonsson M, Karlsson I, Sjogren M, Wallin A, Xilinas M, Gottfries CG. Treatment of Alzheimer's disease with clioquinol. Dement Geriatr Cogn Disord. 2001;12:408–414. doi: 10.1159/000051288. [DOI] [PubMed] [Google Scholar]
- 12.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 13.Zheng J, Lou JR, Zhang XX, Benbrook DM, Hanigan MH, Lind SE, Ding WQ. N-Acetylcysteine interacts with copper to generate hydrogen peroxide and selectively induce cancer cell death. Cancer Lett. 298:186–194. doi: 10.1016/j.canlet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 2007. [Google Scholar]
- 15.Wardman P. Some Reactions and Properties of Nitro Radical-Anions Important in Biology and Medicine. Environmental Health Perspectives. 1985;64:309–320. doi: 10.1289/ehp.8564309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason RP. Redox cycling of radical anion metabolites of toxic chemicals and drugs and the Marcus theory of electron transfer. Environ Health Perspect. 1990;87:237–243. doi: 10.1289/ehp.9087237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs MR, Robinson RG, Koornhof HJ. Antibacterial activity of nitroxoline and sulphamethizole alone and in combination in urinary tract infections. S Afr Med J. 1978;54:959–962. [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics Committee on Drugs Clioquinol (iodochlorhydroxyquin, vioform) and iodoquinol (diiodohydroxyquin): blindness and neuropathy. Pediatrics. 1990;86:797–798. [PubMed] [Google Scholar]
- 19.Andersson DA, Gentry C, Moss S, Bevan S. Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proc Natl Acad Sci U S A. 2009;106:8374–8379. doi: 10.1073/pnas.0812675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbiser JL, Kraeft SK, van Leeuwen R, Hurwitz SJ, Selig M, Dickersin GR, Flint A, Byers HR, Chen LB. Clioquinol-zinc chelate: a candidate causative agent of subacute myelo-optic neuropathy. Mol Med. 1998;4:665–670. [PMC free article] [PubMed] [Google Scholar]
- 21.Shim JS, Matsui Y, Bhat S, Nacev BA, Xu J, Bhang HE, Dhara S, Han KC, Chong CR, Pomper MG, So A, Liu JO. Effect of nitroxoline on angiogenesis and growth of human bladder cancer. J Natl Cancer Inst. 102:1855–1873. doi: 10.1093/jnci/djq457. [DOI] [PMC free article] [PubMed] [Google Scholar]