Abstract
Brain ischemia is a leading cause of death and long-term disabilities worldwide. Unfortunately, current treatment is limited to thrombolysis, which has limited success and a potential side effect of intracerebral hemorrhage. Searching for new cell injury mechanisms and therapeutic interventions has become a major challenge in the field. It has been recognized for many years that intracellular Ca2+ overload in neurons is essential for neuronal injury associated with brain ischemia. However, the exact pathway(s) underlying the toxic Ca2+ loading remained elusive. This review discusses the role of two Ca2+-permeable cation channels, TRPM7 and acid-sensing channels, in glutamate-independent Ca2+ toxicity associated with brain ischemia.
Keywords: acid-sensing ion channel, TRPM7, brain ischemia, neurons
Introduction
Stroke or cerebral ischemia is a leading cause of death and long-term disabilities worldwide. Although major advances have occurred in the past decades in the prevention of brain ischemia, treatment is limited to the use of tissue plasminogen activator (tPA), which has limited success and a major side effect of intracranial hemorrhage1, 2. Searching for new cell injury mechanisms and effective therapeutic strategies therefore constitutes a major challenge for stroke research.
It has been recognized for several decades that excessive Ca2+ entry and resultant cytosolic Ca2+ overload play an important role in neuronal injury associated with stroke/brain ischemia3. In the resting condition, free intracellular Ca2+ concentration ([Ca2+]i) is maintained at nanomolar levels. Following ischemia, [Ca2+]i can reach as high as several micromoles. Excessive [Ca2+]i loading can activate enzymes such as proteases, phospholipases, and endonucleases. Over-activation of these enzymes causes breakdown of proteins, lipids and nucleic acids, which leads to destruction of neurons4, 5, 6. In addition, overloading Ca2+ in mitochondria can cause opening of mitochondria permeability transition pore (PTP), a large conductance channel residing in mitochondrial membrane7, 8, promoting apoptosis through release of cytochrome c and activation of caspases9, 10, 11.
Ca2+ may enter neurons through various Ca2+-permeable ion channels (eg voltage-gated or ligand-gated channels) or through ion exchange systems (eg reverse Na+/Ca2+ exchanger). Accumulation of [Ca2+]i can also occur through Ca2+ release from intracellular stores (eg endoplasmic reticulum, ER). The exact source(s) of Ca2+ loading responsible for ischemic brain injury, however, remains unclear. This review discusses the involvement of two novel Ca2+-permeable cation channels, TRPM7 channels and acid-sensing ion channels, in ischemic brain injury.
Glutamate mediated Ca2+-toxicity
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS)12, 13, 14. Its receptors are widely expressed at soma and dendrites of the CNS neurons. Activation of these receptors is involved in a variety of physiological functions of neurons including synaptic transmission/plasticity, learning/memory, neuronal development and differentiation12, 15. Glutamate receptors are classified into two major categories: ionotropic receptors, which are ligand-gated cation channels; and metabotropic receptors, which are coupled through G proteins to second messenger systems16. One subtype of ionotropic glutamate receptors, the N-methyl-D-aspartate (or NMDA) receptor, is highly permeable to Ca2+ ions. Activation of these receptors has been considered to play a critical role in Ca2+ toxicity associated with ischemic brain injury3, 17, 18, 19, 20. Accordingly, blocking these receptors has been shown to be neuroprotective in cell culture and animal models of brain ischemia. Unfortunately, none of the human trials using the antagonists of glutamate receptors showed a satisfactory protection for stroke patients. Although multiple factors, including difficulty in early initiation of treatment and intolerance of severe side effects, may have contributed to the failure of the trials4, 21, 22, 23, 24, recent studies suggest that Ca2+ entry through glutamate-independent pathways, eg TRPM7 channels and Ca2+-permeable acid-sensing ion channels (ASICs), may contribute to the injury of neurons associated with brain ischemia.
TRPM channels and ischemic neuronal injury
Transient receptor potential (TRP) channels belong to a novel family of cation channels that are highly expressed in various tissues including the brain25, 26. Several members of TRP family can be activated by oxidative stress and oxygen free radicals, both of which play important roles in neuronal injury associated with stroke/brain ischemia. Recent work has indicated that members of the melastatin subfamily (TRPM) of the TRP channels, particularly the TRPM7, play a key role in neuronal cell death associated with brain ischemia27, 28, 29, 30, 31.
The TRP superfamily is a diverse group of voltage-independent calcium-permeable cation channels expressed in mammalian cells25, 26. These channels have been divided into six subfamilies, and two of them, TRPC and TRPM, have members that are widely expressed and activated by oxidative stress. TRPC3 and TRPC4 are activated by oxidants, which induce Na+ and Ca2+ entry into cells through phospholipase C-dependent mechanisms. TRPM2 is activated by oxidative stress or TNFalpha, and the mechanism involves production of ADP-ribose, which binds to an ADP-ribose binding cleft in the TRPM2 C-terminus. Treatment of neurons or HEK 293T cells expressing TRPM2 with H2O2 resulted in Ca2+ influx and increased susceptibility to cell death27. Inhibition of endogenous TRPM2 function, in contrast, protected cell viability27, 32. Nevertheless, the exact role of TRPM2 in Ca2+ toxicity associated with ischemic brain injury remains to be explored.
The potential role of TRPM7 channels in ischemic neuronal death has been described recently30, 31. Aarts and colleagues first examined the mechanism of neuronal cell death in ischemic conditions in the presence of glutamate antagonists. Cultured mouse cortical neurons were exposed to oxygen-glucose deprivation (OGD), an in vitro model of ischemia reported to mediate neuronal death through NMDA receptor activation33, 34. Blocking the glutamate excitotoxicity in these cultures, however, unmasked a potent, previously unappreciated mechanism of non-excitotoxic neuronal cell death, which became increasingly responsible for neurodegeneration as the duration of OGD was prolonged30. Further studies demonstrated that the mechanism of cell death involved activation of a non-selective cation current with high permeability to Ca2+. The current showed outward rectifying properties, was potentiated by reactive oxygen/nitrogen species (ROS), and was blocked by Gd3+. The electrophysiological characteristics and pharmacological properties of the current suggested the involvement of TRPM7 channels. Indeed, molecular biological approaches (eg siRNA) confirmed the involvement of TRPM7 channels in glutamate-independent anoxic neuronal injury30. Although a specific agonist remains to be determined, these studies suggest that, in ischemic conditions, TRPM7 channels could be activated by ROS. Ca2+ entry through these channels participates in neuronal injury. A lethal positive feedback loop is established when Ca2+ influx through TRPM7 channels stimulates additional ROS production, causing further TRPM7 activation30. Blocking TRPM7 channels or suppressing its expression by RNA interference was effective in preventing the death of neurons by OGD.
Very recent studies by Sun and colleagues also demonstrated involvement of TRPM7 channels in the injury of hippocampal neurons in vivo in rat model of global ischemia31. Suppressing TRPM7 expression in CA1 neurons by intrahippocampal injections of viral vectors bearing shRNA specific for TRPM7 channels had no ill effect on animal survival, neuronal and dendritic morphology, neuronal excitability, or synaptic plasticity. However, TRPM7 suppression made neurons resistant to ischemic injury and preserved neuronal morphology and function. Also, it prevented ischemia-induced deficits in long-term potentiation and preserved performance in fear-associated and spatial-navigational memory tasks. Thus, regional suppression of TRPM7 is feasible, well tolerated and inhibits delayed neuronal death in vivo. In addition to Ca2+ toxicity mediated by TRPM7 channels, studies by Inoue and colleagues have suggested that Zn2+ permeability of these channels also plays a role in ischemic brain injury35.
Acid-sensing ion channels and ischemic brain injury
In acute neurological conditions such as brain ischemia, marked reduction of tissue pH takes place36, 37, 38, 39, 40, 41. Following ischemia, shortage of oxygen supply promotes anaerobic glycolysis, leading to lactic acid accumulation and resultant decrease in brain pH42, 43. Increased ATP hydrolysis and release of H+ also contributes to pH drop. At the same time, cessation of local circulation results in carbon dioxide accumulation and carbonic acid build up, which may participate in the decrease of tissue pH39. During ischemia, decreases of brain pH to ∼6.5 are commonly observed. It can also fall to 6.0 or below during severe ischemia or under hyperglycemic conditions37, 40, 41, 44, 45.
Decrease of brain pH or acidosis has long been known to play an important role in ischemic brain injury39, 42, 43, 46, 47, 48. A direct correlation between the degree of brain acidosis and infarct size has also been described39, 49. However, the exact mechanism underlying acidosis-mediated neuronal injury remained vague. Acidosis may cause non-selective denaturation of proteins and nucleic acids50; trigger cell swelling and osmolysis via stimulation of Na+/H+ and Cl-/HCO3− exchangers51; hinder postischemic metabolic recovery by inhibiting mitochondrial energy metabolism and impairing postischemic blood flow via vascular edema52; or stimulate pathologic free radical formation53. At the neurotransmitter level, profound acidosis inhibits astrocytic glutamate uptake, which may contribute to excitatory neuronal injury54.
Interestingly, mild acidosis has been considered to be beneficial in protecting neurons from excitotoxic injury55, 56, 57. This may be explained by proton inhibition of NMDA channel activity58, 59. In contrast to its modulating effect on other ion channels, protons can activate a distinct family of ligand-gated channels, the acid-sensing ion channels (ASICs)60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71. ASICs belong to the amiloride-sensitive epithelial Na+-channel/degenerin (ENaC/Deg) superfamily60, 72, which are formed with homomultimeric or heteromultimeric subunits. Each subunit contains two transmembrane spanning regions (TM1 and TM2) flanked by a large cysteine-rich extracellular loop and short intracellular N and C termini60, 61, 73, 74, 75, 76, 77, 78. To date, four genes encoding seven ASIC subunits have been cloned and characterized. ASIC1a subunits (originally named ASIC or BNaC2) are widely expressed in peripheral sensory neurons and in CNS neurons61, 65, 79, 80. Pertinent to brain ischemia, these channels are activated by moderate decreases of pHo with a threshold pH of ∼7.0 and a pH for half maximal activation (pH0.5) at ∼6.261, 81. In addition to Na+, homomeric ASIC1a channels are permeable to Ca2+ ions61, 82, 83. ASIC1β and its longer form variant ASIC1b are expressed only in sensory neurons84, 85. Similar to ASIC1a, homomeric ASIC1β channels have high sensitivity to H+ with a pH0.5 at ∼5.985. Unlike ASIC1a, however, ASIC1b or ASIC1β has no detectable Ca2+ permeability84, 85. ASIC2a subunits (originally named MDEG, or BNaC1) have widespread distribution in both peripheral sensory and central neurons63, 79, 86. However, homomeric ASIC2a channels have very low sensitivity to H+ with a pH0.5 of 4.463, 86, 87. It is unlikely that homomeric ASIC2a channels can be activated in any physiological or pathological conditions in the brain. Similarly, ASIC2b subunits (originally named MDEG2) are expressed in peripheral sensory and central neurons87. However, they do not form functional homomeric channels, but may associate with other ASIC subunits (eg ASIC3) to form heteromultimeric channels87. ASIC3 subunits (originally also named DRASIC) are predominantly expressed in neurons of dorsal root ganglia88, 89, though its expression in the brain has been reported90. Homomeric ASIC3 channels respond to pH drops biphasically, with a fast desensitizing current followed by a sustained component88, 89, 91. ASIC4 subunits are highly expressed in the pituitary gland92, 93. Similar to ASIC2b, they do not seem to form functional homomeric channels93. ASICs were initially believed to be assembled as tetramers61, 77. However, recent analysis of crystal structure suggested that ASICs existed as trimers94.
ASICs in peripheral sensory neurons are implicated in nociception, mechanosensation, and taste transduction95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107. The presence of ASICs in the brain, which lacks nociceptors, suggests that these channels have functions beyond nociception. Indeed, ASIC1a has been shown to be involved in synaptic plasticity, learning and memory64, 108. Both ASIC1a and ASIC2a are implicated in the maintenance of retinal integrity109, 110, 111. In pathological conditions, activation of Ca2+-permeable ASIC1a is involved in glutamate-independent, acidosis mediated, ischemic brain injury71, 82, 112, and in axon degeneration associated with multiple sclerosis113. In contrast, increased expression of ASIC2a is associated with neuronal survival following global ischemia114, while reduced expression of ASIC1a is associated with neuroprotection elicited by ischemic pre- and post-conditioning115.
The presence of ASIC1a in the brain, its activation by pH drops to the levels commonly seen during brain ischemia, and its permeability to Ca2+ make it a potential player in ischemic brain injury. A series of recent studies, performed in vitro in neuronal cell culture and in vivo in whole animal models of ischemia, have provided strong evidence supporting this hypothesis71, 82, 112. In cultured neurons, for example, brief acid incubations induced significant neuronal injury. This acid-induced neuronal injury was glutamate-independent, but was inhibited by amiloride, a non-specific ASIC blocker, or PcTX1, a specific ASIC1a inhibitor. In contrast to the neurons from ASIC1+/+ mice, neurons cultured from ASIC1−/− mice were resistant to acid injury. Reducing the concentration of extracellular Ca2+, which lowers the driving force for Ca2+ entry through ASICs, also decreased acid-induced injury of CNS neurons71, 82. Thus, activation of ASICs, and subsequent Ca2+ entry, participates in acidosis-mediated injury of neurons.
While homomeric ASIC1a can conduct Ca2+, some studies have suggested that, a significant portion of acid-evoked increases of intracellular Ca2+ is not due to Ca2+ entry directly through ASIC1a homomers. Rather, acidosis might induce Ca2+ accumulation through secondary activation of voltage-gated Ca2+ channels due to ASIC-mediated membrane depolarization and/or Ca2+ release from intracellular stores116, 117, 118.
In vivo studies also support a role for ASIC1a activation in acidosis-mediated, ischemic brain injury71, 112, 119. In rats and mice, intracerebral ventricular injection of ASIC1a inhibitors reduced the infarct volume by up to 60%. Similarly, ASIC1a gene knockout protected the mouse brain from ischemic injury. Furthermore, ASIC1a blockade and ASIC1 gene knockout provided additional protection in the presence of glutamate receptor antagonist71. The protection by ASIC1a blockade has an effective time window of >5 h, and the protection persists for at least 7 d119. Attenuating brain acidosis by intracerebroventricular administration of NaHCO3 is also protective, further suggesting that acidosis is a mediator of ischemic brain injury.
Interactions between ASIC1a and hypoxia/ischemia related signals contribute to ischemic brain injury
In the normal condition, ASIC1a current desensitizes rapidly in the continuous presence of acidosis. This property of ASIC1a argues against its role in brain ischemia in which acidosis is, in general, long-lasting. Recent findings showing that the properties of ASICs, particularly the ASIC1a channels, can be dramatically modulated by ischemia per se and/or ischemia-related signals have provided good explanation supporting the role of ASIC1a channels in ischemic brain injury71, 112, 120, 121, 122. In cultured mouse cortical neurons, for example, brief OGD not only increased the amplitude but also reduced the desensitization of the ASIC current. Accordingly, OGD treatment enhanced acidosis-mediated neuronal injury71. The cellular and molecular mechanisms underlying ischemia-induced increase of ASIC activity has been investigated extensively by several studies. Allen and Attwell demonstrated that arachidonic acid, a lipid metabolite released in ischemia, increased the amplitude of the ASIC current in rat cerebellar Purkinje neurons122. Gao and colleagues demonstrated that an increased phosphorylation of ASIC1a channels by CaMKII, mediated by NMDA receptor activation, was involved in ischemia-induced enhancement of the ASIC responses112. Sherwood and Askwith demonstrated that dynorphins, the most basic neuropeptides abundantly expressed in the central nervous system, could increase the activities of ASIC1a channels and enhance neuronal damage following ischemia120. They do so by reducing the steady-state desensitization of the ASIC1a channels. Very recent studies by Duan and colleagues also showed that, spermine, one of the endogenous polyamines, exacerbated ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis121. Spermine slows down the desensitization of these channels in the open state, shifting steady-state desensitization to more acidic pH, and accelerating recovery of the channels between repeated periods of acid stimulation. Thus, therapeutic interventions for brain ischemia may target ASICs directly by using ASIC blockers/inhibitors or indirectly by blocking the ischemia-related signals which enhance the activation of ASICs.
Perspectives
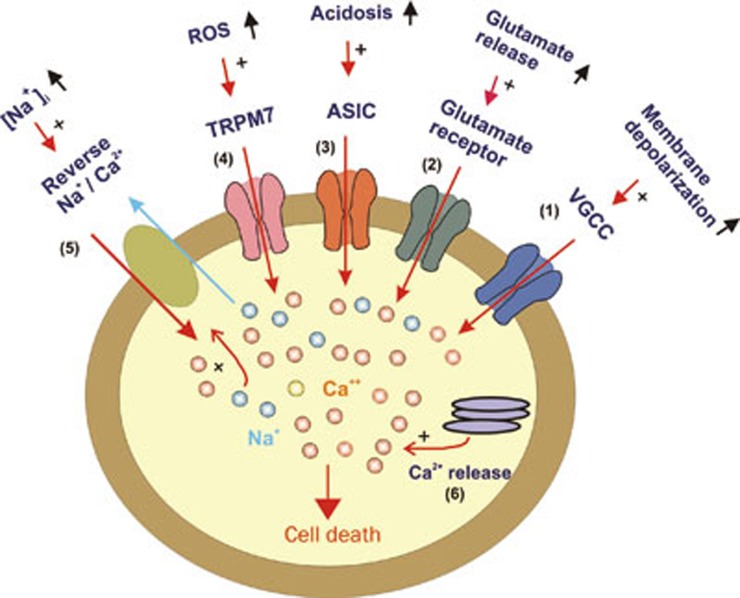
Stroke/brain ischemia is a leading health problem worldwide. Although in recent years enormous progresses have been made in the prevention of stroke, unfortunately, there is still no effective treatment for stroke patients. Searching for new cell injury mechanisms and effective therapeutic strategies is therefore a major challenge in the field. Brain ischemia initiates various biochemical changes such as increased glutamate release, production of oxygen free radicals, lactic acidosis, and reduced ATP synthesis, etc. These changes may facilitate the opening of various Ca2+-permeable ion channels such as glutamate-receptor-gated channels, voltage-gated Ca2+ channels, TRPM7 channels, acid-sensing ion channels, etc. Activation of these channels induces entry of Ca2+ and accumulation of intracellular Ca2+. Intracellular Ca2+ accumulation can also occur through other pathways, eg release of Ca2+ from intracellular stores, or entry of Ca2+ through reversed Na+/Ca2+ exchange system (Figure 1). Overload of neurons with Ca2+ activates a panel of enzymes including proteases, phospholipases and endonucleases, leading to destruction of neurons either through necrotic or apoptotic process. Targeting the pathways responsible for Ca2+ overload may lead to effective neuroprotective interventions for stroke patients. The recent failure of clinical trials using the antagonists of glutamate receptors, however, suggests that future effort should also consider glutamate-independent Ca2+ toxicity in ischemia, eg through activation of TRPM7 channels or ASICs.
Figure 1.
Potential pathways responsible for intracellular Ca2+ accumulation in neurons in ischemic condition. VGCC: voltage-gated Ca2+ channel; ASIC: acid-sensing ion channel; ROS: reactive oxygen species; TRPM7: transient receptor potential melastatin 7; Na+/Ca2+: sodium-calcium exchanger.
Acknowledgments
The work in author's laboratories were supported by grants from National Institute of Health (R01NS47506) and American Heart Association (0840132N)
References
- Weintraub MI. Thrombolysis (tissue plasminogen activator) in stroke: a medicolegal quagmire. Stroke. 2006;37:1917–22. doi: 10.1161/01.STR.0000226651.04862.da. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–30. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–9. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–76. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, et al. The mitochondrial permeability transition. Biofactors. 1998;8:273–81. doi: 10.1002/biof.5520080315. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–9. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Watkins JC. Acidic amino acids with strong excitatory actions on mammalian neurones. J Physiol. 1963;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K. Glutamate and gamma-aminobutyric acid in brain. Nature. 1970;228:119–24. doi: 10.1038/228119a0. [DOI] [PubMed] [Google Scholar]
- Gasic GP, Hollmann M. Molecular neurobiology of glutamate receptors. Ann Rev Physiol. 1992;54:507–36. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–37. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci. 1996;17:348–55. [PubMed] [Google Scholar]
- Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993;13:2085–104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor — Still lethal after eight years. Trends Neurosci. 1995;18:57–8. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–6. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury. Lancet Neurol. 2002;1:383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4:131–6. doi: 10.2174/1566524043479248. [DOI] [PubMed] [Google Scholar]
- Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies--the need for new approaches. Cerebrovasc Dis. 2004;17:153–66. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005;451:1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Kawakami S Hara Y, Wakamori M, Itoh E, Minami T, et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci. 2006;101:66–76. doi: 10.1254/jphs.fp0060128. [DOI] [PubMed] [Google Scholar]
- Aarts MM, Tymianski M. TRPM7 and ischemic CNS injury. Neuroscientist. 2005;11:116–23. doi: 10.1177/1073858404272966. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Bano D. The enemy at the gates. Ca2+ entry through TRPM7 channels and anoxic neuronal death. Cell. 2003;115:768–70. doi: 10.1016/s0092-8674(03)01019-5. [DOI] [PubMed] [Google Scholar]
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–7. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L, Costa E, Wroblewski JT. Glutamate receptor agonists stimulate nitric oxide synthase in primary cultures of cerebellar granule cells. J Neurochem. 1992;58:335–41. doi: 10.1111/j.1471-4159.1992.tb09315.x. [DOI] [PubMed] [Google Scholar]
- Grassi F, Giovannelli A, Fucile S, Eusebi F. Activation of the nicotinic acetylcholine receptor mobilizes calcium from caffeine-insensitive stores in C2C12 mouse myotubes. Pflugers Arch. 1993;422:591–8. doi: 10.1007/BF00374007. [DOI] [PubMed] [Google Scholar]
- Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–9. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell JW, Kaufmann BN. Changes in tissue pH after circulatory arrest. Am J Physiol. 1961;200:743–5. doi: 10.1152/ajplegacy.1961.200.4.743. [DOI] [PubMed] [Google Scholar]
- Ljunggren B, Norberg K, Siesjo BK. Influence of tissue acidosis upon restitution of brain energy metabolism following total ischemia. Brain Res. 1974;77:173–86. doi: 10.1016/0006-8993(74)90782-3. [DOI] [PubMed] [Google Scholar]
- Thorn W, Heitmann R. Hydrogen ion concentration of cerebral cortex of rabbit in situ during peracute total ischemia; pure anoxia and during recuperation. Pflugers Arch. 1954;258:501–10. doi: 10.1007/BF00363713. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Acidosis and ischemic brain damage. Neurochem Pathol. 1988;9:31–88. doi: 10.1007/BF03160355. [DOI] [PubMed] [Google Scholar]
- Rehncrona S. Brain acidosis. Ann Emerg Med. 1985;14:770–6. doi: 10.1016/s0196-0644(85)80055-x. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260:R581–R588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo BK, Katsura K, Kristian T. Acidosis-related damage. Adv Neurol. 1996;71:209–33. [PubMed] [Google Scholar]
- Tombaugh GC, Sapolsky RM. Evolving concepts about the role of acidosis in ischemic neuropathology. J Neurochem. 1993;61:793–803. doi: 10.1111/j.1471-4159.1993.tb03589.x. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Pulsinelli WA, Plum F. Hydrogen ion buffering during complete brain ischemia. Brain Res. 1985;342:281–90. doi: 10.1016/0006-8993(85)91127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemkowicz E, Hansen AJ. Brain extracellular ion composition and EEG activity following 10 minutes ischemia in normo- and hyperglycemic rats. Stroke. 1981;12:236–40. doi: 10.1161/01.str.12.2.236. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Petito CK, Plum F, Pulsinelli WA. Hydrogen ions kill brain at concentrations reached in ischemia. J Cereb Blood Flow Metab. 1987;7:379–86. doi: 10.1038/jcbfm.1987.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian T, Katsura K, Gido G, Siesjo BK. The influence of pH on cellular calcium influx during ischemia. Brain Res. 1994;641:295–302. doi: 10.1016/0006-8993(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Pulsinelli WA, Clarke W Y, Kraig RP, Plum F. The effects of extracellular acidosis on neurons and glia in vitro. J Cereb Blood Flow Metab. 1989;9:471–7. doi: 10.1038/jcbfm.1989.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back T, Hoehn M, Mies G, Busch E, Schmitz B, Kohno K, et al. Penumbral tissue alkalosis in focal cerebral ischemia: relationship to energy metabolism, blood flow, and steady potential. Ann Neurol. 2000;47:485–92. [PubMed] [Google Scholar]
- Kalimo H, Rehncrona S, Soderfeldt B, Olsson Y, Siesjo BK. Brain lactic acidosis and ischemic cell damage: 2. Histopathology. J Cereb Blood Flow Metab. 1981;1:313–27. doi: 10.1038/jcbfm.1981.35. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Barron KD, Bourke RS, Nelson LR, Cragoe EJ. Brain anti-cytoxic edema agents. Prog Clin Biol Res. 1990;361:363–85. [PubMed] [Google Scholar]
- Hillered L, Smith ML, Siesjo BK. Lactic acidosis and recovery of mitochondrial function following forebrain ischemia in the rat. J Cereb Blood Flow Metab. 1985;5:259–66. doi: 10.1038/jcbfm.1985.33. [DOI] [PubMed] [Google Scholar]
- Rehncrona S, Hauge HN, Siesjo BK. Enhancement of iron-catalyzed free radical formation by acidosis in brain homogenates: differences in effect by lactic acid and CO2. J Cereb Blood Flow Metab. 1989;9:65–70. doi: 10.1038/jcbfm.1989.9. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Farrell K, Simon RP. Acidosis causes failure of astrocyte glutamate uptake during hypoxia. J Cereb Blood Flow Metab. 1995;15:417–24. doi: 10.1038/jcbfm.1995.52. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Monyer H, Christine CW, Choi DW. Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen-glucose deprivation neuronal injury in cortical cultures. Brain Res. 1990;506:339–42. doi: 10.1016/0006-8993(90)91276-m. [DOI] [PubMed] [Google Scholar]
- Kaku DA, Giffard RG, Choi DW. Neuroprotective effects of glutamate antagonists and extracellular acidity. Science. 1993;260:1516–8. doi: 10.1126/science.8389056. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Trafton J, Tombaugh GC. Excitotoxic neuron death, acidotic endangerment; and the paradox of acidotic protection. Adv Neurol. 1996;71:237–44. [PubMed] [Google Scholar]
- Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci U S A. 1990;87:6445–9. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–50. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–24. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–7. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–94. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–82. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–77. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- De La Rosa DA, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5:2325–7. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Krishtal OA, Nowycky MC. The proton-activated inward current of rat sensory neurons includes a calcium component. Neurosci Lett. 1990;115:237–42. doi: 10.1016/0304-3940(90)90461-h. [DOI] [PubMed] [Google Scholar]
- Grantyn R, Perouansky M, Rodriguez-Tebar A, Lux HD. Expression of depolarizing voltage- and transmitter-activated currents in neuronal precursor cells from the rat brain is preceded by a proton-activated sodium current. Brain Res Dev Brain Res. 1989;49:150–5. doi: 10.1016/0165-3806(89)90070-9. [DOI] [PubMed] [Google Scholar]
- Ueno S, Nakaye T, Akaike N. Proton-induced sodium current in freshly dissociated hypothalamic neurones of the rat. J Physiol (Lond) 1992;447:309–27. doi: 10.1113/jphysiol.1992.sp019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varming T. Proton-gated ion channels in cultured mouse cortical neurons. Neuropharmacology. 1999;38:1875–81. doi: 10.1016/s0028-3908(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–67. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol. 1999;520:631–44. doi: 10.1111/j.1469-7793.1999.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron. 2002;34:337–40. doi: 10.1016/s0896-6273(02)00687-6. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J. Mechanosensation and the DEG/ENaC ion channels. Science. 1996;273:323–4. doi: 10.1126/science.273.5273.323. [DOI] [PubMed] [Google Scholar]
- Alvarez d. lR, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu Rev Physiol. 2000;62:573–94. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators. Trends Neurosci. 2003;26:477–83. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Roberts JA, Dong J, Zeitouni S, Evans RJ. Analysis of the membrane topology of the acid-sensing ion channel 2a. J Biol Chem. 2004;279:55514–9. doi: 10.1074/jbc.M411849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A. 1997;94:1459–64. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder S, Chen X. Structure, function; pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, et al. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–89. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–7. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Miesch J, Johnson M, Root L, Zhu XM, Chen D, et al. Proton-gated channels in PC12 cells. J Neurophysiol. 2002;87:2555–61. doi: 10.1152/jn.00741.2001. [DOI] [PubMed] [Google Scholar]
- Bassler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Grunder S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–7. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–5. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem. 1996;271:10433–6. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, De Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, et al. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–83. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–8. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–6. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QY, Wang W, Chen XN, Xu TL, Zhou JN. Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience. 2009;159:1126–34. doi: 10.1016/j.neuroscience.2009.01.069. [DOI] [PubMed] [Google Scholar]
- De Weille J, Bassilana F, Lazdunski M, Waldmann R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett. 1998;433:257–60. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–22. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11:1607–11. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–23. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–8. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Bevan S, Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J Physiol (Lond) 1991;433:145–61. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6:2599–601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–90. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–39. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992–7. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, et al. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–24. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–11. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–83. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–15. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, et al. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J Neurosci. 2003;23:3616–22. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S. Identification of sour-taste receptor genes. Anat Sci Int. 2003;78:205–10. doi: 10.1046/j.0022-7722.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- Lin W, Ogura T, Kinnamon SC. Acid-activated cation currents in rat vallate taste receptor cells. J Neurophysiol. 2002;88:133–41. doi: 10.1152/jn.2002.88.1.133. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci. 2004;24:1005–12. doi: 10.1523/JNEUROSCI.4698-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J Neurosci. 2006;26:5800–9. doi: 10.1523/JNEUROSCI.0344-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Render JA, Howe KR, Wunsch AM, Guionaud S, Cox PJ, Wemmie JA. Histologic examination of the eye of acid-sensing ion channel 1a knockout mice. Int J Physiol Pathophysiol Pharmacol. 2010;2:69–72. [PMC free article] [PubMed] [Google Scholar]
- Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–46. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–9. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab. 2001;21:734–40. doi: 10.1097/00004647-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Cuomo O, Esposito E, Sirabella R, Di Renzo G, Annunziato L. ASIC1a contributes to neuroprotection elicited by ischemic preconditioning and postconditioning. Int J Physiol Pathophysiol Pharmacol. 2011;3:1–8. [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103:16556–61. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samways DS, Harkins AB, Egan TM. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium. 2009;45:319–25. doi: 10.1016/j.ceca.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, et al. Sigma-1 receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther. 2008;327:491–502. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–8. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–80. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Wang YZ, Yang T, Chu XP, Yu Y, Huang Y, et al. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of asic1a channels to extracellular acidosis. J Neurosci. 2011;31:2101–12. doi: 10.1523/JNEUROSCI.4351-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Attwell D. Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischemia-related signals. J Physiol (Lond) 2002;543:521–9. doi: 10.1113/jphysiol.2002.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]