Abstract
This study was designed to examine the prospective relations of life stress and genetic status with increases in drug use. African Americans (N = 399) in rural Georgia (Wave 1 mean age = 17 years) provided 3 waves of data across 27.5 months and a saliva sample from which the dopamine receptor gene DRD4 was genotyped. Multilevel growth curve modeling analysis indicated that emerging adults manifested the highest escalations in drug use when they reported high life stress and carried an allele of DRD4 with 7 or more repeats (7+R allele). In addition, emerging adults who reported high life stress and carried the 7+R allele evinced the largest increases in two proximal risk factors for drug use, affiliations with drug-using companions and drug use vulnerability cognitions. Furthermore, when the G×E effects on increases in affiliations with drug-using companions and vulnerability cognitions were entered into the model forecasting drug use, the life stress × DRD4 status interaction on drug use became nonsignificant in the presence of the risk mechanisms. This finding provides an example of “second generation” G×E interaction research in which the interaction's effects on proximal risk mechanisms account for its effects on outcomes.
Keywords: African Americans, drug use, early adulthood, genetics, stress
Nearly 10 million African American families live in the rural coastal plain that stretches across South Carolina, Georgia, Alabama, Mississippi, and Louisiana. This region is one of the most economically disadvantaged areas in the United States (Proctor & Dalaker, 2003; Wimberly & Morris, 1997). The socioeconomic challenges that characterize the rural South are particularly consequential for rural African Americans as they make the transition from adolescence to emerging adulthood. When they leave school, many rural African Americans have no jobs; eventually, they find part-time or full-time employment performing simple functions in retail and service-sector jobs that offer little training and no opportunity for advancement (Offner & Holzer, 2002; Sum et al., 2002). Many rural African American emerging adults are thus confronted with challenging environments that provide minimal resources to help them embark on beneficial life paths (Fuligni & Hardway, 2004). Some who see no pathway to adequate subsistence, much less the attainment of life course goals, cope by increasing their use of drugs (Paschall, Flewelling, & Faulkner, 2000). Escalation of drug use has prognostic significance for rural African American youths' educational and occupational opportunities and attainment, involvement with the criminal justice system, mental health, and physical health (Centers for Disease Control and Prevention, 2000). These circumstances and the resulting need for a better scientific understanding of the processes that account for escalations in drug use among African Americans in the rural South served as the motivation for this study. The identification of etiologic processes among this population will advance knowledge while informing the design of preventive interventions.
The primary purpose of the study was to test multilevel predictors regarding the genetic moderation of the hypothesized association between life stress and increases in drug use. We did not expect genetic variation to have a direct linear association with drug use escalation; instead, we expected genetic status to predict variation in emerging adults' responses to life stress. This perspective is consistent with resilience and differential susceptibility theories, in which genetic variations are hypothesized to render individuals more or less susceptible to environmental risks (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Cicchetti & Blender, 2006; Kim-Cohen & Gold, 2009). One such genetic factor is variation at the dopamine receptor gene DRD4. We tested a hypothesis involving the presence of an allele with 7 or more repeats (7+R). We proposed that emerging adults who carry the 7+R allele would display higher rates of drug use over time when they experienced greater life stress. We also expected trajectories of drug use among emerging adults who experience high life stress and carry an allele of DRD4 with 6 or fewer repeats (6−R) to evince relatively more modest increases in drug use.
The aforementioned hypothesis is an example of “first generation” G×E research, which involves hypotheses designed to document that a G×E interaction forecasts a phenotype. Although this is an important and necessary step in understanding the etiology of drug use and abuse, it does not further understanding of the reasons why or the processes through which the G×E interaction operates to influence a phenotype like drug use trajectories. Research designed to lead to an understanding of the locus of a G×E effect can be termed “second generation” research. Such research is underdeveloped in the G×E literature in developmental psychopathology, particularly that which pertains to drug use etiology. To address this need, the present study also addressed a second generation G×E hypothesis. Specifically, we hypothesized a G×E interaction in which emerging adults who experience high levels of life stress and carry the 7+R allele of DRD4 would evince increases in affiliation with drug-using companions and vulnerability cognitions for drug use, two proximal risk factors for drug use escalation. We further proposed that these interactions would account for the association between the DRD4 status × life stress interaction and drug use. In the following sections, we discuss the hypothesized roles of life stress, DRD4 genotype, and their interaction in the development of drug use and the hypothesized G×E effects on the proposed mediational processes.
A basic premise of this study is that life stresses in combination with genetic sensitivity sponsor increases in drug use. Life stress is a demonstrated risk factor for adolescents' high-intensity drug use and other risk behavior because it precipitates emotional distress and perceptions of limited efficacy and control (Brody et al., 2006). Cross-sectional surveys, prospective surveys, and experience-sampling studies with adolescents in the United States and other countries have found initiation and escalation of drug use to be positively associated with life stress (Aseltine & Gore, 2000; Castro, Maddahian, Newcomb, & Bentler, 1987; Cooper, Shapiro, & Powers, 1998; He, Kramer, Houser, Hacker, & Chomitz, 2004; Patton et al., 1996; Unger, Hamilton, & Sussman, 2004; Whalen, Jamner, Henker, & Delfino, 2001; Windle, Mun, & Windle, 2005). Baumeister and Scher (1988) advanced a parsimonious interpretation of this link. People desire the quickest possible escape from life stress and the negative affect that accompanies it; this increases the attraction of activities that provide short-term relief. Thus, the “quick fix” that risk behavior offers becomes appealing regardless of possible long-term costs to health and well-being.
An individual's reaction to life stress clearly has multiple determinants, including genetic inheritance. Genes and their interactions with life experiences have been conjectured to play an important role in drug use and abuse (Brody, Beach, Philibert, Chen, & Murry, 2009); knowledge to date, however, is more theoretical than empirical, particularly for African Americans. We focused on DRD4 in our effort to understand the operation of such interactions among rural African American emerging adults. The 48bp Variable Number Tandem Repeats (VNTRs) in DRD4 that we examined form a polymorphism in exon 3 that codes for a 16 amino acid insert in the dopamine D4 receptor. The VNTR contains 2 to 11 repeats, with the 4-repeat and 7-repeat alleles being most common. The 7+R alleles function in a way that yields a protein structure that produces less reactive D4 receptors in both in vitro and in vivo tests of responsiveness, resulting in weaker transmission of intracellular signals for those with a 7+R allele versus a 6−R allele (for example, see Levitan et al., 2006). The 7+R allele has been found to be associated with attention deficit/hyperactivity disorder (Li, Sham, Owen, & He, 2006), alcoholism (Laucht, Becker, Blomeyer, & Schmidt, 2007), pathological gambling (Pérez de Castro, Ibáñez, Torres, Sáiz-Ruiz, & Fernández-Piqueras, 1997), and impulsivity (Eisenberg et al., 2007). DRD4 has also been associated with novelty seeking, which is characterized by impulsivity, excitement, and approach responses to novel stimuli (Cloninger, 1987). Novelty seeking has been found to be associated with smoking (Heath, Madden, Slutske, & Martin, 1995), alcohol use disorder (Flory et al., 2006), and drug misuse (Gabel, Stallings, Schmitz, Young, & Fulker, 1999). We propose that emerging adults experiencing high life stress along with the prospect of limited future opportunities in rural southern contexts will be particularly drawn to the effects of drugs if they carry the DRD4 7+R. Conversely, we do not expect emerging adults confronted with similar levels of life stress and limited opportunities who carry the DRD4 6−R allele to evince increases in drug use. The latter youths would demonstrate the protective properties of the DRD4 6−R allele.
A second purpose of this study was to examine the extent to which the interaction of life stress and genotype-associated trajectories of drug use is mediated by two proximal risk factors, affiliation with drug-using companions and the development of vulnerability cognitions. We expect that a significant proportion of the effect of the interaction of life stress and genotype on escalation of drug use will be mediated by the aforementioned proximal risk factors, operating as mediated moderators. We are aware of only one study (Simons et al., in press) in which researchers have tested a second generation G×E hypothesis, that G×E effects on proximal risk factors account for G×E effects on an outcome, in that case antisocial behavior. Our rationale for the selection of these risk factors and for the G×E mediated moderation hypothesis is presented next.
We conjectured that African American emerging adults experiencing high levels of life stress may come to believe that they have little to lose by abandoning planful, conventional orientations in favor of a present orientation that promotes “living in the moment.” These emerging adults are at heightened risk of discounting short- and long-term consequences, increasing their affiliations with drug-using peers and romantic partners, and developing cognitions that increase their likelihood of drug use. Empirical evidence justifies a focus on these particular proximal risk mechanisms. Because emerging adults are not randomly assigned to friends and romantic partners, the observed similarities in self-regulation, future orientation, and drug use they share with their companions is due partly to young people's tendency to seek like-minded companions (Caspi & Herbener, 1990; Connell & Dishion, 2006; Simons, Stewart, Gordon, Conger, & Elder, 2002). Hopelessness and disinterest in long-term goals accompany negative life stress (American Psychiatric Association, 1994). This perspective can be expected to influence emerging adults' selection of companions who share and support their norms, attitudes, and behavior. Consistent with this idea, several studies demonstrate that persons experiencing high life stress select as friends, and begin romantic relationships with, individuals who are less conventional and more likely to use drugs (Brody, Chen, & Kogan, 2010; Daley & Hammen, 2002; Meeus, Branje, & Overbeek, 2004). Thus, we predict that emerging adults reporting relatively higher life stress will increase their affiliations with companions who use drugs if the emerging adults carry the DRD4 7+R allele. Such companions are likely to model, sanction, and encourage drug use.
Intentions and willingness to use drugs start to develop at an early age and continue to develop during emerging adulthood, serving as a proximal risk mechanism in longitudinal, etiological research forecasting drug use escalation (Chassin, Tetzloff, & Hershey, 1985; Cleveland, Gibbons, Gerrard, Pomery, & Brody, 2005). Behavioral willingness is defined as an openness to using drugs given an opportunity—that which an emerging adult might do under certain circumstances such as the presence of substance-using friends (Cleveland et al., 2005). Intentions to use drugs predict use more strongly with increasing age, as drug use becomes more intentional and, in some cases, habitual (Pomery, Gibbons, Reis-Bergan, & Gerrard, 2009). To maximize predictive power, both willingness and intentions to use drugs were included in the current study in a construct labeled vulnerability cognitions. We hypothesized that emerging adults who carry the 7+R allele of DRD4 would display larger increases in vulnerability cognitions, especially when they are contending with high levels of life stress. The allure that drug use offers as a short-term escape from stressful circumstances, particularly for persons who are rendered somewhat more prone to novelty, sensation seeking, and impulsiveness by the7+R allele, are expected to increase their thoughts of using drugs if the opportunity presents itself, even planning scenarios for drug use.
Summary of the Present Study
This study was conducted with rural African American youths as they transitioned out of secondary school, using procedures that have been shown to yield reliable data from longitudinal, epidemiological research focusing on drug use. These procedures include computer-based interviewing, matching of interviewers and participants by ethnicity, and extensive reassurances concerning data confidentiality (Brody et al., 2006; Kotchick, Shaffer, & Forehand, 2001; Patrick et al., 1994). We predicted that (a) emerging adults who report high life stress and carry the 7+R allele of DRD4 would evince more drug use across the 27.5 months that separated the first and third waves of data collection, and (b) the effect of life stress × DRD4 interactions on increases in affiliations with drug-using companions and drug use vulnerability cognitions would operate as mediators of life stress × DRD4 interaction effects on increases in drug use.
Method
Participants
A total of 494 youths were recruited randomly from public school lists in six rural counties. The data used in the present study were collected in 2006, 2008, and 2009; they were analyzed in 2011. Youths were enrolled in the study when they were about 17 years of age (Brody, Chen, Kogan, Smith, & Brown, 2010) and provided self-report data at ages 17, 19, and 20 years. The genetic data were collected when the youths were 17 years of age. Data were collected in the context of a family-based prevention study. Assignment to the prevention or control condition was controlled in all data analyses. At Wave 1, median household gross monthly income was $2016.00 (SD = 4353.86) and mean monthly per capita gross income was $887.54 (SD = 1578.98). Although youths' caregivers worked an average of 38.5 hours per week, 42% of the families lived below federal poverty standards and another 15% lived within 150% of the poverty threshold; they could be described as working poor (Boatright, 2005).
Procedures
Families were contacted and enrolled in the study by community liaisons who resided in the counties where the participants lived. The community liaisons were African American community members, selected on the basis of their social contacts and standing in the community, who worked with the researchers on participant recruitment and retention. At all data collection points, parents gave written consent to minor youths' participation, and youths gave written assent or consent to their own participation. Each family was paid $100 after each assessment.
To enhance rapport and cultural understanding, African American university students and community members who did not know the families' condition assignments served as field researchers to collect data. During each assessment, one home visit lasting 2 hr was made to each family. At the home visit, self-report questionnaires were administered to the youth on a laptop computer in a private place in each home.
Measures
Demographics
Monthly income, maternal age, and number of children in the household were recorded from parent report. Poverty status was based on per capita income and federal guidelines.
Life stress
Life stress was assessed at Wave 1; youths completed a checklist of 12 events (e.g., acute economic stressor, death of a friend, parental divorce, serious injury or illness; Brody, Chen, Kogan, et al., 2010), indicating whether each had occurred during the previous 6 months. Because this index is composed of count data, internal consistency was not computed.
Companions' drug use
At each wave, youths completed measures focusing on peer and romantic partner drug use. Youths reported the proportions of their close friends (none, some, all) who engaged in drug use (cigarettes, alcohol, marijuana, excessive drinking [consumption of 3+ drinks on one occasion]). Cronbach's alphas ranged from .83 to .86 across waves. On a scale ranging from 1 (never) to 3 (often), youths also reported how often their current or last romantic partners engaged in such drug use. Cronbach's alphas ranged from .75 to .84 across waves. Because friends and romantic partners typically belonged to the same peer groups, the items from both scales were summed to form a companions' drug use scale. Cronbach's alphas for the combined measure ranged from .79 to .82 across the study.
Vulnerability cognitions
Youths' willingness to use drugs was measured with three items, worded as in previous studies (Brody et al., 2004). The items began with the stem “Suppose you were with a group of friends and there were some drugs there that you could have if you wanted. How willing would you be to do the following things: (a) take some and use it; (b) use enough to get high; and (c) take some with you to use later?” Item responses ranged from 1 (not at all) to 3 (very); Cronbach's alphas ranged from .73 to .89 across the study. Vulnerability also included items measuring intentions to smoke cigarettes, smoke marijuana, drink alcohol, and drink alcohol excessively: “Do you plan to use (drug) in the next year?” and “How likely is it that you will use (drug) in the next year?” (Warshaw & Davis, 1985). Cronbach's alpha for the eight-item intention measure ranged from .77 to .82 across waves. The intention and willingness items were then combined and used as an indicator of vulnerability cognitions. Cronbach's alphas for the combined indicator were .78 to .88 across waves.
Drug use
Four items were used to assess past-month drug use (Johnston, O'Malley, & Bachman, 2000). Youths were asked whether they had engaged in each of the forms of drug use included in the study during the past month. Because drug use rates were low, the data were coded into two categories: 0 (no use of drugs in any form) and 1 (any drug use).
Genotyping
Youths' DNA was obtained using Oragene™ DNA kits (Genetek, Calgary, AB, Canada). Youths rinsed their mouths with tap water, then deposited 4 ml of saliva in the Oragene sample vial. The vial was sealed, inverted, and shipped via courier to a central laboratory in Iowa City, where samples were prepared according to the manufacturer's specifications. Genotype at DRD4 was determined for each youth as described by Bradley, Dodelzon, Sandhu, and Philibert (2005) using the primers F-GGCGTTGCCGCTCTGAATGC and R-GAGGGACTGAGCTGGACAACCAC, standard Taq polymerase and buffer, standard dNTPs with the addition of 100 μM 7-deaza GTP, and 10% DMSO. The resulting PCR products were electrophoresed on a 6% non-denaturing polyacrylamide gel and products visualized using silver staining. Genotype was then called by two individuals blind to the study hypotheses and other information about the participants. For tests of the G×E hypotheses, DRD4 status was dummy coded; participants with at least one 7+R allele were assigned a code of 1 (41.9% of the sample), and participants who were homozygous for the 6−R allele were assigned a code of 0 (58.1% of the sample). None of the alleles deviated from Hardy-Weinberg equilibrium (p = .87, ns).
Plan of Analysis
Growth curve models (GCMs) embedded in a multilevel modeling framework were used (Raudenbush & Bryk, 2002; Singer & Willett, 2003). Three steps of analyses were conducted to test the proposed hypotheses regarding DRD4 × life stress effects on the developmental trajectories of past month drug use and two mediators that were hypothesized to link life stress with increases in drug use (companion's drug use and vulnerability cognitions) during emerging adulthood. At the first step, unconditional GCMs were estimated. Individual developmental trajectories of companions' drug use and vulnerability cognitions were modeled as a function of age (centered at age 17) as the Level 1 model:
| (1) |
The notation i was used to index targets and the notation t was used to index different time points of the study nested in targets; π0i and π1i were the intercept and slope, respectively, of growth in the outcome variables; and eti was the Level 1 error. Because of its dichotomous nature, the Level 1 model for past month drug use was executed as a logistic regression model. The log odds of past month drug use was regressed on targets' age (centered at 17 years) as the Level 1 model with the same notation presented in equation (1):
| (2) |
The Level 2 model treated π0i and π1i as random variables. In the unconditional growth curve model, the Level 2 parameters were:
| (3) |
| (4) |
where γ00 is the “grand mean” of the outcomes of interest in log odds ratio across targets and γ10 is the slope of age regressed on the outcomes, indicating the rate of change across time. U0 and U1 were the errors of π0i and π1i, respectively.
At the second step, DRD4, life stress, DRD4 × life stress, and two control variables (intervention status and youth gender) were included in the Level 2 models. By regressing the DRD4 × life stress interaction on the rate of change of past month drug use, companions' drug use, and vulnerability cognitions, we tested the exacerbating effect of DRD4 on the influence of life stress.
At the third step, we first fitted several cross-lagged models to ensure the direction of causality between companions' drug use and past month drug use and between vulnerability cognitions and past month drug use. With the direction of causality established, companions' drug use and vulnerability cognitions were introduced as time-varying predictors of past month drug use. Both time-varying predictors were first centered within each individual (person-mean-centering) before their inclusion in the Level 1 model, and then the time-averaged levels of the predictors were included in the Level 2 model along with life stress, DRD4, DRD4 × life stress, and the control variables. This approach was introduced in (Hoffman & Stawski, 2009) and (Shaw, Agahi, & Krause, 2011) for separating between- and within-individual effects of time-varying predictors.
The aforementioned analyses were re-executed with monthly family income added to the model as a control. This addition did not change any of the results. All the GCMs were conducted in STATA 12 (StataCorp, 2011) with its XT modules. The cross-lagged models were executed in Mplus 6.11 (Muthén & Muthén, 1998–2010) with appropriate distribution functions.
Results
Attrition Analysis
Study hypotheses were tested with 399 youths (80% of the Wave 1 sample, N = 494) who agreed to provide DNA during a follow-up assessment. Mean comparisons based on retention revealed no differences on any study or demographic variables between youths who left the study and those who continued to participate. Table 1 presents the means, standard deviations, and intercorrelations among the research variables. Independent samples t-tests on youths who did or did not agree to provide DNA revealed one difference: Youths who did not provide DNA reported fewer affiliations with drug-using companions at all three data collection waves (all ts, p < .05).
Table 1.
Means, Standard Deviations, and Correlations for the Research Variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. DRD4 status | - | ||||||||||||
| 2. Male gender | −.00 | - | |||||||||||
| 3. Intervention | .08 | −.07 | - | ||||||||||
| 4. Life stress | −.03 | .02 | .09 | - | |||||||||
| Cognitive Vulnerability | |||||||||||||
| 5. Wave 1 | .06 | 15*** | −.05 | 15*** | - | ||||||||
| 6. Wave 2 | .08 | 15** | −.05 | .10* | 48*** | - | |||||||
| 7. Wave 3 | .13* | .21*** | −.02 | 14** | 48*** | 67*** | - | ||||||
| Companions' Drug Use | |||||||||||||
| 8. Wave 1 | .00 | .02 | .08 | .28*** | .35*** | .28*** | .24*** | - | |||||
| 9. Wave 2 | .03 | .03 | −.04 | .19*** | .30*** | .47*** | .41*** | .43*** | - | ||||
| 10. Wave 3 | .02 | −.07 | .02 | .20*** | .24*** | .28*** | .43*** | .34*** | .47*** | - | |||
| Drug Use | |||||||||||||
| 11. Wave 1 | .01 | .11* | −.01 | .22*** | .49*** | .39*** | .33*** | .35*** | .32*** | .30*** | - | ||
| 12. Wave 2 | .08 | .09 | .04 | .21*** | .28*** | .45*** | .40*** | .30*** | .40*** | .33*** | .37*** | - | |
| 13. Wave 3 | .07 | .20*** | .02 | .15** | .30*** | .37*** | .50*** | .18*** | .33*** | .41*** | .31*** | .44*** | - |
|
| |||||||||||||
| Mean | 0.42 | 0.41 | 0.64 | 2.31 | 11.52 | 12.81 | 13.30 | 8.85 | 9.78 | 10.21 | 0.24 | 0.39 | 0.45 |
| SD | 0.49 | 0.49 | 0.48 | 1.62 | 3.51 | 4.99 | 5.10 | 3.71 | 3.80 | 3.77 | 0.43 | 0.49 | 0.50 |
p < .05,
p < .01,
p < .001.
Unconditional Growth Curve Models
Results from unconditional GCMs showed a significant and positive growth trend across time in past month drug use (βage = 5.94, p < .001) and companions' drug use (βage = 5.47, p < .001); the mean slope of vulnerability cognitions, however, was not significant (βage = .19, p = .64). All the variance estimates for the slopes in the three drug use-related variables were significant (p = .05). This facilitated further investigation of predictors of personal change.
DRD4 × Life Stress Interaction Predicts Growth of Research Outcomes
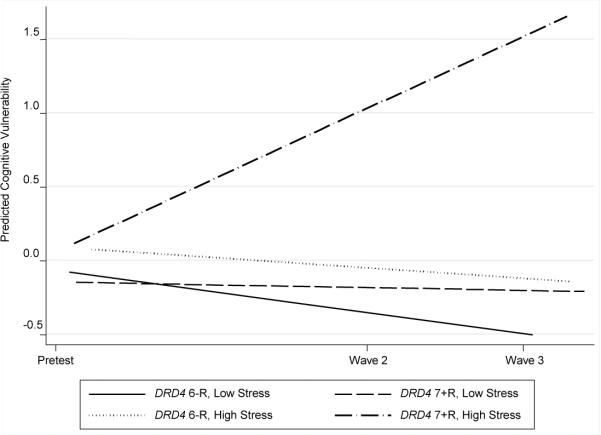
Table 2 presents the results of DRD4 × life stress effects on individual changes in past month drug use, companions' drug use, and vulnerability cognitions with age, gender, and intervention status controlled. The interaction of DRD4 × life stress significantly predicted individual changes in the three drug use variables. Figures 1 to 3 present individual changes across time for different combinations of DRD4 and life stress levels. All figures show that participants who carry at least one 7+R allele and are exposed to high levels of life stress reported the greatest increases in the predicted values of the three drug use variables.
Table 2.
Life Stress, DRD4 Status, and the Interaction between Life Stress and DRD4 Status Predicting the Slope of Research Variables with Time-varying Effects of Two Intermediate Drug Use Predictors
| Past month drug useb |
||||||||
|---|---|---|---|---|---|---|---|---|
| Companions' drug usea |
Cognitive vulnerabilitya |
Model 1 |
Model 2c |
|||||
| Predictors | Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE |
| On slope | ||||||||
| Life stress | −1.49 | 0.86 | −0.05 | 0.50 | −1.26 | 0.88 | −1.52 | 0.81 |
| DRD4 | 0.33 | 1.60 | 1.22 | 0.91 | 1.42 | 1.66 | 0.78 | 1.54 |
| Life stress × DRD4 | 2.41** | 0.91 | 1.28* | 0.58 | 1.82* | 0.92 | 0.84 | 0.74 |
| Within-individual time-varying effects | ||||||||
| Companions' drug use | 0.13** | 0.04 | ||||||
| Cognitive vulnerability | 0.59*** | 0.09 | ||||||
Gender and intervention status were controlled.
Multilevel logistic regression was used; coefficients presented are in log odds metric.
Gender, intervention status, and time-averaged levels of companions' drug use and cognitive vulnerability were controlled.
p <.05
p <.01
p <.001.
Figure 1.
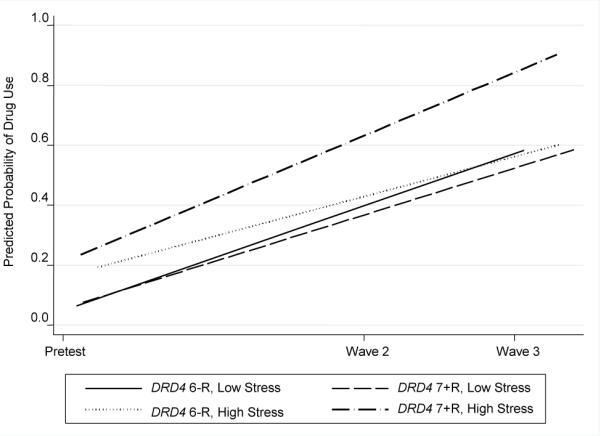
Growth in probability of past month drug use by DRD4 status and life stress. Low stress = 1 SD below the mean; high stress = 1 SD above the mean.
Figure 3.
Growth in cognitive vulnerability by DRD4 status and life stress. Low stress = 1 SD below the mean; high stress = 1 SD above the mean.
Time-varying Effects of Companions' Drug Use and Vulnerability Cognitions
Before investigating time-varying effects of the two hypothesized mediators, we executed two cross-lagged models to determine whether the direction of causality among the variables conformed to the study hypotheses. The path from companions' drug use at age 17 to past month drug use at age 20 was .61 (p < .001) and the path from drug use at age 17 to companions' drug use at age 20 was .31 (p < .001). A Wald test was performed to assess the equality of the two paths. The results showed that the paths differed significantly [χ2(1) = 5.07, p = .024], suggesting that the direction of causality from companions' drug use to past month drug use was stronger than the reverse. Similarly, the path from vulnerability cognitions at age 17 to past month drug use at age 20 was .61 (p < .001) and the path from drug use at age 17 to vulnerability cognitions at age 20 was .33 (p < .001). A Wald test again showed a significant difference between the two path coefficients [χ2(1) = 18.61, p < .001], suggesting the direction of causality proceeded from vulnerability cognitions to past month drug use rather than the reverse.
The last column of Table 2 presents estimates of the time-varying effects of the two hypothesized drug use mediators on past month drug use. Within-individual variations in both companions' drug use and vulnerability cognitions significantly predicted the probability of past month drug use (β = .13, p < .01 for companions' drug use; β = .59, p < .001 for vulnerability cognitions). For one unit change in companions' drug use across time, the odds of past month drug use increased by 14%; whereas for one unit change in vulnerability cognitions across time, the odds of past month drug use increased by 80%. Furthermore, after including the time-varying effects of the drug use mediators, the DRD4 × life stress interaction effect on the slope of past month drug use became nonsignificant, confirming the hypothesized mediational role of the two predictors.
Discussion
Drawing on differential susceptibility and resilience theories (Belsky et al., 2007; Caspi et al., 2010; Cicchetti & Blender, 2006; Kim-Cohen & Gold, 2009), we predicted that high levels of life stress would interact with the 7+R allele of DRD4 to predict increases in emerging adults' drug use. Consistent with this hypothesis, a clear link emerged between life stress and a rise in drug use across 27.5 months among emerging adults with this genotype. This finding is consistent with the proposition, informed by prior G×E research involving DRD4, that the genetic makeup of emerging adults who carry the 7+R allele renders them more likely to be affected by life stress than are those with the DRD4 6−R allele. The finding that carrying the 6−R allele buffered emerging adults from escalating drug use when they experienced high levels of life stress is pertinent to research on resilience. The literature has addressed the reasons why some youths who experience many discrete and chronic stressors do not succumb to their negative effects (Cicchetti & Blender, 2006). Typically, such resilience is attributed to contextual processes at various levels of analysis (family, peer, school, or neighborhood) that alter several types of pathways, including reduction of risk factor effects. The present results reinforce suggestions that genetic status can also contribute to resilience (Kim-Cohen & Gold, 2009; Moffitt, Caspi, & Rutter, 2006; Rutter & Silberg, 2002). The observed buffering effects of the 6−R allele suggest a self-regulatory mechanism in which genotype contributes to planfulness and a reflective consideration of consequences during decision making. Further research is needed to test this hypothesis.
Caution should be used, however, in interpreting and generalizing the protective effects of the 6−R genotype to all rural African American emerging adults. Some who seem unfazed by high levels of life stress may not be as adaptable as they appear; still others may be resilient in some areas but experience distress in others, such as health or aspects of social relationships that do not involve drug use (Brody et al., 2011; Brody et al., in press). Research with children who have been maltreated (Farber & Egeland, 1987), whose mothers have depression (Hammen, 2003), and whose parents have alcoholism (Zucker, Wong, Puttler, & Fitzgerald, 2003) support this caveat. Even emerging adults who are well-adjusted behaviorally can have their resilient trajectories disrupted by the introduction of other risk factors that diminish the protective capacities that the 6−R genotype may confer.
The second purpose of this study was to address a second generation G×E research hypothesis concerning the processes responsible for the G×E interaction effect on drug use. Tests of the mediated moderation hypothesis revealed two pathways through which the life stress × DRD4 interaction effects occurred. Paths from increases in affiliations with drug-using companions and increases in vulnerability cognitions to escalation of drug use accounted for the interaction effects on the outcome variable. To our knowledge, the present study is the first to show that the effects of G×E interactions on proximal risk factors for increases in drug use are responsible for the interaction effects on drug use escalation. This finding is important because it not only begins to pinpoint the locus of G×E interaction effects on drug use but also suggests targets for genetically informed prevention programs. Knowing that life stress in combination with carrying a 7+R allele of DRD4 leads to increases in associations with drug-using companions and vulnerability cognitions enables prevention scientists to formulate interventions targeting protective mechanisms that can interrupt this sequence. Such mechanisms may include relationships with family members and natural mentors, along with enhancements of self-regulation that, together, will buffer reactivity to stress (Brody, Chen, Kogan, et al., 2010).
The mediated moderation analyses are also noteworthy because they add to knowledge about the processes that are subject to the life stress × DRD4 interactions. Consistent with the idea that “birds of a feather flock together” (Glueck & Glueck, 1950), this mechanism implies that emerging adults select companions with risk-related characteristics similar to their own and that this process is partly under the control of interactions involving both contextual and genetic factors. These interactions influence the choice of companions, producing a social environment that encourages drug use. After such differential affiliations and vulnerability cognitions become established, behavior contagion processes activate (Dishion, Eddy, Haas, Li, & Spracklen, 1997). These processes include companions' explicit reinforcement of drug use and disapproval of conventional conduct (Dishion, Spracklen, Andrews, & Patterson, 1996).
The effect of G×E processes on escalation in vulnerability cognitions for drug use is another important finding. The sense of hopelessness that accompanies intractable life stress affects cognitive orientations toward drug use among emerging adults who carry the 7+R allele. The lure of drugs as a respite from inescapable burdens increases, a process that the propensity for novelty and sensation seeking associated with the 7+R allele enhances. An increase in vulnerability cognitions is likely to be reinforced by the affiliation process described previously, as increases in willingness and intentions to use drugs attract emerging adults to like-minded companions. A reciprocal influence process then ensues in which vulnerability cognitions and affiliation processes become intertwined to produce ongoing drug use. From a developmental perspective, these mediated moderation processes probably assume greater importance during adolescence and emerging adulthood, when youths become more autonomous. Future research should focus on mediated moderation processes involving the provision of protective parenting to preadolescents who carry DRD4 7+R alleles. Such parenting is hypothesized to carry forward, at least through adolescence, to deter these youths from affiliating with substance-using companions and thinking about drug use as a means of coping with life stress.
Some limitations of the research should be noted. Only one genetic polymorphism was examined; this does not represent all of the variation that could place emerging adults at risk for drug use. Many genetic variants may alter risk, the expression of which may emerge only under particular contextual conditions. A corollary of this limitation is the perception that genetic variation confers only risk. Genetic effects many also be protective, and that which is conceptualized as a risk-promoting genetic effect may actually be the absence of protective genes. In this study, the DRD4 6−R allele protected emerging adults who experienced high levels of life stress from escalating drug use. This is an important finding that should be explored in future research. Studies are also needed to examine processes that, in interaction with genetic status, have been found to protect emerging adults from the costs of life stress, such as harmonious family and romantic relationships, affiliation with prosocial friends, and self-regulation (Brody, Chen, Kogan, et al., 2010). In this way, understanding of the processes and conditions that account for variation in links between life stress and escalation of drug use can be refined. Future research with larger samples will be able to address this issue. These limitations notwithstanding, the present study demonstrates the ways in which life stress and DRD4 status combine to create different drug use trajectories among rural African American emerging adults.
Figure 2.
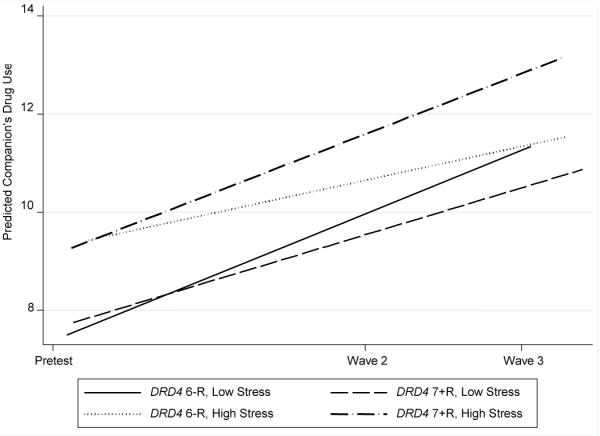
Growth in companions' drug use by DRD4 status and life stress. Low stress = 1 SD below the mean; high stress = 1 SD above the mean.
Acknowledgments
This research was supported by Awards Numbers P30DA027827 and R01DA019230 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Aseltine RH, Jr., Gore SL. The variable effects of stress on alcohol use from adolescence to early adulthood. Substance Use and Misuse. 2000;35:643–668. doi: 10.3109/10826080009148415. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Scher SJ. Self-defeating behavior patterns among normal individuals: Review and analysis of common self-destructive tendencies. Psychological Bulletin. 1988;104:3–22. doi: 10.1037/0033-2909.104.1.3. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Boatright SR. The Georgia county guide. Center for Agribusiness and Economic Development; Athens, GA: 2005. [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;136:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Philibert RA, Chen Y.-f., Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene × environment hypotheses tested via a randomized prevention design. Child Development. 2009;80:645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Chen Y.-f., Kogan SM. A cascade model connecting life stress to risk behavior among rural African American emerging adults. Development and Psychopathology. 2010;22:667–678. doi: 10.1017/S0954579410000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y.-f., Kogan SM, Smith K, Brown AC. Buffering effects of a family-based intervention for African American emerging adults. Journal of Marriage and Family. 2010;72:1426–1435. doi: 10.1111/j.1741-3737.2010.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y.-f., Murry VM, Ge X, Simons RL, Gibbons FX, et al. Perceived discrimination and the adjustment of African American youths: A five-year longitudinal analysis with contextual moderation effects. Child Development. 2006;77:1170–1189. doi: 10.1111/j.1467-8624.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, et al. The Strong African American Families program: Translating research into prevention programming. Child Development. 2004;75:900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen Y.-f., Kogan SM, Evans GW, Beach SRH, et al. Cumulative socioeconomic status risk, allostatic load, and adjustment: A prospective latent profile analysis with contextual and genetic protective factors. 2011. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen Y.-f., Kogan SM, Evans GW, Windle M, et al. Supportive family environments, sensitivity genes, and allostatic load among rural African American emerging adults: A prospective analysis. Journal of Family Psychology. doi: 10.1037/a0027829. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Herbener ES. Continuity and change: Assortative marriage and the consistency of personality in adulthood. Journal of Personality and Social Psychology. 1990;58:250–258. doi: 10.1037//0022-3514.58.2.250. [DOI] [PubMed] [Google Scholar]
- Castro FG, Maddahian E, Newcomb MD, Bentler PM. A multivariate model of the determinants of cigarette smoking among adolescents. Journal of Health and Social Behavior. 1987;28:273–289. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Youth risk behavior surveillance—United States, 1999. Morbidity and Mortality Weekly Report. 2000;49 [PubMed] [Google Scholar]
- Chassin LA, Tetzloff C, Hershey M. Self-image and social-image factors in adolescent alcohol use. Journal of Studies on Alcohol. 1985;46:39–47. doi: 10.15288/jsa.1985.46.39. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Cleveland MJ, Gibbons FX, Gerrard M, Pomery EA, Brody GH. The impact of parenting on risk cognitions and risk behavior: A study of mediation and moderation in a panel of African American adolescents. Child Development. 2005;76:900–916. doi: 10.1111/j.1467-8624.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: A proposal. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Connell AM, Dishion TJ. The contribution of peers to monthly variation in adolescent depressed mood: A short-term longitudinal study with time-varying predictors. Development and Psychopathology. 2006;18:139–154. doi: 10.1017/S0954579406060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Shapiro CM, Powers AM. Motivations for sex and risky sexual behavior among adolescents and young adults: A functional perspective. Journal of Personality and Social Psychology. 1998;75:1528–1558. doi: 10.1037//0022-3514.75.6.1528. [DOI] [PubMed] [Google Scholar]
- Daley SE, Hammen C. Depressive symptoms and close relationships during the transition to adulthood: Perspectives from dysphoric women, their best friends, and their romantic partners. Journal of Consulting and Clinical Psychology. 2002;70:129–141. doi: 10.1037//0022-006x.70.1.129. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Eddy JM, Haas E, Li F, Spracklen KM. Friendships and violent behavior during adolescence. Social Development. 1997;6:207–223. [Google Scholar]
- Dishion TJ, Spracklen KM, Andrews DW, Patterson GR. Deviancy training in male adolescents' friendships. Behavior Therapy. 1996;27:373–390. [Google Scholar]
- Eisenberg DTA, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: A DRD2 TaqI A and DRD4 48-bp VNTR association study. Behavioral and Brain Functions. 2007;3 doi: 10.1186/1744-9081-3-2. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber EA, Egeland B. Invulnerability among abused and neglected children. In: Anthony EJ, Cohler BJ, editors. The invulnerable child. Guilford Press; New York: 1987. pp. 253–288. [Google Scholar]
- Flory K, Brown TL, Lynam DR, Miller JD, Leukefeld C, Clayton RR. Developmental patterns of African American and Caucasian adolescents' alcohol use. Cultural Diversity and Ethnic Minority Psychology. 2006;12:740–746. doi: 10.1037/1099-9809.12.4.740. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Hardway C. Preparing diverse adolescents for the transition to adulthood. Future of Children. 2004;14:99–119. [Google Scholar]
- Gabel S, Stallings MC, Schmitz S, Young SE, Fulker DW. Personality dimensions and substance misuse: Relationships in adolescents, mothers and fathers. American Journal on Addictions. 1999;8:101–113. doi: 10.1080/105504999305901. [DOI] [PubMed] [Google Scholar]
- Glueck S, Glueck E. Unraveling juvenile delinquency. Commonwealth Fund; Oxford, UK: 1950. [Google Scholar]
- Hammen C. Risk and protective factors for children of depressed parents. In: Luthar SS, editor. Resilience and vulnerability: Adaptation in the context of childhood adversities. Cambridge University Press; New York: 2003. [Google Scholar]
- He K, Kramer E, Houser RF, Hacker KA, Chomitz VR. Defining and understanding healthy lifestyles choices for adolescents. Journal of Adolescent Health. 2004;35:26–33. doi: 10.1016/j.jadohealth.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Slutske WS, Martin NG. Personality and the inheritance of smoking behavior: A genetic perspective. Behavior Genetics. 1995;25:103–107. doi: 10.1007/BF02196921. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–120. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG. Monitoring the Future national survey results on drug use, 1975–1999. Volume I: Secondary school students. National Institute on Drug Abuse; Bethesda, MD: 2000. NIH Publication No. 00-4802. [Google Scholar]
- Kim-Cohen J, Gold AL. Measured gene-environment interactions and mechanisms promoting resilient development. Current Directions in Psychological Science. 2009;18:138–142. [Google Scholar]
- Kotchick BA, Shaffer A, Forehand R. Adolescent sexual risk behavior: A multisystem perspective. Clinical Psychology Review. 2001;21:493–519. doi: 10.1016/s0272-7358(99)00070-7. [DOI] [PubMed] [Google Scholar]
- Laucht M, Becker K, Blomeyer D, Schmidt MH. Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: Results from a high-risk community sample. Biological Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Levitan RD, Masellis M, Lam RW, Kaplan AS, Davis C, Tharmalingam S, et al. A birth-season/DRD4 gene interaction predicts weight gain and obesity in women with seasonal affective disorder: A seasonal thrifty phenotype hypothesis. Neuropsychopharmacology. 2006;31:2498–2503. doi: 10.1038/sj.npp.1301121. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Human Molecular Genetics. 2006;15:2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- Meeus W, Branje SJT, Overbeek G. Parents and partners in crime: A six-year longitudinal study on changes in supportive relationships and delinquency in adolescence and young adulthood. Journal of Child Psychology and Psychiatry. 2004;45:1288–1298. doi: 10.1111/j.1469-7610.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter ML. Measured gene-environment interactions in psychopathology: Concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspectives on Psychological Science. 2006;1:5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. 6th ed. Authors; Los Angeles, CA: 1998–2010. [Google Scholar]
- Offner P, Holzer H. Left behind in the labor market: Recent employment trends among young Black men. Brookings Institution; Washington, DC: 2002. [Google Scholar]
- Paschall MJ, Flewelling RL, Faulkner DL. Alcohol misuse in young adulthood: Effects of race, educational attainment, and social context. Substance Use and Misuse. 2000;35:1485–1506. doi: 10.3109/10826080009148227. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Hibbert M, Rosier MJ, Carlin JB, Caust J, Bowes G. Is smoking associated with depression and anxiety in teenagers? American Journal of Public Health. 1996;86:225–230. doi: 10.2105/ajph.86.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez de Castro I, Ibáñez A, Torres P, Sáiz-Ruiz J, Fernández-Piqueras J. Genetic association study between pathological gambling and a functional DNA polymorphism at the D4 receptor gene. Pharmacogenetics. 1997;7:345–348. [PubMed] [Google Scholar]
- Pomery EA, Gibbons FX, Reis-Bergan M, Gerrard M. From willingness to intention: Experience moderates the shift from reactive to reasoned behavior. Personality and Social Psychology Bulletin. 2009;35:894–908. doi: 10.1177/0146167209335166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor BD, Dalaker J. Poverty in the United States: 2002 (Current Population Reports, P60-222) U.S. Bureau of the Census; Washington, DC: 2003. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage; Newbury Park, CA: 2002. [Google Scholar]
- Rutter ML, Silberg J. Gene-environment interplay in relation to emotional and behavioral disturbance. Annual Review of Psychology. 2002;53:463–490. doi: 10.1146/annurev.psych.53.100901.135223. [DOI] [PubMed] [Google Scholar]
- Shaw BA, Agahi N, Krause N. Are changes in financial strain associated with changes in alcohol use and smoking among older adults? Journal of Studies on Alcohol and Drugs. 2011;72:917–925. doi: 10.15288/jsad.2011.72.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei M-K, Beach SRH, Brody GH, Philibert RA, Gibbons FX. Social environmental variation, plasticity genes, and aggression: Evidence for the differential susceptibility hypothesis. American Sociological Review. doi: 10.1177/0003122411427580. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Stewart EA, Gordon LC, Conger RD, Elder GH., Jr. A test of life-course explanations for stability and change in antisocial behavior from adolescence to young adulthood. Criminology. 2002;40:401–434. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- StataCorp . Stata statistical software: Release 12. Author; College Station, TX: 2011. [Google Scholar]
- Sum A, Khatiwada I, Pond N, Trubsky M, Fogg N, Palma S. Left behind in the labor market: Labor market problems of the nation's out-of-school, young adult populations. Center for Labor Market Studies, Northeastern University; Chicago, IL: 2002. [Google Scholar]
- Unger JB, Hamilton JE, Sussman S. A family member's job loss as a risk factor for smoking among adolescents. Health Psychology. 2004;23:308–313. doi: 10.1037/0278-6133.23.3.308. [DOI] [PubMed] [Google Scholar]
- Warshaw PR, Davis FD. Disentangling behavioral intention and behavioral expectation. Journal of Experimental Social Psychology. 1985;21:213–228. [Google Scholar]
- Whalen CK, Jamner LD, Henker B, Delfino RJ. Smoking and moods in adolescents with depressive and aggressive dispositions: Evidence from surveys and electronic diaries. Health Psychology. 2001;20:99–111. [PubMed] [Google Scholar]
- Wimberly RC, Morris LV. The Southern Black Belt: A national perspective. TVA Rural Studies Press; Lexington, KY: 1997. [Google Scholar]
- Windle M, Mun EY, Windle RC. Adolescent-to-young adult heavy drinking trajectories and their prospective predictors. Journal of Studies on Alcohol. 2005;66:313–322. doi: 10.15288/jsa.2005.66.313. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Wong MW, Puttler LI, Fitzgerald HE. Resilience and vulnerability among sons of alcoholics: Relationship to developmental outcomes between early childhood and adolescence. In: Luthar SS, editor. Resilience and vulnerability: Adaptation in the context of childhood adversities. Cambridge University Press; New York: 2003. pp. 76–103. [Google Scholar]