Abstract
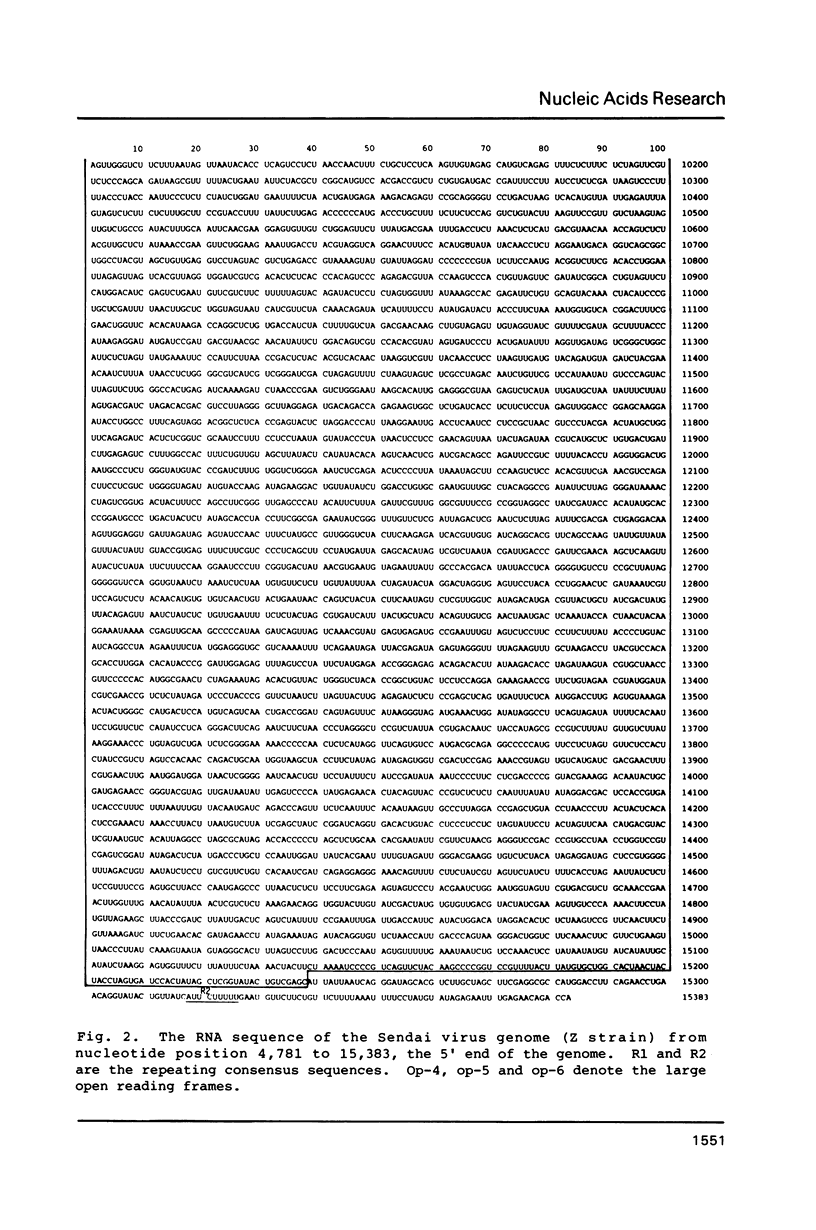
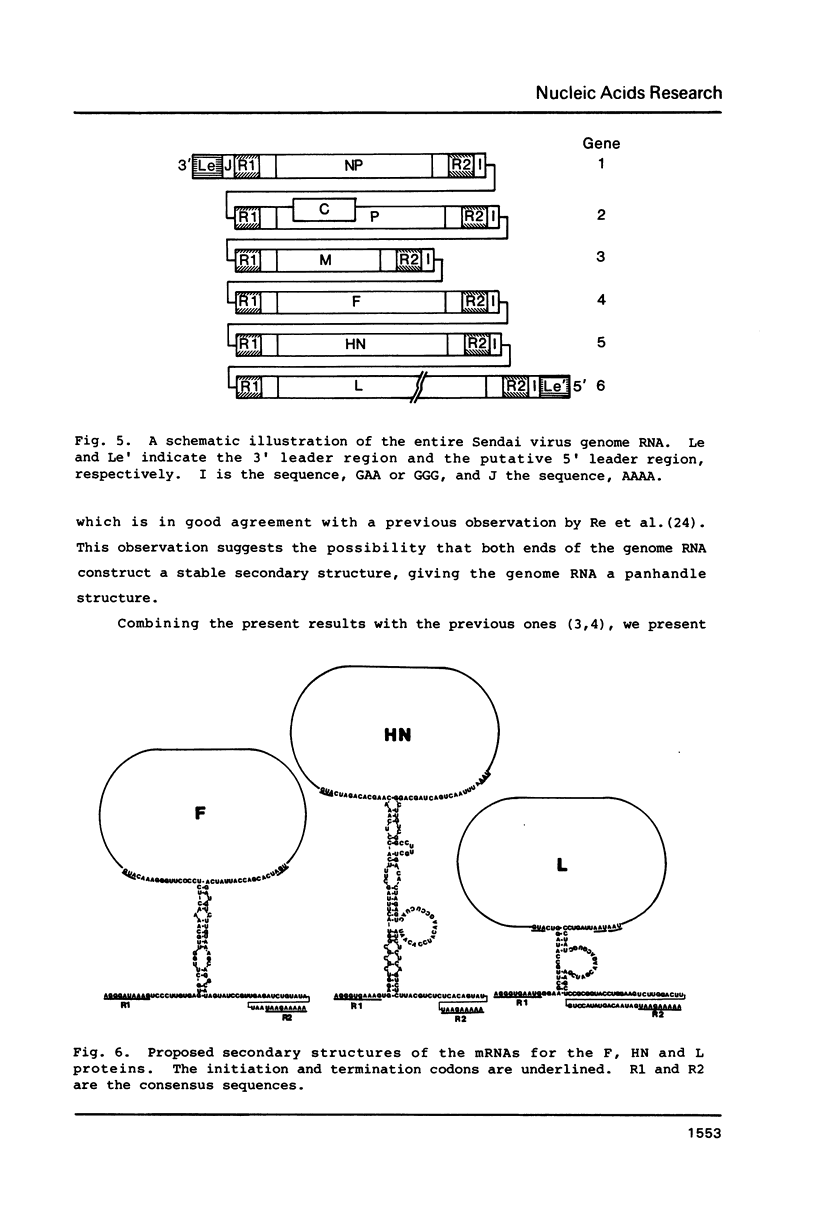
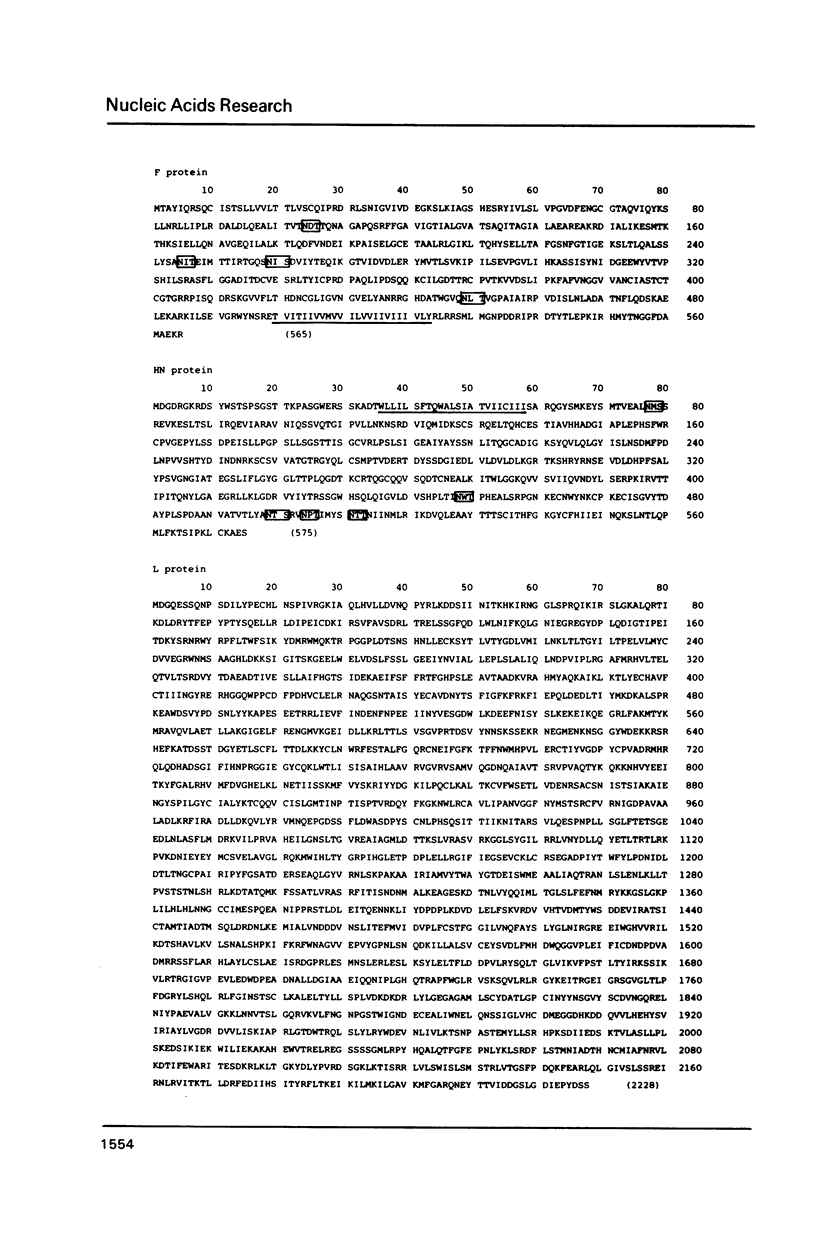
We previously determined the 3' proximal 5,824 nucleotides of the Sendai virus genome RNA (Nucleic Acids Res. 11, 7317-7330, 1983; Nucleic Acids Res. 12, 7965-7973, 1984), and present here the sequence of the remaining 5' proximal 9,559 nucleotides. Thus, this is the first paramyxovirus to have its genome organization elucidated. The set of complementary DNA clones used was prepared by the method of Okayama and Berg from polyadenylylated viral genome RNA. We sequenced the region containing the 5' proximal half of the F gene, and the subsequent HN and L genes, and predicted the complete amino acid sequence of the products of these genes. Sequence analyses confirmed that all the genes are flanked by consensus sequences and suggest that the viral mRNAs are capable of forming stem-and-loop structures. Comparison of the F and HN glycoproteins of Sendai virus with those of simian virus 5 strongly suggests that the cysteine residues are highly important for maintenance of the molecular structures of these glycoproteins.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Preparation and analysis of the nucleocapsid proteins of vesicular stomatitis virus and sendai virus, and analysis of the sendai virus leader-NP gene region. J Gen Virol. 1984 Apr;65(Pt 4):769–779. doi: 10.1099/0022-1317-65-4-769. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Sequence determination of the Sendai virus fusion protein gene. J Gen Virol. 1985 Feb;66(Pt 2):317–331. doi: 10.1099/0022-1317-66-2-317. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Rose K., Simona M. G., Roux L., Giorgi C., Kolakofsky D. Analysis of the Sendai virus M gene and protein. J Virol. 1984 Nov;52(2):656–663. doi: 10.1128/jvi.52.2.656-663.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B., Giorgi C., Roux L., Raju R., Dowling P., Chollet A., Kolakofsky D. Sequence determination of the Sendai virus HN gene and its comparison to the influenza virus glycoproteins. Cell. 1985 May;41(1):269–278. doi: 10.1016/0092-8674(85)90080-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Davis A. R., Nayak D. P. NH2-terminal hydrophobic region of influenza virus neuraminidase provides the signal function in translocation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2327–2331. doi: 10.1073/pnas.81.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Dowling P. C., Giorgi C., Roux L., Dethlefsen L. A., Galantowicz M. E., Blumberg B. M., Kolakofsky D. Molecular cloning of the 3'-proximal third of Sendai virus genome. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5213–5216. doi: 10.1073/pnas.80.17.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Satake M., Coligan J. E., Norrby E., Camargo E., Venkatesan S. Respiratory syncytial virus fusion glycoprotein: nucleotide sequence of mRNA, identification of cleavage activation site and amino acid sequence of N-terminus of F1 subunit. Nucleic Acids Res. 1985 Mar 11;13(5):1559–1574. doi: 10.1093/nar/13.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka Y., Kanda T., Iwasaki K., Nomoto A., Shioda T., Shibuta H. Nucleotide sequence of a Sendai virus genome region covering the entire M gene and the 3' proximal 1013 nucleotides of the F gene. Nucleic Acids Res. 1984 Nov 12;12(21):7965–7973. doi: 10.1093/nar/12.21.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Paterson R. G., Lamb R. A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985 Apr;54(1):1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M., Choppin P. W. Analysis of Sendai virus mRNAs with cDNA clones of viral genes and sequences of biologically important regions of the fusion protein. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7732–7736. doi: 10.1073/pnas.81.24.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi Y., Hirashima A., Watanabe I. Comparison of the nucleotide sequences at the 3'-terminal region of RNAs from RNA coliphages. J Mol Biol. 1982 Jul 15;158(4):711–730. doi: 10.1016/0022-2836(82)90256-x. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama T., Shimizu K., Ishida N. Carbohydrate composition of the envelope glycoproteins of Sendai virus. Virology. 1978 Oct 15;90(2):226–234. doi: 10.1016/0042-6822(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miura N., Nakatani Y., Ishiura M., Uchida T., Okada Y. Molecular cloning of a full-length cDNA encoding the hemagglutinin-neuraminidase glycoprotein of Sendai virus. FEBS Lett. 1985 Aug 19;188(1):112–116. doi: 10.1016/0014-5793(85)80885-1. [DOI] [PubMed] [Google Scholar]
- Morgan E. M., Re G. G., Kingsbury D. W. Complete sequence of the Sendai virus NP gene from a cloned insert. Virology. 1984 May;135(1):279–287. doi: 10.1016/0042-6822(84)90137-5. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Homma M., Compans R. W. Effect of tunicamycin on the replication of Sendai virus. Virology. 1982 Jun;119(2):474–487. doi: 10.1016/0042-6822(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Okada Y., Richardson M. A., Ikegami N., Nomoto A., Furuichi Y. Nucleotide sequence of human rotavirus genome segment 10, an RNA encoding a glycosylated virus protein. J Virol. 1984 Sep;51(3):856–859. doi: 10.1128/jvi.51.3.856-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re G. G., Gupta K. C., Kingsbury D. W. Sequence of the 5' end of the Sendai virus genome and its variable representation in complementary form at the 3' ends of copy-back defective interfering RNA species: identification of the L gene terminus. Virology. 1983 Oct 30;130(2):390–396. doi: 10.1016/0042-6822(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Richardson C. D., Scheid A., Choppin P. W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980 Aug;105(1):205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J. Sequences of vesicular stomatitis virus RNA in the region coding for leader RNA, N protein mRNA, and their junction. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4793–4797. doi: 10.1073/pnas.76.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Kanda T., Adachi A., Yogo Y. Characterization of bovine parainfluenza virus type 3. Microbiol Immunol. 1979;23(7):617–628. doi: 10.1111/j.1348-0421.1979.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]