Abstract
Molecule targeting therapy using somatostatin (SS) analogues has become a widely accepted modality to treat neuroendocrine tumors (NETs), particularly gastrointestinal (GI) and pancreatic endocrine tumors. On the other hand, little is known about the expression of somatostatin receptor (SSTR) subtypes in neuroendocrine carcinomas (NECs). We investigated the expression of SSTR subtypes (SSTR-1, 2A, 3, 4 and 5) using real-time reverse transcription polymerase chain reaction (RT-PCR) method and immunohistochemistry in 32 neuroendocrine neoplasms (9 NET G1, 2 NET G2, 18 NECs G3 and 3 mixed NEC G3) of various primary sites. Expression of more than two SSTR subtypes was detected in all neuroendocrine neoplasms examined. Expression of SSTR-2A mRNA was significantly higher than other subtypes. In addition, mRNA expression of SSTR-3 and SSTR-5 was significantly low or below the detection level except for gastroduodenal NET G1. No significant difference of the expression of SSTR subtypes was observed between the NET and NEC groups. The expression of protein and mRNA was generally well correlated. In conclusion, NECs would be a good candidate for molecule targeting therapy using SS analogues, and the expression of SSTR-2A can be useful as a biomarker of neuroendocrine differentiation. We have demonstrated that NEC G3 small cell type shows a different expression profile of SSTR subtypes compared with NET and NEC non-small cell type.
Keywords: neuroendocrine tumor (NET), neuroendocrine carcinoma (NEC), somatostatin receptor, real-time RT-PCR, immunohistochemistry
I. Introduction
It is well known that neuroendocrine tumors (NETs) can develop in almost all tissues or organs in the body, and are characterized by a wide range of histological appearances and biological behavior [9, 12, 17]. While the nomenclature of neuroendocrine neoplasms has changed several times in the past [1, 4–6, 9], the recent WHO classification of the neuroendocrine neoplasms of gastroentropancreatic (GEP) system puts them into three major categories: neuroendocrine tumor (NET) G1, G2 and neuroendocrine carcinoma (NEC) G3, and others including mixed adenoneuroendocrine carcinoma, some rare tumors of special types and hyperplastic/preneoplastic lesions, based on histological differentiation (well differentiated and poorly differentiated), proliferative activity (G1, G2 and G3) and TNM factors (size, infiltration/invasion, metastasis) [2]. NET G1 and NET G2 correspond to what were formerly called well differentiated endocrine tumor (WDET) and well differentiated endocrine carcinoma, respectively. NEC G3 is nearly the same as poorly differentiated endocrine carcinoma (PDEC) and highly malignant [2]. Nowadays, this classification seems to be gradually accepted in the neuroendocrine neoplasms of other primary sites besides GEP system.
From the standpoint of molecule targeting therapy for NETs, somatostatin (SS) analogues have been applied to recurrent and/or metastatic diseases of well differentiated NETs and well differentiated NECs, because such tumors express somatostatin receptor (SSTR) subtypes on their cell membrane and/or in cytoplasm, particularly gastrointestinal (GI) tract primary and pancreatic islet cell tumors [7, 11]. In addition, previous clinical studies showed preventive effect on tumor growth and symptoms due to hormone over-secretion even in inoperable cases of NETs and NECs [8, 19, 21]. Furthermore, the potential clinical utility of SS analogue therapy was pointed out in the hepatic metastasis of neuroendocrine tumors with high expression of SSTR subtypes [14].
Up to now, 5 subtypes of SSTR (SSTR-1, 2A, 3, 4, and 5) have been cloned and characterized [18, 22, 23]. SSTR-2A is known to be closely related with regulation of hormone synthesis and secretion, and control of cell cycle including cell proliferation and apoptosis induction [20]. Although the affinity of SS analogues to SSTR subtypes is quite different, the highest affinity to SSTR-2A and the quite low affinity to SSTR-4 have been demonstrated [3]. On the basis of such clinical background, it is important to know the precise expression status of SSTR subtypes in NETs and NECs. Therefore, we investigated the expression status of SSTR subtypes in NET G1, G2, and NEC G3 of various primary sites, using real-time RT-PCR method and immunohistochemistry.
II. Materials and Methods
Neuroendocrine tumors and neuroendocrine carcinomas
Thirty-two neuroendocrine neoplasms were selected from the surgical pathology files of the Pathology Division, Nihon University Itabashi Hospital. All the diagnosis of the selected materials were confirmed by more than two pathologists on the basis of H-E histopathology and positivity for more than two of the following neuroendocrine markers, such as synaptophysin, chromogranin A, CD56, and neuron specific enolase (NSE) on the previously immunostained sections. At the selection of neuroendocrine neoplasms, we used a criterion of more than 50% immunoreactivity of tumor cells in the previously stained slides of at least two markers mentioned above. The selected tumors were reclassified into NET G1, NET G2, NEC G3, and mixed NEC according to the new WHO classification. All the tissue samples were handled according to the Ethical Guidelines for Clinical Studies (July 30, 2003, amended December 28, 2004, Ministry of Health, Labour and Welfare), and the study was approved by the Ethical committee, Nihon University Itabashi Hospital for clinical investigation using human material. Summary of the pathology information of the material is shown in Table 1.
Table 1.
Summary of the pathology profiles of surgical materials
| WHO classification | Original pathological diagnosis | Tissue/Organs | |
|---|---|---|---|
| 1 | NET G1 | carcinoid tumor | Duodenum |
| 2 | NET G1 | carcinoid tumor | Appendix |
| 3 | NET G1 | carcinoid tumor | Colon |
| 4 | NET G1 | carcinoid tumor | Colon |
| 5 | NET G1 | carcinoid tumor | Colon |
| 6 | NET G1 | carcinoid tumor | Rectum |
| 7 | NET G1 | carcinoid tumor | Rectum |
| 8 | NET G1 | carcinoid tumor | Lung |
| 9 | NET G1 | carcinoid tumor | Lung |
| 10 | NET G2 | atypical carcinoid tumor | Lung |
| 11 | NET G2 | atypical carcinoid tumor | Stomach |
| 12 | NEC G3 | small cell carcinoma | Esophagus |
| 13 | NEC G3 | small cell carcinoma | Esophagus |
| 14 | NEC G3 | neuroendocrine carcinoma | Stomach |
| 15 | NEC G3 | neuroendocrine carcinoma | Vater’s papilla |
| 16 | NEC G3 | small cell carcinoma | Colon |
| 17 | NEC G3 | small cell carcinoma | Lung |
| 18 | NEC G3 | large cell neuroendocrine carcinoma | Lung |
| 19 | NEC G3 | large cell neuroendocrine carcinoma | Lung |
| 20 | NEC G3 | large cell neuroendocrine carcinoma | Lung |
| 21 | NEC G3 | large cell neuroendocrine carcinoma | Lung |
| 22 | NEC G3 | thymic neuroendocrine carcinoma | Thymus |
| 23 | NEC G3 | small cell carcinoma | mediastinum |
| 24 | NEC G3 | neuroendocrine carcinoma | Prostate |
| 25 | NEC G3 | carcinoma with NE differentiation | Prostate (lung meta) |
| 26 | NEC G3 | non-invasive neuroendocrine carcinoma | Breast |
| 27 | NEC G3 | invasive ductal carcinoma with NE differentiation | Breast |
| 28 | NEC G3 | neuroendocrine carcinoma | Breast |
| 29 | mixed NEC G3 | invasive ductal carcinoma with NE differentiation | Breast |
| 30 | mixed NEC G3 | mucinous carcinoma with NE differentiation | Breast |
| 31 | NEC G3 | carcinoma with NE differentiation | Uterine cervix |
| 32 | mixed NEC G3 | combined squamous cell and small cell carcinoma | Uterine cervix |
NET: neuroendocrine tumor, NEC: neuroendocrine carcinoma.
Immunohistochemistry for SSTR subtypes
Immunohistochemistry was carried out on 10% formalin fixed paraffin embedded tissue sections using EnVision/HRP-labelled polymer system (DAKO, Tokyo, Japan) and an autostainer (Histostainer, Nichirei Biosciences Inc., Tokyo, Japan). For antigen retrieval, dewaxed 4 µm tissue sections were immersed in citrate buffer pH 6.0 and boiled in water bath for 40 min at 95°C and cooled down at room temperature. After washing several times in PBS pH 7.2, the sections were processed for quenching the endogenous peroxidase activity with 0.3% hydrogen peroxide and for blocking the non-specific binding with 1% goat serum. Sections were then processed in the usual manner, and incubated with primary antibodies of anti-SSTR-1, 2A, 3 and 5 (Gramsch Laboratories, Germany) at the working dilution of 1:400 to 1:1000 for 30 min at room temperature. For the positive control of SSTR immunohistochemistry, we used normal pancreas islets. For Ki 67 immunohistochemistry, we used anti-human Ki 67 mouse monoclonal antibody (clone MIB-1, DakoCytomation, Denmark) and LSAB method (Histofine SAB-PO (M) kit, Nichirei Biosciences Inc., Japan). Negative controls were done by omitting the specific primary antibodies and processed in the same way. The tissue-bound HRP activity was visualized by immersing the sections in 0.005% 3,3'-diaminobenzidine tetrahydrochloride (DAB) in PBS containing hydrogen peroxide (10 µl/150 ml DAB solution). After the completion of the immunohistochemical process, the sections were stained lightly with hematoxylin, and processed and mounted in the usual manner.
Laser assisted microdissection for tissue sections
Eight-µm thick paraffin sections were mounted on the membrane film-coated slide glasses. After dewaxing with xylene, the sections were stained lightly with toluidine blue, then the target tumor areas were microdissected using a laser assisted microdissection system (PALM MBIII-N, Zeiss, Germany) by ultraviolet laser beam under a light microscope. The microdissected target tumor cells were retrieved precisely into an Eppendorf lid with mineral oil. Details of the procedure of laser assisted microdissection have been described elsewhere [15].
Total RNA extraction from the tumor tissue
Target tumor cell sample was mixed with 200 µl of denaturing buffer containing with 2% SDS, 0.1 mM EDTA, 10 mM Tris-HCl. They were incubated at 55°C with proteinase K until sections were dissolved completely. Total RNA was purified with 20 µl 2M sodium acetate (pH 4.0), 220 µl citrate saturated phenol (pH 4.3), and 60 µl chloroform-isoamyl alcohol, centrifuged for 15 min at 15,000 rpm and the upper aqueous layer transferred into new tubes. Two hundred µl isopropanol and 2 µl glycogen were added as a carrier and stored at −80°C for more than 30 min. The pellets were corrected by centrifugation for 30 min at 14,000 rpm, washed with 70% ethanol and air dried on ice. They were then dissolved with 5–10 µl RNase free water and quantified with a spectrophotometer at the 260-nm optical density using Nanodrop 1000 (Thermo Fisher Scientific, Waltham, MA, U.S.A.). Total RNA samples were stored at −80°C until use. Both Genomic DNA elimination and synthesizing cDNA was done by QuantiTect Reverse Transcription Kit (QIAGEN, Tokyo, Japan) according to the manufacture’s instructions.
Quantitative real-time RT-PCR for measurement SSTR subtypes
Quantity of mRNA for SSTR-1, 2A, 3, 4, 5 and GAPDH as an internal control were measured by real-time RT-PCR method. Real-time RT-PCR was performed with SYBR Green PCR Master Mix (Life Technologies Japan, Tokyo, Japan) and the primers used in this study are shown in Table 2. RT-PCR amplification and data analysis was performed using ABI Prism 7000 Sequence Detection System (Life Technologies Japan), with a 20 µl final reaction mixture containing 900 nmol/L each primer, 1×SYBR Green PCR Master Mix (Life Technologies). The reaction mixture was preheated at 95°C for 10 min, followed by 45 cycles at 95°C for 15 sec and 60°C for 1 min. Each SSTR subtype mRNA relative value was measured by ΔΔCt method with threshold cycle times of each target SSTR and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [13].
Table 2.
Primer sequences for RT-PCR assay
| Target | Sequence | Products (bp) | |
|---|---|---|---|
| SSTR 1 | forward | tgagtcagctgtcggtcatc | 93 |
| reverse | ggaaagagcgcttgaagttg | ||
| SSTR 2 | forward | ctttgtggtggtcctcacct | 100 |
| reverse | gcagaggacattctggaagc | ||
| SSTR 3 | forward | ttcctctcctaccgcttcaa | 123 |
| reverse | ctcctcctcatcctcctcct | ||
| SSTR 4 | forward | tctttgtgctctgctggatg | 96 |
| reverse | ggataagggacacgtggttg | ||
| SSTR 5 | forward | cccttcttcaccgtcaacat | 102 |
| reverse | gttggcgtaggagaggatga | ||
| GAPDH | forward | ggaaggtgaaggtcggagtca | 101 |
| reverse | gtcattgatggcaacaatatccact |
Statistical analysis
Statistical analysis was performed using SPSS software for Windows version 14, using Mann-Whitney’s U test to assess the significance of the difference between the means±SD of two samples. A p value of <0.05 was considered to be significant.
III. Results
Expression of SSTR subtypes in neuroendocrine neoplasms
Prior to the quantitative analysis of mRNA expression of SSTR subtypes, the expression of SSTR-1, 2A, 3, 4 and 5 was confirmed in representative cases of NET G1, G2 and NEC G3 by RT-PCR method. Representative expression profiles of SSTR subtypes in NET G1 (SSTR-1=0.08, SSTR-2A=0.55, SSTR-3=0.03, SSTR-4=0.02, SSTR-5=0.28), G2 (SSTR-1=0.88, SSTR-2A=14.42, SSTR-4=0.18, SSTR-5=10.63) and NEC G3 (SSTR-2A=4.00, SSTR-4=2.79, SSTR-5=0.51) are shown in Figure 1.
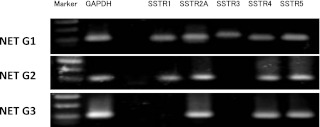
Fig. 1.
Representative expression patterns of SSTR subtypes in NET G1, G2 and NEC G3. Differences of mRNA expression of SSTR subtypes were shown by RT-PCR. Carcinoid tumor of the appendix (NET G1) expressed all the SSTR subtypes. Atypical carcinoid of the stomach (NET G2) expressed SSTR 1, 2A, 4 and 5. In the LCNEC of the lung expressed SSTR 2A, 4 and 5.
Expression of SSTR subtypes in NET G1 and G2
All NETs (G1 and G2) expressed more than two SSTR subtypes, and the expression of SSTR-1 and SSTR-2A was 100%, respectively. SSTR-4 expression was also frequently observed. The expression of SSTR-3 was below the detection level in all NETs examined in this study. The expression of SSTR-5 was also quite low or below the detection level in NETs of large intestine, appendix vermiformis and lung primaries, though it was high in the gastroduodenal primary (not significant). The expression of SSTR-2A in gastrointestinal primaries was significantly higher than lung primary (p=−0.02). No expression of SSTR-3 and SSTR-5 was observed in lung primaries (Fig. 2).
Fig. 2.
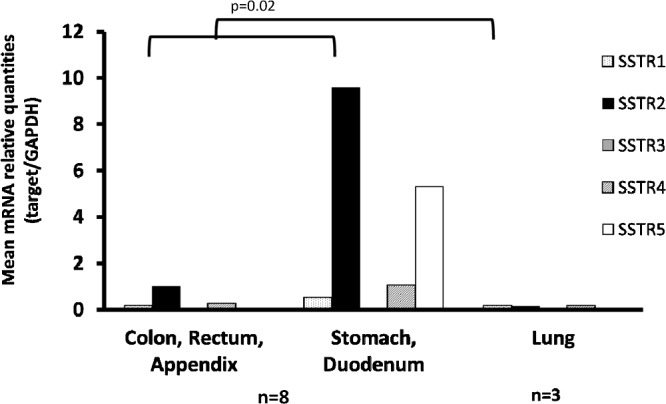
Expression of SSTR subtypes in NET G1 and G2. Expression of SSTR2A mRNA was significantly high in the NETs of gastrointestinal origin compared with lung primary.
Expression of SSTR subtypes in NEC G3 and mixed NEC
In G3 (NEC and mixed NEC) group, more than two SSTR subtype expression was observed in all the primary sites, though the quantity of mRNA of each SSTR subtype was quite varied. However, the majority of NEC G3 constantly expressed SSTR-1 and SSTR-2A (85.7% and 95.2%, respectively). A statistical difference was obtained between the expression of SSTR-2A and the expression of other subtypes in the lung and breast primaries (p<0.05), Table 3. Except for stomach and duodenal primary, the expression of SSTR-3 was very low or below the detection level in other primary sites. Gastroduodenal tumors showed relatively high expression of SSTR subtypes except for SSTR-4.
Table 3.
mRNA expression of SSTR subtypes in neuroendocrine carcinoma G3
| Primary sites | Number | mRNA relative quantities (mean±SD) | ||||
|---|---|---|---|---|---|---|
| SSTR1 | SSTR2 | SSTR3 | SSTR4 | SSTR5 | ||
| Colon | 1 | 0.74 | 0.06 | 0 | 0 | 1.80 |
| Stomach, Duodenum | 2 | 0.41±0.19 | 1.02±0.86 | 1.71±2.41 | 0.02±0.02 | 0.29±0.13 |
| Esophagus | 2 | 0.005±0.007 | 0.04±0.04 | 0.002±0.003 | 0 | 0.02±0.02 |
| Thymus | 1 | 0.79 | 0.54 | 0 | 0 | 0.70 |
| Mediastinum | 1 | 0.06 | 0.18 | 0.02 | 0.01 | 0.28 |
| Lung | 5 | 0.31±0.30 | * 1.01±2.98 | 0.00±0.01 | 0.46±1.06 | 0.00±0.23 |
| Prostate | 2 | 0.13±0.13 | 0.21±0.11 | 0 | 0.41±0.28 | 0.00±0.00 |
| Breast | 5 | 0.11±0.36 | * 0.23±1.67 | 0.00±0.01 | 0.00±0.23 | 0.00±0.43 |
| Uterine cervix | 2 | 0.05±0.08 | 0.15±0.12 | 0 | 0.22±0.31 | 0.11±0.15 |
* p<0.05: Mann-Whitney’s U-test between SSTR2 and other subtypes.
Expression status of SSTR subtypes in NET (G1, G2) and NEC (G3 NEC and mixed NEC)
The expression status of SSTR subtypes was compared between the NET (G1, G2) group and NEC (NEC G3 and mixed NEC) group. In both groups, SSTR-2A expression was significantly higher (p=0.0003 and p=0.0001, respectively) than other subtypes (Fig. 3). The expression of SSTR-3 was very low or below the detection level in both groups. In addition, SSTR-5 expression was also quite low in the NET group.
Fig. 3.
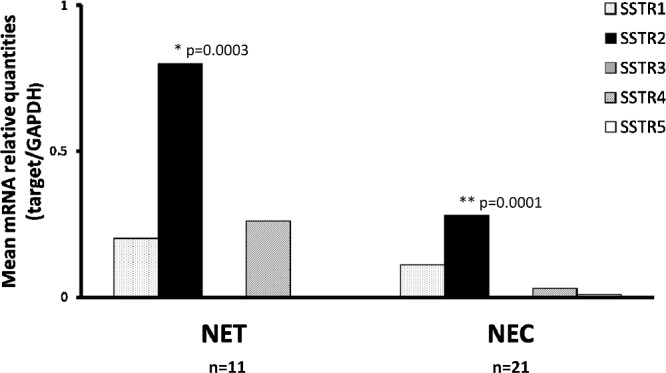
Comparison of expression status of SSTR subtypes in NET (G1, G2) and NEC (G3 NEC and mixed NEC). Expression of SSTR2A was significantly higher than other subtypes in both NET and NEC groups.
Expression status of SSTR subtypes in NEC G3 small cell type and NEC G3 non-small cell type
The expression status of SSTR subtypes was compared between the NEC G3 small cell type (small cell carcinoma) and non-small cell type (large cell neuroendocrine carcinoma; LCNEC and other non-small cell carcinoma). The expression of SSTR-1 and SSTR-2A was significantly low in NEC G3 small cell type. On the other hand, small cell carcinomas showed high expression of SSTR-5 compared with non-small cell carcinomas (not significant) (Fig. 4).
Fig. 4.
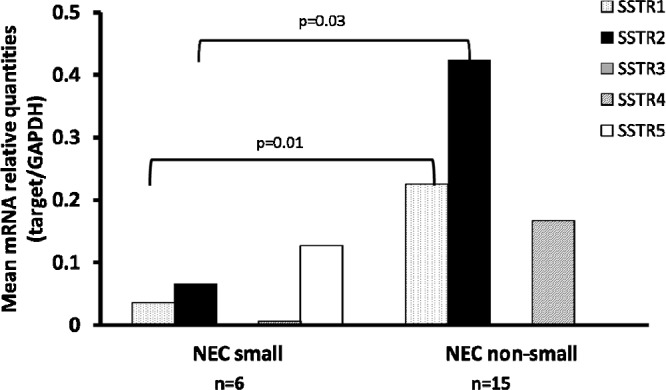
Comparison of the expression status of SSTR subtypes in NEC G3 small cell type and NEC G3 non-small cell type. The expression of SSTR1 and 2A subtypes was significantly high in NEC G3 non-small cell type compared with NEC G3 small cell type.
Immunohistochemistry for SSTR subtypes
Overall protein expression of SSTR-1, 2A, 3 and 5 was 93.8%, 65.6%, 53.1% and for 6.2%, respectively.
SSTR-1
Immunohistochemical expression of SSTR-1 was cytoplasmic and/or membranous localization along the plasma membrane, though the cytoplasmic localization was predominant. SSTR-1 expression was the most constant in NET G1, G2 group (100%). On the other hand, in NEC G3 and mixed NEC group it was 85.7%. Immunohistochemical intensity varied greatly from case to case, and there was heterogeneous distribution of SSTR-1 positive cells even in the same tumor tissue (Figs. 5, 6, 7, 8, 9, 10).
Fig. 5.
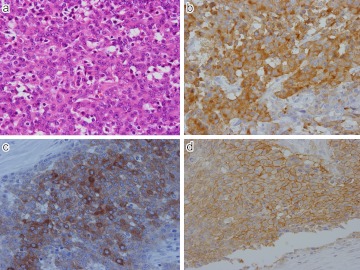
Expression of SSTR-1, 2, 3 and 5 in NET G1 (carcinoid tumor) of the appendix vermiformis. a) SSTR-1; Diffuse and intense positive staining identified mostly in cytoplasm of the tumor cells. b) SSTR-2; Weak but positive staining seen along the plasma membrane, c) SSTR-3; Diffuse cytoplasmic staining present, d) SSTR-5; very weak, but membranous staining seen along the plasma membrane. Original magnification ×400 (a–d).
Fig. 6.
Expression of SSTR-1, 2 and 3 in NET G1 (carcinoid tumor) of rectum. a) H-E; Ribbon-likearrangement of relatively uniform tumor cells. b) SSTR-1; Diffuse cytoplasmic positive staining in tumor cells. c) SSTR-2; Membranous and/or cytoplasmic localization seen. d) SSTR-3; Cytoplasmic staining identified in tumor cells. Positive staining present in some interstitial cells. Original magnification ×200 (a), ×400 (b–d).
Fig. 7.
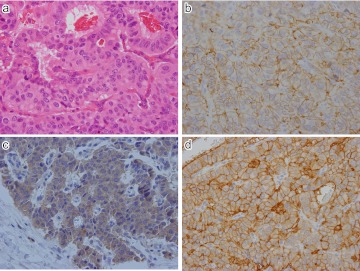
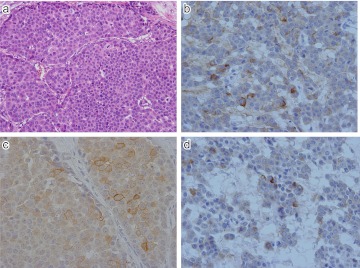
Expression of SSTR-1 and 2 in NET G2 (atypical carcinoid) of stomach. a) Ribbon-like arrangement of tumor cells of nuclear atypia. b) CD56; Intense membranous staining seen along the plasma membrane. c) SSTR-1; Mostly cytoplasmic positivity in tumor cells. d) SSTR-2; Intense membranous staining along the plasma membrane of tumor cells. Original magnification ×400 (a–d).
Fig. 8.
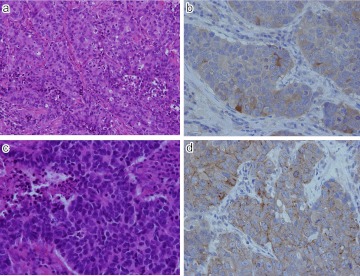
Expression of SSTR1 and 2 in NEC G3 (neuroendocrine carcinoma) of stomach. a) H-E; Rather solid growth of poorly differentiated tumor cells. b) Synaptophysin; Diffuse and intense cytoplasmic positive staining seen in tumor cells. c) SSTR-1; Diffuse cytoplasmic positivity seen in tumor cells. d) SSTR-2; Intense membranous staining along the plasma membrane. Original magnification ×400 (a–d).
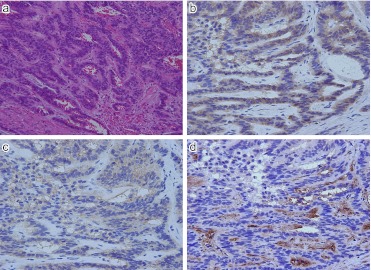
Fig. 9.
Expression of SSTR-1 in two different NEC G3 (large cell neuroendocrine carcinoma; LCNEC) of lung. a) H-E; Rather solid growth of poorly differentiated tumor cells with incomplete peripheral nuclear palisading. b) SSTR-1; In this case cytoplasmic positive staining seen. c) H-E; Another case of LCNEC. d) Intense membranous and occasional cytoplasmic positivity identified in the tumor cells. Original magnification ×200 (a), ×400 (b–d).
Fig. 10.
Expression of SSTR-1, 2 and 3 in NEC G3 (mixed neuroendocrine carcinoma) of breast. a) H-E; Solid growth of poorly differentiated tumor cells. b) SSTR-1; Cytoplasmic and/or membranous positivity identified in scattered tumor cells. c) SSTR-2; Intense membranous and/or cytoplasmic localization present. d) SSTR-3; Some tumor cells show cytoplasmic positive reaction. Original magnification ×200 (a), ×400 (b–d).
SSTR-2A
The expression of SSTR-2A was the second most common in NET G1, G2 group (81.8%) and in NEC G3 and mixed NEC group (61.9%). Immunohistochemical localization of SSTR-2 was membranous in most cases. However, cytoplasmic localization was also seen in some cases. As with SSTR-1, heterogeneous distribution was also frequently observed (Figs. 5, 6, 7, 8 and 10).
SSTR-3
Approximately 50% of neuroendocrine neoplasms examined in this study expressed SSTR-3 in both NET G1, G2 group (54.5%) and NEC G3 group (52.4%). The immunohistochemical expression of SSTR-3 was usually located in the cytoplasm (Figs. 5, 6 and 10).
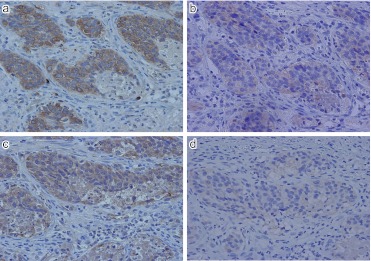
SSTR-5
The immunohistochemical expression of SSTR-5 was quite low in both NET G1, G2 group (9.0%) and NEC G3 (4.8%). Very weak membranous staining along the plasma membrane was observed (Fig. 5).
IV. Discussion
In this study, we investigated the expression of SSTR in neuroendocrine neoplasms of various primary sites with different biological behavior, particularly focused on its expression profiles of SSTR subtypes, because the expression pattern of SSTR subtypes is vary greatly even in the same tumor types or same primary sites. Particularly in high grade neuroendocrine neoplasms (NEC G3 and mixed NEC), little is known about the expression profiles of SSTR subtypes. Furthermore, the expression patterns of SSTR subtypes are directly related to the choice of molecule targeting therapy using SS analogues.
Today molecule targeting therapy using SS analogues to low grade NETs (G1 and G2) of GEP system, particularly in cases of recurrent or unresectable tumors, has come to be widely accepted as standard therapy [14, 19, 21]. In addition, the immunohistochemical detection of SSTR-2A has emphasized their role in the response of growth hormone-producing pituitary adenomas to SS analogues [16]. Although it is well known that neuroendocrine neoplasms express various SSTR subtypes, the frequency and patterns of expression are very different even in the same tumor type [10]. Such heterogeneous expression was also observed in the NETs and NECs of the same primary sites, and their frequency of expression were quite varied. Such heterogeneous expression of SSTR subtypes in the same tumor type is not clearly understood, but it seems to depend greatly on the individual tumor character rather than universal features of neuroendocrine neoplasms.
In the NET G1, G2 group, SSTR-2A expression was significantly higher in the gastrointestinal primaries than the lung primaries. This may suggest that SSTR-2A is a rather characteristic subtype in low grade neuroendocrine neoplasms of GI origin. Among the SSTR subtypes, the expression of SSTR-2A was significantly high in both the NET and NEC groups. No significant difference was observed in the expression pattern of SSTR subtypes in the NET and NEC groups. However, we obtained a significant difference in the expression profiles of SSTR-1 and 2A between NEC G3 small cell type and non-small cell type. In NEC G3 small cell type, the expression of SSTR-1 and 2A was significantly low. On the other hand, the expression of SSTR-5 was rather high (not significant). Therefore we conclude NEC G3 should be classified into small cell type and non-small cell type like lung cancer.
It has been shown that each SS analogue binds with different affinity to SSTR subtypes, and in particular they bind with quite low affinity to SSTR-4 compared with other subtypes [3]. From such findings, it is thought that SSTR-4 may figure less importantly in clinical practice using SS analogues.
All the specific antibodies against SSTR-1, 2A, 3 and 5 used in this study showed specific and satisfactory immunolocalization of SSTR subtypes in the tumor cells. The immunolocalization of SSTR-2A was usually membranous and intensely stained. Regarding the expression of SSTR-2A in the NET G1, G2 group, there was relatively good correlation between the expressions of mRNA (100%) and protein (>80%). However, in the NEC G3 group the expression of SSTR-2A protein was rather low (61.9%) compared with the expression of mRNA (95.2%). Such discrepant results may be caused by the different sensitivities of each detection system. The immunohistochemical localization of SSTR-1, 3 and 5 was usually cytoplasmic, but membranous localization was also seen occasionally, and these findings were nearly the same as those of previous studies [3, 10]. No significant correlation was observed between the expression of neuroendocrine markers (synaptophysin, chromogranin A, CD56, and NSE) and the expression patterns of SSTR subtypes.
In our study, the expression of SSTR-3 mRNA was quite low or below the detection level in some cases. In these cases, the immunolocalization of SSTR-3 was always quite restricted in the tumor tissue. Therefore, such discrepancy between the expression of mRNA and protein was thought to be derived from the tissue sampling for molecular analysis. Similar discrepancy was also obtained in the expression of SSTR-5 in both NET and NEC groups, and the frequency of this subtype was low compared with a previous report [14].
In this study, we examined the expression status of SSTR subtypes in the neuroendocrine neoplasms, which were re-classified into NET G1, G2 and NEC G3 according to the new WHO classification. This classification is much more reliable to evaluate biological behavior, due to the introduction of the Ki-67 labeling system and the sub-classification of small cell and large cell types in NEC G3.
In both the NET and NEC groups, the expression patterns and frequency of SSTR subtypes were quite similar, however, the expression of SSTR-2A was significantly high compared with others.
In conclusion, high grade neuroendocrine neoplasms (NEC G3 and mixed NEC) would be a good candidate for molecule targeting therapy using SS analogues. We have demonstrated that NEC G3 small cell type show a different expression profile of SSTR subtypes compared with NEC non-small cell type. In addition, the expression of SSTR-2A could serve as a good biomarker for neuroendocrine differentiation.
V. Acknowledgments
We are grateful to all the staff members of the Pathology Division, Nihon University Itabashi Hospital, for their assistance. This work was supported in part by the Strategic Research Base Development program for Private Universities subsidized by Ministry of Education, Culture, Sports, Science, and Technology (2010) and by Grants-in-Aid for National Cancer Center Research and Development Fund (23-A-35).
VI. References
- 1.Arnold R. Endocrine tumors of the gastrointestinal tract. Introduction; definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract. Res. Clin. Gastroenterol. 2005;19:491–505. doi: 10.1016/j.bpg.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Bosman F., Carneriro F., Hruban R., Theise N. In “WHO Classification of Tumors of the Digestive System”, ed. by F. Bosman, F. Carneriro, R. Hruban and N. Theise. IARC Press; France: 2010. WHO classification of tumors of the stomach; pp. 46–47. [Google Scholar]
- 3.Burns C., Lewis I., Briner U., Meno-Tetang G., Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatostatin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002;146:707–716. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 4.Capella C., Solcia E., Sobin L. H., Arnold R. In “Pathology and Genetics of Tumors of the Digestive System”, ed. by S. R. Hamilton, L. A. Aaltonen. IARC Press; Lyon, France: 2000. Endocrine tumor of the stomach; pp. 53–57. [Google Scholar]
- 5.Capella C., Solcia E., Sobin L. H., Arnold R. In “Pathology and Genetics of Tumors of the Digestive System”, ed. by S. R. Hamilton, L. A. Aaltonen. IARC Press; Lyon, France: 2000. Endocrine tumor of the small intestine; pp. 77–82. [Google Scholar]
- 6.Capella C., Solcia E., Sobin L. H., Arnold R. In “Pathology and Genetics of Tumors of the Digestive System”, ed. by S. R. Hamilton, L. A. Aaltonen. IARC Press; Lyon, France: 2000. Endocrine tumor of the colon and rectum; pp. 137–141. [Google Scholar]
- 7.Hofland L. J., Lamberts S. W. The patholophysiological consequence of somatostatin receptor internalization and resistance. Endocr. Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 8.Janson E. T., Oberg K. Long-term management of the carcinoid syndrome: treatment with octreotide alone and in combination with alpha-interferon. Acta Oncol. 1993;32:225–229. doi: 10.3109/02841869309083916. [DOI] [PubMed] [Google Scholar]
- 9.Jensen R. Pancreatic endocrine tumors: Recent advances. Ann. Oncol. 1999;10:S170–176. [PubMed] [Google Scholar]
- 10.Kulaksiz H., Eissele R., Rossler D., Schulz S., Hollt V., Cetin Y., Arnold R. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumors with subtype specific antibodies. Gut. 2002;50:52–60. doi: 10.1136/gut.50.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kvols L. K., Reubi J. C., Horisberger U., Moertel C. G., Rubin J., Charboneau J. W. The presence of somatostatin receptors in malignant neuroendocrine tumor tissue predicts responsiveness to octreotide. Yale J. Biol. Med. 1992;65:505–536. [PMC free article] [PubMed] [Google Scholar]
- 12.Macabeo-Ong M., Ginzinger D. G., Dekker N., McMillan A., Regezi J. A., Wong D. T. W., Jordan R. C. K. Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analysis. Mod. Pathol. 2002;15:979–987. doi: 10.1097/01.MP.0000026054.62220.FC. [DOI] [PubMed] [Google Scholar]
- 13.Modlin I. M., Oberg K., Chung D. C., Jensen R. T., de Herder W. W., Thakker R. V., Caplin M., Fave G. D., Kaltsas G. A., Krenning E. P., Moss S. F., Nilsson O., Rindi G., Salazar R., Ruszniewski P., Sundin A. Gastroenteropancreatic neuroendocrine tumors. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 14.Nasir A., Stridsberg M., Strosberg J., Su P-H., Livingston S., Malik H. A., Kelly S. T., Centeno B. A., Coppola D., Malafa M. E., Yeatman T. J., Kvols L. K. Somatostatin receptor profiling in hepatic metastases from small intestinal and pancreatic neuroendocrine neoplasms: immunohistochemical approach with potential clinical utility. Cancer Control. 2006;13:52–60. doi: 10.1177/107327480601300108. [DOI] [PubMed] [Google Scholar]
- 15.Ohno C., Nakanishi Y., Honma T., Henmi A., Sugitani M., Kanai Y., Nemoto N. Significance of system L amino acid transporter 1 (LAT-1) and 4F2 heavy chain (4F2hc) expression in human developing intestines. Acta Histochem. Cytochem. 2009;42:73–81. doi: 10.1267/ahc09010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osamura R. Y., Egashira N., Kajiya H., Takei M., Tobita M., Miyakoshi T., Inomoto C., Takekoshi S., Teramoto A. Pathology, pathogenesis and therapy of growth hormone (GH)-producing pituitary adenomas: technical advances in histochemistry and their contribution. Acta Histochem. Cytochem. 2009;42:95–104. doi: 10.1267/ahc.09004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearse A. G. E. The diffuse neuroendocrine system and the APUD concept: related ‘endocrine’ peptides in brain, intestine, pituitary, placenta and human cutaneous glands. Med. Biol. 1977;55:115–125. [PubMed] [Google Scholar]
- 18.Petel Y. C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 19.Ruszniewski P., Ducreux M., Charyvialle J. A., Blumberg J., Cloarec D., Michel H., Raymond J. M., Dupas J. L., Gouerou H., Jian R., Genestin E., Bemades P., Rougier P. Treatment of the carcinoid syndrome with the long acting somatostatin analogue lanreotide: a prospective study in 39 patients. Gut. 1996;39:279–283. doi: 10.1136/gut.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Susihi C., Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann. Oncol. 2006;17:1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 21.Wymenga A. N. M., Erksson B., Salmela P. I., Jacobsen M. B., Van Cutsem E. J. D. G., Fiasse R. H., Valimaki M. J., Renstrup J., de Vries E. G. E., Oberg K. E. Efficacy and safety of prolonged-release lanreotide in patients with gastrointestinal neuroendocrine tumors and hormone-related symptoms. J. Clin. Oncol. 1999;17:1111–1117. doi: 10.1200/JCO.1999.17.4.1111. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc. Natl. Acad. Sci. U S A. 1992;89:251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada Y., Reisine T., Law S. F., Ihara Y., Kubota A., Kagimoto S., Seino M., Seino Y., Bell G. I., Seino S. Somatostatin receptors, an expanding gene family: Cloning and functional characterization of human SSTR3, a protein coupled to adenylate cyclase. Mol. Endocrinol. 1992;6:2136–2142. doi: 10.1210/mend.6.12.1337145. [DOI] [PubMed] [Google Scholar]