Abstract
The transplantation of myogenic cells is a potentially effective therapy for muscular dystrophy. However, this therapy has achieved little success because the diffusion of transplanted myogenic cells is limited. Hepatocyte growth factor (HGF) is one of the primary triggers to induce myogenic cell migration in vitro. However, to our knowledge, whether exogenous HGF can trigger the migration of myogenic cells (i.e. satellite cells) in intact skeletal muscles in vivo has not been reported. We previously reported a novel in vivo real-time imaging method in rat skeletal muscles. Therefore, the present study examined the relationship between exogenous HGF treatment and cell migration in rat intact soleus muscles using this imaging method. As a result, it was indicated that the cell migration velocity was enhanced in response to increasing exogenous HGF concentration in skeletal muscles. Furthermore, the expression of MyoD was induced in satellite cells in response to HGF treatment. We first demonstrated in vivo real-time imaging of cell migration triggered by exogenous HGF in intact soleus muscles. The experimental method used in the present study will be a useful tool to understand further the regulatory mechanism of HGF-induced satellite cell migration in skeletal muscles in vivo.
Keywords: skeletal muscle, HGF, cell migration, in vivo, real-time imaging
I. Introduction
Since myoblast transplantation was initially shown as a potential therapy for muscular dystrophy in mice in 1989 [20], active attempts have been made to cure muscular dystrophy using therapy with myoblast transplantation. However, these clinical trials have had limited success. This has been mainly attributed to immune rejection, poor survival and limited diffusion of the transplanted myoblasts [21]. In particular, the limited diffusion problem remains unresolved because the regulatory mechanisms of the migration of the transplanted myoblasts and the existing satellite cells in skeletal muscle have been poorly understood in vivo.
Multiple factors such as myofibers, extracellular matrix, secreted growth factors and cytokines are complexly related to the regulation of satellite cell migration in skeletal muscles [11, 22]. Among these related factors, it has been thought that hepatocyte growth factor (HGF) is one of the primary triggers to regulate the migration of satellite cells [4, 25]. When HGF binds to its tyrosine kinase receptor c-met, c-met is autophosphorylated [3, 5, 10, 17–19]. Autophosphorylated c-met stimulates the intracellular signaling pathway via phosphatidylinositol 3-kinase (PI3K), and thereby promotes cell migration in response to increasing HGF concentration in vitro [2, 4, 6, 7, 17]. Moreover, recently, it was reported that both N-WASP and WAVE2 activated via HGF/PI3K signaling pathway played an important role in regulation of the migration through the lamellipodial formation of C2C12 cells derived from satellite cells in vitro [14]. On the hand, to our knowledge, there is a lack of information about whether exogenous HGF can trigger the satellite cell migration in intact skeletal muscle in vivo because it is technically very difficult to investigate the migration of living satellite cells localized within skeletal muscles, and hence the regulatory mechanism of HGF-triggered satellite cell migration in skeletal muscles in vivo remains unclear. However, recently, we reported that a novel in vivo real-time imaging method devised in our laboratory enabled us to investigate the migration of living satellite cells localized within skeletal muscles [12]. Therefore, in the present study, we tried to investigate whether exogenous HGF-triggered cell migration could be observed in rat intact skeletal muscles using this in vivo real-time imaging method. This study will provide important information to lead to an effective experimental design to provide further understanding of the regulatory mechanism of the migration of satellite cells in skeletal muscles.
II. Materials and Methods
Animals
Female Fischer 344 rats (7 weeks of age) were used in the present study. They were housed in individual cages at 22°C under a 12:12 hr light-dark cycle and were provided with food and water ad libitum. All experiments were carried out with the approval of Aichi University of Education Animal Ethics Committee.
Optical imaging system with epifluorescent microscope
The optical system for observation of the fluorescence consisted primarily of an epifluorescent microscope (BX-51, Olympus, Tokyo, Japan) with modification and electron multiplier-type CCD camera (Ixon DV887, Andor Technology, Belfast, Northern Ireland). The objective lens used was a UPLSAO (60×, 1.20 NA, water) for in vivo imaging. 4',6-diamidino-2-phenylindole (DAPI) was used to visualize the nuclei and illuminated at an excitation wavelength of 360–370 nm. When the target cell was focused in soleus muscles, depth in the range of 5–10 µm from its point was observed concomitantly with the target cell in one picture.
In vivo imaging
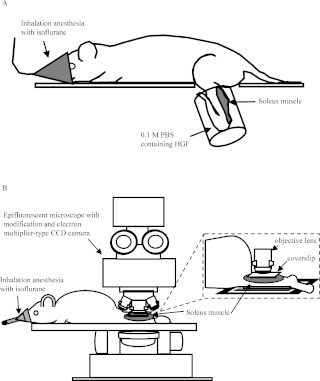
For in vivo imaging, four rats were used in each group. In vivo real-time imaging was performed by modifying the method reported previously [12]. Briefly, under pentobarbital sodium anesthesia (60 mg/kg i.p.), the skin of the left lower leg was peeled for minimal bleeding. The gastrocnemius muscle tendon was cut, and the soleus muscle was exposed by carefully everting the gastrocnemius muscle. The motor nerve and blood vessel supplying the soleus muscle and other lower-limb muscles remained intact. As shown in Fig. 1A, the left lower legs were incubated with 0.1 M phosphate-buffered saline (PBS, pH 7.4) containing DAPI and recombinant human HGF (R&D Systems, Inc., Minneapolis, USA) in a silicon tube at 37°C for 6 hr, by modifying the method reported previously [12]. HGF was tested at concentrations of 25 and 100 ng/ml on the basis of previous studies [2, 4, 6]. On the other hand, the left lower legs incubated with 0.1 M PBS/DAPI without HGF were used as control group. The left lower leg was fully washed in 0.1 M PBS, and then rats were set in an optical imaging system in a prone position (Fig. 1B). Rats were placed under inhalation anesthesia with isoflurane (air flow rate 300 ml/min, isoflurane concentration 0.7%), and the anesthetized condition was maintained for the course of the incubation and imaging session. The migration distance for 10 min was measured from the gap of the position of 0 min phase and 10 min later phase regardless of a direction, and then the migration velocity was calculated from that. A total of approximately 20 migrating cells were measured in 6 rats per group using image analyzing software ImageJ (National Institutes of Health, Bethesda, MD).
Fig. 1.
Experimental diagram of in vivo real-time imaging. A: Schematic illustration of exogenous HGF incubation methods for the left soleus muscle. Under inhalation anesthesia with isoflurane, the exposed soleus muscle was incubated with 0.1 M PBS with HGF in a silicon tube for 6 hr at 37°C. B: Schematic illustration of the optical imaging system with epifluorescent microscope for in vivo real-time imaging.
Immunohistochemical staining
After the incubation of 0.1 M PBS with or without HGF, rats were sacrificed under anesthesia (n=3/group). Then, the soleus muscles were removed. The isolated whole soleus muscles were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 15 min and washed with 0.1 M PBS. To block any non-specific reaction, the isolated whole soleus muscles were incubated with 0.1 M PBS containing 10% normal serum and 1% Triton X-100 for 1 hr. The isolated whole soleus muscles were simultaneously incubated for 16 hr to 48 hr at 4°C with the primary antibodies. The primary antibodies were diluted as required with 0.1 M PBS containing 5% normal serum and 0.3% Triton X-100. The primary antibodies used in the present study were as follows: mouse monoclonal anti-MyoD (a marker for activated satellite cells [8, 11]) (1:100; DAKO, Carpentaria, CA) and goat polyclonal anti-M-cadherin (a marker for satellite cells) (1:200; Santa Cruz Biotech, Santa Cruz, CA). The muscles were washed in 0.1 M PBS and then simultaneously incubated overnight at 4°C with the secondary antibodies. The secondary antibodies were diluted as required with 0.1 M PBS containing 5% normal serum and 0.1% Triton X-100. The secondary antibodies used in the present study were fluorescein-labeled horse anti-mouse IgG (1:300; Vector Laboratories, Burlingame, CA) and Alexa Fluor 568-labeled donkey anti-goat IgG (1:300; Molecular Probes, Eugene, OR). The isolated whole soleus muscles were washed in 0.1 M PBS and mounted in Vectashield mounting medium with DAPI (Vector Labs) to visualize the nuclei. All image analyses of immunostained whole soleus muscles were carried out using an epifluorescent microscope (BX-51, Olympus) with modification and electron multiplier-type CCD camera (Ixon DV887, Andor Technology).
Statistical analysis
All data are presented as the mean±SD and were analyzed with STATVIEW (SAS Institute Inc., Cary, NC). Differences among the groups were tested by one-way ANOVA using Scheffé’s S-test. Differences were considered to be significant at the 0.05 confidence level.
III. Results
Exogenous HGF-triggered cell migration in intact soleus muscles
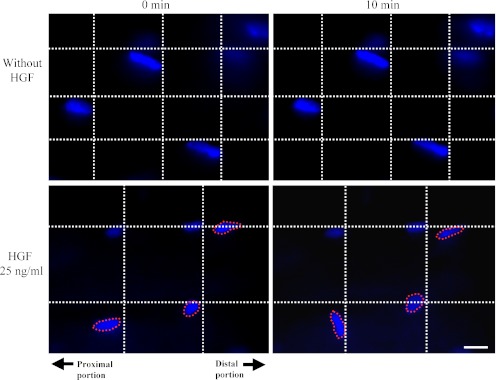
In the present study, in vivo real-time imaging was performed to clarify the effects of exogenous HGF on intact soleus after 0 and 10 min. As a result, cell migration was observed in the intact soleus muscle with HGF treatment while no spatiotemporal changes of any cells localized within intact soleus muscles were observed in intact soleus muscles without HGF treatment (Fig. 2). These results indicated that exogenous HGF could trigger the cell migration in intact soleus muscles.
Fig. 2.
Photomicrographs showing exogenous HGF-triggered cell migration in intact soleus muscles. The localizations of the nuclei in the intact soleus muscles without HGF treatment (upper panels) and with 25 ng/ml HGF (lower panels) are shown. The left and right panels indicate the localizations of nuclei after 0 and 10 min, respectively. The nuclei surrounded by a broken line indicate the migrating cells. In each panel, the right and left sides indicate the distal and proximal portions of the intact soleus muscle, respectively. Bar=10 µm.
The relationship between cell migration velocity and exogenous HGF concentration in intact soleus muscles
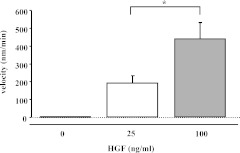
To investigate whether the velocity of cell migration was affected by the difference of exogenous HGF concentration, the cell migration velocity was quantitated in each HGF concentration. As a result, the velocity of 100 ng/ml HGF-triggered cell migration was significantly higher than that of 25 ng/ml HGF-triggered cell migration (p<0.05) (Fig. 3).
Fig. 3.
The cell migration velocity in response to increasing concentration of exogenous HGF in the intact soleus muscles. Note: In the intact soleus muscles without HGF treatment, no cell migration was detected. Values are expressed as mean±SD. *p<0.05.
Exogenous HGF triggered satellite cell activation in intact soleus muscles
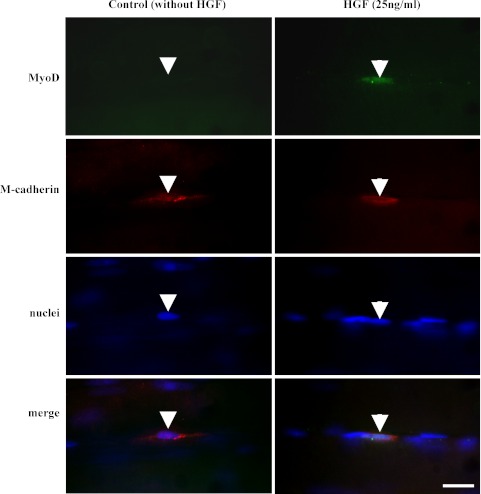
To confirm whether the activation of the satellite cells was triggered by the exogenous HGF, we performed immunostaining using MyoD, M-cadherin antibodies and DAPI on the intact soleus muscles with or without HGF. In Fig. 4, it is indicated that the MyoD-positive immunoreactivities were detected in the nuclei of M-cadherin-positive satellite cells in the intact soleus with HGF treatment (25 ng/ml) while the expression of MyoD was not detected in the intact soleus muscle without HGF treatment. The expression of MyoD was also observed in the satellite cell nuclei of the intact soleus muscles with 100 ng/ml HGF treatment. These findings indicated that exogenous HGF could trigger the activation of satellite cells in the intact soleus muscles.
Fig. 4.
Photomicrographs showing the expression of MyoD in satellite cells in exogenous HGF-stimulated intact soleus muscles. Immunostaining was performed to visualize the localizations of MyoD, M-cadherin and nuclei, and the three images were merged. The left panels show the MyoD-negative nucleus of satellite cell (arrowheads) in intact soleus muscles without HGF treatment. The right panels show the MyoD-positive nucleus of satellite cell (arrows) in the intact soleus muscle with HGF treatment (25 ng/ml). Bar=10 µm.
IV. Discussion
It is well known the exogenous HGF stimulates the chemotaxis of myogenic cells such as primary myoblasts, muscle satellite cells and C2C12 cells in vitro [2, 4, 14, 23, 25]. On the other hand, to our knowledge, there is a lack of information about whether exogenous HGF can trigger cell migration in intact skeletal muscles in vivo. The present study is the first to demonstrate that exogenous HGF triggered cell migration in intact soleus muscles using in vivo real-time imaging methods.
In the present study, cell migration was not detected in the intact soleus muscles stimulated with 0.1 M PBS but without HGF. This finding was consistent with our previous study, which indicated that no spatiotemporal changes of any cells localized within intact soleus muscles were observed for 90 min by in vivo real-time imaging [12]. On the other hand, cell migration was observed in the intact soleus muscles stimulated with 0.1 M PBS and HGF. These results are consistent with previous studies, which indicated that HGF was a trigger factor in the chemotaxis of myogenic cells (e.g. primary myoblasts, muscle satellite cells, C2C12 cells) in vitro [2, 4, 14, 23, 25]. Furthermore, in the present study, the velocity of cell migration triggered by 100 ng/ml HGF was significantly higher than that triggered by 25 ng/ml HGF. This result was reasonable because HGF-induced chemotaxis of myogenic cells was enhanced in response to increasing HGF concentration in vitro [2, 4, 6]. On the other hand, to our knowledge, there is a lack of information about the in vivo effect of a difference of incubation time of exogenous HGF on the cell migration in the intact muscles. The present study is the first to demonstrate the effect of the incubation time of 6 hr on cell migration in the intact soleus muscles in vivo, but did not investigate the effect of a difference of incubation time of exogenous HGF. Therefore, further studies are needed to elucidate the relationship between the incubation time of exogenous HGF and the cell migration in intact muscles in vivo.
We previously reported in vivo real-time imaging of satellite cells localized within rat skeletal muscles by applying anti-M-cadherin antibody-conjugated Quantum dots (anti-Mcad Qdots) [12]. However, under the incubation medium conditions used in the present study, anti-Mcad Qdot-positive immunoreactivities could not be clearly detected in rat skeletal muscles, but the reasons for this remain unknown. Therefore, unfortunately, the kinds of migrating cell could not be directly identified in the present study. In general, HGF stimulates a variety of cellular responses including cell migration and activation via its receptor c-met [3, 5, 10, 17–19]. It is well known that c-met is specifically expressed on the surface of satellite cells in skeletal muscles, and in fact in many previous studies, c-met has been generally used as a marker protein of satellite cells in skeletal muscles in vivo [8, 9, 11, 13, 15, 24, 26]. Moreover, it was previously reported that exogenous HGF induced the activation of quiescent skeletal muscle satellite cells in vitro [1]. In fact, in the present study, the expression of MyoD (a marker for activated satellite cells [8, 11]) was detected in the satellite cells in intact soleus muscles in response to exogenous HGF. For these reasons, the migrating cells detected in the intact soleus muscle with HGF were probably the activating satellite cells in the present study. On the other hand, the present study could not rule out the possibility that other cell types may also be stimulated by exogenous HGF because it was reported that the expression of c-met was detected in pericytes but not CD31-positive microvascular endothelial cells in vivo [16].
In conclusion, the present study is the first to demonstrate in vivo real-time imaging of exogenous HGF-triggered cell migration in rat intact soleus muscles. It was indicated that the cell migration velocity was enhanced in response to increasing exogenous HGF concentration in skeletal muscles. Furthermore, it was suggested that migrating cells may be satellite cells in intact soleus muscles because the expression of MyoD was induced in satellite cells in response to HGF. Therefore, the combination of exogenous HGF treatment and in vivo real-time imaging method used in the present study may provide beneficial information for understanding the regulatory mechanism of satellite cell migration in skeletal muscles and helping to establish satellite cell-based therapies.
V. Acknowledgments
This work was supported by Grants-in-Aid for Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency (JST).
VI. References
- 1.Allen R. E., Sheehan S. M., Taylor R. G., Kendall T. L., Rice G. M. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 2.Barbero A., Benelli R., Minghelli S., Tosetti F., Dorcaratto A., Ponzetto C., Wernig A., Cullen M. J., Albini A., Noonan D. M. Growth factor supplemented matrigel improves ectopic skeletal muscle formation—a cell therapy approach. J. Cell. Physiol. 2001;186:183–192. doi: 10.1002/1097-4652(200102)186:2<183::AID-JCP1020>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Birchmeier C., Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff R. Chemotaxis of skeletal muscle satellite cells. Dev. Dyn. 1997;208:505–515. doi: 10.1002/(SICI)1097-0177(199704)208:4<505::AID-AJA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 6.Cai W., Rook S. L., Jiang Z. Y., Takahara N., Aiello L. P. Mechanisms of hepatocyte growth factor-induced retinal endothelial cell migration and growth. Invest. Ophthalmol. Vis. Sci. 2000;41:1885–1893. [PubMed] [Google Scholar]
- 7.Cain R. J., Ridley A. J. Phosphoinositide 3-kinases in cell migration. Biol. Cell. 2009;101:13–29. doi: 10.1042/BC20080079. [DOI] [PubMed] [Google Scholar]
- 8.Cornelison D. D., Wold B. J. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 9.Cornelison D. D., Filla M. S., Stanley H. M., Rapraeger A. C., Olwin B. B. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 10.Giordano S., Di Renzo M. F., Narsimhan R. P., Cooper C. S., Rosa C., Comoglio P. M. Biosynthesis of the protein encoded by the c-met proto-oncogene. Oncogene. 1989;4:1383–1388. [PubMed] [Google Scholar]
- 11.Hawke T. J., Garry D. J. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 12.Ishido M., Kasuga N. In situ real-time imaging of the satellite cells in rat intact and injured soleus muscles using quantum dots. Histochem. Cell Biol. 2011;135:21–26. doi: 10.1007/s00418-010-0767-x. [DOI] [PubMed] [Google Scholar]
- 13.Kami K., Senba E. In vivo activation of STAT3 signaling in satellite cells and myofibers in regenerating rat skeletal muscles. J. Histochem. Cytochem. 2002;50:1579–1589. doi: 10.1177/002215540205001202. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura K., Takano K., Suetsugu S., Kurisu S., Yamazaki D., Miki H., Takenawa T., Endo T. N-WASP and WAVE2 acting downstream of phosphatidylinositol 3-kinase are required for myogenic cell migration induced by hepatocyte growth factor. J. Biol. Chem. 2004;279:54862–54871. doi: 10.1074/jbc.M408057200. [DOI] [PubMed] [Google Scholar]
- 15.Lindstrom M., Pedrosa-Domellof F., Thornell L. E. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: application to a cohort of power lifters and sedentary men. Histochem. Cell Biol. 2010;134:371–385. doi: 10.1007/s00418-010-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Wilkinson F. L., Kirton J. P., Jeziorska M., Iizasa H., Sai Y., Nakashima E., Heagerty A. M., Canfield A. E., Alexander M. Y. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J. Pathol. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto K., Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023–2038. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- 18.Naldini L., Vigna E., Narsimhan R. P., Gaudino G., Zarnegar R., Michalopoulos G. K., Comoglio P. M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- 19.Naldini L., Weidner K. M., Vigna E., Gaudino G., Bardelli A., Ponzetto C., Narsimhan R. P., Hartmann G., Zarnegar R., Michalopoulos G. K. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 21.Peault B., Rudnicki M., Torrente Y., Cossu G., Tremblay J. P., Partridge T., Gussoni E., Kunkel L. M., Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 22.Seale P., Rudnicki M. A. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 23.Siegel A. L., Atchison K., Fisher K. E., Davis G. E., Cornelison D. D. W. 3D timelapse analysis of muscle satellite cell motility. Stem Cells. 2009;27:2527–2538. doi: 10.1002/stem.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatsumi R., Anderson J. E., Nevoret C. J., Halevy O., Allen R. E. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 25.Villena J., Brandan E. Dermatan sulfate exerts an enhanced growth factor response on skeletal muscle satellite cell proliferation and migration. J. Cell Physiol. 2004;198:169–178. doi: 10.1002/jcp.10422. [DOI] [PubMed] [Google Scholar]
- 26.Wozniak A. C., Pilipowicz O., Yablonka-Reuveni Z., Greenway S., Craven S., Scott E., Anderson J. E. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J. Histochem. Cytochem. 2003;51:1437–1445. doi: 10.1177/002215540305101104. [DOI] [PMC free article] [PubMed] [Google Scholar]