Abstract
Aims
To investigate the distribution of beta-3 adrenergic receptors (β3ARs) in the rat bladder and to examine the contribution of urothelial β3ARs to agonist-induced suppression of bladder reflexes and relaxation of smooth muscle.
Methods
Bladder tissue was collected from 8-10 months old female SD rats. In some samples, the urothelium was surgically separated from the smooth muscle. The expression and localization of βAR mRNA and β3AR protein were determined using RT-PCR and immmunohistochemistry. Contractile responses to the specific β3AR agonists TAK-677 and BRL37344 were measured in bladder strips with or without the urothelium. The contribution of urothelial β3AR to the micturition reflex was assessed in continuous cystometry in urethane anesthetized rats using intravesical delivery of β3AR agonists.
Results
RT-PCR detected mRNA of all βARs in urothelium and smooth muscle. Immunostaining detected β3ARs throughout the urothelium, in the smooth muscle, myofibroblast-like cells, and in the peripheral nerves. Ovariectomy did not change the distribution of β3ARs in any bladder structure. Intravesical administration of TAK-677 and BRL37344 (1 - 5×10-4 M) decreased voiding frequency and amplitude of bladder contractions. In bladder strips in vitro both β3AR agonists (10-12 - 10-4 M) relaxed the smooth muscle in a concentration-dependent manner to the same extent in strips with and without the urothelium.
Conclusions
In addition to their presence in bladder smooth muscle, β3ARs are present in the urothelium where their activation may alter reflex voiding via release of factor(s) that act on non-myocyte structures including the afferent and/or efferent nerves to influence bladder contractility.
Keywords: urethane anesthesia, bladder strips, immunohistochemistry, cystometry, intravesical application, RT-PCR, BRL37344, TAK-677
Introduction
Beta-3 adrenergic receptor (β3AR) agonists are being developed to treat voiding dysfunctions including urinary frequency, urgency or incontinence [Yamaguchi and Chapple, 2007]. The traditional view is that βARs are expressed in the bladder smooth muscle and their activation by norepinephrine released from sympathetic nerves relaxes the detrusor [Yamaguchi and Chapple, 2007]. Nonselective βAR agonists (isoproterenol) and selective β3AR agonists including BRL37344, CL316243, FK175, YM178 (mirabegron) or TAK-677 relax bladder smooth muscle strips from rat [Frazier et al., 2006], [Fujimura et al., 1999], [Kullmann et al., 2009], [Takeda et al., 2000], [Woods et al., 2001], human [Biers et al., 2006] [Otsuka et al., 2008] [Tyagi et al., 2009] or pig [Badawi et al., 2005; Masunaga et al., 2010], and act in vivo to decrease voiding frequency and voiding pressure in rats [Kullmann et al., 2009] [Fujimura et al., 1999; Kaidoh et al., 2002; Leon et al., 2008; Woods et al., 2001] and in other species including humans [Chapple et al., 2008]. Although most of the effects of β3AR agonists have been attributed to activation of receptors expressed in the smooth muscle, recent studies using RT-PCR and/or immunohistochemistry detected all subtypes of βARs (β1ARs, β2ARs and β3AR) in the urothelium [Otsuka et al., 2008] [Tyagi et al., 2009]. Very few studies addressed the role of urothelial βARs in smooth muscle relaxation or in bladder reflexes. One study showed that isoproterenol-induced relaxation of pig bladder strips was urothelium independent [Murakami et al., 2007]. Conversely, the presence of urothelium caused a right-ward shift in the isoproterenol-induced relaxation of human bladder strips [Otsuka et al., 2008]. A recent study in pig bladder showed that β3ARs in the urothelium contribute to the inhibitory action of βAR agonists on the smooth muscle via the urothelium [Masunaga et al., 2010].
We have shown previously that systemic administration of β3AR agonists alters reflex voiding and that hormonal depletion induced by ovariectomy (OVX) does not affect whole-bladder expression of β3ARs or efficacy of β3AR agonists [Kullmann et al., 2009]. We observed intense staining in the urothelium, raising the possibility that activation of urothelial β3AR could contribute to the effects of β3AR agonists on bladder activity. This is explored further in this study by: (1) testing the effects of intravesical administration of β3AR agonists on reflex voiding in anesthetized animals, (2) testing the effects of β3AR agonists on bladder strips with and without the urothelium, (3) examining the distribution of β1, β2 and β3ARs in the urothelium, suburothelial region and muscle layers using qPCR and immunhistochemistry.
Material and Methods
Animals
Female Sprague Dawley rats (8-10 mo old; Harlan, Indianapolis, IN) were handled according to the University of Pittsburgh Institutional Animal Care and Use Committee protocol which requires strict adherence to all NIH guidelines. In some rats the ovaries were removed bilaterally (OVX) or inspected and left in place (SHAM), as previously described [Kullmann et al., 2009].
qPCR
Whole bladders were collected from 8 SHAM and 8 OVX rats, sacrificed 6 weeks after surgery. Each bladder was cut into four sections lengthwise, pinned into a sylgard-coated dish containing Hanks buffer (Invitrogen, Carlsbad, CA) and the urothelium and lamina propria separated from the detrusor muscle using a dissection microscope. The urothelial and detrusor tissues from individual rats were pooled. RNA was separated using TRIzol® Reagent and mRNA Catcher™ PLUS Kit (Invitrogen). cDNA generation and quantitative RT-PCR were performed as previously described [Kullmann et al., 2009]. βARs mRNA was normalized to 18SmRNA. There was no change in 18SmRNA expression with +/-OVX (data not shown).
Immunohistochemistry
Whole bladders were collected from 3 SHAM and 3 OVX rats, fixed in formalin and sent for immunohistochemistry to LifeSpan (LifeSpan BioSciences, Seattle, WA). Tissue was oriented to preserve anatomical landmarks (Fig. 1D), bisected, embedded in paraffin and sectioned at 4 microns. The primary antibodies were CH-AB15688 (Millipore, Billerica, MA; catalog #AB15688; chicken antibody; 5μg/ml) and LS-A4198 (MBL International, Woburn, MA; catalog #LS-A4198; rabbit polyclonal antibody; 5μg/ml). The detection system was a Vector anti-rabbit secondary (BA-1000; Vector Laboratories, Burlinghame, CA) and a Vector ABC-AP kit (AK-5000) with a Vector Red substrate kit (SK-5100), which produced a fuchsia-colored deposit. We have shown that both primary antibodies specifically recognized the rat and human β3ARs, respectively, and did not recognize either β1ARs or β2ARs when expressed in CHOK1 cells (figure 4 and supplemental figure 1 from [Kullmann et al., 2009]). In rat bladder tissue, these antibodies produced a similar staining pattern (supplemental figure 2 from [Kullmann et al., 2009]). Only tissues stained with positive control antibodies for the intermediate filament protein vimentin, a prevalent stromal marker, and for the adhesion molecule CD31, a blood vessel marker, were selected for this study. The negative control consisted of performing the entire immunohistochemistry procedure on adjacent sections in the absence of primary antibody. Slides were imaged by LifeSpan with a DVC 1310C digital camera coupled to a Nikon microscope, stored as tiff files and processed similarly using Photoshop (Adobe Systems Incorporated, San Jose, CA).
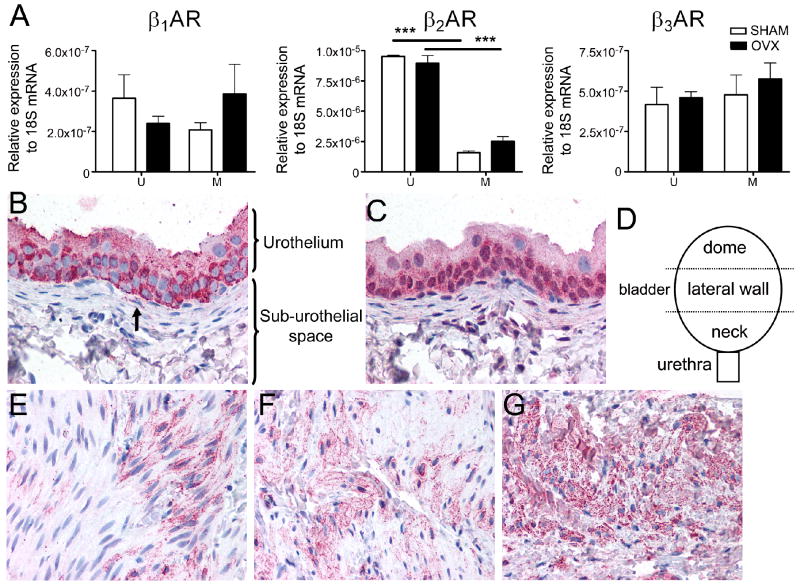
Figure 1. βARs are expressed throughout the rat bladder and their expression is maintained after ovariectomy.
A. mRNA of β1AR, β2AR and β3AR is present in the urothelium (U) and smooth muscle (M) of the bladder from SHAM (white bars) and OVX (black bars) rats. Expression levels are relative to 18S. Asterisks indicate statistically significant differences between urothelium and smooth muscle (ANOVA followed by Tukey-Kramer test; p<0.001). No statistically significant differences were found between SHAM and OVX (ANOVA, p>0.05). Data are the average from three rats; values are expressed as mean ± SEM. Similar results were obtained in an additional experiment.
B, C. Staining for β3AR (fuchsia) with the LS-A4198 antibody (B) and the CH-AB15688 antibody (C) shows moderate to strong staining in the urothelium, whereas the sub-urothelial layer showed faint to no staining. The arrow in B indicates staining of a probable sub-urothelial fibroblast. These samples are serial sections taken from an OVX animal. Similar results were obtained in 5 additional SHAM and OVX rats.
D. Schematic of the bladder regions: dome, lateral wall and neck from which tissue was selected for staining.
E-G. Antibody LS-A4198 showed frequent focal faint to moderate staining (fuchsia) in the detrusor, with the staining often being more intense in the sample taken near the bladder neck (G) than from the lateral wall of the bladder (F) or from the bladder dome (E). This sample was taken from a SHAM rat. Similar results were obtained in additional SHAM and OVX rats, with the stronger intensity and more prevalent staining observed in the bladder neck area vs. the other regions observed in a total of 5/6 rats.
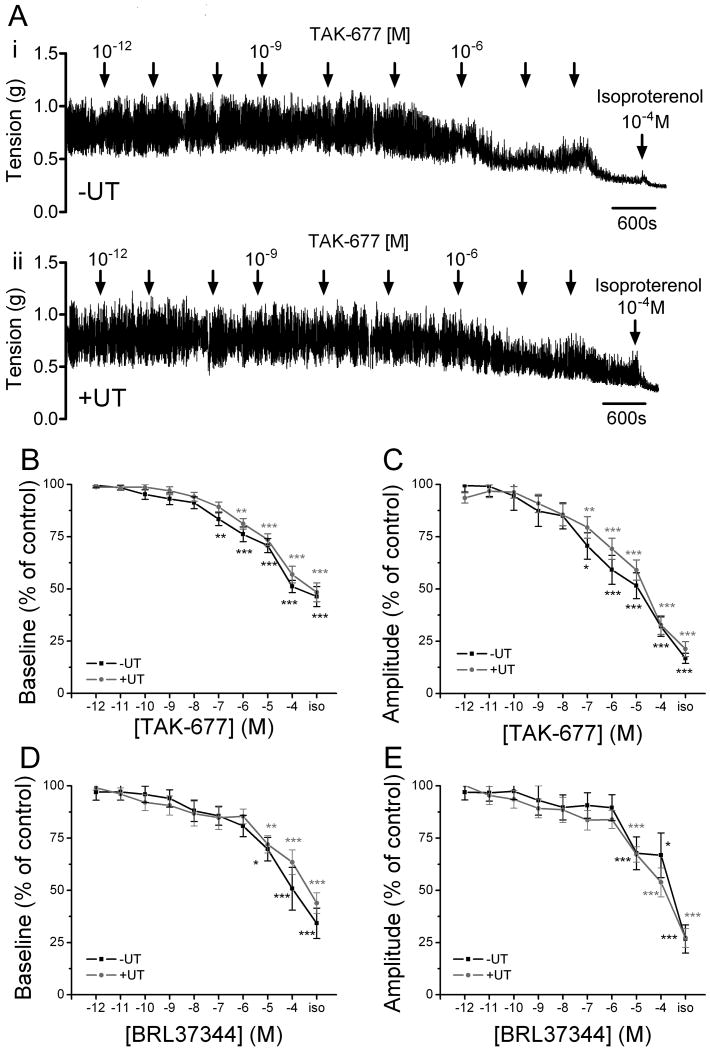
Figure 4. β3AR agonists decrease baseline tone and spontaneous activity of bladder strips with and without the urothelium.
A. TAK-677 decreases baseline tone and amplitude of spontaneous contractions in a concentration dependent manner in strips without (i) and with (ii) the urothelium. Isoproterenol (iso), a nonspecific βAR agonist, further decreases the baseline tone and the amplitude of the contractions in the presence of a maximal concentration of TAK-677. Examples are from strips from the same bladder. B-C. Summary of the effects of TAK-677 on baseline tone (B) and on amplitude of spontaneous contractions (C) in strips without (n=23 strips from 13 animals; black bars) and with (n=27 strips from 17 animals; grey bars) the urothelium. D-E. Summary of the effects of BRL37344 on baseline tone (D) and on amplitude of spontaneous contractions (E) in strips without (n=11 strips from 7 animals; black bars) and with (n=22 strips from 11 animals; grey bars) the urothelium. Asterisk indicates statistically significant differences between the effect of the agonist and control (ANOVA followed by Tukey-Kramer Multiple Comparisons Test; ***p<0.001; **p<0.01; *p<0.05). Values are expressed as mean ± SEM.
Continuous infusion cystometry (CMG) was performed in 17 urethane anesthetized rats (1-1.2g/kg, subcutaneous), as previously described [Kullmann et al., 2009]. Voiding responses were elicited by continuously infusing saline at 0.04ml/min through a catheter implanted at the bladder dome and connected to infusion pump and pressure transducer. BRL37344 ((±)-(R*,R*)-[4-[2-[[2-(3-Chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetic acid sodium hydrate; Sigma) was dissolved in saline; TAK-677 ([3-[(2R)-[[(2R)-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1H-indol-7-yloxy]-acetic acid; Procter&Gamble) was dissolved in DMSO and diluted in saline. Neither saline [Kullmann et al., 2008] nor DMSO (up to 2.5%; data not shown) delivered intravesically altered CMG parameters. Concentration-response curves were constructed by instilling drugs intravesically at increasing concentrations, every 3-4 voidings (∼50-120min). Data were recorded using Windaq (DATAQ Instruments Inc, Akron, OH) and analyzed using Excel, Origin7 (OriginLab Corporation, Northampton, MA) and Prism 4 (GraphPad Software, Inc, San Diego, CA). The parameters analyzed were: inter-contraction interval (ICI; interval between two voidings), amplitude of contractions (A; difference between bladder pressure at peak contraction minus baseline pressure), pressure threshold (PTh; bladder pressure necessary to evoke voiding contractions) and baseline intravesical pressure (BP; lowest bladder pressure just after voiding). For each parameter, at least 3 measurements in control period and after drug administration were averaged. Data are reported as percentage change relative to control (set to 100%).
Bladder strips were prepared from 48 rats as previously described [Kullmann et al., 2009]. The bladder was placed in Krebs (in mM: NaCl 118, KCl 4.7, CaCl2 1.9, MgSO4 1.2, NaHCO3 24.9, KH2PO4 1.2, dextrose 11.7; pH. 7.4 when aerated with 95%O2, 5%CO2) and cut into three or four longitudinal strips. In some strips the urothelium and lamina propria (suburothelium) were surgically removed by careful dissection. Strips were mounted into a 15ml organ bath in Krebs solution (37°C), washed several times and allowed to equilibrate for more than 2h prior to drug testing. A 10mN force was set as baseline tension and stimulus evoked changes were measured with force displacement transducers (Grass, Astromed, RI). Drugs from concentrated stock solutions were added to the bath every 12-15 min. BRL37344 and TAK-677 were dissolved in water or DMSO, respectively at 10-2M and further diluted in Krebs to 10-12-10-4M. Vehicles including saline and DMSO (up to 1%) had no significant effects on bladder strip activity [Kullmann et al., 2009]. Isoproterenol hydrochloride was dissolved in Krebs at 0.1-1×10-4M. Data were recorded and analyzed using Windaq, Excel and Prism. Baseline tone and amplitude of spontaneous contractions were measured in a 3 min window selected at ∼3-4 min after drug application (maximal effect). In this window, 4-8 measurements of baseline tension and amplitude of contractions were averaged and taken as one data point. The threshold for amplitude of spontaneous activity was set to 0.05g. Results are reported relative to values before drug application, which were set to 100%. Curve fitting for obtaining EC50 was performed using the sigmoidal dose-response curve model in Prism (Y=Bottom+(Top-Bottom)/(1+10ˆ((LogEC50-X))); X is the logarithm of concentration; Y is the response).
Statistics
Statistical significance was analyzed using paired, unpaired t-test or ANOVA followed by Tukey-Kramer Multiple Comparisons Test (significance p<0.05).
Results
Expression of βAR mRNA in urothelium and smooth muscle
qPCR identified mRNA of all βARs in urothelium and bladder smooth muscle (Fig. 1A). As standard curves for βAR primer sets were not identical (Y-intercept values for β1, β2, and β3 were 36.0, 37.4, and 33.5 respectively, data not shown), conclusions about the relative receptor levels could not be made. However, it is reasonable to compare levels of the same receptor between different bladder regions. In both groups (OVX and SHAM) the levels of β1AR and β3AR mRNA were similar in the urothelium (mucosa) and the smooth muscle (ANOVA, p>0.05), whereas the level of β2AR mRNA was significantly higher in the urothelium (ANOVA, p<0.001). Hormonal depletion after ovariectomy did not significantly alter the expression of any of the βARs in either the detrusor or urothelium.
Localization of β3AR protein in the rat bladder
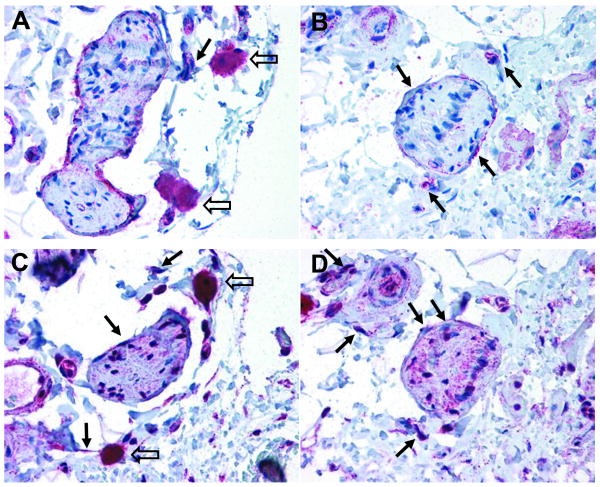
To investigate the localization of β3AR protein in the bladder, we used two specific antibodies, LS-A4198 and CH-AB15688, both validated previously [Kullmann et al., 2009]. The results were not detectably different with the two antibodies, both of them yielding a similar pattern of receptor expression in the detrusor and urothelium (Figs. 1B-C, 2). β3ARs were detected in the urothelium, in certain cells within the sub-urothelial region, and the detrusor smooth muscle (Figs. 1B-G). The urothelium was intensely stained compared to the muscle. Strong staining occurred in the innermost cell layers (i.e. basal and intermediate cell layers), while the outermost layer (i.e. umbrella cell layer) was only moderately stained (Figs. 1B-C), regardless of the sample location in the bladder (bladder neck versus dome). In the sub-urothelial space, staining of the cytoplasm of myofibroblast-like cells was detected (arrow in Fig. 1A), while smooth muscle cells in this region lacked staining. This lack of staining is in contrast to the clear staining encountered in the detrusor smooth muscle (Figs. 1E-G). The detrusor staining was not uniform. Intense staining was detected near the bladder neck (Fig. 1G), a densely innervated area [Fowler et al., 2008], while weaker staining was detected at the bladder dome (Fig. 1E). The functional significance of this differential distribution of β3ARs remains to be investigated. The antibody LS-A4198 produced a lower background staining thus allowing the staining of certain cell types in the sub-urothelial space, identified as myofibroblast-like cells (arrow in Fig. 1B), to be more readily resolved. β3AR staining was observed in presumed perineural fibroblasts surrounding some presumptive peripheral nerves (solid arrows in Fig. 2), in addition to faintly positive staining inside the nerve bundle (Fig. 2). However, the precise identification of neuronal fiber type cannot be determined in the absence of double labeling experiments with specific neuronal markers. Strong positive immunoreactivity in mast cells was also detected (open arrows in Figs. 2A,C), but this should be interpreted with caution since mast cells are frequently positive with polyclonal antibodies [Harley et al., 2002]. No qualitative differences were noted between OVX and SHAM rats, extending our previous observations that hormonal depletion does not alter β3AR distribution in the rat bladder [Kullmann et al., 2009].
Figure 2. Expression of the β3AR is apparent in peripheral nerves, perineural fibroblasts, and mast cells.
Staining of the perineural fibroblasts and peripheral nerves was observed with the LS-A4198 antibody in SHAM (A) and OVX (B) rats. Similar staining patterns were observed with the CH-AB15688 antibody (serial sections C, D from the same animals as in A, B, respectively). The solid arrows indicate immunoreactivity in perineural fibroblasts and open arrows indicate immunoreactivity in mast cells. Similar results were obtained in 5 additional SHAM and OVX rats.
Effects of β3AR agonists on voiding function
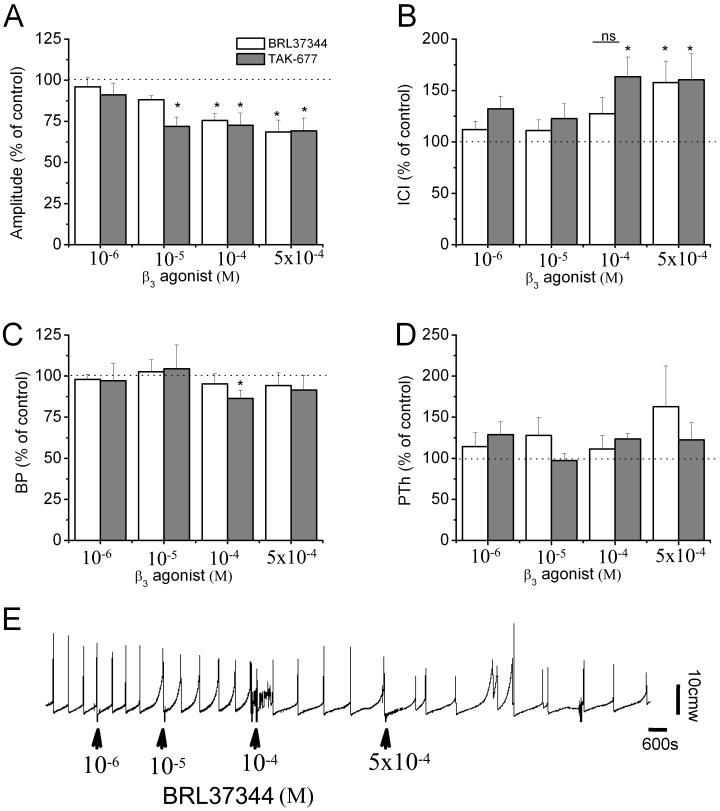
Because β3ARs were strongly expressed in the urothelium we evaluated whether their activation alters reflex voiding. The specific β3AR agonists BRL37344 (n=9 rats) and TAK-677 (n=8 rats) were administered intravesically at increasing concentrations: 1, 10, 100 and 500 ×10-6M (Fig. 3, table 1). Both agonists significantly reduced the amplitude of voiding contractions and increased the ICI in a concentration-dependent manner. The effects were visible generally after the first or second ICI. The effects on baseline intravesical pressure were not significant except for one concentration of TAK-677 (10-4M). For amplitude and ICI, TAK-677 seems to be more potent than BRL37344. This is evidenced by TAK-677 reaching the maximal response for amplitude at 10-5M whereas BRL37344 reaches the maximal response at 10-4M. Similarly, for ICI, TAK-677 reaches the maximal response at a lower concentration than BRL37344 does (10-4M vs. 5×10-4M). To determine whether systemic administration of β3AR agonists can further affect the bladder, BRL37344 or TAK-677 were administered intravenously (i.v.) after the highest intravesical concentration of either agonist (5×10-4M). In two rats BRL37344 (10-4M/kg) or TAK-677 (2.5×10-4M/kg) had no further significant effect on any parameter. In another two rats TAK-677 (2.5×10-4M/kg) impaired voiding (producing overflow), similar to results obtained previously with high i.v. doses of β3AR agonists [Kullmann et al., 2009].
Figure 3. Intravesical instillation of the β3AR agonists BRL37344 and TAK-677 decreases voiding frequency and amplitude of voiding contractions.
A-D. Percentage change in the amplitude of bladder contractions (A), intercontraction interval (ICI; B), baseline intravesical pressure (BP; C) and pressure threshold (PTh; D) relative to control (before drug application) induced by application of BRL37344 (white bars; n=9 rats) and TAK-677 (grey bars; n=8 rats). Each rat served as its own control. *=p<0.05, when comparing each parameter before and after drug application, using paired t-test; ns=no significant difference (p>0.05). Values are expressed as mean ± SEM.
E. Example of the effects of the intravesical administration of BRL37344. Arrows indicate when the drug was administered.
Table 1.
CMG parameters of rats during BRL37344 (n=9 rats) and TAK-677 (n=8 rats) administered intravesically.
| control | β3AR agonists | 10-6M | 10-5M | 10-4M | 5×10-4M | |
|---|---|---|---|---|---|---|
| ICI (s) | 558.6±112.6 | BRL37344 | 596.5±107.3 | 586.1±112.8 | 611.5±87.2 | 671.5±57.0* |
| 635.3±90.3 | TAK-677 | 797.8±86.1 | 710.2±56.9 | 885.6±85.0* | 851.1±88.2* | |
| A (cmH2O) |
30.0±5.0 | BRL37344 | 27.9±3.8 | 26.4±4.4* | 22.5±3.6* | 21.2±3.2* |
| 24.1±2.0 | TAK-677 | 21.1±1.2* | 16.6±0.8* | 16.5±1.6* | 15.4±1.3* | |
| PTh (cmH2O) |
6.3±1.7 | BRL37344 | 6.7±2.3 | 7.1±1.7 | 6.5±2.1 | 5.9±0.8 |
| 4.7±0.7 | TAK-677 | 6.1±1.6 | 4.2±0.4 | 5.3±0.7 | 4.9±0.6 | |
| BP (cmH2O) |
4.4±0.4 | BRL37344 | 4.3±0.5 | 4.5±0.5 | 4.0±0.4 | 4.6±0.3 |
| 3.0±0.3 | TAK-677 | 2.9±0.3 | 3.0±0.3 | 2.6±0.1* | 2.7±0.2 | |
p <0.05; paired t-test when compare the effect of the drug with control
Effects of β3AR activation in bladder strips
To further investigate the role of urothelial β3ARs in smooth muscle relaxation, we compared the effects of β3AR agonists in strips with and without the urothelium. Bladder strips had intrinsic spontaneous activity when placed in the organ bath (Fig. 4A). Removal of the mucosa did not alter the amplitude of this spontaneous activity (0.48 ± 0.03 mN, n=49 strips with urothelium vs. 0.42 ± 0.04 mN, n=34 strips without urothelium, unpaired t-test p>0.05). BRL37344 and TAK-677 decreased basal smooth muscle tone and amplitude of spontaneous activity in a concentration-dependent manner (Fig. 4). Potency pEC50 values for baseline relaxation were not statistically different in strips with or without urothelium (TAK677: 6.06±0.2 (EC50 8.6×10-7M) with UT, n=27 vs. 6.48±0.3 (EC50 3.3×10-7M) without UT, n=23; BRL36344: 6.91±0.6 (EC50 1.22×10-7M) with UT, n=22 vs. 6.42±0.5 (EC50 3.76×10-7M) without UT, n=11). Isoproterenol administered in the presence of a β3AR agonist further decreased baseline tone and amplitude of spontaneous contractions independent of the presence of the urothelium.
Discussion
This study demonstrated that β3ARs are distributed at various sites in the bladder including the smooth muscle and the urothelium. In vitro bladder strip experiments indicated that β3AR agonists relax the smooth muscle independent of the presence of the urothelium and other cell types present in the lamina propria. In vivo experiments suggested that activation of β3ARs located in the urothelium or near the bladder lumen contributes to agonist-induced suppression of bladder reflexes. Thus, the action of β3AR agonists on voiding reflex may involve receptors located not only in the smooth muscle but also in the sensory system including the urothelium.
RT-PCR showed mRNA for all βARs in the urothelium (mucosa) at levels comparable to or exceeding those expressed in the smooth muscle (Fig. 1A) [Barendrecht et al., 2009]. Immunohistochemistry demonstrated the presence of β3AR protein in the urothelium, detrusor smooth muscle, nerves and other non-neuronal cell types including myofibroblast-like cells and mast cells (Figs. 1B-G, 2). Activation of β3ARs relaxed the bladder smooth muscle (Fig. 4), as previously reported [Yamaguchi and Chapple, 2007], and reduced spontaneous contractile activity of bladder strips (Fig. 4).
Activation of urothelial β3ARs using intravesical administration of β3AR agonists decreased voiding frequency and micturition contraction amplitude without significantly changing baseline intravesical pressure (Fig. 3). The changes in voiding frequency are consistent with an action on the sensory system including the afferent nerves. The magnitude of these changes were comparable to those produced by the i.v. application of agonists (i.e. TAK-677 1×10-4M and 5×10-4M intravesically and 2.5 and 5 ×10-4M/kg i.v. produced ∼50% increase in ICI - compare Fig. 3B in this study with Figs. 8C,F from [Kullmann et al., 2009]; similar comparisons hold for BRL37344). The mechanisms by which activation of urothelial β3ARs alters the voiding reflex in vivo might involve the release of factors from the urothelium [Birder and de Groat, 2007] [Hawthorn et al., 2000] [Chaiyaprasithi et al., 2003] [Masunaga et al., 2010]. For example, a recent study using pig bladder strips has shown that isoprenaline caused a right-shift the carbachol concentration response curve which was smaller in denuded strips and was abolished by the β3AR antagonist SR59230A [Masunaga et al., 2010]. Our results are similar to results obtained in carbachol pre-contracted pig bladder strips where urothelium did not play a role in βAR-induced smooth muscle relaxation [Murakami et al., 2007], but somewhat different from results obtained in human bladder strips where the presence of urothelium caused a rightward shift in the concentration-response curve for isoproterenol-induced relaxation [Otsuka et al., 2008]. These discrepancies may be related to species differences (rat vs. human) and also to the experimental conditions for bladder strips (carbachol pre-contracted strips vs. strips at rest; [Michel and Sand, 2009]). Urothelial-derived factors may act on the smooth muscle but more importantly on afferent nerves because removal of the urothelium together with the lamina propria in bladder strips did not alter β3AR-induced smooth muscle relaxation (Fig. 4). Further studies are required to investigate the mechanisms underlying urothelial β3ARs involvement in reflex voiding.
β3ARs agonists, TAK-677 and BRL37344, decreased bladder contraction amplitude (by ∼25% when administered intravesically and by ∼25-38% when administered i.v.; Fig. 3A in this study and Figs. 8C, F from [Kullmann et al., 2009]). Changes in the amplitude of contractions are often related to changes in the efferent system or the properties of the smooth muscle. Because the agonists were applied intravesically and did not significantly affect baseline pressure, it is less likely that the observed changes were due to a direct action on the smooth muscle resulting from drug penetration into the muscle. Since the efferent pathways are activated reflexively by the afferent system it is possible that some of the changes in the amplitude were due to an inhibitory action of β3AR agonists on the afferent nerves. Alternatively, β3ARs may be located at efferent nerve terminals where their activation may reduce transmitter release. Staining for β3ARs was detected in bladder nerves (Fig. 2), although the identification of nerve types (afferents or efferents) was not performed. Together, these results suggest that activation of β3ARs located at multiple sites in the bladder, including the urothelium but also possibly the interstitial cells or nerves, plays a role in reflex voiding in vivo.
Conclusions
β3AR agonists are currently undergoing clinical trials for the treatment of overactive bladder [Yamaguchi and Chapple, 2007]. β3ARs agonists decreased voiding frequency and amplitude of bladder contractions when administered intravesically in rats in vivo and decreased basal tone in bladder strips with or without the urothelium (mucosa) in vitro. These results imply that clinical efficacy of β3AR agonists may result from an action on bladder smooth muscle, as well as on non-myocyte structures that could influence bladder contractility. Furthermore, hormonal depletion did not alter the distribution of β3AR in the bladder (Figs 1,2), suggesting that these agonists may be clinically effective in the postmenopausal population.
Acknowledgments
The authors wish to thank members of W.C. de Groat laboratory and Dr. Karl-Erik Andersson for valuable discussions. We also would like to thank the staff of LifeSpan BioSciences, Inc. (Seattle, WA) for excellent technical assistance with the IHC samples.
Grants
This work was supported by Procter & Gamble Pharmaceuticals, Inc., now Warner Chilcott Pharmaceuticals Inc.; the American Urology Association (Research Scholar Award to F.A. Kullmann (Negoita)); and the National Institutes of Health (NIDDK 49430).
References
- Badawi JK, Uecelehan H, Hatzinger M, Michel MS, Haferkamp A, Bross S. Relaxant effects of beta-adrenergic agonists on porcine and human detrusor muscle. Acta Physiol Scand. 2005;185:151–9. doi: 10.1111/j.1365-201X.2005.01474.x. [DOI] [PubMed] [Google Scholar]
- Barendrecht MM, Frazier EP, Vrydag W, Alewijnse AE, Peters SL, Michel MC. The effect of bladder outlet obstruction on alpha1- and beta-adrenoceptor expression and function. Neurourol Urodyn. 2009;28:349–55. doi: 10.1002/nau.20642. [DOI] [PubMed] [Google Scholar]
- Biers SM, Reynard JM, Brading AF. The effects of a new selective beta3-adrenoceptor agonist ( GW427353) on spontaneous activity and detrusor relaxation in human bladder. BJU Int. 2006;98:1310–4. doi: 10.1111/j.1464-410X.2006.06564.x. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyaprasithi B, Mang CF, Kilbinger H, Hohenfellner M. Inhibition of human detrusor contraction by a urothelium derived factor. J Urol. 2003;170:1897–900. doi: 10.1097/01.ju.0000091870.51841.ae. [DOI] [PubMed] [Google Scholar]
- Chapple CR, Yamaguchi O, Ridder A, Liehne J, Carl S, Mattiasson A, Aramburu MAL, Lucas M, Everaert K. Clinical proof of concept study (BLOSSOM) shows novel β3 adrenoceptor agonist YM178 is effective and well tolerated in the treatment of symptoms of overactive bladder. European Association of Urology Meeting; Milan, Italy. 2008. poster # 674. [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–66. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier EP, Schneider T, Michel MC. Effects of gender, age and hypertension on beta-adrenergic receptor function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:300–9. doi: 10.1007/s00210-006-0077-y. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y, Kobayashi M, Yamaguchi O. Expression and possible fucntional role of the beta 3-adrenoreceptor in human and rat detrusor muscle. J Urol. 1999;161:680–85. [PubMed] [Google Scholar]
- Harley R, Gruffydd-Jones TJ, Day MJ. Non-specific labelling of mast cells in feline oral mucosa--a potential problem in immunohistochemical studies. Journal of comparative pathology. 2002;127:228–31. doi: 10.1053/jcpa.2002.0583. [DOI] [PubMed] [Google Scholar]
- Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–9. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidoh K, Igawa Y, Takeda H, Yamazaki Y, Akahane S, Miyata H, Ajisawa Y, Nishizawa O, Andersson KE. Effects of Selective [beta]2 and [beta]3-Adrenoceptor Agonists on Detrusor Hyperreflexia in Conscious Cerebral Infarcted Rats. J Urol. 2002;168:1247–52. doi: 10.1016/S0022-5347(05)64634-4. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–87. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann FA, Limberg BJ, Artim DE, Shah M, Downs TR, Contract D, Wos J, Rosenbaum JS, de Groat WC. Effects of beta3-adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. J Pharmacol Exp Ther. 2009;330:704–17. doi: 10.1124/jpet.109.155010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LA, Hoffman BE, Gardner SD, Laping NJ, Evans C, Lashinger ES, Su X. Effects of the beta 3-adrenergic receptor agonist disodium 5-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzo dioxole-2,2-dicarboxylate (CL-316243) on bladder micturition reflex in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008;326:178–85. doi: 10.1124/jpet.108.138651. [DOI] [PubMed] [Google Scholar]
- Masunaga K, Chapple CR, McKay NG, Yoshida M, Sellers DJ. The beta(3)-adrenoceptor mediates the inhibitory effects of beta-adrenoceptor agonists via the urothelium in pig bladder dome. Neurourol Urodyn. 2010 doi: 10.1002/nau.20838. [DOI] [PubMed] [Google Scholar]
- Michel MC, Sand C. Effect of pre-contraction on beta-adrenoceptor-mediated relaxation of rat urinary bladder. World J Urol. 2009;27:711–15. doi: 10.1007/s00345-009-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Chapple CR, Akino H, Sellers DJ, Chess-Williams R. The role of the urothelium in mediating bladder responses to isoprenaline. BJU Int. 2007;99:669–73. doi: 10.1111/j.1464-410X.2006.06679.x. [DOI] [PubMed] [Google Scholar]
- Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S. Expression and functional role of beta-adrenoceptors in the human urinary bladder urothelium. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:473–81. doi: 10.1007/s00210-008-0274-y. [DOI] [PubMed] [Google Scholar]
- Takeda H, Yamazaki Y, Akahane M, Igawa Y, Ajisawa Y, Nishizawa O. Role of the beta 3-adrenoceptor in urine storage in the rat: comparison between the selective beta 3-adrenoceptor agonist, CL316,243, and various smooth muscle relaxants. J Pharmacol Exp Ther. 2000;293:939–45. [PubMed] [Google Scholar]
- Tyagi P, Thomas CA, Yoshimura N, Chancellor MB. Investigations into the presence of functional Beta1, Beta2 and Beta3-adrenoceptors in urothelium and detrusor of human bladder. Int Braz J Urol. 2009;35:76–83. doi: 10.1590/s1677-55382009000100012. [DOI] [PubMed] [Google Scholar]
- Woods M, Carson N, Norton NW, Sheldon JH, Argentieri TM. Efficacy of the beta3-adrenergic receptor agonist CL-316243 on experimental bladder hyperreflexia and detrusor instability in the rat. J Urol. 2001;166:1142–47. [PubMed] [Google Scholar]
- Yamaguchi O, Chapple CR. Beta3-adrenoceptors in urinary bladder. Neurourol Urodyn. 2007;26:752–6. doi: 10.1002/nau.20420. [DOI] [PubMed] [Google Scholar]