Abstract
Rationale
Experimental research has shown that 3,4-methylenedioxymethamphetamine (MDMA) can improve some psychomotor driving skills when administered during the day. In real life, however, MDMA is taken during the night, and driving may likely occur early in the morning after a night of “raving” and sleep loss.
Objectives
The present study assessed the effects of MDMA on road-tracking and car-following performance in on-the-road driving tests in normal traffic.
Methods
Sixteen recreational MDMA users participated in a randomized double-blind placebo-controlled four-way cross-over design. They received single, evening doses of 0, 25, 50, and 100 mg MDMA on separate occasions. Actual driving tests were conducted in the evening when MDMA serum concentrations were maximal and in the morning after a night of sleep loss.
Results
The primary measure of driving, i.e., standard deviation of lateral position (SDLP, a measure of weaving) was significantly increased during driving tests in the morning in all treatment conditions, irrespective of MDMA dose and concentration. The increments in SDLP were of high clinical relevance and comparable to those observed for alcohol at blood alcohol concentrations >0.8 mg/mL. These impairments were primarily caused by sleep loss.
Conclusions
In general, MDMA did not affect driving performance nor did it change the impairing effects of sleep loss. It is concluded that MDMA cannot compensate for the impairing effects of sleep loss and that drivers who are under the influence of MDMA and sleep deprived are unfit to drive.
Keywords: Driving under the influence of drugs, DUID, MDMA, Ecstasy, Sleep deprivation, Oral fluid
Introduction
3,4-Methylenedioxymethamphetamine (MDMA) is the main psychoactive constituent of ecstasy. Ecstasy has stimulant and hallucinogenic effects and has often been described as an entactogenic drug because of its characteristic that it makes people feel close to each other. Ecstasy is a popular drug: in 2007 9.5 million European adults had ever used ecstasy, which is 2.8% of the general population (EMCDDA 2008). In the USA a trend of increasing ecstasy use among adults in 2006–2007 is noticeable, which remained stable in 2008 (SAMHSA 2009). In Europe as well as in the USA, ecstasy is one of the most commonly used illicit drugs after cannabis.
The widespread use of ecstasy might have implications for traffic safety. Epidemiological studies show an increased risk of accidents while driving under the influence of drugs and/or alcohol (EMCDDA 2008). Data on illicit substances such as cannabis, amphetamines (including MDMA), and other stimulants in traffic seem to point out that driving home after a party under the influence of so-called “party drugs” is increasing (Morgan 2000; Ojaniemi et al. 2009; Walsh et al. 2004). A recent report of the National Highway and Traffic Safety Association reported that 10.5% of drivers during nighttime were under the influence of illicit drugs (Lacey et al. 2009).
Driving is a complex task that requires several cognitive functions. There is ample evidence that long-term use as well as acute use of MDMA detrimentally affects cognition. Multiple reviews have indicated that chronic MDMA use impairs cognitive performance on tasks measuring working and episodic memory, attention, frontal-executive functions, impulsiveness, and psychomotor speed (e.g., Kalechstein et al. 2007; Morgan 2000; Parrott 2006; Zakzanis et al. 2007). Acute studies demonstrated attentional and memory deficits during MDMA intoxication. For example, Kuypers and Ramaekers (2005, 2007) reported impairments in verbal and spatial memory performance after a single dose of 75 mg MDMA. Dumont et al. (2008) also described acute impairment in memory as well as in attention after a single dose of 100 mg MDMA. In contrast, psychomotor performance improved after a single acute dose of MDMA (Lamers et al. 2003), showing that MDMA also possesses stimulating properties.
A number of studies have assessed the effects of MDMA on driving performance in actual driving studies and driving simulator studies. Studies that assessed on-the-road driving performance showed that acute administration of regular recreational doses of 75 or 100 mg MDMA improved road-tracking performance (Kuypers et al. 2006; Ramaekers et al. 2006). However, MDMA also impaired other aspects of driving such as car-following performance (Ramaekers et al. 2006). Subjects overreacted to speed decelerations of a leading car as indicated by a significant “overshoot” in their adaptive response. Another on-the-road driving study indicated that the stimulating effects of MDMA on driving performance were only mild and not sufficient to counteract the impairing effect of alcohol when used in combination (Kuypers et al. 2006). The latter finding was also reported by Brookhuis et al. (2004) who assessed simulated driving performance of rave party visitors before and after the party in a quasi-controlled study. All of the participants used multiple drugs, including MDMA and alcohol. Drug users clearly had higher accidents rates and displayed more risk-taking behaviors early in the morning, when compared to non-drug-using controls. However, it was difficult to determine whether these impairments in the drug-using group resulted from MDMA use, polydrug use, or sleep deprivation.
The present study was designed to assess effects of MDMA and sleep deprivation on actual driving performance, separately and in combination. In addition, the present study assessed the association between MDMA concentration in blood and oral fluid. In order to cover a wide range of concentrations, three doses of MDMA (25, 50, and 100 mg) were included in the design. Driving performance was measured in the evening after administration of placebo or MDMA and in the morning after a night of sleep loss. It was expected that MDMA would produce stimulant effects in the evening, but impairment in the morning after a night of sleep loss.
Method
Subjects
Eight males and eight females participated in this study (N = 16). Their mean (SE) age was 22.0 (0.41) years, and their mean (SE) lifetime MDMA use was 27.0 (8.4) times. Participants were recruited by advertisements at Maastricht University and were paid upon completion of the study. Before enrollment all subjects were screened by means of a telephone interview to determine whether they qualified for the study. The inclusion criteria were: experience with MDMA, i.e., at least one time in the last year; free from psychotropic medication; good physical health as determined by a medical examination; absence of any major medical, endocrine, and neurological condition; normal weight, i.e., BMI between 18 and 28; valid driving license; and written informed consent. The exclusion criteria were history of drug abuse or addiction; pregnancy or lactation; cardiovascular abnormalities on electrocardiogram; excessive drinking, i.e., more than 20 alcoholic consumptions a week; hypertension, i.e., systolic blood pressure over 170 mmHg or diastolic blood pressure over 100 mmHg; and history of or current psychiatric disorder. If subjects met the inclusion criteria, they received a medical history and a drug questionnaire to get a more precise view on their health and drug use. Finally, participants underwent a medical examination and took part in a training session.
The study was conducted according to the code of ethics on human experimentation established by the Declaration of Helsinki (1964) and amended in Seoul (2008). Approval for the study was obtained from the Medical Ethics Committee of the Academic Hospital of Maastricht and Maastricht University. A permit for obtaining, storing, and administering MDMA was obtained from the Dutch drug enforcement administration.
Study design
The study was conducted according to a double-blind, placebo-controlled, randomized, four-way cross-over design. Treatments consisted of single doses of placebo and 25, 50, and 100 mg MDMA. Treatment orders were balanced over subjects and treatment periods. Placebo and MDMA were administered orally in identically appearing formulations. MDMA was dissolved in 25-mL bitter orange peel syrup, and placebo consisted of only the bitter orange peel syrup. The syrup was mixed with 200 mL juice before it was given to the participants. The wash-out period between treatments was at least 1 week.
Procedure
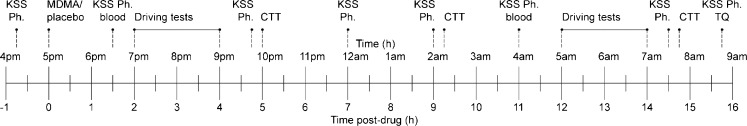
Subjects were asked to refrain from any drugs 1 week before the medical examination until 2 weeks after study completion. Subjects were not allowed to drink alcohol and caffeine or smoke tobacco during a 24-h period prior to testing. Subjects were always tested for alcohol and drugs in breath and urine upon arrival (4:30 p.m.) at the laboratory on test days. In case of a positive result, subjects were sent home and asked to come back on another day. This happened in the case of one subject. After repeated violation this subject was excluded and replaced. At 5:00 p.m. participants received a light, standard dinner, and at 5:15 p.m. MDMA or placebo was administered. Driving performance was assessed in the evening and in the morning after a night of sleep loss and psychomotor performance in the evening, in the middle of the night, and in the morning. Karolinska sleepiness scale was administered throughout the night. The timeline for performance testing, questionnaires, and blood draws is displayed in Fig. 1. An additional blood sample was drawn 1 week after each testing day to monitor renal and liver function. A test day ended at 9:00 a.m. the next morning at which time participants were driven home.
Fig. 1.
Timeline for blood samples, questionnaires, and performance tests relative to drug administration. KSS Karolinska sleepiness scale, Ph physiological measures, CTT critical tracking task, TQ treatment questionnaire
Actual driving tests
The road-tracking test (O'Hanlon 1984) consists of driving in a specially instrumented car with a constant speed of 95 km/h and as straight as possible on the right lane of primary highway during a 1-h test ride. A video camera mounted on the rear end of the car registers its lateral position relative to the road delineation. The images are recorded onto a hard drive in the car with a frequency of 4 Hz and are transformed into a file containing the measures of the lateral position. An offline editing routine is applied for removal of all data segments that reveal signal loss, disturbance, or occurrence of passing maneuvers. The edited dataset is then used to calculate means and variances for lateral position. The primary dependent measure of this test is the standard deviation of lateral position (SDLP; i.e., a measure of weaving). Speed and standard deviation of speed are recorded as secondary control measures. The highway driving test has been calibrated in a manner allowing expression of any sedative drug effect in terms of the blood alcohol concentration (BAC) required to achieve the equivalent level of driving impairment (Louwerens et al. 1987). The alcohol calibration curve demonstrates that drinkers' mean SDLP rises exponentially with BAC. Results from the alcohol calibration study can be used for describing drugs' effects on SDLP in terms of respective BAC equivalencies. The change in SDLP at a BAC of 0.5 mg/ml (i.e., 2.4 cm) has been used as a criterion level to quantify drug effects. Any drug-induced changes in SDLP that exceed this criterion value are defined as clinically relevant impairing drug effect in the present study.
The car-following test (Brookhuis et al. 1994; Ramaekers et al. 1995) consists of two cars driving in tandem on a secondary road. The leading vehicle is operated by a study staff member; the following vehicle is operated by the subject who is accompanied by a driving instructor. The test begins with the two vehicles traveling in tandem at speeds of 70 km/h on a secondary highway. Subjects attempt to drive 15–30 m behind the preceding vehicle and to maintain that headway as it executes a series of deceleration maneuvers. During the test, the speed of the leading car is automatically controlled by a modified cruise-control system. At the beginning it is set to maintain a constant speed of 70 km/h, and by activating a microprocessor, the investigator can start sinusoidal speed changes reaching an amplitude of −10% and returning to the starting level within 50 s. The maneuver is repeated six to ten times. Speed signals collected during speed maneuvers enter a power spectral analysis for yielding phase delay between the vehicle's velocities at the maneuver cycle frequency (0.02 Hz). Phase delay converted to a measure of time to speed adaptation (TSA, in seconds) is the primary measure. Gain and coherence are secondary control measures. Gain is the amplification factor between both speed signals collected from the leading and following vehicle and indicates the magnitude of overshoot in reaction. Coherence is a measure to control for correspondence between both speed signals. Test duration is 25 min.
Critical tracking task
The critical tracking task measures the participant's ability to control a displayed error signal in a 1st-order compensatory tracking task. Error appears as horizontal deviation of the cursor from midpoint on a horizontal, linear scale. Compensatory joystick movements null the error by returning the cursor to the midpoint. The frequency of cursor deviations and therefore its velocity increase as a stochastic, linear function of time. The participant is required to make compensatory movements with a progressively higher frequency until the participant loses control. The frequency at which control loss occurs is λ c (the critical frequency). The reciprocal of this frequency is theoretically the perceptual/motor delay lag for humans operating in a closed-loop system. The participant performs this test in five trials, and the mean λ c is recorded as the final score (Jex et al. 1966).
Karolinska sleepiness scale
The Karolinska sleepiness scale is a subjective rating scale with scores that range from 1, “extremely alert,” to 9, “very sleepy, great effort to keep alert, fighting sleep” (Åkerstedt and Gillberg 1990). Participants are instructed to report their experienced sleepiness during the preceding 10 min. Reyner and Horne (1998) modified the original scale by adding verbal descriptions to intermediate steps, which do not have any descriptions in the original version.
Pharmacokinetic assessment
Blood samples (10 mL) and oral fluid (1–2 mL) samples were collected throughout a testing day/night, i.e., at 1.5 and 11 h post drug. The blood sample was centrifuged immediately, and the resulting serum was frozen at −20°C until analyses for pharmacokinetic assessments. MDMA concentrations and its main metabolite 3,4-methylenedioxyamphetamine (MDA) were determined in the corresponding serum samples using solid phase extraction and gas chromatography with mass spectrometric (GC-MS) detection with a limit of quantification of 16.8 ng/mL. Oral fluid was collected with the Orasure Intercept® device for a quantitative analysis of MDMA concentrations by GC-MS.
Statistical analyses
All statistical analyses were conducted by means of SPSS 16.0 for Mac. Statistical analyses consisted of two steps: (1) assessment for overall treatment effects by means of superiority testing, (2) equivalence or non-inferiority testing of drug effects based on difference scores from placebo (within group) relative to the pre-established alcohol criterion, and (3) determination of concentration-effect relations. Steps 2 and 3 were only conducted in case of treatment effects and only for the primary measures of driving performance.
During step 1 all data entered the general linear model repeated measures ANOVA procedures with MDMA (four levels) and hours of sleep loss (two levels for driving tests, three levels for cognitive test, and eight levels for subjective test) as main within-subject factors. If the sphericity assumption was violated or not applicable, the Greenhouse–Geisser correction was used. In the case of an overall effect of MDMA, separate drug-placebo contrast analyses were conducted for each MDMA dose.
Step 2 assessed whether a pre-established alcohol criterion falls within the 95% confidence interval (CI) of the drug effect. If yes, than the drug effect was considered to be equivalent or bigger than a BAC of 0.5 mg/mL and thus relevant for traffic safety. If the 95% CI was lower than the alcohol criterion value, than a drug effect was considered of no clinical relevance.
In step 3, concentration-effect relations were determined according to the following procedure. Data collected during different doses of a drug were converted to change scores from placebo for analyses of the association between drug concentration and performance. A linear regression analysis was conducted to establish linear relationships between changes (from placebo) in task performance during drug treatment and log-transformed drug concentrations in serum. The total number of data points included in these equations was defined by the number of subjects × maximal number test repetitions × the number of drug doses. Individual drug concentrations in serum prior to performance assessments in each of the drug dose conditions were divided over three mutually exclusive categories covering the full range of drug concentrations. The concentration ranges in serum were 0–50, 50–100, and >100 ng/mL during evening sessions and 0–25, 25–50, and >50 ng/mL during morning sessions. The concentration ranges in oral fluid were 0–250, 250–1,000, and >1,000 ng/mL during evening sessions and 0–100, 100–500, and >500 ng/mL during morning sessions. Corresponding change scores of task performance were then classified either as showing “impairment” or “no impairment” for all individual cases within each of these categories. Impairment was defined as a positive change score from placebo. Binomial tests were applied to measure whether the proportion of observations showing impairment or no impairment significantly differed from the hypothesized proportion. It was hypothesized that in the case of no effect of a drug on task performance, the proportion of observations showing impairment or no impairment would be equal, i.e., 50%.
Results
Dropouts and missing data
Two participants dropped out. One was positive for ∆9-tetrahydrocannabinol in urine. The other indicated that the sleep loss interfered too much with his daily life. Both dropouts were replaced. Data were missing in the car-following test on some occasions. These missing values were replaced by the overall group mean for the respective treatments.
Driving tests prematurely terminated
Table 1 shows the highway driving tests that were prematurely terminated, the drug condition in which the test was terminated, the reasons for termination, the initiator of termination, the measured SDLP, and distance traveled before termination.
Table 1.
Drug conditions of and reasons for dropouts in highway driving test, mean SDLP and distance traveled until drop out
| Subject number | Drug condition (mg MDMA) | Time of testing | Reason/terminated by | Mean SDLP (cm) | Distance traveled (km) |
|---|---|---|---|---|---|
| 3 | 25 | Morning | Sleepiness/instructor | 26.59 | 92 |
| 3 | 100 | Morning | Sleepiness/subject | 22.50 | 64 |
| 4 | 0 | Morning | Sleepiness/instructor | 23.88 | 99 |
| 4 | 50 | Morning | Sleepiness/instructor | 23.30 | 29 |
| 6 | 50 | Morning | Sleepiness/subject | 18.69 | 31 |
| 6 | 100 | Morning | Sleepiness/instructor | 22.25 | 88 |
| 7 | 0 | Morning | Sleepiness/subject | 24.94 | 66 |
| 7 | 100 | Evening | Anxiousness/subject | 20.02 | 61 |
| 7 | 100 | Morning | Sleepiness/instructor | 33.72 | 11 |
| 9 | 0 | Morning | Sleepiness/instructor | 29.53 | 25 |
| 9 | 50 | Morning | Sleepiness/instructor | 28.95 | 32 |
| 9 | 100 | Morning | Sleepiness/instructor | 23.49 | 43 |
| 10 | 0 | Morning | Sleepiness/subject | 22.57 | 48 |
| 10 | 25 | Morning | Sleepiness/subject | 21.23 | 31 |
| 10 | 50 | Morning | Sleepiness/instructor | 17.25 | 32 |
| 10 | 100 | Morning | Sleepiness/instructor | 31.24 | 13 |
| 11 | 25 | Morning | Sleepiness/instructor | 21.83 | 59 |
| 11 | 50 | Evening | Sleepiness/instructor | 28.62 | 63 |
| 11 | 50 | Morning | Sleepiness/instructor | 28.85 | 54 |
| 15 | 0 | Morning | Sleepiness/instructor | 29.43 | 14 |
| 15 | 25 | Morning | Sleepiness/instructor | 26.60 | 31 |
| 15 | 50 | Evening | Sleepiness/instructor | 23.30 | 61 |
| 15 | 50 | Morning | Sleepiness/instructor | 25.76 | 29 |
| 15 | 100 | Morning | Sleepiness/instructor | 22.61 | 9 |
| 16 | 0 | Morning | Sleepiness/instructor | 25.89 | 47 |
| 16 | 25 | Morning | Sleepiness/subject | 19.74 | 65 |
The car-following test was prematurely terminated in two cases (1.6%), once in the placebo condition and once in the 25-mg MDMA condition. Both tests were terminated in the morning and by the instructor because of sleepiness of the subject. In case of a premature stop, available data till time of test termination were used for statistical analysis.
Driving tests
Mean (SE) performances on the primary (SDLP and TSA) and secondary measures of the highway driving and car-following test are shown in Table 2. MDMA did not affect any of the driving measures. Sleep loss significantly affected SDLP (F 1,15 = 40.833, p < 0.001) and SD speed (F 1,15 = 29.905, p < 0.001). SDLP and SD speed were markedly higher in the morning as compared to the evening. The interaction between MDMA and sleep loss only reached significance SD speed (F 3,45 = 3.607, p = 0.020). SD speed was higher in the morning as compared to the evening, particularly after treatment with MDMA 100 mg (p = 0.017). In general, variations in mean SD speed were very small and ranged between 1 and 2.5 km/h.
Table 2.
Mean (SE) of the driving and psychomotor tests and subjective measure for the treatment conditions and measuring times
| Test | Test repetitions | Drug conditions | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | 25 mg MDMA | 50 mg MDMA | 100 mg MDMA | Sleep deprivation | MDMA | MDMA × sleep deprivation | ||
| Road tracking | ||||||||
| SDLP (cm) | 1 | 18.2 (0.7) | 18.4 (0.8) | 18.1 (1.0) | 18.0 (0.6) | <0.001 | NS | NS |
| 2 | 22.8 (0.8) | 22.9 (0.6) | 21.7 (0.9) | 22.4 (1.1) | ||||
| SD speed (km/h) | 1 | 1.70 (0.13) | 1.74 (0.14) | 1.88 (0.17) | 2.14 (0.14) | <0.001 | NS | 0.020 |
| 2 | 2.68 (0.23) | 2.62 (0.19) | 2.66 (0.18) | 2.46 (0.19) | ||||
| Car following | ||||||||
| TSA (s) | 1 | 2.8 (0.2) | 3.0 (0.2) | 3.0 (0.3) | 2.7 (0.2) | NS | NS | NS |
| 2 | 3.3 (0.4) | 3.3 (0.4) | 3.2 (0.3) | 3.1 (0.5 | ||||
| Coherence | 1 | 0.9 (0.01) | 0.9 (0.01) | 0.9 (0.01) | 0.9 (0.01) | NS | NS | NS |
| 2 | 0.9 (0.01) | 0.9 (0.01) | 0.9 (0.01) | 0.9 (0.01) | ||||
| Gain | 1 | 1.1 (0.04) | 1.2 (0.03) | 1.0 (0.03) | 1.1 (0.03) | NS | NS | NS |
| 2 | 1.1 (0.03) | 1.1 (0.03) | 1.1 (0.04) | 1.2 (0.06) | ||||
| Critical tracking task | ||||||||
| λ c (rad/s) | 1 | 3.77 (0.13) | 3.83 (0.16) | 3.70 (0.15) | 3.78 (0.19) | <0.001 | NS | NS |
| 2 | 3.63 (0.16) | 3.56 (0.18) | 3.73 (0.16) | 3.77 (0.16) | ||||
| 3 | 3.09 (0.19) | 2.87 (0.22) | 3.09 (0.19) | 3.36 (0.17) | ||||
| Karolinska sleepiness scale | ||||||||
| Score | 1 | 2.94 (0.21) | 2.94 (0.27) | 2.88 (0.36) | 2.94 (0.25) | <0.001 | NS | NS |
| 2 | 3.13 (0.32) | 2.69 (0.25) | 2.19 (0.21) | 1.81 (0.25) | ||||
| 3 | 3.62 (0.32) | 3.69 (0.29) | 3.62 (0.38) | 3.62 (0.41) | ||||
| 4 | 4.44 (0.35) | 4.62 (0.38) | 4.25 (0.41) | 5.13 (0.40) | ||||
| 5 | 5.31 (0.40) | 5.81 (0.45) | 5.44 (0.35) | 6.12 (0.42) | ||||
| 6 | 5.88 (0.39) | 6.50 (0.45) | 6.19 (0.38) | 5.81 (0.44) | ||||
| 7 | 7.38 (0.30) | 7.69 (0.37) | 7.50 (0.32) | 7.56 (0.33) | ||||
| 8 | 7.38 (0.44) | 7.88 (0.36) | 7.62 (0.40) | 7.62 (0.42) | ||||
Significance is indicated by p value
SDLP standard deviation of lateral position, NS not significant, TSA time to speed adaptation
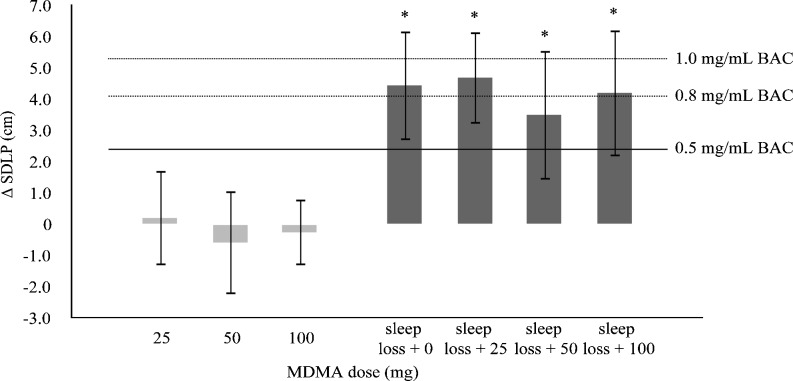
Equivalence testing demonstrated that increments in SDLP in the morning in all treatment conditions were equivalent to a BAC of 0.8 mg/mL when compared to placebo performance in the evening. The upper limits of the 95% CI of changes induced by sleep loss clearly exceeded the pre-established inferiority margin of 2.4 cm. Mean change SDLP and 95% CI in every treatment condition are shown in Fig. 2.
Fig. 2.
Mean (95% CI) SDLP difference from placebo after single doses of MDMA during the road-tracking test in the evening and in the morning after a night of sleep loss. (* = non-inferiority not shown; the upper bound of the 95% CI is above the non-inferiority margin of 2.4 cm)
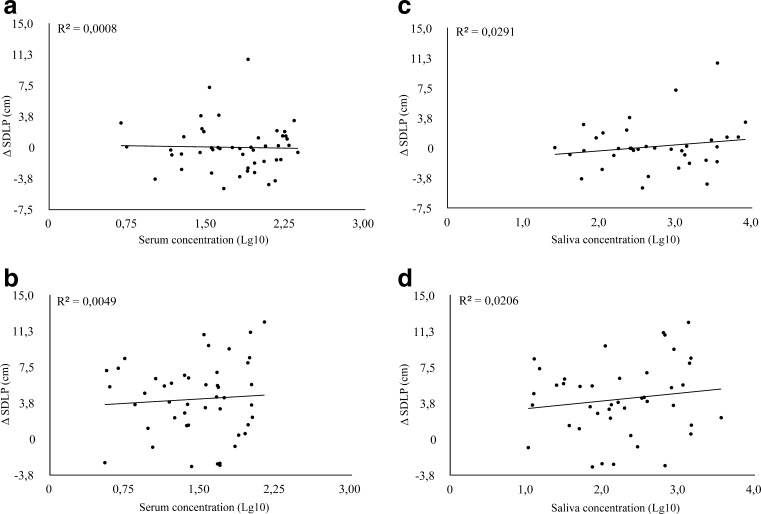
Regression analysis of MDMA levels in serum and SDLP change scores showed a general lack of correlation between the measures. Scatterplots showing the linear relationship between MDMA levels in serum and oral fluid and changes in SDLP are shown in Fig. 3.
Fig. 3.
Correlations between change SDLP and log-converted MDMA concentrations in serum (left panels, a–b) and saliva (right panels, c–d) during driving tests in the evening (upper panels, a and c) and in the morning (lower panels, b and d)
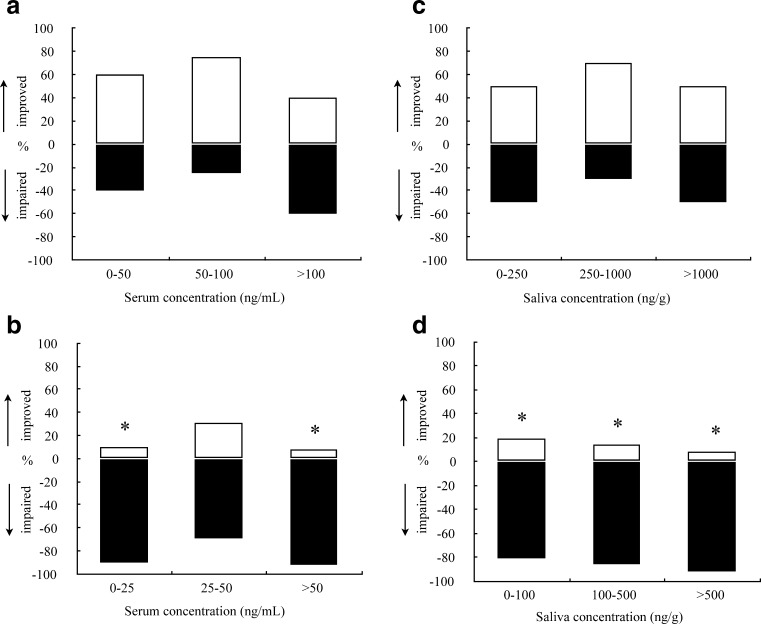
Binomial tests showed a significant increase in the proportion of observations showing impairment in the highway driving test when conducted in the morning for serum MDMA concentrations between 0–25 and >50 ng/mL (p < 0.05). In oral fluid all concentration ranges were associated with impairment (p < 0.05). Impairments were only apparent when compared to placebo SDLP during evening sessions. Distributions of observations showing “impairment” and “no impairment” in the highway driving test as a function of MDMA in serum and oral fluid are shown in Fig. 4.
Fig. 4.
Percentage of observations showing MDMA-induced impairment or MDMA-induced improvement of SDLP in the road-tracking task, as a function of MDMA concentrations in serum (left panels, a–b) and saliva (right panels, c–d) during driving tests in the evening (upper panels, a and c) and in the morning after a night of sleep loss (lower panels, b and d). * p < 0.05
Critical tracking task
Mean λ c (critical frequency) of the critical tracking task was significantly affected by sleep deprivation (F 2,30 = 34.516, p < 0.001). Mean critical frequency generally decreased during the night, indicating diminished psychomotor control. Critical tracking was not affected by MDMA or MDMA × sleep loss.
Karolinska sleepiness scale
The Karolinska sleepiness scale showed a significant effect of sleep loss (F 2.972,44.576 = 108.717, p < 0.001). The Karolinska sleepiness scale also demonstrated a significant dose-related MDMA effect at T max (F 3,45 = 6.006, p = 0.002). Contrasts indicated that the 100-mg as well as 50-mg MDMA conditions differed significantly from placebo (p = 0.009 and p = 0.023, respectively).
Pharmacokinetic assessment
Pharmacokinetic analysis in serum revealed mean (SE) MDMA concentrations of 25.8 (3.3), 63.9 (6.4), and 157.2 (9.5) ng/mL at 1.5 h after administration of a 25-, 50-, and 100-mg dose, respectively. At 11 h post-drug, mean MDMA concentrations were 14.2 (2.7), 34.0 (3.9), and 84.3 (6.7) ng/mL, respectively. MDA concentrations were 3.5 (0.1), 3.9 (0.4), and 5.8 (0.2) ng/mL 1.5 h post drug and 2.9 (0.5), 5.8 (0.2), and 9.7 (0.6) ng/mL after 11 h for 25, 50, and 100 mg MDMA, respectively.
Mean (SE) MDMA concentrations in oral fluid were 208.2 (72.4), 833.0 (270.9), and 3417.8 (694.2) ng/g, and MDA concentrations were 3.2 (1.5), 13.9 (4.2), and 56.4 (13.5) ng/g for 25, 50, and 100 mg MDMA, respectively, at 1.5 h post drug. The concentrations at 11 h after drug intake were respectively 57.0 (16.3), 292.6 (79.0), and 925.3 (224.7) ng/g for MDMA and 3.6 (1.4), 18.9 (4.4), and 56.8 (12.8) ng/g for MDA.
Discussion
The present study demonstrated that sleep deprivation produced severe impairment in actual driving performance as expressed by a significant rise in SDLP and a large number of prematurely terminated driving tests during early morning sessions. In general, MDMA did not affect actual driving performance and did not interact with the effects of sleep deprivation.
On average, SDLP increased with 4.2 cm in the morning after sleep deprivation, relative to SDLP before sleep deprivation. This increment is about 1.5–2 times greater than found in two recent driving under the influence of alcohol studies with blood alcohol concentrations between 0.29 and 0.5 mg/mL (Kuypers et al. 2006; Ramaekers et al. 2000). From a previous alcohol study that was conducted in order to calibrate SDLP for the dose-related effects of alcohol (Louwerens et al. 1987), it can be concluded that a mean increase in SDLP of 4.2 cm is equivalent to a blood alcohol concentration of approximately 0.8 mg/mL. Equivalence testing even demonstrated that the upper limit of the 95% CI associated with the mean change in SDLP after sleep deprivation widely exceeded the criterion level of 1.0 mg/mL BAC. Together, this indicates that sleep deprivation caused severe driving impairment comparable to driving under the influence of high to very high BAC.
These findings were corroborated by results from the critical tracking task. Critical tracking performance significantly decreased over the night, as a function of hours of sleep loss. Similar findings have previously been reported by Dawson and Reid (1997). They measured tracking performance as a function of hours of sleep loss and BAC intoxication. According to their model, tracking performance of subjects after 17–24 h of wakefulness is equivalent to that observed at BAC between 0.5 and 1.0 mg/mL. This again indicates that a night of sleep deprivation causes serious impairment of driving skills. In terms of BAC equivalents, these impairments exceeded those observed at legal BAC limits of 0.5 and 0.8 mg/mL that are currently in place for driving under influence of alcohol in most of Europe and the USA.
The car-following test was the only driving measure that did not show an effect of sleep loss. This diverging effect between the two driving tests, i.e., road tracking and car following, could be explained by a study of Harrison and Horne (2000). They conducted a study in which they showed that dull and monotonous tasks are more sensitive to sleep deprivation than more complex, rule-based tasks, because the latter ones generate more interest and effort to compensate for the effects of sleep deprivation. The highway driving test is monotone, and subjects reported that it was more boring than the car-following test, which is more complex. This is corroborated by the fact that 20% of road-tracking and 2% of car-following tests were prematurely terminated, of which the majority were in the morning, i.e., after sleep deprivation. Therefore, this divergence between the two driving tests are in line with the results of Harrison and Horne (2000), in that the more monotone road-tracking test is more sensitive to sleep deprivation than the car-following test.
In general, MDMA did not produce any significant effects on driving parameters independent of dose or concentration. Stimulating effects of MDMA on actual driving parameters or psychomotor measures that have been demonstrated before (e.g., Dumont et al. 2008; Kuypers et al. 2006, 2007; Lamers et al. 2003; Ramaekers et al. 2006) could not be clearly replicated in the present study. This discrepancy could be due to the fact that the present study also included lower doses of MDMA, i.e., 25 and 50 mg, that may be less likely to produce stimulatory effects than MDMA between 75 and 125 mg that have been tested in the studies mentioned above. However, even in the present study, some measures tended to show stimulatory effects of MDMA. For example, SD speed was significantly affected by an interaction between sleep loss and MDMA. Overall, SD speed increased after a night of sleep loss, but this increase was somewhat less after 100 mg MDMA. Also, binomial tests of SDLP change scores in the evening demonstrated that the majority of observations indicated a reduction in SDLP, particularly at lower MDMA concentrations. However, this effect failed to reach statistical significance.
It is also apparent from the present study that the stimulant effects of MDMA, if any, could not compensate for the impairing effect of sleep loss on driving performance. None of the primary driving measures demonstrated any significant MDMA x sleep loss interaction. The effects of sleep deprivation on driving were highly prominent during MDMA treatments and did not change as a function of dose and concentration. These findings are in line with those in a previous study that assessed the stimulant effects of MDMA on SDLP during alcohol intoxication (Kuypers et al. 2006). That study demonstrated that stimulatory effects of MDMA did not compensate for driving impairment caused by alcohol. Together, these data indicate that the effects of MDMA on psychomotor functions are neutral or mildly stimulating, but that these effects are not sufficient to overcome impairments caused by other factors such as sleep deprivation or alcohol intoxication.
The latter notion is of crucial importance when evaluating driving under the influence (DUI) offenders involving the use of MDMA. Subjects in the present study were significantly impaired during MDMA treatments when deprived of sleep for one night. However, the prime factor causing these impairments was sleep deprivation rather than the use of MDMA itself. When applied in courts, one could rightfully pose the argument that such drivers should not be prosecuted for DUI since MDMA did not contribute to the impairments of the driver. There are a number of counter arguments that should be taken into consideration when evaluating MDMA cases in traffic. First, it has been demonstrated in previous studies that single doses of MDMA increase subjective feelings of arousal and mood (Bosker et al. 2010; Kuypers et al. 2008) and the current study showed a decrease in sleepiness from baseline at T max in the MDMA conditions. Such feelings may affect the subjective judgment of MDMA users on whether or not it is safe to drive home after spending a night at a rave party. During MDMA intoxication, they may not be able to subjectively experience the debilitating effects of sleep loss to the same degree as drug-free drivers, because they feel energetic. As a consequence, they may decide to drive because they feel alert, thereby neglecting the impairing effects of other impairing factors such as sleep deprivation or even alcohol use. Secondly, several studies have also demonstrated that cognitive functions such as working memory, spatial memory, and timing of moving objects (Kuypers and Ramaekers 2005; Lamers et al. 2003; Ramaekers et al. 2009) are impaired during MDMA intoxication. Such cognitive impairments may also affect a person's ability to reflect on his/her fitness to drive as well driving ability in general.
It is concluded from this study that drivers who are under the influence of MDMA and are sleep deprived are unfit to drive. The impairing effects of sleep deprivation during MDMA intoxication occurred independent of MDMA dose and concentration.
Acknowledgments
This work was conducted as part of the DRUID research consortium funded by EU grant TREN-05-FP6TR-S07.61320-518404-DRUID.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Bosker WM, Kuypers KPC, Conen S, Ramaekers JG. Dose-related effects of MDMA on psychomotor function and mood before, during, and after a night of sleep loss. Psychopharmacology (Berl) 2010;209:69–76. doi: 10.1007/s00213-009-1767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookhuis KA, de Waard D, Mulder B. Measuring driving performance by car-following in traffic. Ergonomics. 1994;37:427–434. doi: 10.1080/00140139408963661. [DOI] [Google Scholar]
- Brookhuis KA, de Waard D, Samyn N. Effects of MDMA (ecstasy), and multiple drugs use on (simulated) driving performance and traffic safety. Psychopharmacology (Berl) 2004;173:440–445. doi: 10.1007/s00213-003-1714-5. [DOI] [PubMed] [Google Scholar]
- Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Wezenberg E, Valkenberg MM, de Jong CA, Buitelaar JK, van Gerven JM, Verkes RJ. Acute neuropsychological effects of MDMA and ethanol (co-)administration in healthy volunteers. Psychopharmacology (Berl) 2008;197:465–474. doi: 10.1007/s00213-007-1056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA . EMCDDA insights series no. 8: drug use, impaired driving and traffic accidents. Luxembourg: Office for Official Publications of the European Communities; 2008. [Google Scholar]
- Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–249. doi: 10.1037/1076-898X.6.3.236. [DOI] [PubMed] [Google Scholar]
- Jex HR, McDonnell JD, Phatak AV (1966) A “critical” tracking task for man-machine research related to the operator’s effective delay time. I. Theory and experiments with a first-order divergent controlled element. NASA CR-616. NASA Contract Rep NASA CR: 1–105 [PubMed]
- Kalechstein AD, De La Garza R, 2nd, Mahoney JJ, 3rd, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology. 2007;189:531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Ramaekers JG. Transient memory impairment after acute dose of 75 mg 3.4-methylene-dioxymethamphetamine. J Psychopharmacol. 2005;19:633–639. doi: 10.1177/0269881105056670. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Ramaekers JG. Acute dose of MDMA (75 mg) impairs spatial memory for location but leaves contextual processing of visuospatial information unaffected. Psychopharmacology (Berl) 2007;189:557–563. doi: 10.1007/s00213-006-0321-7. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Samyn N, Ramaekers JG. MDMA and alcohol effects, combined and alone, on objective and subjective measures of actual driving performance and psychomotor function. Psychopharmacology (Berl) 2006;187:467–475. doi: 10.1007/s00213-006-0434-z. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Wingen M, Samyn N, Limbert N, Ramaekers JG. Acute effects of nocturnal doses of MDMA on measures of impulsivity and psychomotor performance throughout the night. Psychopharmacology (Berl) 2007;192:111–119. doi: 10.1007/s00213-006-0679-6. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Wingen M, Ramaekers JG. Memory and mood during the night and in the morning after repeated evening doses of MDMA. J Psychopharmacol. 2008;22:895–903. doi: 10.1177/02698811080220081401. [DOI] [PubMed] [Google Scholar]
- Lacey JH, Kelley-Baker T, Furr-Holden D, Voas RB, Romano E, Ramirez A, Brainard K, Moore C, Torres P, Berning A (2009) 2007 national roadside survey of alcohol and drug use by drivers: drug results (DOT HS 811 249). US Department of Transportation, National Highway Traffic Safety Administration, Washington
- Lamers CT, Ramaekers JG, Muntjewerff ND, Sikkema KL, Samyn N, Read NL, Brookhuis KA, Riedel WJ. Dissociable effects of a single dose of ecstasy (MDMA) on psychomotor skills and attentional performance. J Psychopharmacol. 2003;17:379–387. doi: 10.1177/0269881103174015. [DOI] [PubMed] [Google Scholar]
- Louwerens J, Gloerich A, de Vries G, Brookhuis K, O’Hanlon J. The relationship between drivers’ blood alcohol concentration (BAC) and actual driving performance during high speed travel. Alcohol Drugs Traffic Saf. 1987;86:183–186. [Google Scholar]
- Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology (Berl) 2000;152:230–248. doi: 10.1007/s002130000545. [DOI] [PubMed] [Google Scholar]
- O’Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br J Clin Pharmacol. 1984;18(Suppl 1):121S–129S. doi: 10.1111/j.1365-2125.1984.tb02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaniemi KK, Lintonen TP, Impinen AO, Lillsunde PM, Ostamo AI. Trends in driving under the influence of drugs: a register-based study of DUID suspects during 1977–2007. Accid Anal Prev. 2009;41:191–196. doi: 10.1016/j.aap.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA in humans: factors which affect the neuropsychobiological profiles of recreational ecstasy users, the integrative role of bioenergetic stress. J Psychopharmacol. 2006;20:147–163. doi: 10.1177/0269881106063268. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Muntjewerff ND, O’Hanlon JF. A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. Br J Clin Pharmacol. 1995;39:397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Robbe HW, O’Hanlon JF. Marijuana, alcohol and actual driving performance. Hum Psychopharmacol. 2000;15:551–558. doi: 10.1002/1099-1077(200010)15:7<551::AID-HUP236>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KPC, Samyn N. Stimulant effects of 3,4-methylenedioxymethamphetamine (MDMA) 75 mg and methylphenidate 20 mg on actual driving during intoxication and withdrawal. Addiction. 2006;101:1614–1621. doi: 10.1111/j.1360-0443.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KP, Wingen M, Heinecke A, Formisano E. Involvement of inferior parietal lobules in prospective memory impairment during acute MDMA (ecstasy) intoxication: an event-related fMRI study. Neuropsychopharmacology. 2009;34:1641–1648. doi: 10.1038/npp.2008.219. [DOI] [PubMed] [Google Scholar]
- Reyner LA, Horne JA. Falling asleep whilst driving: are drivers aware of prior sleepiness? Int J Legal Med. 1998;111:120–123. doi: 10.1007/s004140050131. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2009) Results from the 2008 National Survey on Drug Use and Health: national findings. Office of Applied Studies, NSDUH series H-36, HHS publication no. SMA 09–4434). Office of Applied Studies, NSDUH series H-36, HHS publication no. SMA 09–4434), Rockville, MD
- Walsh JM, de Gier JJ, Christopherson AS, Verstraete AG. Drugs and driving. Traffic Inj Prev. 2004;5:241–253. doi: 10.1080/15389580490465292. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z, Jovanovski D. The neuropsychology of ecstasy (MDMA) use: a quantitative review. Hum Psychopharmacol. 2007;22:427–435. doi: 10.1002/hup.873. [DOI] [PubMed] [Google Scholar]