Abstract
Adolescence is a critical age for addiction formation as a large percentage of pathological drug-seeking behaviors manifest during this time. The extent to which neurotoxic effects of drugs of abuse influence subsequent drug seeking behaviors and impulsivity is an understudied area of research. Methamphetamine (METH) is a widely abused drug that produces locomotor responses ranging from behavioral sensitization to tolerance, both of which are behaviors that may relate to risk of abuse. Here we investigated the effects of age, genotype, METH dose, including a neurotoxic dose, and METH metabolism on open-field activity (OFA) to gain insight into the complex disease of drug abuse. C57Bl/6 (B6), DBA/2 (D2), and 129S6SvEv/Tac (129) mouse strains were administered saline or either a high dose (4 × 5 mg/kg in 2h intervals for 2 days) or low dose (2 × 1 mg/kg in 24h intervals) METH pretreatment during adolescence (post natal day (PND) 40) or early adulthood (PND 80) followed by behavioral testing with a METH (1 mg/kg) or saline challenge 40 days later. Striatal concentrations of METH and AMPH were also determined. Significant findings include: 1) METH pretreated adolescent B6 mice displayed significant sensitization for horizontal locomotion due to high dose METH pretreatment; 2) METH pretreated B6 adults showed significant tolerance for the vertical activity measure caused by low dose METH pretreatment; 3) METH pretreated adult D2 mice exhibited significant sensitization for vertical activity induced by low dose METH pretreatment, and 4) 129 mice metabolized METH significantly faster than the B6 and D2 mice, but METH pretreatment did not alter metabolism. No significant behavioral responses to either METH pretreatment dose were observed for the D2 adolescent studies or either 129 age group. Our results highlight the importance of the interactions of age, strain and METH dose on locomotor behavioral outcomes.
Keywords: Methamphetamine, B6, D2, 129, Locomotor activity, Methamphetamine metabolism, Age
1.1 INTRODUCTION
Methamphetamine (METH) is a common drug of abuse that poses a serious world-wide health problem (Davidson et al., 2001; Mirecki et al., 2004). METH use has increased drastically in the last decade within the United States as well as other developed countries. Psychosis of METH users is typically characterized by symptoms of hallucinations and delusions, as well as mood and anxiety disorders. Users also exhibit symptoms of social withdrawal, stereotypy and irrationally hostile behavior (Darke et al., 2008; Harris and Batki, 2000).
There is significant evidence linking initiation of drug use during adolescence to the formation of a drug addiction in adulthood (Chambers et al., 2003; Crews et al., 2007; Frost and Cadet, 2000; Koob and Le Moal, 2001.) The risk of illicit drug experimentation can be greatly increased by the impulsive decision-making of adolescence (Kalivas and Volkow, 2005). Self-reporting from adults with substance use disorders placed the median age of illicit drug use initiation at 16 years of age, with rare initiation after 20 years (Anthony and Helzer, 1990). Two reasons why adolescents may be more susceptible to adult drug dependence are that: 1) they are significantly less sensitive to withdrawal effects of METH when compared to adults, and 2) adolescents run the risk of developing greater drug sensitization if experimentation begins during early- to mid-adolescence (Schramm-Sapyta et al, 2009). Twin studies have documented a progression for drug abuse which begins with drug use being strongly influenced by familial environmental factors during adolescence and a shift to genetic factors driving drug use during adulthood (Kendler et al., 2008).
It is becoming apparent that chronic exposure to the neurotoxic effects of drugs of abuse can lead to addiction-related behavioral changes. Alterations in dopamine (DA) pathways perpetuate drug use, ultimately directing users to display greater impulsivity and to also place a greater reward value on drugs than non-users (Crews and Boettiger, 2009; de Wit, 2009; Jentsch and Taylor, 1999a). These behavioral changes have been observed in both human abusers and animal models of abuse (Hoffman et al, 2006; Jentsch and Taylor, 1999b). While METH is a known neurotoxicant to both humans and rodents, the precise mechanism of its neurotoxicity within the DA system has yet to be elucidated (Davidson et al., 2001; Deng et al., 2001; Imam et al., 2001a, 2001b; Jayanthi, et al., 2004). The mechanism of action for METH is complex and causes decreases in the number of high-affinity DA uptake sites (Cadet and Brannock, 1998; Fumagalli et al., 1998; Wagner et al., 1980), as well as decreases in activity of tyrosine hydroxylase (TH), the rate limiting step in DA synthesis (Sulzer et al., 2005). METH-induced neurotoxicity can also result from the oxidation of extracellular DA to dopa-quinones and the consequent formation of free radicals (Acikgoz et al., 2000; Deng et al., 2002, Pubill et al., 2005; Yamamoto et al., 1998). These free radicals primarily target the striatal dopaminergic neurons to produce DNA damage, lipid peroxidation and apoptotic as well as necrotic cell death (Davidson et al., 2001; Hirata et al., 1996; Imam et al., 2001a; Jayanthi et al., 2004).
A variety of METH doses and dosing schedules have been used in previous rodent behavioral and toxicity studies. A continuum of effects ranging from neurotoxicity to acute hyperactivity and behavioral sensitization have been observed. These regimens have spanned from single large doses of 40 mg/kg METH (Imam et al., 2001a) and 4 × 5 mg/kg METH every 2 hours (Zhu et al., 2006a), to acute low doses ranging between 1 and 5 mg/kg METH (Phillips et al., 2008. The use of a 7.5 mg/kg METH challenge 8 days after a single session of 4 × 10 mg/kg METH every 2 hours produced a significant decrease in spontaneous activity, an increase in stereotypic behavior, and a significant decline in the concentration of DA in the caudate nucleus and the nucleus accumbens of rats (Wallace et al., 1999). Large single doses of 30 mg/kg METH and greater, or repeated doses of 4 × 10 mg/kg, can produce neurotoxic effects such as activation of caspase cascades (Zhu et al., 2005 & 2006b).
Physiological and behavioral responses to METH administration are influenced by genotype and age variables. For example, drug naïve ICR, dd, BALB/c, C57Bl/6 (B6), C3H/H3 and DBA/2 (D2) strains exhibited a wide range of activity when administered 1, 2, 3 or 4 mg/kg METH. The B6 and BALB/c mice showed markedly lower activity over 3 hours than the other four strains for all four METH doses (Kuribara and Tadokoro, 1989). This response profile was again observed during the repeated dosing regimen of 2 mg/kg, administered 5 times at 3 day intervals. Quantitative trait locus mapping and targeted gene mutation studies have begun to identify and characterize genes that confer genetic variation to METH responses in the mouse (Bryant et al., 2009; Grisel et al., 1997; Phillips et al., 2008; Uhl et al., 2008). Effects of age were observed in a study in which rats aged 1, 6 and 12 months were exposed to four different METH doses ranging from 5 to 40 mg/kg. The 40 mg/kg dose produced no deaths in the 1 and 6 month old animals, but 100% mortality was witnessed for the 12 month animals (Imam et al., 2001a). Post-natal day (PND) 90 rats exhibited decreased striatal DA in response to either 4 × 10 mg/kg or 4 × 5 mg/kg METH while PND 40 animals did not (Truong et al., 2005). B6/J and B6/N mice, aged 1 month to 23 months were given 4 × 10, 5, or 2.5 mg/kg METH in 2 hour intervals, and glial fibrillary acidic protein (GFAP), DA and DA metabolites were assessed 72 hours after the final injection. While all four of the METH doses were able to increase the body temperature of all age groups, only the 1 month animals displayed little to no elevation in striatal GFAP levels or depletion in DA and its metabolites. All other age groups demonstrated significant neurotoxicity (Miller et al., 2000).
Differences in the behavioral responses to varying doses of METH and its major metabolite amphetamine (AMPH) have been observed. A comparison of the behavioral effects of the two drugs in rats demonstrated similar open-field locomotor activation in response to 1 and 4 mg/kg METH or AMPH doses, but not a 2 mg/kg dose. Within 1 hour, the 2 mg/kg AMPH produced significantly greater total activity counts than the 2 mg/kg METH dose (Shoblock et al., 2003). Cytochrome P450 enzymes are the primary means of removal of METH and AMPH (Lin et al., 1997). It was not reported by Shoblock et al. (2003) if enzyme kinetics influenced their results, leading to the question of whether dose, age and strain variables can influence changes in METH metabolism in a behavioral mouse model.
We investigated the effects of age and mouse genotype on the long-term consequences of a low dose and a high dose of METH on open-field activity (OFA). Our initial interest was in determining if a potentially neurotoxic dosing regimen, which could be considered a binge episode, would have long-lasting effects on behavior. Upon confirmation of significant behavioral findings from this study follow-up experiments were performed utilizing a low non-toxic dosing regimen. We also investigated whether METH pretreatment would have long-lasting effects on METH metabolism. The results demonstrated the importance of dose, age, and genotype on OFA and METH metabolism.
1.2 METHODS
1.2.1 Animals
Male B6, D2, 129S6SvEv/Tac (129), C3H/H3 (C3H), and A/J (A) mice were obtained either from the Institute for Behavioral Genetics (Boulder, CO), Jackson Laboratories (Bar Harbor, Maine), or Taconic Farms (Cranbury, New Jersey). Equal numbers of each strain were obtained from the vendors and were equally distributed among the experimental groups. Animals were acclimated to the animal facility at the University of Colorado Health Sciences Center (Denver, CO) for 10 to 12 days before the dosing regimen was initiated. Animals were maintained in an environment of constant temperature and humidity (22° C, 40% humidity) and on a 12h L: 12h D cycle.
1.2.2 Methamphetamine Administration
Methamphetamine hydrochloride (METH) was obtained from Sigma Aldrich (St. Louis, MO). METH was dissolved in a 0.9% saline solution at concentrations of 0.5 mg/ml or 0.1 mg/ml and administered at a 0.01 ml/g volume for the two doses. Pretreatment was administered on PND 40-41 (adolescent) or PND 80-81 (adult). The high dose pretreatment consisted of 2 days of 4 × 5 mg/kg METH administered in 2 hour intervals and the low dose pretreatment consisted of 2 single injections of 1.0 mg/kg METH spaced 24 hours apart. Saline was administered as a control for both the high and low dose studies. Animals were dosed between 0800 and 1730 hours. The behavioral challenge dose, given 40 days following initial dosing, was a single injection of either 1.0 mg/kg METH or saline. Treatment groups are defined in Table 1.
Table 1.
Treatment groups.
| Treatment | Pretreatment | Challenge |
|---|---|---|
| SS | Saline | Saline |
| MS | METH | Saline |
| SM | Saline | METH |
| MM | METH | METH |
1.2.3 Open-Field Behavior
OFA testing was conducted 40 days after the first day of pretreatment on PND 80 and PND 120 for the adolescent and adult groups, respectively. Behavioral testing was conducted between 0800 and 1300 hours. Animals naïve to the behavioral test were transferred to the testing room where they were weighed 45 minutes before test initiation. Subjects were injected with their respective challenge dose (1 mg/kg METH or saline) and immediately placed inside an automated open-field testing apparatus (Omnitech, Columbus, OH) consisting of a clear Plexiglas square box (40 × 40 × 56 cm) crisscrossed by eight photobeam cells on each side. Activity was recorded for 1 hour in 6 minute bins. Subjects were returned to their home cage upon test completion. The Plexiglas boxes were cleaned with a 10% bleach solution between subjects. Two behaviors were analyzed: 1) total distance – cm traveled in the horizontal plane, and 2) vertical activity – total number of interruptions of vertical sensors.
1.2.4 Methamphetamine Metabolism
Adolescent and adult B6, D2 and 129 mice were given the high dose METH or saline pretreatment as described in section 1.2.2. Forty days later the animals were administered a 1 mg/kg METH challenge. METH concentrations are thought to peak in rodents at 10 minutes and AMPH concentrations at 20 minutes (Segal and Kuczenski, 2006). Striatal tissues were collected 10 minutes and 60 minutes following challenge administration and immediately frozen in liquid nitrogen. Tissues were homogenized in a 50% acetonitrile / 50% water solution at a 1:17 w/v ratio. Homogenates were centrifuged for 10 minutes at 13,000 rpm at room temperature. Supernatant was removed for LC/MS analysis conducted by a contract laboratory (Forensic Laboratories Inc., Aurora, CO). Briefly, a LEAP HTS PAL autosampler was connected to a Varian HPLC system, powered by two Prostar 210 pumps. A Varian Monochrom column separated the analytes from the bulk components via a gradient of mobile phases, and detection of METH and AMPH was conducted using a Varian 320 Mass Spectrometer and electrospray ionization.
1.2.5 Data Analysis
For the behavioral study each of the age and treatment-dose combinations were considered to be independent experiments and were analyzed accordingly; i.e., by 3-way ANOVA to test for effects of strain, treatment, and time. In all cases significant main effects and numerous complex interactions were observed (p<0.01). Our primary interest was in whether the pretreatment affected a subsequent METH challenge-induced behavioral response within each strain. Therefore in order to simplify interpretations a 2-way ANOVA (treatment × time) was conducted within each strain. The results of the analyses are shown in the figure legends. The ANOVA was followed by Tukey’s post hoc where appropriate. Strain effects were also examined by 2-way ANOVA (strain × time) within each treatment group followed by Tukey’s post hoc where appropriate. All significant treatment group comparisons exhibited p<0.05.
The METH metabolism data was assessed with a 3-way ANOVA (strain, age, and treatment). Significant main effects were observed only for strain (p<0.05). An ANOVA was conducted to further investigate this finding; Tukey’s post-hoc analyses were conducted where appropriate. Significant strain comparisons of METH turnover demonstrated p<0.05. Analyses were performed using SPSS for Windows (v. 17.0).
1.3 RESULTS
1.3.1 Preliminary experiments
As there are many dosing regimens available in the literature we needed to select one that would be best suited for our study conditions (Imam et al., 2001a, Phillips et al., 2008, Zhu et al., 2006a). A pilot strain survey was conducted using a single 2 mg/kg METH dose to assess whether different OFA behavioral profiles would be elicited across 5 inbred strains (results not shown). The 2 mg/kg dose was initially selected for the strain survey as it was not neurotoxic and would also be our challenge dose, but was ultimately reduced to 1 mg/kg as this dose had been used previously (Itzhak and Ali, 2002). Male C3H, A, D2, B6 and 129 genotypes, age PND 90, were studied for 30 minutes following administration of the 2 mg/kg METH dose. The B6 genotype was the most activated, followed by the D2 exhibiting moderate locomotor activation, then C3H and A genotypes eliciting similar low activation profiles, and finally the 129 strain, which showed little horizontal locomotor activation in response to the drug. The B6, D2 and 129 strains were selected for the study as they displayed the three most diverse locomotor responses to the 2 mg/kg METH administration. Our results corresponded well with previously reported results (Kuribara and Tadokoro, 1989).
Additional pilot studies were conducted to determine the optimal high dose paradigm. Initially, two 40 mg/kg METH doses were administered 24 hours apart. The regimen proved lethal to greater than 50% of the D2 genotype and 25% of the B6 animals. The doses were scaled down to a single 30 mg/kg METH dose for two days. Again this regimen proved lethal to 50% of the D2 mice; however, all of the B6 animals tolerated this lower dose. Instead of a single 20 mg/kg dosing schedule, it was decided to divide the single injections of 20 mg/kg METH into 4 doses of 5 mg/kg METH administered 2 hours apart (Sonsalla and Heikkila, 1988), repeating this procedure again on the second day of dosing. This dose did not prove to be lethal in either strain and was therefore selected for the study; the selected dosing regimen was also not lethal to the 129 strain.
1.3.2 Experiment 1 – High dose METH pretreatment in adolescent mice
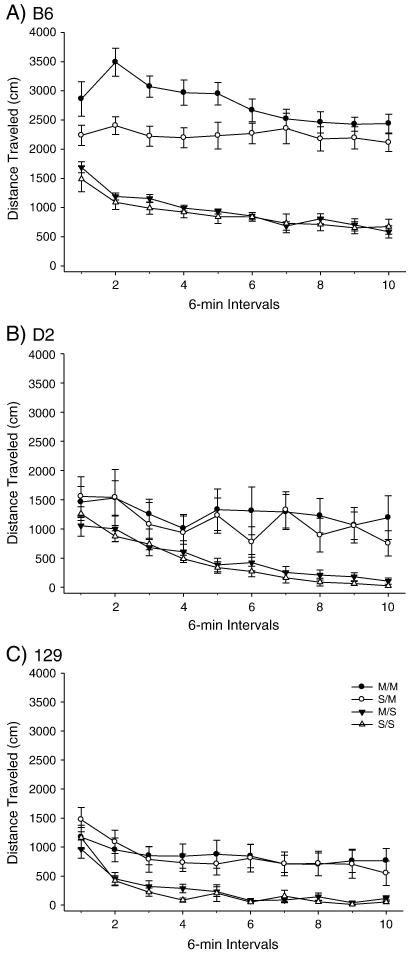
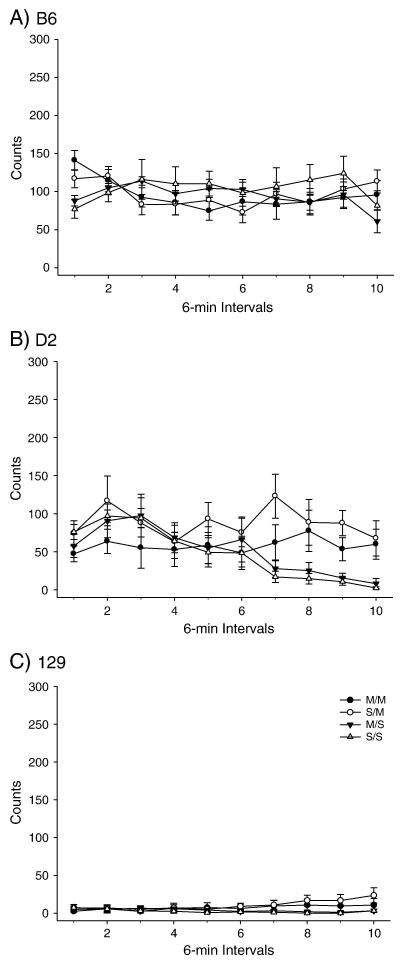
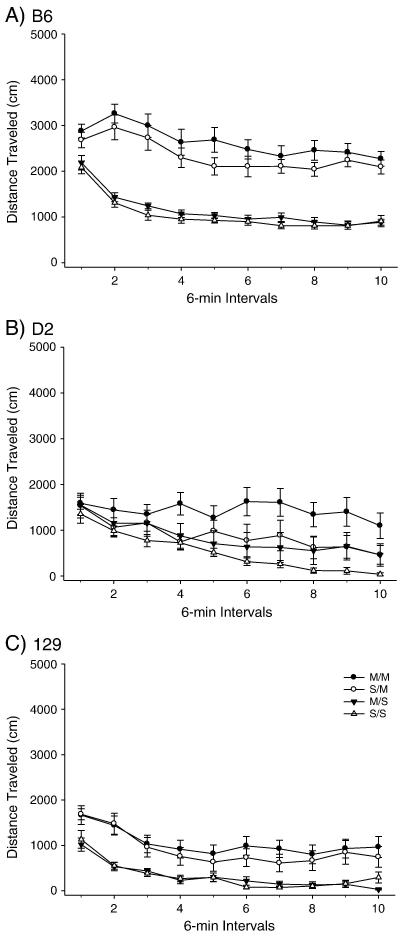
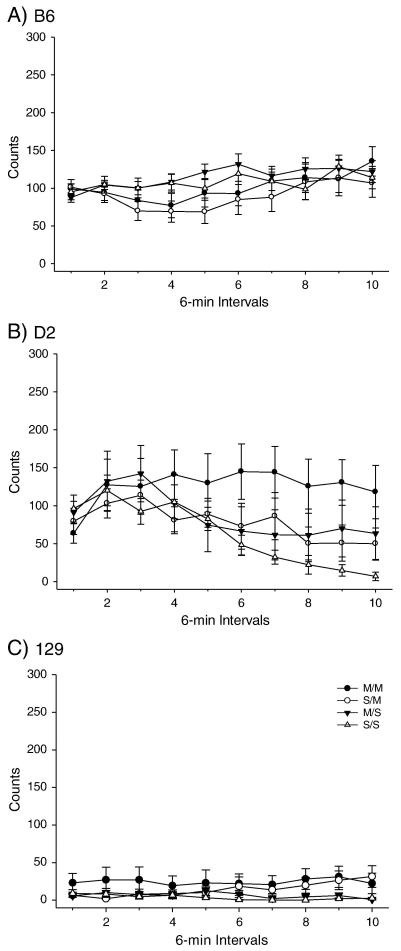
PND 40 B6, D2 and 129 mice were administered the high dose METH pretreatment (Figures 1-2) and OFA was assessed on PND 80. A two-way ANOVA produced a significant effect of treatment among the strains for total distance traveled. Post-hoc tests indicated that the total distance SS/MS comparison was not significant for any strain, while the SS/SM and MS/SM comparisons produced significant differences in total distance traveled for both the B6 and 129 strains but not the D2 strain. Importantly, the B6 strain exhibited an effect of METH pretreatment for the SM/MM comparison, while the D2 and 129 strains did not (Figure 1). No effect was observed for the high dose pretreatment or acute challenge dose for vertical activity in any strain (Figure 2).
Figure 1.
Long-term behavioral effects of high dose METH pretreatment during adolescence on adult total distance traveled in B6, 129 and D2 strains. A) B6 within-subjects effect of time F(9,288) = 15.931 p<0.001; time × treatment interaction F(27,288)=2.920, p<0.001. Between subjects effect of treatment F(3,32) = 63.179, p< 0.001. (SS: n= 9; MS: n=9; SM: n=9; MM: n=9). B) D2 within-subject effect of time F(9,243) = 11.583, p<0.001. Between-subjects treatment effect of treatment F(3,27) = 4.263, p<0.05. (SS: n= 7; MS: n=7; SM: n=8; MM: n=9). C) 129 within-subjects effect of time F(9,306) = 22.319, p<0.001. Between-subjects effect of treatment F(3,34) = 6.597, p<0.005. (SS: n= 9; MS: n=9; SM: n=8; MM: n=12).
Figure 2.
Long-term behavioral effects of high dose METH pretreatment during adolescence on adult vertical activity. A) B6 within-subjects time × treatment interaction F(27,288)=2.505, p<0.001. (SS: n= 9; MS: n=9; SM: n=9; MM: n=9). B) D2 within-subjects effect of time F(9,243) = 6.020, p<0.001; within-subjects time × treatment interaction F(27, 243) = 2.505, p<0.001. (SS: n= 7; MS: n=7; SM: n=8; MM: n=9). C) 129 within-subjects effect of time F(9,306) = 2.054, p<0.05; within-subjects time × treatment interaction F(27,306) = 2.352, p<0.001 (SS: n= 9; MS: n=9; SM: n=8; MM: n=12).
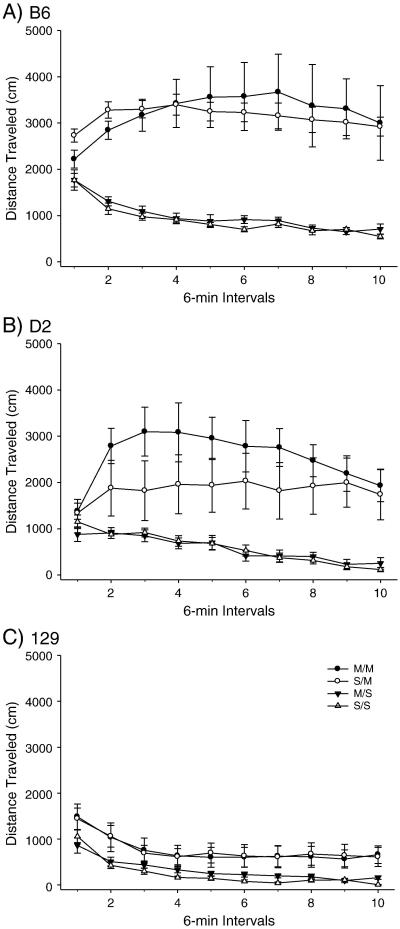
1.3.3. Experiment 2 – High dose METH pretreatment in adult mice
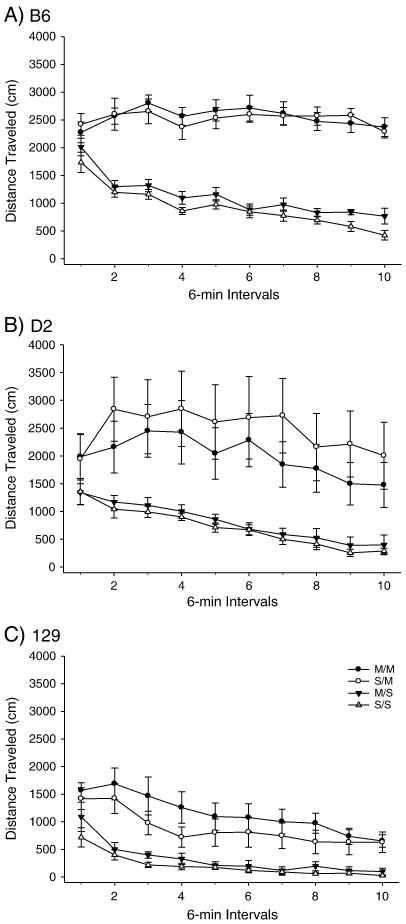
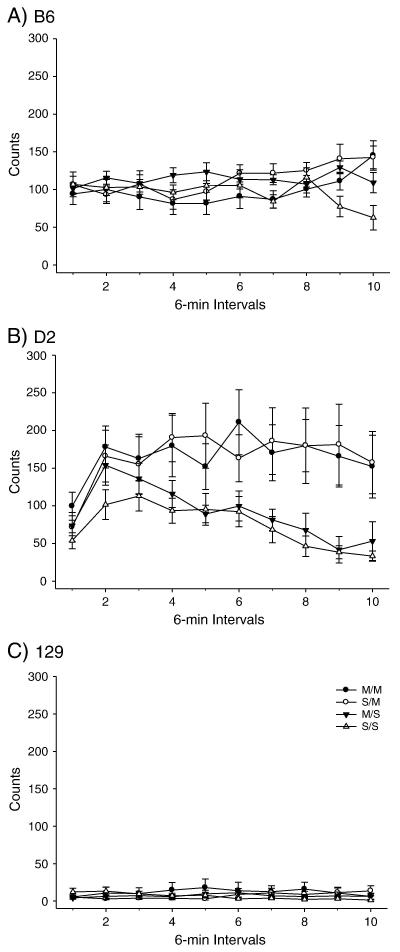
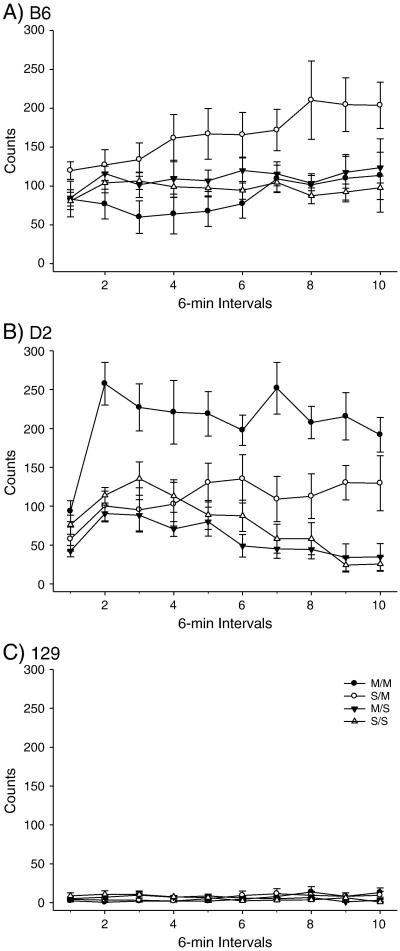
PND 80 B6, D2 and 129 mice were administered the high dose METH pretreatment or saline; OFA was conducted 40 days later (Figure 3-4). Two-way ANOVA yielded a significant effect of treatment on total distance. Post-hoc assessment of total distance traveled yielded significant SS/SM and SS/MM treatment group comparisons for all three strains. The B6 strain demonstrated significant MS/SM and MS/MM treatment comparisons for total distance; the D2 strain also produced a significant MS/SM treatment comparison. The 129 total distance comparison yielded a significant MS/MM treatment comparison (Figure 3). There were no significant effects of treatment on vertical activity in any of the three strains. However, there were trends towards significant differences in vertical activity for the D2 SM and MM treatment groups, compared to the SS treatment group (p<0.068 and p<0.056 respectively) (Figure 4).
Figure 3.
Long-term behavioral effects of high dose METH pretreatment on adult total distance traveled in B6, 129 and D2 strains. A) B6 within-subjects effect of time F(9,288)=12.220, p<0.001; time × treatment interaction F(27,288)=4.791, p<0.001. Between subjects effect of treatment F(3,32) = 58.69, p< 0.001. (SS: n= 8; MS: n=10; SM: n=10; MM: n=8). B) D2 within-subjects effect of time F(9,306) = 10.051, p<0.001. Between-subjects effect of treatment F(3,34) = 5.803, p<0.005. (SS: n= 9; MS: n=10; SM: n=9; MM: n=10). C) 129 within-subjects effect of time F(9,288) = 30.307, p<0.001. Between-subjects effect of treatment F(3,32) = 8.750, p<0.001. (SS: n= 8; MS: n=10; SM: n=9; MM: n=9).
Figure 4.
Long-term behavioral effects of high dose METH pretreatment on adult vertical activity. A) B6 within-subjects effect of time × treatment interaction F(27,288)=3.110, p<0.001. (SS: n= 8; MS: n=10; SM: n=10; MM: n=8). B) D2 within-subjects effect of time F(9,306) = 6.692, p<0.001; within-subjects time × treatment interaction F(27,306) = 1.813, p<0.01. Between-subjects effect of treatment F(3,34) = 3.847, p<0.05. (SS: n= 9; MS: n=10; SM: n=9; MM: n=10). C) 129 within-subjects time × treatment interaction (27,288) = 2.144, p<0.001. (SS: n= 8; MS: n=10; SM: n=9; MM: n=9).
1.3.4 Experiment 3 – Low dose METH pretreatment in adolescent mice
Low dose METH or saline was administered to PND 40 B6, D2 and 129 animals; OFA was recorded 40 days after pretreatment (Figures 5-6). Two-way ANOVA revealed a significant effect of treatment on total distance. Post-hoc analyses showed that all three strains exhibited significant SS/MM differences for total distance traveled. B6 and 129 strains also yielded significant differences in total distance traveled for the following comparisons: SS/SM, MS/SM and MS/MM (Figure 5). No significant treatment effects were found for vertical activity in any strain (Figure 6).
Figure 5.
Long-term behavioral effects of low dose METH pretreatment during adolescence on adult total distance traveled in B6, D2 and 129 strains. A) B6 within-subjects effect of time F(9,378) = 47.291, p<0.001; within-subjects time × treatment interaction F(27,378)=3.818, p<.001. Between-subjects effect of treatment F(3,42) = 32.202, p<0.001. (SS: n= 11; MS: n=11; SM: n=11; MM: n=13). B) D2 within-subjects effect of time F(9,288) = 17.796, p<0.001; within-subjects time × treatment interaction F(27,288) = 2.039, p<0.00. Between-subjects effect of treatment F(3,32) = 3.044, p<0.05. (SS: n= 8; MS: n=11; SM: n=7; MM: n=10). C) 129 within-subjects effect of time F(9,306) = 36.536, p<0.001. Between-subjects effect of treatment F(3,34) = 8.012, p<0.001. (SS: n= 9; MS: n=9; SM: n=10; MM: n=10).
Figure 6.
Long-term behavioral effects of low dose METH pretreatment during adolescence on adult vertical activity. A) B6 within-subjects effect of time F(9,378) = 6.622, p<0.001; within-subjects effect of time × treatment interaction F(27,378) = 1.581, p<0.05. (SS: n= 11; MS: n=11; SM: n=11; MM: n=13). B) D2 within-subjects effect of time F(9,288) = 8.337, p<0.001; within-subjects time × treatment interaction F(27,288) = 2.703, p<0.001. (SS: n= 8; MS: n=11; SM: n=7; MM: n=10). C) 129 within-subjects time × treatment interaction F(27,306) = 2.295, p<0.001. (SS: n= 9; MS: n=9; SM: n=10; MM: n=10).
1.3.5 Experiment 4 – Low dose METH pretreatment in adult mice
PND 80 B6, D2 and 129 mice were pretreated with either the low dose METH or saline; OFA was recorded on PND 120. Two-way ANOVA demonstrated significant treatment effects for total distance and vertical activity (Figures 7-8). Post-hoc analyses yielded significant SS/MM and MS/MM treatment comparisons for total distance traveled for the B6 and D2 strains. The D2 animals did not display any other significant treatment responses, while the B6 animals exhibited significant responses for the SS/SM and MS/SM comparisons. The treatments did not produce any significant changes in the 129 total distance response (Figure 7). Post-hoc analysis of the vertical activity response produced surprising results. The B6 and the D2 strains demonstrated significant opposing pretreatment effects for the SM/MM comparison; the B6 animals demonstrated significant behavioral tolerance to the METH pretreatment, and the D2 mice displayed significant sensitization. In addition, the D2 animals also demonstrated significant SS/MM and MS/MM treatment comparisons and the B6 a significant SS/SM comparison. The 129 mice showed no significant changes for vertical activity (Figure 8).
Figure 7.
Long-term behavioral effects of low dose METH pretreatment on total distance traveled in adult B6, 129 and D2 strains. A) B6 within-subjects effect of time F(9,216) = 1.979, p<0.05; within-subjects time × treatment interaction F(27,216) = 4.029, p<0.001. Between-subjects effect of treatment F(3,24) = 31.521, p<0.001. (SS: n= 8; MS: n=5; SM: n=10; MM: n=5). B) D2 within-subjects effect of time F(9,306) = 8.530, p<0.001; within-subjects time × treatment interaction F(27,306) = 4.255, p<0.001. Between-subjects effect of treatment F(3,34) = 6.799, p<0.005. (SS: n= 10; MS: n=9; SM: n=11; MM: n=8). C) 129 within-subjects effect of time F(9,396) = 32.259, p<0.001. Between-subjects effect of treatment F(3,44) = 2.956, p<0.05. (SS: n= 10; MS: n=12; SM: n=13; MM: n=13).
Figure 8.
Long-term behavioral effects of low dose METH pretreatment on vertical activity. A) B6 within-subjects effect of time F(9,216) = 2.337, p<0.05. Between-subjects effect of treatment F(3,24) = 4.169, p<0.05. (SS: n= 8; MS: n=5; SM: n=10; MM: n=5). B) D2 within-subjects effect of time F(9,306) = 8.875, p<0.001; within-subjects time × treatment interaction F(27,306) = 3,910, p<0.001. Between-subjects effect of treatment F(3,34) = 12.979, p<0.001. (SS: n= 10; MS: n=9; SM: n=11; MM: n=8). C) 129 within-subjects time × treatment interaction F(27 396) = 2.864, p<0.001. (SS: n= 10; MS: n=12; SM: n=13; MM: n=13).
1.3.6 Strain Effects – All Experiments
A two-way ANOVA (strain × time) yielded significant strain effects within many of the treatment groups. As seen in Tables 2 and 3, the B6 exhibited a greater behavioral response for all treatments and behaviors with a few exceptions. An example of where this pattern deviated is the low dose MS total distance response; the B6 adult activity was equal to the D2 but still greater than the 129 response. It is particularly interesting to note that the trends in greatest to least activity measured did not deviate from B6 > D2 > 129 in the adolescent high dose vertical activity measure. Also, the D2 and 129 strains had equal responses for all treatments for the adolescent high dose total distance behavior. It should be noted that within our study conditions the three genotypes never exhibited equal responses for a given behavior.
Table 2.
Strain comparison of adolescent behavior a.
| HIGH DOSE | LOW DOSE | |||
|---|---|---|---|---|
| Total Distance | Vertical Activity | Total Distance | Vertical Activity | |
| SS | B6 > D2 = 129 | B6 > D2 > 129 | B6 > D2 = 129 | B6 > D2 > 129 |
| MS | B6 > D2 = 129 | B6 > D2 > 129 | B6 > 129 B6=D2; D2=129 |
B6 = D2 > 129 |
| SM | B6 > D2 = 129 | B6 > D2 > 129 | B6 > D2 = 129 | B6 = D2 > 129 |
| MM | B6 > D2 = 129 | B6 > D2 > 129 | B6 > D2 = 129 | B6 =D2 > 129 |
Strain comparisons shown according to treatment group and locomotor behavior. = denotes no statistical difference. > indicates significant difference between the two compared strains for the specific pretreatment effect on a behavior (p<0.05). As an example, read B6 > D2 = 129 as the B6 exhibited significantly greater activity than the D2 and 129 strains. No significant difference existed between the D2 and 129 strains.
Table 3.
Strain comparison of adult behavior b.
| HIGH DOSE | LOW DOSE | |||
|---|---|---|---|---|
| Total Distance |
Vertical Activity |
Total Distance |
Vertical Activity |
|
| SS | B6 = D2 > 129 | B6 = D2 > 129 | B6 > D2 > 129 | B6 = D2 > 129 |
| MS | B6 = D2 > 129 | B6 = D2 > 129 | B6 > D2 > 129 | B6 > D2 > 129 |
| SM | B6 =D2 > 129 | B6 = D2 > 129 | B6 > D2 = 129 | B6 = D2 > 129 |
| MM | B6 > 129 B6=D2; D2=129 |
B6 = D2 > 129 | B6 = D2 > 129 | D2 > B6 > 129 |
Strain comparisons shown according to treatment group and locomotor behavior. = denotes no statistical difference. > indicates significant difference between the two compared strains for the specific pretreatment effect on a behavior (p<0.05). As an example, read B6 > D2 = 129 as the B6 exhibited significantly greater activity than the D2 and 129 strains. No significant difference existed between the D2 and 129 strains.
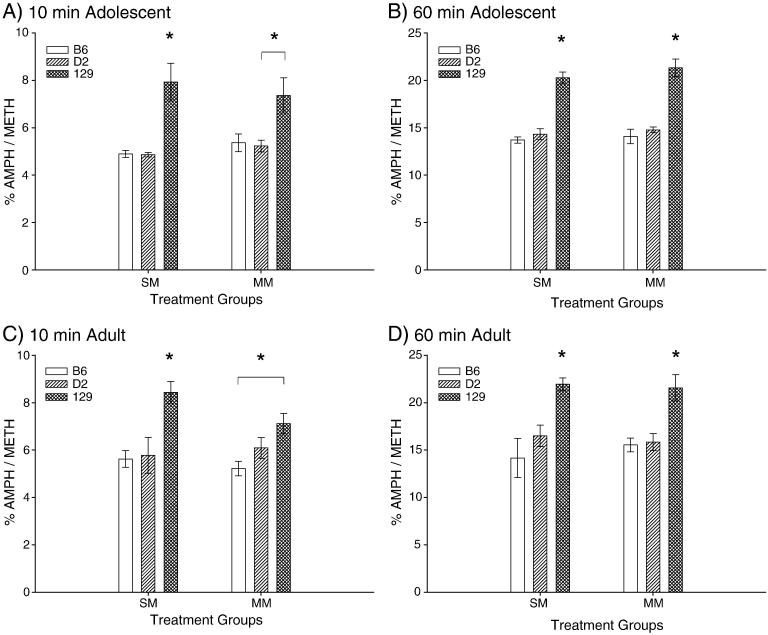
1.3.7 Methamphetamine Metabolism
The results of our striatal METH metabolism study indicate that there were few pretreatment effects for age, strain, or treatment group at the 10 and 60 minute time points for METH or AMPH concentrations, primarily with the 129 genotype exhibiting significantly less METH and AMPH than the B6 or D2 genotypes (Table 4). However, significant strain effects for METH metabolism were found (Figure 9). Post-hoc assessment showed that the 129 adolescents metabolized METH to AMPH significantly faster at both the 10 and 60 minute time points than the D2 mice for both treatments, as viewed by the percent AMPH/METH turnover. The same was true for the adolescent B6 and 129 comparison with the exception of the 10 minute MM time point (p<0.054). For all other adolescent METH turnover comparisons the 129 mice metabolized METH significantly faster that the B6. The adult 129 mice demonstrated a greater rate of metabolism than the B6 for the 10 minute SM comparison, and a faster metabolism rate than the B6 and D2 for both SM and MM 60 minute comparisons. There were no differences between the B6 or D2 animals for METH metabolism rates.
Table 4.
| 10 Minute |
60 Minute |
|||||
|---|---|---|---|---|---|---|
| Strain | Age | Pretreatment | METH ± SEM (ng/mg) |
AMPH ± SEM (ng/mg) |
METH ± SEM (ng/mg) |
AMPH ± SEM (ng/mg) |
| B6 | Adolescent | SM | 18.33 ± 0.66t | 0.90 ± 0.02 | 10.23 ± 0.38 | 1.40 ± 0.06 |
| MM | 17.46 ± 1.20 | 0.88 ± 0.02 | 10.07 ± 0.45 | 1.40 ± 0.02 | ||
| Adult | SM | 15.80 ± 0.81 | 0.91 ± 0.04 | 7.56 ± 1.70 | 1.23 ± 0.09 | |
| MM | 16.57 ± 1.48 | 0.85 ± 0.04 | 9.29 ± 0.30 | 1.48 ± 0.12 | ||
| D2 | Adolescent | SM | 16.72 ± 1.14 | 0.86 ± 0.04 | 9.09 ± 0.53 | 1.29 ± 0.04 |
| MM | 16.82 ± 1.28 | 0.87 ± 0.03 | 8.74 ± 0.39 | 1.29 ± 0.04 | ||
| Adult | SM | 14.79 ± 1.82 | 0.85 ± 0.02 | 8.46 ± 1.01 | 1.35 ± 0.13 | |
| MM | 14.63 ± 1.54 | 0.87 ± 0.05 | 7.65 ± 0.33 | 1.21 ± 0.08 | ||
| 129 | Adolescent | SM | 13.03 ± 0.97t‡ | 1.01 ± 0.05 | 7.70 ± 0.71 | 1.54 ± 0.11 |
| MM | 14.56 ± 1.68 | 1.02 ± 0.05 | 6.71 ± 0.33tt‡ | 1.42 ± 0.06 | ||
| Adult | SM | 14.17 ± 1.00 | 1.18 ± 0.07‡ | 7.59 ± 0.39 | 1.66 ± 0.06‡ | |
| MM | 14.67 ± 0.56 | 1.03 ± 0.03 | 7.45 ± 0.61 | 1.58 ± 0.10 | ||
METH and AMPH concentrations for adolescent and adult B6, D2 and 129 subjects at the 10 and 60 minute time points.
indicates significant differences between the B6 and D2 genotypes.
indicates significant differences between the D2 and 129 genotypes.
indicates significant differences between the B6 and 129 genotypes.
ANOVA results for the adolescent METH and AMPH comparisons at the 10 and 60 minute time points are as follows: SM 10 min METH F(2,13) = 11.961, p<0.005; MM 60 min METH F(2,14) = 15.080, p<0.005. Significant adult comparisons for the two time points include: SM 10 min AMPH F(2,11) = 6.089, p<0.05; SM 60 min AMPH F(2,13) = 4.749, p<0.05.
Figure 9.
Percent turnover of METH to AMPH in striatal tissues 10 minutes and 60 minutes after challenge dose. A) Adolescent 10 minute time point between-subjects analysis of SM and MM treatment groups. Effect of strain for SM comparison F(2,11) = 12.367, p<0.005 (B6: n=5; D2: n=4; 129: n=5). Effect of strain for MM comparison F(2,11) = 5.514, p< 0.05 (B6: n=4; D2: n=5; 129: n=5). B) Adolescent 60 minute time point between-subjects effect of strain for SM and MM treatment groups: SM comparison F(2,12) = 47.025, p< 0.001 (B6: n=5; D2: n=5; 129: n=5); MM comparison F(2,12) = 31.245, p<0.001 (B6: n=5; D2: n=5; 129: n=5). C) Adult 10 minute time point between-subjects analysis of strain effects for SM and MM treatment groups: SM comparison F(2,9) = 8.208, P<0.01 (B6: n=4; D2: n=4; 129: n=4); MM comparison F(2,10) = 6.160, p<0.05 (B6: n=4; D2: n=4; 129: n=5). D) Adult 60 minute time point between-subjects analysis of SM and MM treatment group analysis for strain effects: SM comparison F(2,11) = 9.470, p<0.005 (B6: n=4; D2: n=5; 129: n=5); MM comparison F(2,10) = 10.016, p<0.005 (B6: n=4; D2: n=4; 129: n=5). Asterisks indicate where 129 results are significantly greater than B6 and D2 results, with the exception of the adolescent and adult MM 10 minute time points, which are clarified with asterisks and bars to denote where the 129 exhibited greater turnover compared to D2 or B6 strains.
1.4 DISCUSSION
The diverse B6 and D2 behavioral effects produced by the high and low dose METH pretreatments merits attention. Behavioral sensitization was observed in pretreated adolescent B6 mice for high dose total distance traveled. The adult pretreated B6 mice demonstrated tolerance for vertical activity in response to low dose METH. No other significant effects for either the high or low dose METH pretreatments were determined in the B6 adults. Traditionally, acute exposure to high dose METH produces sensitization in stereotypy behavior and acute low dose METH administration increases ambulation (Schramm-Sapyta et al., 2009). However, these predicted responses may not be observed 40 days after METH pretreatment and not within our age parameters. Interestingly, the adult low dose vertical activity measure demonstrated sensitization in the D2 animals and tolerance in the B6 mice. These high and low dose METH pretreatment-induced behavioral outcomes may result from a combination of possibilities, such as neurotoxicity and inherent differences in the dopamine systems of the B6, D2 and 129 genotypes.
The effects of three different dosing schedules and two METH doses were previously assessed across 12 days to determine the locomotor changes in B6 and D2 male mice, aged 8-16 weeks. The chronic saline group received METH on day 11 but saline on all other test days, while the chronic drug group received METH before behavioral testing on days 3, 5, 7, 9 and 11 and saline at the other time points. The chronic drug control group received METH before behavioral testing on days 7 and 9 and saline on days 1, 2, 3, 5, 7 and 12 (Phillips et al., 1994). These experimental parameters were tested using both a 1 mg/kg and a 2 mg/kg METH dose. Results indicated that the chronic 1 mg/kg dose elicited locomotor sensitization in both the B6 and D2 strains. The D2 animals exhibited greater stereotypy and locomotor activation than the B6 strain. The chronic 2 mg/kg dose produced equal sensitization for the two strains, but the rate of achieving the sensitization was different. The B6 showed a gradual increase in sensitization which may have not peaked by day 11. However, the D2 mice exhibited a plateau for sensitization with a sudden large increase in horizontal distance on day 5 which remained constant throughout the remainder of the study. Interestingly, this study did not find behavioral changes in vertical activity for either dose or treatment group, while our low dose study indicated tolerance for the B6 adults and sensitization for the D2 adults. The chronic drug control group demonstrated sensitized increases in horizontal distance and stereotypy number. These results are of interest as the 8 – 16 week age range fell between our age parameters.
Although the 129 mice did not show pretreatment effects for either dose, they did demonstrate behavioral activation to the METH challenge in patterns that mimicked both the B6 and D2 strains albeit to a lesser extent. The low behavioral response to dopaminergic stimulation has been witnessed before with both cocaine (Schlussman et al., 1998) and amphetamine (Chen et al., 2007). The B6 genotype exhibited no differences in basal locomotor activity or dopamine levels when compared to the 129S2/SvHsd (Chen et al., 2007). However in the same study the B6 did display greater locomotion as well as greater DA efflux in response to 2.5 mg/kg or 5 mg/kg AMPH. These AMPH-induced differences between the two mouse strains could not be explained by striatal DA concentrations, surface and total DAT expression, or DAT activity. It has been postulated that alterations in the DA D2-long receptor, as well as other discrepancies within the DA pathway, may be responsible for the observed low locomotor activity for the 129 (Kelly et al., 1998). Another study assessing behavioral differences between the B6 and 129 noted that the 129 strain spent significantly less time in the center of an open field, possibly due to their circular movement patterns which differ from the darting motions of the B6 animals (Paulus et al., 1999).
Our observation that the adolescent and adult 129 mice metabolize METH to AMPH faster than the B6 and D2 animals is intriguing. This finding may be partially responsible for why we did not observe pretreatment effects in these animals. METH and AMPH have been shown to influence DAT function differently (Goodwin et al., 2009). In vivo rat voltammetry experiments in the nucleus accumbens showed that METH inhibited DAT-mediated DA clearance more efficiently than AMPH. The 129 mice in our study metabolized METH to AMPH significantly faster. Perhaps the decreased exposure to METH led to less DAT inhibition and less neurotoxicity, consequently diminishing the down-stream effects on DA uptake.
There are many studies investigating various mouse strains and the behavioral responses to different dosing schedules of METH. Six-week old male ICR mice were administered 1 mg/kg METH on three schedules: 1) saline only; 2) METH on days 2-6 and METH challenge on day 11; 3) METH only on day 11. The acute group exhibited behavioral activation to the single dose of METH while the chronic group reached a plateau in behavioral sensitization four days into the study (Kitanaka et al., 2003). In the same study, significant reductions in 3,4-dihydroxyphenylacetic acid (DOPAC)/DA were described for both the acute and chronic dosing groups. In comparison with our own adolescent results, the stratification of activity counts for the SS, SM and MM ICR treatment groups were similar to our low dose METH pretreated B6 adolescent total distance response. Male BALB/C mice (age 12-14 weeks) assessed at 10 days, 3 months and 5 months post METH administration (single day of i.p. 4 × 7.5 mg/kg, every 2 hours) for neurotoxicity and novelty-seeking behavior did not exhibit decreases in novelty-seeking behavior until 5 months, while decreases in DA were apparent after 10 days. Tunel staining 3 days after METH administration showed drug-induced cell death. DA levels recovered to control levels by the 3 and 5 month time points (Krasnova et al., 2009). Even though both the ICR and BALB/C strains exhibited METH-induced neurotoxicity from doses similar to our dosing regimen, we cannot assume that neurotoxicity would follow these same timelines in the B6, D2 or 129 genotypes at PND 40 or 80.
The complexity of METH’s neurochemical interactions generates many pharmacological, genetic and toxicity factors that may be influencing the DA system. METH produces several toxic responses within DA neurons by formation of quinone radicals and hydroxyl radicals (Cadet et al., 2003; Zhu et al., 2006b). A single i.p. injection of 30 mg/kg METH in male ICR mice (age 10-11 weeks) determined a timeline for apoptosis versus loss of dopaminergic markers (Zhu et al., 2005). It was shown that the 30 mg/kg dose induced apoptosis in 25% of striatal neurons after 24 hours. TH depletion occurred in 80% of neurons at 48 hours. Depletion of dopamine transporter (DAT) sites began to appear 24 hours after METH exposure but did not peak (60% versus control values) until 48 hours. GFAP increased at 48 hours and peaked at 72 hours. These results indicate that striatal apoptosis occurs before the depletion of DA terminal markers (Zhu et al., 2005). In addition, the ability of the striatum to cope with toxic substances changes throughout a lifetime due to the effects of ageing and oxidative stress related damage (Cadet and Brannock, 1998; Yamamoto et al., 1998). A study of B6 mice ranging in age from 1 month to 24 months determined that 72 hours following a 4 × 10 mg/kg dosing schedule, only DA is significantly decreased in 1 month old B6 mice while 12 month old B6 mice displayed significant losses to DA, DOPAC and homovanillic acid. The 2, 5 and 12 month age groups all demonstrated significant increases in GFAP expression 72 hours post dosing while the 1 month animals did not (Miller et al., 2000). As our B6 animals were closer in age to the 1 month than 2 month animals and our adults were almost 3 months old when initially dosed, we could hypothesize that we would see similar trends in both GFAP expression and DA losses in our high dose experiments. These age-related differences in toxic responses could relate to why we saw significant changes to the high dose adolescent B6 total distance measure and no significant changes to any of the high dose adult B6 behavior.
The behavioral differences we observed may also be due to influences of age and genotype upon the DA system’s rate of maturation. It has been shown in a rat model that DA receptors were over-produced prior to PND 40 and then pruned back to adult levels as the animals aged (Teicher et al., 1995). In the striatum the D1 and D2 DA receptors peaked at PND 40 but then showed two different patterns of pruning between PND 60-120. The D1 receptor density declined from PND 40 levels by 53% until PND 80 when they began to increase up to 35% of the PND 40 levels at PND 120. The D2 receptor density also declined by approximately 35% after PND 40 but remained stable during PND 60-120. The nucleus accumbens demonstrated a different timeline. There was a great increase in the D1 and D2 receptors between PND 25 and PND 40, but the levels dropped between PND 60-80 and then recovered up to PND 40 levels by PND 120. With this in mind, the three genotypes used in our study may exhibit varied rates of D1 and D2 receptor maturation in addition to different degrees of pharmacological responses to the high and low METH doses. It has been reported that there are endogenous differences in the baseline concentrations of both D1 and D2 receptors for the B6 and D2 mice. Between 7 - 15 weeks of age, B6 mice expressed significantly fewer D2 receptors than D2 mice, but the discrepancy averaged out by 31-45 weeks of age as the D2 mice demonstrated an overall decrease in receptor density (Leprohon-Greenwood and Cinader, 1987). Another age study assessing 7 week old mice found that B6 mice exhibited both higher D1 and D2 mRNA and also higher D1 and D2 receptor densities in the striatum when compared to D2 animals. The B6 mice demonstrated adenylyl cyclase activity that was much more sensitive to DA activity, as well as a stronger D1 – D2 receptor link than the D2 mice (Ng et al., 1994).
Methamphetamine is capable of producing alterations to various locomotor behaviors in the B6 and D2 strains dependent upon pretreatment, dose, and age parameters. The 129 mice did display faster rates of METH metabolism but did not show any effects of METH pretreatment. It remains to be determined whether the observed variance in behavioral responses between the strains is due to METH-induced neurotoxicity, different rates of neuronal maturation, or changes within DA regulatory mechanisms. Assessments of DA metabolism and levels of TH at our challenge time point will begin to answer these questions. The present study demonstrates that the complex interactions of strain, age, METH pretreatment, and METH metabolism are all important variables to consider when investigating the behavioral effects of METH exposure in mouse models.
Research Highlights.
B6 adolescent mice pretreated with high dose METH 40 days prior to behavioral testing (4 × 5 mg/kg × 2 hours × 2 days) exhibited significant sensitization for total distance traveled while the D2 and 129 adolescents did not show behavioral changes.
Forty days following initial treatment with low dose METH (2 × 1 mg/kg × 24 hours) adult pretreated B6 mice demonstrated significant tolerance for vertical activity in response to a 1 mg/kg METH challenge.
Significant sensitization was observed in the D2 adults for vertical activity 40 days following a low dose METH pretreatment; no effects were seen in the 129 adults.
Adolescent and adult 129 mice tended to metabolize METH faster than both the B6 and D2 strains.
Acknowledgements
We are grateful for our support from NIDA Training Grant 1543765 (RLG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.6 References
- Açikgöz O, Gönenç S, Kayatekin BM, Pekçetin C, Uysal N, Dayi A, et al. The effects of single dose of methamphetamine on lipid peroxidation levels in the rat striatum and prefrontal cortex. Eur Neuropsychopharmacol. 2000;10(5):415–8. doi: 10.1016/s0924-977x(00)00103-6. [DOI] [PubMed] [Google Scholar]
- Anthony J, Helzer J. Syndromes of drug abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric disorders of america: the epidemiologic catchment area study. Free Press; New York: 1990. pp. 116–54. [Google Scholar]
- Bryant CD, Chang HP, Zhang J, Wiltshire T, Tarantino LM, Palmer AA. A major QTL on chromosome 11 influences psychostimulant and opioid sensitivity in mice. Genes Brain and Behav. 2009;8(8):795–805. doi: 10.1111/j.1601-183X.2009.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32(2):117–31. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17(13):1775–88. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang M, Park S, Gnegy ME. C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in striatum than 129S2/SvHsd mice. Pharmacol Biochem Behav. 2007;87(1):158–63. doi: 10.1016/j.pbb.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93(3):237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27(3):253–62. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36(1):1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93(1):64–9. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL. Methamphetamine induced apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology. 2002;42(6):837–45. doi: 10.1016/s0028-3908(02)00034-5. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Brain Res Rev. 2000;34(3):103–18. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18(13):4861–9. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284(5):2978–89. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Belknap JK, O’Toole LA, Helms ML, Wenger CD, Crabbe JC. Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J Neurosci. 1997;17:745–754. doi: 10.1523/JNEUROSCI.17-02-00745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Batki SL. Stimulant psychosis: symptom profile and acute clinical course. Am J Addict. 2000;9(1):28–37. doi: 10.1080/10550490050172209. [DOI] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 1996;714(1-2):95–103. doi: 10.1016/0006-8993(95)01502-7. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188(2):162–70. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Aging increases the susceptibility to methamphetamine-induced dopaminergic neurotoxicity in rats: correlation with peroxynitrite production and hyperthermia. J Neurochem. 2001a;78(5):952–9. doi: 10.1046/j.1471-4159.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, el-Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W, Jr, et al. Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann N Y Acad Sci. 2001b;939:366–80. doi: 10.1111/j.1749-6632.2001.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Behavioral consequences of methamphetamine-induced neurotoxicity in mice: Relevance to the psychopathology of methamphetamine addiction. Ann N Y Acad Sci. 2002;965:127–135. doi: 10.1111/j.1749-6632.2002.tb04156.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PH, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18(2):238–51. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 1999a;24(1):66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999b;146(4):373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Kessov CN, Burkhart-Kasch S, Zhang G, et al. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background and developmental adaptations. J Neurosci. 1998;18(9):3470–9. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65(6):674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka N, Kitanaka J, Takemura M. Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. Eur J Pharmacol. 2003;474(1):63–70. doi: 10.1016/s0014-2999(03)02015-6. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Hodges AB, Ladenheim B, Rhoades R, Phillip CG, Cesena A, et al. Methamphetamine treatment causes delayed decrease in novelty-induced locomotor activity in mice. Neurosci Res. 2009;65(2):160–5. doi: 10.1016/j.neures.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H, Tadokoro S. Reverse tolerance to ambulation-increasing effects of methamphetamine and morphine in 6 mouse strains. Jpn J Pharmacol. 1989;49(2):197–203. doi: 10.1254/jjp.49.197. [DOI] [PubMed] [Google Scholar]
- Leprohon-Greenwood CE, Cinader B. Variations in age-related decline in striatal D2-dopamine receptors in a variety of mouse strains. Mech Ageing Dev. 1987;38(2):199–206. doi: 10.1016/0047-6374(87)90079-0. [DOI] [PubMed] [Google Scholar]
- Lin LY, Di Stefano EW, Schmitz DA, Hsu L, Ellis SW, Lennard MS, et al. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos. 1997;25(9):1059–64. [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP, Ali SF. Age as a susceptibility factor in the striatal dopaminergic neurotoxicity observed in the mouse following substituted amphetamine exposure. Ann N Y Acad Sci. 2000;914:194–207. doi: 10.1111/j.1749-6632.2000.tb05196.x. [DOI] [PubMed] [Google Scholar]
- Mirecki A, Fitzmaurice P, Ang L, Kalasinsky KS, Peretti FJ, Aiken SS, et al. Brain antioxidant systems in human methamphetamine users. J Neurochem. 2004;89(6):1396–408. doi: 10.1111/j.1471-4159.2004.02434.x. [DOI] [PubMed] [Google Scholar]
- Ng GY, O’Dowd BF, George SR. Genotypic differences in brain dopamine receptor function in the DBA/2J and C57BL/6J inbred mouse strains. Eur J Pharmacol. 1994;269(3):349–64. doi: 10.1016/0922-4106(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Geyer MA. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835(1):27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108(4):789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Kamens HM, Wheeler JM. Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev. 2008;32(4):707–59. doi: 10.1016/j.neubiorev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubill D, Chipana C, Camins A, Pallas M, Camarasa J, Escubedo E. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol Appl Pharmacol. 2005;204(1):57–68. doi: 10.1016/j.taap.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ. Effects of “binge” pattern cocaine on stereotypy and locomotor activity in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 1998;60(2):593–9. doi: 10.1016/s0091-3057(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206(1):1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Human methamphetamine pharmacokinetics simulated in the rat: single daily intravenous administration reveals elements of sensitization and tolerance. Neuropsychopharmacology. 2006;31(5):941–55. doi: 10.1038/sj.npp.1300865. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165(4):359–69. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Heikkila RE. Neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and methamphetamine in several strains of mice. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(2-3):345–54. doi: 10.1016/0278-5846(88)90054-1. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Gallia A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75(6):406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89(2):167–72. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, et al. Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: Implications for neurotoxicity. J Pharmacol Exp Ther. 2005;314(3):1087–92. doi: 10.1124/jpet.105.085951. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Fatusin OO, Liu QR, Contoreggi C, Li CY, Buck K, Crabbe J. Higher order” addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochem Pharmacol. 2008;75:98–111. doi: 10.1016/j.bcp.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–60. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19(20):9141–8. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287(1):107–14. [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Disparity in the temporal appearance of metamphetamine induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res. 2005;1049(2):171–81. doi: 10.1016/j.brainres.2005.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo N, Angulo JA. Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration. Neurotoxicology. 2006a;27(1):131–36. doi: 10.1016/j.neuro.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum of mice. Neuroscience. 2006b;140(2):607–22. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]