Abstract
We have selectively bred mice that reach very high blood ethanol concentrations (BECs) after drinking from a single bottle of 20% ethanol. High Drinking in the Dark (HDID-1) mice drink nearly 6 g/kg ethanol in 4 hr and reach average BECs of more than 1.0 mg/ml. Previous studies suggest that DID and two-bottle preference for 10% ethanol with continuous access are influenced by many of the same genes. We therefore asked whether HDID-1 mice would differ from the HS/Npt control stock on two-bottle preference drinking. We serially offered mice access to 3 – 40% ethanol in tap water vs tap water. For ethanol concentrations between 3 and 20%, HDID-1 and HS/Npt controls did not differ in two-bottle preference drinking. At the highest concentrations, the HS/Npt mice drank more than the HDID-1 mice. We also tested the same mice for preference for two concentrations each of quinine, sucrose, and saccharin. Curiously, the mice showed preference ratios (volume of tastant / total fluid drunk) of about 50% for all tastants and concentrations. Thus, neither genotype showed either preference or avoidance for any tastant after high ethanol concentrations. Therefore, we compared naive groups of HDID-1 and HS/Npt mice for tastant preference. Results from this test showed that ethanol-naive mice preferred sweet fluids and avoided quinine but the genotypes did not differ. Finally, we tested HDID-1 and HS mice for an extended period for preference for 15% ethanol vs water during a 2 hr access period in the dark. After several weeks, HDID-1 mice consumed significantly more than HS. We conclude that Drinking in the Dark shows some genetic overlap with other tests of preference drinking, but that the degree of genetic commonality depends upon the model used.
Keywords: Selected mouse lines, High Drinking in the Dark (HDID) mice, ethanol preference, pharmacogenetics, binge drinking, limited access
Introduction
The excessive alcohol (ethanol) drinking that is diagnostic of alcohol use disorders including alcohol dependence has been modeled with many different assays in rodents. The oldest historical method is the two-bottle preference test, where individually housed animals are offered a choice between a bottle containing only water and one containing a concentration of ethanol, usually 10% (Richter and Campbell, 1940). Early in the history of alcohol research, studies clearly showed that rat and mouse genotypes differed markedly in their willingness to drink (i.e., their preference for) ethanol. These seminal studies established that there was a substantial influence of some (unknown) genes to increase or decrease preference (Williams et al., 1949) (McClearn and Rodgers, 1959; Mardones and Segovia-Riquelme, 1983). Research since has established that the genetic contributions to two-bottle preference drinking in mice have remained remarkably stable across laboratories for 50 years (Wahlsten et al., 2006). A great deal of research in recent years has addressed the identification of those genes and has seen some success [for review see (Crabbe et al., 2010)].
The alcohol research field has long benefitted from a wealth of genetic animal models devoted to exploring two-bottle preference drinking. Notably, selective breeding over many generations has been used to develop 6 pairs of rat lines and three pairs of mouse lines that differ in two-bottle ethanol preference. Each selection has employed nearly identical procedures, a major feature of which is an initial four-day period of forced access to 10% ethanol (except for the HAP/LAP lines), followed by continuous access to 10% ethanol versus water for three to four weeks. Animals are selected for breeding based on high (or low) preference across the three-week two-bottle choice period. Each high drinking line (UChB, P, AA, HAD-1, HAD-2, sP, and msP rats; HAP-1, HAP-2 and HAP-3 mice) was developed in parallel with a low-drinking line (UChA, NP, ANA, LAD-1, LAD-2, sNP, LAP-1, LAP-2 and LAP-3; no low drinking line exists for the msP). The similarity of the selection trait across experiments has facilitated the discovery of some neurobiological correlates of high vs low preference drinking [for a summary of recent reviews, see (Crabbe, 2008); see also Oberlin et al, 2010, epub]. There has been less congruence in the gene-finding area, but some genes have been suggested across multiple selections [see, for example (Mulligan et al., 2006)].
One limitation of the standard two-bottle preference test is that by spacing their intake over the day, even genetically high drinking rodents generally drink only amounts of alcohol sufficient to reach a blood ethanol concentrations (BECs) of approximately 0.6–0.7 mg/ml (e.g. Dole and Gentry, 1984). Even limiting access to alcohol in high-drinking genotypes results in mean BECs of about 0.6 mg/ml (Grahame et al., 1999b; Grahame and Grose, 2003; Oberlin et al., 2010). This is one reason that investigators have also used many other ways to assess alcohol consumption. Another reason is that in studies where animals are started at a fixed ethanol concentration (usually 10% in water), even genetically predisposed genotypes can require more than a week to reach a stable plateau of drinking (e.g., for C57BL, see McClearn & Rodgers, 1959; for HAP mice, see Oberlin et al, 2010). This occurs because while some individual mice may start out drinking with >90% preference for alcohol, there are always individual differences within a genotype, and others may require several days before their drinking reaches a plateau. This can make such studies time-consuming. Another paradigm that has been employed is the so-called “alcohol acceptance” test. In the earliest version of this test, six inbred strains of mice were offered access to a single bottle of alcohol for a 24 hr period, alternating with a single bottle of water for 24 hr. Inbred mouse strains differed in how much alcohol they would accept (McClearn, 1968b). Alternating access for 6 days was then switched to two-bottle preference (10% ethanol vs water) for 6 days, and the 12 day schedule was then repeated; order was counterbalanced. The rank order of strain differences in two-bottle preference did not appear to be correlated with the strain differences in acceptance, suggesting that different genes influence these two measures of alcohol intake. The acceptance test has been performed with or without a period of fluid deprivation (with or without “thirst motivation”). In the former version of the test, mice were scored daily for water drinking for two days. All fluid was removed for 24 hr, and on the 4th day, a single bottle of 10% ethanol was offered for 24 hr. The index of alcohol acceptance was the ratio of ethanol intake to water intake averaged across the first two days. When inbred strains’ acceptance under thirst motivation was compared with two-bottle preference drinking, strain rank orders were identical across the 6 strains (McClearn, 1968b), suggesting that some genes affect drinking across these latter two drinking paradigms. Lines of mice were subsequently selectively bred for High (HEA) vs Low Ethanol Acceptance (LEA) using the thirst motivation paradigm, and this experiment showed that the response was heritable (Anderson and McClearn, 1981).
Another powerful strategy for assessing whether there may be some genetic overlap in contributions to alcohol acceptance and preference is to compare alcohol acceptance drinking across the lines selected for high vs low preference drinking. If lines are selected for one trait and naive animals from the selected lines are subsequently found to differ on another, the two traits are likely to be affected by some common genes (Crabbe et al., 1990). Several studies have examined rat lines selected for preference drinking using many different schedules of limited or scheduled access to alcohol [e.g., (Murphy et al., 1986), and see Discussion] but these studies typically only examine genetically high drinkers. One exception is a study where P and NP rats were offered daily 1 hr access to increasing concentrations of ethanol vs water, and P rats drank more ethanol beginning at quite low concentrations. Few studies include periods where alcohol alone is offered, as in the “alcohol acceptance” methods. Rats have also been bred for high vs low drinking of 12% alcohol during a 20 minute limited access session. Water was always an alternative. The high-drinking HARF rats also drank more alcohol than LARF rats when ethanol alone was offered for 4 days in a single bottle test (Le et al., 2001). When the single-bottle test was followed by a two-bottle preference drinking test, HARF rats also drank more than LARF rats. Together these results suggest some common genetic influences on limited access and continuous access two-bottle drinking, as well as acceptance (Le et al., 2001).
In order to develop a new animal model for limited-access drinking in an acceptance paradigm that would lead to intoxicating blood alcohol levels, we recently developed the Drinking in the Dark (DID) assay. Starting with a method that involved exposing genetically high-drinking C57BL/6J mice to several days of gradually increased ethanol concentrations during their circadian dark phase (Ryabinin et al., 2003; Sharpe et al., 2005), we developed a 4 day test in which mice drank intoxicating amounts of ethanol (Rhodes et al., 2005). After showing that inbred strains differed substantially in DID (Rhodes et al., 2007), we shortened the test to 2 days and developed pairs of mouse lines selected for high blood ethanol concentrations at the end of a 4 hr period of access in the circadian dark to a single bottle containing 20% ethanol. High Drinking in the Dark mice (HDID-1 and HDID-2 lines) achieve blood alcohol levels of greater than 100 mg% and show visible signs of intoxication (Crabbe et al., 2010; Crabbe et al., 2009). The inbred strain studies suggested that there was partial genetic overlap between Drinking in the Dark and two-bottle preference drinking (Rhodes et al., 2007). In the current report, we compared the HDID-1 line of mice with their control line to see whether they would drink more ethanol when it was offered continuously in a standard, 24 hr two bottle preference test. We also tested their preference for different tastants. Finally, we compared the lines for two-bottle preference in an extended 2 hr/day limited access procedure.
Methods
Animals and husbandry
All procedures were approved by the Portland VA Medical Center Institutional Animal Care and Use Committee and were performed according to NIH Guidelines for the Care and Use of Laboratory Animals. Mice from the High Drinking in the Dark - 1 (HDID-1) line were selectively bred to reach high blood ethanol concentrations (BECs) at the end of a second, limited-access drinking period where a single bottle of 20% ethanol was offered for 2–4 hr early in the circadian dark phase (Crabbe et al., 2009). Mice used in the current studies were from the 13th, 16th and 17th selected generations (S13, S16 and S17). The control population for the selection is the genetically heterogeneous stock (HS/Npt, or HS) created by Dr. Robert Hitzemann at the State University of New York - Stony Brook and moved to the OHSU Department of Comparative Medicine animal facility in 2000. The HS/Npt stock was created by the systematic intercrossing of 8 inbred mouse strains (A/J, AKR/J, BALB/cJ, C3H/HeJ, C57BL/6J, CBA/J, DBA/2J, and LP/J) derived from 4 of the 8 inbred mouse strain lineages (Petkov, Ding, Cassell, Zhang, Wagner, Sargent, Asquith, Crew, Johnson, Robinson, Scott, and Wiles 2004). It is maintained by rotational mating of approximately 48 breeding pairs.
We obtained pregnant females from 25 families of the 50th filial generation (G50) of HS mice and moved them into a separate colony room at the OHSU facility in 2004. Their offspring became the founder population for the HDID-1 selected line. Generations S0 - S2 were maintained and tested at OHSU within the Department of Comparative Medicine. The S2 breeding pairs and their pre-weaning S3 offspring were moved to the Portland VA Medical Center Veterinary Medical Unit in 2005. In 2008, we obtained pregnant HS/Npt females from 20 families of the G61 generation and moved them to the VA Medical Center facility to establish a control colony. This control subcolony of HS has been maintained since by rotational mating among 20 breeding pairs, and the mice tested here were from the 64th, 68th and 69th filial generations. All mice were naive at the time of testing (except as noted below).
Mice were maintained in standard polycarbonate or polysulfone (the two plastic types were used interchangeably) cages (19 X 31 X 13 cm) on Bed-o-cob bedding (Andersons, Maumee, OH, USA) with stainless steel wire bar tops with a recess for chow. Until the beginning of the drinking tests, male and female mice were maintained in groups of 2–5. Cages were changed once weekly. Animals were maintained on a 12 hr:12 hr light:dark schedule at a temperature of 21±1°C, and Purina 5001 chow (PMI Nutrition Internatio nal, Brentwood, MO, USA) was available at all times. Until entering the studies, most animals were housed on Thoren racks with automatic lixit water spouts always available. Some generations were housed on flat racks with square, stoppered polycarbonate water bottles placed on the cage tops. Water was obtained from these bottles by licking a pinhole in the bottom side, through the wire cage top. Three weeks before experiments, mice in Experiments 1 and 2 were transferred to a procedure room with the same environmental conditions, with lights on at 0600 and lights off at 1800. Mice in Experiment 3 remained in the primary colony, with lights on at 21:30 and off at 09:30. Mice in Experiment 4 were transferred 2 weeks before their experiment to a procedure room with the same environmental conditions except that lights were off at noon and on at midnight. Mice were individually housed 5 – 7 days before beginning a drinking study in the same type of caging with a water bottle with a stainless steel drinking spout.
Experiment 1: Two bottle ethanol consumption and preference
Mice were 65–74 days old at the start of testing. Thirty male and 30 female mice of the HDID-1 and HS/Npt lines were used. Mice were randomly distributed across the rack with respect to genotype and sex. The normal water bottle was removed and mice were offered two 25ml graduated cylinders with stainless steel drinking spouts, both containing tap water, for two days. Food pellets were always available. On subsequent days, one bottle always contained tap water and the other contained an ethanol solution. Fluid levels were recorded at 1100 hours each day. All mice were first offered 3% ethanol (v/v) in tap water on the left side vs water on the right side, and all tube volumes were recorded. Twenty-four hr later, levels were read again, and the bottles were left in place. After 48 hr and another reading, tube positions were switched.
Mice were tested for 40 days, and were offered 4 days each of choice between 3%, 6%, 9%, 12%, 15%, 20%, 25%, 30%, 35%, and finally 40% ethanol (Decon Laboratories, Inc., King of Prussia, PA) vs water. All solutions were freshly prepared every other day. Each concentration was tested for two consecutive days on one side, then two more days after a position switch to control for any individual differences in bottle position preference. Body weights were taken the day the experiment started, and every 4 days thereafter. Cages were changed every 8 days starting on day 5 of the experiment. Two cages without mice were placed on the beginning and end of each rack and were treated exactly the same: the volumes of these solutions served as spillage controls for evaporative loss and accidental disturbances of the rack.
Each day's volumes of ethanol and water drunk were first corrected for the average loss from the two control cages. Data analyzed were consumption (g ethanol / kg body weight, where body weight was taken to be the average of the nearest weight before and after that day) and preference ratio (volume from the ethanol tube / total fluid volume consumed from ethanol + water). We also report water consumption and body weight. As is our custom for assessing genetic differences in preference drinking studies [e.g., see Phillips et al., 1994, to simplify the data reported, we condensed the data for each concentration of each solution (ethanol or tastant) to the average consumption and preference for the second day at each position for each concentration. For example, consumption of 3% ethanol was taken to be the average of Day 2 and 4 intakes. We believe that this procedure reduces the influence of position preferences and allows a better estimate of a genotype's actual interest in the solutions offered. Data from all 3 continuous access preference experiments were similarly treated; in no case did statistical analyses that employed all days’ data change the results or lead to different interpretations of outcomes. Occasionally a tube leaked, yielding a wildly inaccurate reading. Such data were removed from the data set and treated as missing in the analyses. Across the 40 day test with ethanol in Experiment 1, we eliminated fewer than 2% (72 /4800) of the data points. These were not systematically related to either sex or genotype.
Experiment 2: Tastant consumption
Mice from Experiment 1 rested from Days 41–46, with water freely available from their original water bottles. Half of the mice (14 female and 15 male HDID-1 and 14 female and 16 male HS/Npt mice, chosen at random) then progressed to Experiment 2. They did not differ in ethanol consumption or preference from the half not chosen (data not shown). They were divided into 6 groups by random assignment. There were no significant differences among groups in g/kg ethanol consumed (Fs < 1.40, NS). Using the same procedures described for Experiment 1, they were serially tested between Days 47 and 70 for drinking three tastants dissolved in tap water versus tap water. Each tastant was offered for four days at a low and then 4 days at a higher concentration. Mice immediately progressed to a different tastant. All six possible tastant orders were tested. We offered: quinine hemisulfate salt monohydrate (Sigma-Aldrich, St. Louis, MO), at 0.03 mM (0.0012%) and then 0.10 mM (0.004 %); saccharin sodium salt hydrate (Sigma-Aldrich, St. Louis, MO) at 1.6 mM (0.033%) then 3.2 mM (0.066%); and sucrose (Fisher Scientific, Pittsburgh, PA) at 49.7 mM (1.7%) then 124 mM (4.25%). As in Experiment 1, bottle positions were changed each two days. Data were reduced as described for Experiment 1 for analysis. Only preference ratios were analyzed (see Results).
Experiment 3: Tastant consumption in naïve mice
Given the results of Experiment 2 (see Results), we tested additional mice for tastant preference. Twelve female and 12 male HDID-1 mice from the S17 generation and 12 female and 12 male mice from the HS/Npt population (G69) were tested. All mice were 63 – 70 days old and naive at the start of the tastant test. Each tastant was offered for four days at a low and then 4 days at a higher concentration. Concentrations different from those used in Experiment 2 were offered based on Experiment 2 results and the literature, as well as some pilot testing with HDID-1. Upon completion of exposure to a tastant at both concentrations, mice had a respite of 6 days with access to only food and water before progressing to the next tastant. We offered quinine hemisulfate at 0.1 mM (0.004%) and then 0.8 mM (0.032%), saccharin sodium salt hydrate at 3.2 mM (0.066%) then 10 mM (0.205%), and sucrose at 49.7mM (1.7%) then 124.0 mM (4.25 %). All mice received the tastants in the stated order.
Experiment 4: Long-term, limited access 2-bottle ethanol consumption in naïve mice
This experiment was undertaken for a different purpose. Several recent studies have reported that C57BL/6J mice show increased ethanol intake during the withdrawal period after they have experienced cycles of physical dependence induced by ethanol vapor inhalation. We initiated such a study to compare HDID-1 and HS/Npt mice using the protocol established by the Becker group (Lopez & Becker, 2005; Griffin 3rd et al., 2009). This protocol first exposes mice to a prolonged period of daily, limited access, two-bottle preference drinking early in their circadian dark phase before initiating vapor inhalation. Unfortunately, a procedural error after the initial drinking phase compromised our experiment, but we thought the initial drinking data were relevant and so report them here.
Thirty female HDID-1 mice from the S16 generation and 30 female mice from the HS/Npt population (G68) were tested (male mice were unavailable in sufficient numbers). All mice were 81 – 90 days old and naive at the start of the study. Mice were weighed and supplied with a clean cage on Mondays. Throughout the 68-day test and beginning at 30 minutes prior to the onset of the dark cycle, mice were given access to two tubes: one containing a 15% v/v ethanol solution and the other containing tap water. The position of the ethanol tube varied over the time course. For days 1–5, it was on the left, and for days 6–8 it was on the right. During days 9 – 64, the ethanol tube was on the left for two days, then the right for two days. During the last four days (65 – 68), the tube positions were alternated daily. To simplify the data reported, we condensed the data into blocks of 9 to 10 days’ duration. This did not change the interpretation from outcomes from of data analyses using all days.
Statistics
Data were analyzed with ANOVA using Systat (version 13). For Experiment 1, initial analyses of the alcohol drinking data included the factors genotype and sex with alcohol concentration as a repeated measure. As effects of sex did not significantly interact with genotype (see Results), we then collapsed on sex and reanalyzed the data with only genotype and concentration. For post hoc comparisons following significant interactions, we used the Tukey HSD procedure. For Experiment 2, we initially analyzed the tastant data for genotype, sex, concentration, and tastant order. After finding no significant interactions with order or sex (see Results), we collapsed on these factors and analyzed the tastant data with three separate ANOVAs, one for each tastant. For Experiment 3, we first analyzed the data for genotype, sex and concentration. Finding no significant interactions with sex (see Results), we collapsed the data on sex and reanalyzed the three tastants with separate ANOVAs. For Experiment 4, we employed repeated measures ANOVAs.
For all statistical outcomes, we report significant main effects and interactions. Those main effects and interactions not mentioned in the text were found to be not statistically significant (P > .05). Where significant interactions were found, we generally do not report the statistics in support of the significant main effects to improve clarity.
Results
Experiment 1: Two bottle ethanol consumption and preference
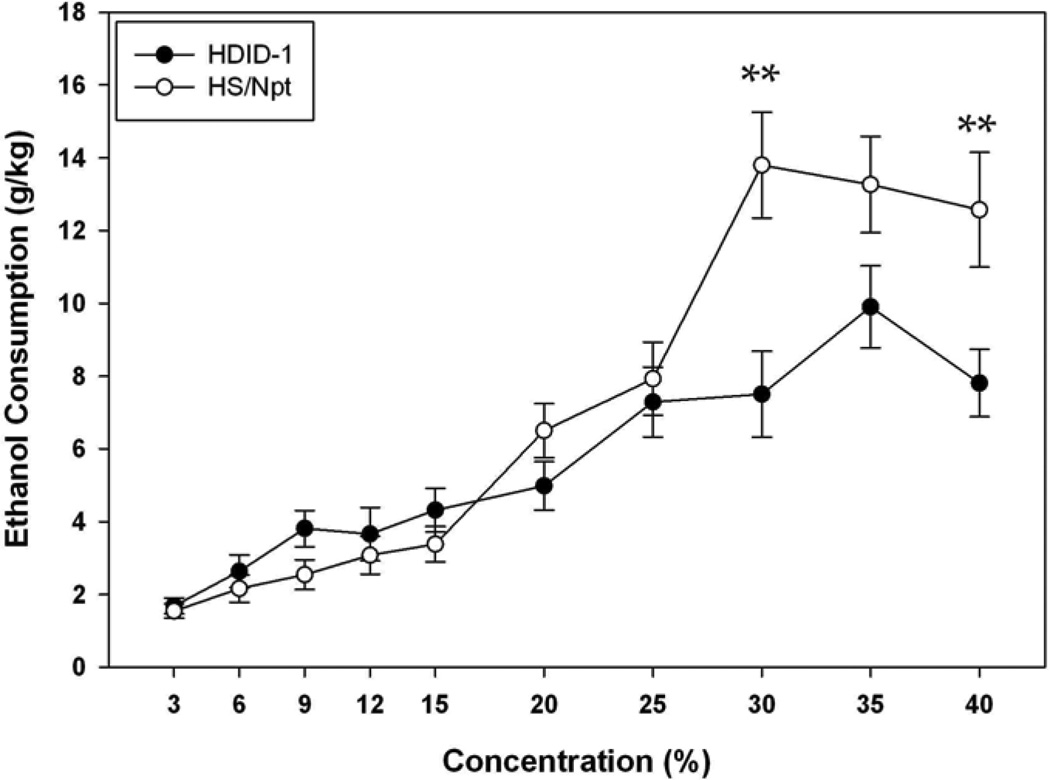
Consumption is shown in Fig. 1. Ethanol consumption increased as the concentration of offered ethanol increased (F[9, 1026] = 50.96, P < 0.0001). As expected, females drank more ethanol than males (F[1, 114] = 5.79, P < 0.05). Neither genotype (F[1, 114] = 3.08, P = 0.08) nor the genotype X sex interaction (F[1, 114] = 0.24, NS) was significant. There was a significant interaction of genotype X concentration (F[9, 1026] = 6.15 , P < 0.0001), but concentration did not interact significantly with sex or sex X genotype (Fs < 1.45, Ps > 0.16). Post hoc tests revealed that while HDID-1 and HS mice did not differ significantly at most concentrations up to 25%, the HDID-1 mice tended to drink more 9% ethanol than did HS mice (P = 0.05). The HS mice drank more ethanol than HDID-1 at 30%, and 40% (Ps < 0.01), and non-significantly more at 35% (P = 0.06). Thus, the large, selected-for difference between genotypes in limited access DID for 20% ethanol (Crabbe et al., 2009) was not paralleled by similar differences in consumption during two-bottle choice.
Figure 1.
Twenty-four hour, two-bottle choice ethanol consumption in HDID-1 vs HS/Npt mice. Mean ± standard error of the mean (S.E.M.) consumption (Y-axis, g/kg) of ethanol at 10 concentrations (X-axis, %) is shown. HDID-1 = High Drinking in the Dark-replicate 1; HS/Npt = heterogeneous stock. ** Significant difference between genotypes (P < 0.01).
Ethanol preference ratio results generally paralleled the consumption differences (see Table 1); however, fewer effects were statistically significant (for example, there was no main effect of sex on preference (F[1, 113] = 0.08, NS]). Across ethanol concentrations, preference for the ethanol solution ranged roughly between 15 and 40% (ie, ethanol was not absolutely preferred over water). There were significant within-subjects effects of concentration (F[9, 1017] = 2.52, P < 0.01) and the interaction of genotype X concentration (F [9, 1017] = 2.41, P < 0.01). While HDID-1 and HS mice did not differ significantly in preference for most concentrations, HDID-1 mice showed significantly greater preference than HS mice for 9% ethanol (F[1, 117] = 4.97, P < 0.05) with a trend for greater preference for 15% (P = 0.07). Conversely, HS mice showed greater preference than HDID-1 mice for 30% ethanol (F[1, 117] = 5.74, P < 0.05).
Table 1.
Ethanol preference ratios and body weights for Experiment 1 (mean +/− S.E.M.a).
| Line | N | Body Weight (g) | Body Weight (g) | Ethanol Preference Ratio (%, by ethanol concentration) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| at start (Day 1) | at end (Day 37) | 3% | 6% | 9% | 12% | 15% | 20 % | 25% | 30% | 35% | 40% | |||
| HDID-1 | ||||||||||||||
| Female | 29 | 21.34 ± 0.48 | 23.69 ± 0.43 | 33 | 28 | 33 | 26 | 30 | 16 | 30 | 24 | 28 | 19 | |
| Male | 30 | 26.48 ± 0.50 | 28.16 ± 0.47 | 41 | 24 | 34 | 21 | 27 | 26 | 27 | 27 | 23 | 32 | |
| Both | 59 | 37 | 26 | 34 * | 24 | 29 | 21 | 29 | 25 * | 26 | 26 | |||
| HS/Npt | ||||||||||||||
| Female | 30 | 20.77 ± 0.41 | 23.11 ± 0.46 | 29 | 19 | 20 | 23 | 16 | 28 | 26 | 33 | 32 | 21 | |
| Male | 30 | 25.74 ± 0.55 | 28.16 ± 0.51 | 29 | 20 | 22 | 16 | 22 | 26 | 23 | 42 | 29 | 23 | |
| Both | 60 | 29 | 19 | 21 | 19 | 19 | 27 | 25 | 37 | 31 | 22 | |||
Standard error of the mean (S.E.M.) of Ethanol Preference ratios ranged from 3.0 – 6.8%.
Significantly different from HS, p < 0.05.
Although the preference ratios generally paralleled ethanol consumption, water consumption per 30 g body weight was greater in HS than in HDID-1 animals (F[1, 114] = 31.5, P = 0.0001) and greater in females than in males (F [1, 114] = 50.1, P = 0.0001) with no significant interaction (F < 0.1). Water consumption increased significantly over time during this experiment (F[9, 1026] = 21.0, P < 0.0001) and interacted significantly with genotype (F[9, 1026] = 2.85, P < 0.01). There were no significant interactions of time with sex or sex X genotype (all Fs < 1.31). These data are shown in Table 2, along with total fluid intake data. For total fluid consumption, significant main effects of sex (F[1,114] = 57.1, P < 0.0001), genotype (F[1,114] = 20.1, P < 0.0001), and time (F[9,1026] = 10.6, P < 0.0001) were observed. Post hoc analyses showed that the main effect of time was primarily due to lower total fluid consumption during the 3% ethanol test (Ps < 0.0001) relative to that during the rest of the experiment. A significant interaction of time and genotype on total fluid intake was also observed (F[9,1044] = 2.37, P = 0.01). Neither of the interactions of time with sex or sex X genotype were significant (both Fs < 1.70). A series of one-way ANOVAs of each time revealed that HS/Npt mice consumed significantly more total fluid per 30 g of body weight than did HDID-1 mice during all ethanol concentrations offered except 3%, 20%, 30% and 35%. Body weights of HDID-1 mice and HS/Npt did not differ at the beginning or the end of the experiment for either sex (Ps > 0.23). Body weights (see Table 1) increased slightly over time (from 20.9 to 23.3 g in females and from 25.1 to 28.0 g in males).
Table 2.
| Experiment 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Consumption [Total Fluid Consumption] (ml/30g of Body Weight) (by ethanol concentration) |
|||||||||||
| Line | 3% | 6% | 9% | 12$ | 15% | 20% | 25% | 30% | 35% | 40% | |
| HDID-1 | |||||||||||
| Female | 3.40 [5.62] | 5.09 [7.01] | 4.50 [6.41] | 5.35 [6.97] | 5.35 [6.63] | 6.58 [7.52] | 5.75 [7.28] | 6.01 [7.05] | 5.73 [7.43] | 5.93 [7.05] | |
| Male | 2.14 [4.39] | 4.02 [5.49] | 3.60 [4.91] | 4.37 [5.13] | 4.42 [5.33] | 4.81 [5.76] | 4.39 [5.09] | 4.69 [5.59] | 4.23 [5.16] | 3.75 [4.33] | |
| Both | 2.76 [4.99] | 4.55 [6.24] | 4.04 [5.65] | 4.85 [6.04] | 4.87 [5.97] | 5.68 [6.63] | 5.06 [6.17] | 5.34 [6.31] | 4.97 [6.29] | 4.82 [5.67] | |
| HS/Npt | |||||||||||
| Female | 3.11 [5.23] | 6.50 [8.00] | 6.41 [7.57] | 6.81 [7.96] | 7.51 [8.31] | 6.54 [8.06] | 6.94 [8.29] | 6.62 [8.55] | 6.28 [7.77] | 7.48 [8.79] | |
| Male | 2.64 [4.35] | 5.35 [6.59] | 5.15 [6.14] | 5.86 [6.65] | 5.61 [6.53] | 5.32 [6.24] | 5.64 [6.71] | 4.41 [6.00] | 5.43 [6.76] | 4.97 [6.05] | |
| Both | 2.88 [4.79] | 5.93 [7.30] | 5.78 [6.85] | 6.33 [7.31] | 6.56 [7.42] | 5.93 [7.15] | 6.29 [7.50] | 5.52 [7.28] | 5.85 [7.27] | 6.23 [7.42] | |
| Experiment 2 | |||||||
|---|---|---|---|---|---|---|---|
| Water Consumption [Total Fluid Consumption] (ml/30g of Body Weight) (by tastant concentration) |
|||||||
| Quinine | Saccharin | Sucrose | |||||
| Line | 0.03mM | 0.10mM | 1.6mM | 3.2mM | 49.7mM | 124.0mM | |
| HDID-1 | |||||||
| Female | 3.20 [6.27] | 3.16 [6.53] | 3.02 [6.32] | 3.94 [6.91] | 3.53 [6.51] | 3.56 [7.73] | |
| Male | 2.59 [5.13] | 2.71 [5.53] | 2.76 [5.54] | 3.11 [5.61] | 2.85 [5.81] | 3.21 [5.56] | |
| Both | 2.88 [5.68] | 2.93 [6.01] | 2.89 [5.92] | 3.51 [6.24] | 3.18 [6.15] | 3.38 [6.61] | |
| HS/Npt | |||||||
| Female | 4.15 [8.35] | 3.46 [7.81] | 4.85 [8.83] | 4.16 [7.76] | 3.88 [8.58] | 5.18 [8.82] | |
| Male | 3.41 [5.97] | 2.67 [5.72] | 2.72 [5.70] | 3.08 [6.11] | 2.47 [6.08] | 3.34 [6.59] | |
| Both | 3.76 [7.04] | 3.04 [6.69] | 3.71 [7.16] | 3.58 [6.88] | 3.13 [7.25] | 4.19 [7.63] | |
In Experiment 1, the standard error of the mean (S.E.M.) of water consumption values ranged from 0.25 – 0.77 ml/30g BW. S.E.M. of fluid consumption values ranged from 0.20 – 060 ml/30g BW.
In Experiment 2, the standard error of the mean (S.E.M.) of water consumption values ranged from 0.20 – 0.75 ml/30g BW. S.E.M. of fluid consumption values ranged from 0.20 – 0.77 ml/30g BW.
Experiment 2: Tastant consumption
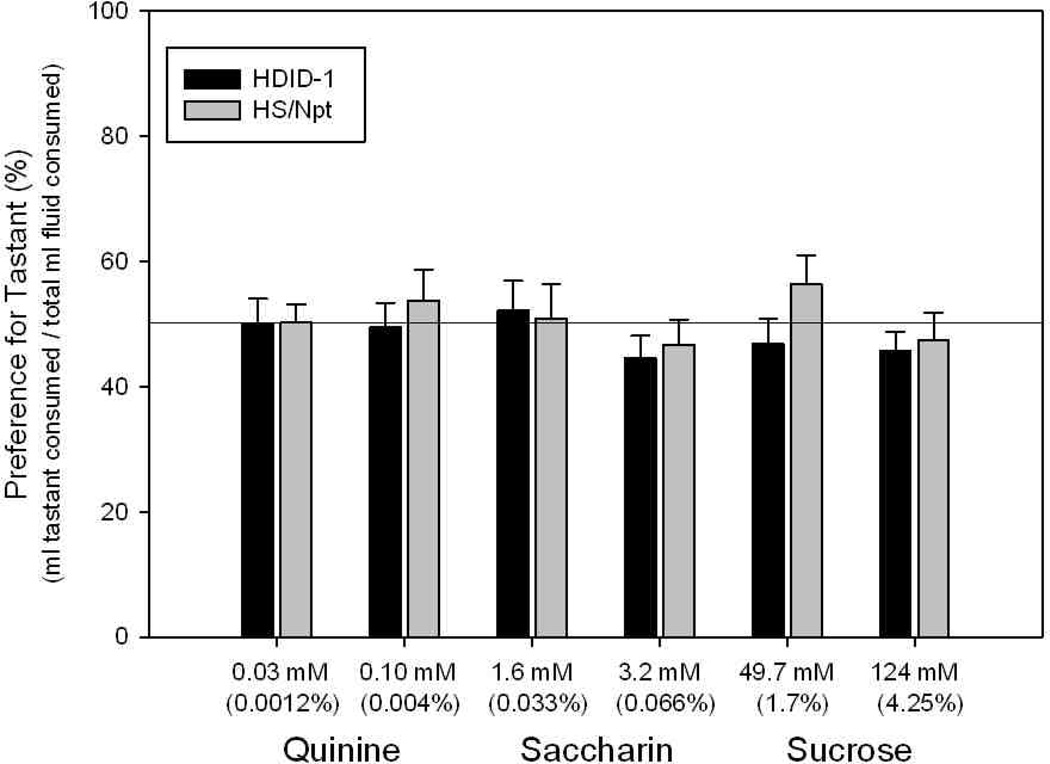
Tastant data are shown in Fig. 2 and Table 2. Because there were very few mice (n = 2–3) in each genotype X sex X order condition, we did not formally analyze the effects of order of tastant testing. However, visual inspection of the data from the six different orders did not reveal any apparent influence of order of tastant exposure. We therefore report data collapsed over order. There were no significant differences between genotypes or sexes, nor an interaction for preference ratios of any tastant at either concentration (all Fs[1,54 – 55] < 2.27, Ps > 0.13). Visual inspection of the preference ratios from this experiment (Fig. 2) revealed that the animals did not show preference for either sweet tastant as expected; nor did they avoid quinine. Indeed, all preference ratios were approximately 50%. Thus, the preference ratio data offered no evidence that the animals could any longer make taste discriminations after their extensive exposure to ethanol solutions, providing the rationale for repeating this experiment with naïve mice.
Figure 2.
Tastant preferences (%) during 24 hr, two-bottle choice in HDID-1 vs HS/Npt mice tested after ethanol consumption (see Figure 1). Bars depict mean ± S.E.M. preference (Y-axis, %) for the stated concentration of each of three tastants: quinine, saccharin, and sucrose (X-axis). HDID-1 = High Drinking in the Dark-replicate 1; HS/Npt = heterogeneous stock. No differences between genotypes were observed. No preference or avoidance of any tastant was observed.
Experiment 3: Tastant consumption in naïve mice
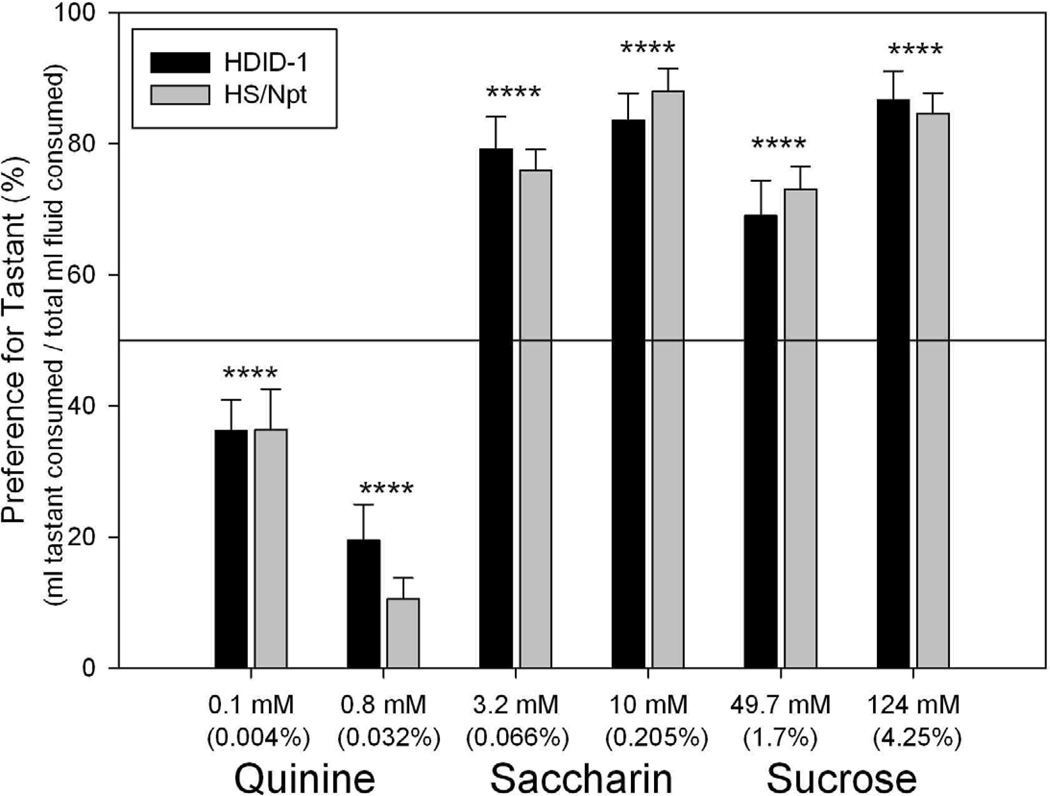
Tastant data are shown in Fig. 3 and Table 3. One male HDID-1 mouse died on day 33 of the experiment (during sucrose consumption), so its data were eliminated. In contrast to the results of Experiment 2, visual inspection of Figure 3 shows that the animals preferred both sweet tastants and avoided quinine. Paired t-tests vs a preference ratio of 50% supported this interpretation (|t|s > 2.40, Ps < 0.05). However, there were no significant differences between genotypes, sexes, or their interaction in preference for or avoidance of any tastant at either concentration (all Fs[1, 43 – 44] < 2.23, Ps > 0.14). There were also no significant interactions of concentration of tastant with genotype, sex, or their interaction (all Fs[1, 43 – 44] < 0.91, Ps > 0.34). The 10 mM saccharin and 124 mM sucrose solutions were most preferred (and mice drank significantly more water when these were offered relative to a lower concentration of the same tastant). The 0.8 mM quinine solution was the most avoided (where mice drank more water than they did with the lower concentration of quinine). No effects of genotype were observed on water intake during any tastant test. Analysis of total fluid consumption (per 30g of body weight) showed significant main effects of sex (F[1,43] = 21.7, P < 0.0001) and tastant period (F[5,215] = 10.6, P < 0.0001), but no other main or interaction effects were significant (all Fs[1–5, 43 – 215] < 1.0, Ps > 0.36). Females drank more than males, and total fluid consumption increased over the course of the experiment (Table 3).
Figure 3.
Tastant preferences (%) during 24 hr, two-bottle choice in ethanol-naïve HDID-1 vs HS/Npt mice. Bars depict mean ± S.E.M. preference (Y-axis, %) for the stated concentration of each of three tastants: quinine, saccharin, and sucrose (X-axis). HDID-1 = High Drinking in the Dark-replicate 1; HS/Npt = heterogeneous stock. No differences between genotypes were observed. Significant preferences for both sweet tastants and significant avoidance of both quinine concentrations were observed (|ts| > 4.79, P < 0.0001).
Table 3.
Water and Total Fluid Intakes for Experiment 3 (mean +/− S.E.M. a).
| Water Consumption [Total Fluid Consumption] (ml/30g of Body Weight) (by tastant concentration) |
|||||||
|---|---|---|---|---|---|---|---|
| Line | 0.1mM quinine |
0.8mM quinine |
3.2mM saccharin |
10.0mM saccharin |
49.7mM sucrose |
124.0mM sucrose |
|
| HDID-1 | |||||||
| Female | 4.18 [6.38] | 5.81 [6.76] | 1.98 [7.49] | 0.88 [7.53] | 2.17 [7.26] | 0.95 [9.11] | |
| Male | 2.77 [4.71] | 4.19 [5.78] | 1.26 [6.08] | 1.19 [7.09] | 2.21 [6.28] | 0.57 [6.81] | |
| Both | 3.47 [5.55] | 5.00 [6.27] | 1.62 [6.79] | 1.04 [7.31] | 2.19 [6.77] | 0.76 [8.01] | |
| HS/Npt | |||||||
| Female | 4.00 [6.49] | 6.01 [6.85] | 2.13 [7.31] | 1.09 [7.26] | 2.12 [6.87] | 1.05 [8.06] | |
| Male | 3.15 [5.02] | 4.59 [5.20] | 1.02 [5.29] | 0.47 [6.14] | 1.07 [5.08] | 0.88 [6.04] | |
| Both | 3.57 [5.75] | 5.30 [6.02] | 1.57 [6.30] | 0.78 [6.70] | 1.60 [5.98] | 0.96 [7.05] | |
Standard error of the mean (S.E.M.) of water consumption values ranged from 0.18 – 0.75 ml/30g BW. S.E.M. of fluid consumption values ranged from 0.26 – 1.09 ml/30g BW.
Experiment 4: Long-term, limited access 2-bottle ethanol consumption in naïve mice
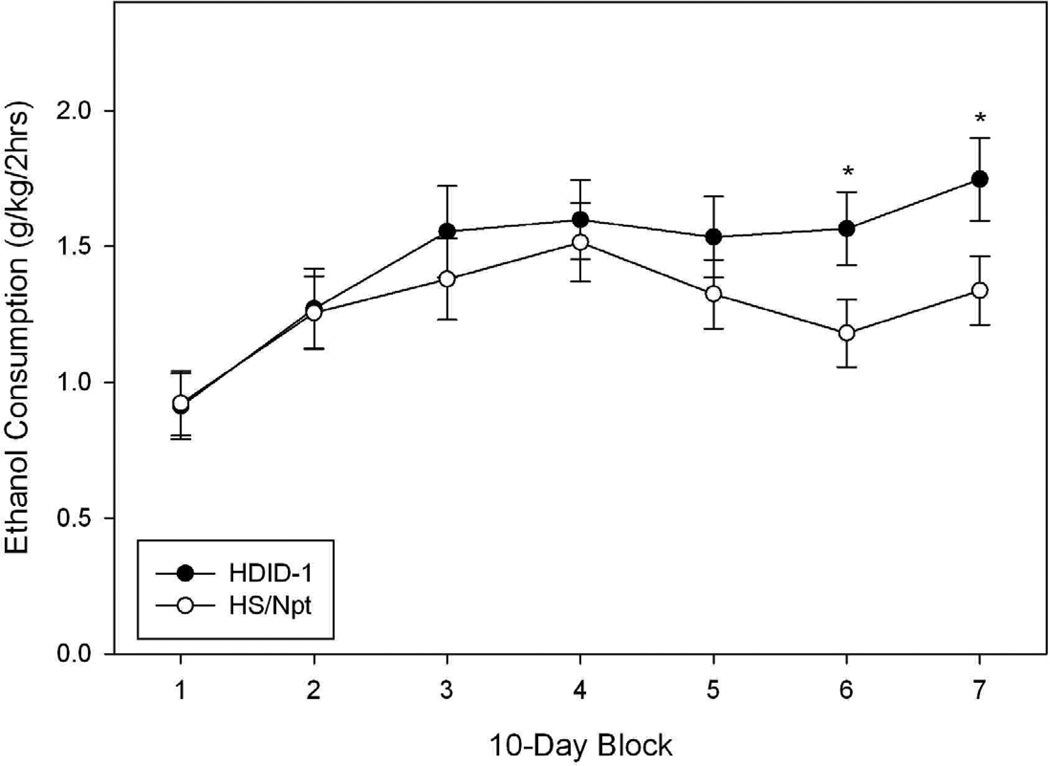
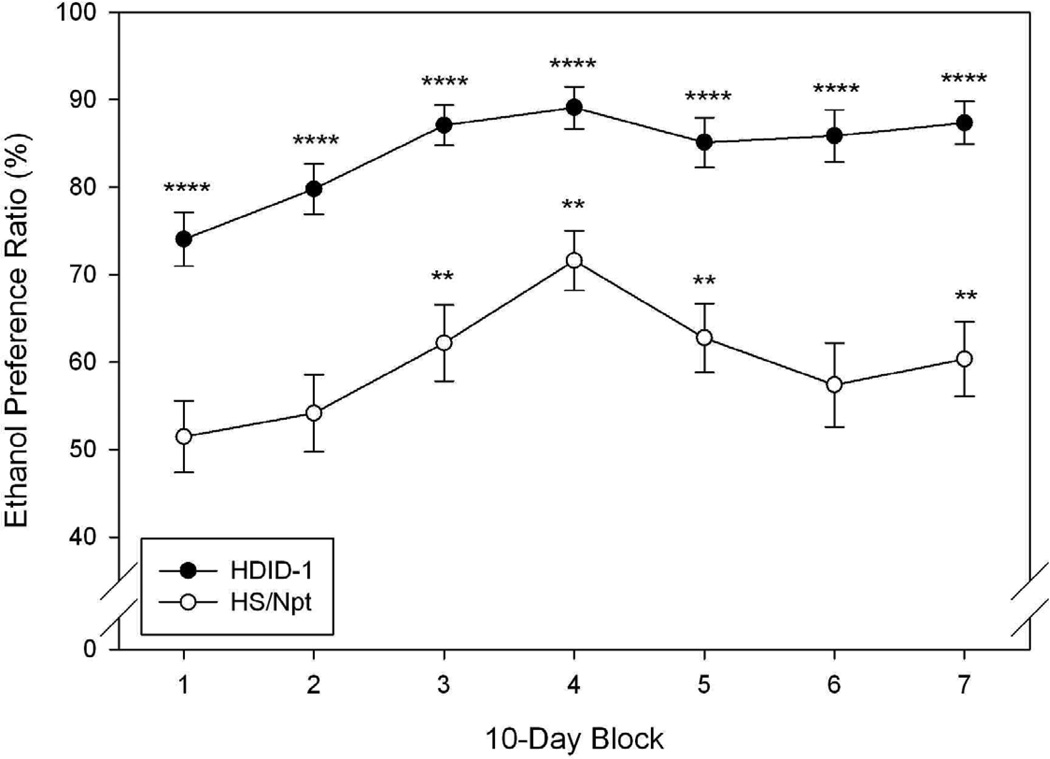
Data from this experiment are shown in Fig. 4, presented as the average daily value in 9 to 10 day blocks. Blocks 1 and 7 represent the average daily value of days 1 – 9 and 60 – 68, respectively, while blocks 2 – 6 each represent the average daily values of 10 consecutive days (i.e., block 2: days 10 – 19, block 3: days 20 – 29, etc.). Fig. 4A shows that the average daily consumption of 15% v/v ethanol in the presence of water generally increased from 0.9 g/kg/2 hr to as high as 1.75 g/kg/2 hr over the course of the study. A significant main effect of block was detected using a repeated measures ANOVA (F[6, 348] = 14.35, P < 0.0001). The main effect of genotype was not significant (F[1, 58] = 1.2, NS), but there was a trend toward a significant interaction of genotype and block (F[6, 348] = 2.05, P = 0.06). Post hoc tests showed significant differences between HDID-1 and HS in blocks 6 and 7 (block 6: F[1,58] = 4.36, P < 0.05; block 7: F[1,58] = 4.30, P < 0.05).
Figure 4.
Limited access (2 hr), two bottle choice ethanol consumption (A) and preference ratio (B) in HDID-1 vs HS/Npt female mice. A: Mean ± standard error of the mean (S.E.M.) daily consumption (Y-axis, g/kg/2hrs) of 15% ethanol over a 68-day period (averaged over 9–10 day blocks, X-axis) is shown. HDID-1 = High Drinking in the Dark-replicate 1; HS/Npt = heterogeneous stock. * Significant difference between genotypes (P < 0.05). B: Mean ± S.E.M. daily ethanol preference (Y-axis, calculated as the ratio of ethanol (mls)/total fluid consumption (mls)) over blocks (X-axis). Significant preference for ethanol (**, |ts| > 2.4, P ≤ 0.01; ***, |ts| > 7.84, P <0.0001). The HDID-1 ethanol preference ratios were significantly greater than those of HS/Npt (Fs > 17.5, Ps ≤ 0.0001).
From Fig. 4B, it is apparent that the HDID-1 mice displayed greater preference for 15% ethanol than did the HS/Npt mice. Analysis of preference scores by repeated measures revealed significant main effects of genotype (F[1, 58] = 32.59, P < 0.0001) and block (F[6, 348] = 15.35, P < 0.0001), but no significant interaction (F = 1.57, P > 0.05). While HDID-1 mice showed significant preference for 15% ethanol during all blocks vs a preference ratio of 50% (|ts| > 7.8, Ps < 0.0001 using one sample t-tests, HS/Npt mice showed significant preference only during blocks 3, 4, 5, and 7 (|ts| > 2.4, Ps < 0.05). Analysis of water consumption data showed a pattern of results similar to ethanol preference in that greater ethanol preference was associated with less water and total fluid consumption (see Table 4). Analysis of total fluid consumption (per 30g of body weight) showed significant main effects of genotype (F[1,58] = 5.04, P < 0.05) and block (F[6,348] = 8.3, P < 0.0001), but the interaction was not significant (F[6, 3485] = 1.91, P = 0.08). HS/Npt mice drank more fluid than did HDID-1 mice (Table 4).
Table 4.
Water and Total Fluid Intakes for Experiment 4 (mean +/− S.E.M. a).
| Water Consumption [Total Fluid Consumption] (ml/30g of Body Weight/2hrs) (by 10-day blockb) |
|||||||
|---|---|---|---|---|---|---|---|
| Line | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| HDID-1 | 0.09 [0.33] | 0.11 [0.46] | 0.06 [0.45] | 0.07 [0.47] | 0.09 [0.47] | 0.09 [0.49] | 0.10 [0.54] |
| HS/Npt | 0.28** [0.52] | 0.37** [0.69] | 0.26** [0.61] | 0.19** [0.58] | 0.27** [0.60] | 0.29** [0.59] | 0.31** [0.64] |
Standard error of the mean (S.E.M.) of Water consumption values ranged from 0.02 – 0.05 ml/30g BW/2 hrs. S.E.M. of Fluid consumption values ranged from 0.04 – 0.07 ml/30g BW/2hrs.
Blocks 1 & 7 were calculated as the average of 9 days; Blocks 2 – 6 were calculated as the average of 10 days.
HS mice drank significantly more water than did HDID-1 mice (P < 0.01).
Discussion
Considering first the g/kg consumption of ethanol during 24 hr two-bottle choice, these results suggest that the genes influencing two-bottle preference for ethanol are generally somewhat distinct from those that lead HDID-1 mice to drink large amounts during a limited access, single bottle test with 20% ethanol in the circadian dark. This appears to be true at EtOH concentrations between 3% and 25% v/v. At concentrations of 30 and 40%, HS mice drank significantly greater amounts of ethanol than did HDID-1.
When we consider the preference ratio data in this experiment, the picture becomes a little more complicated. Neither genotype showed particularly high preference for ethanol vs water in Experiment 1; no average preference ratio exceeded 37% (see Table 1). In comparison, after similar two-bottle tests of escalating concentrations of ethanol, several inbred strains of the C57/C58 lineage typically show preference ratios > 90% for ethanol concentrations between 3 and 12–15% (Yoneyama et al., 2008; Wahlsten et al., 2006). In Experiment 1, the differences in preference between HDID-1 and HS mice were small, were only seen at two concentrations, and were not in the same direction. Ethanol preference ratios do not always follow consumption data exactly, and are well known to show an inverted-U relationship with increasing concentration (McClearn, 1968a). This is thought to reflect the joint influences of motivation to experience ethanol's post-ingestive effects [e.g., (Belknap et al., 1977)] and the different taste sensations elicited by different concentrations [e.g. (Belknap et al., 1993; Bachmanov et al., 2003)].
We earlier reported a fairly strong genetic correlation between g/kg ethanol intake on the 4th day of the standard DID test (Rhodes et al, 2007) and g/kg intake on the final two days of a brief two-bottle preference study (Belknap et al., 1993). Yet, in the current experiments, we found only modest evidence supporting such a correlation (i.e., higher preference in HDID than HS only at 9%). There are several potential reasons for this. Data from Belknap et al (1993) were taken from the final two days of a protocol where male mice first drank 3% ethanol or water for 3 days, then 6% ethanol or water for 3 days, and finally, 10% ethanol or water for 4 days. There were 7 inbred strains in common between the two studies, including C57BL/6J, and the strain mean correlation was r = 0.70 (P = .08). Removal of the C57BL/6J strain from this correlation lowered it to r = 0.30 (NS). Thus, the correlation among inbred strain means could have been a false positive due to low N. However, the Belknap et al data are quite representative of other inbred strain ethanol preference data, and correlate with other extensive data sets with r values exceeding 0.90 (Wahlsten et al, 2006). We have since tested 17 additional inbred strains for DID, and there are now 14 strains in common between the Belknap et al (1993) data set and our DID data (Crabbe et al., unpublished data and Rhodes et al., 2007). The genetic correlation across 14 strains remained r = 0.70, P < 0.01); removal of the C57BL/6J strain from the analysis lowered the correlation coefficient slightly, but not significantly (r = 0.59, P < 0.05). Thus, the preponderance of evidence from inbred strains suggests genetic overlap between DID for 20% and two-bottle preference for 10% ethanol.
An additional consideration is the genetic animal model employed to estimate the strength of the genetic association between DID and preference. All inbred mouse strains lack heterozygosity at all genes. Hence, dominance genetic variation (which can reflect the interactive effects between an individual’s pair of alleles at a single gene) is absent in such populations. Dominance has been shown to influence continuous access two-bottle preference drinking (Blednov et al., 2005, 2010) and DID limited access drinking (Phillips et al, 2010). In the HDID selected lines, dominance may have played a role in either type of drinking, but has not been assessed.
Our test for genetic correlation using a selected line was able to ask the question only unidirectionally: selection for genes affecting DID appeared to have only modest effects on preference drinking. The parallel experiment would be a test of mice selectively bred for high vs low preference (e.g., HAP vs LAP mice, see Grahame et al, 1999a, Oberlin et al., 2010) for DID. HAP mice drink significantly more ethanol than LAP mice during sessions where access to alcohol or water is limited to 2-hr periods, a situation resembling the 2-bottle version of the DID test (Grahame et al, 1999b). In late 2003, we tested male HAP-1 and LAP-1 mice (generation 46, obtained from Dr. Nick Grahame) using a modified 4 day, 20%, 1-bottle DID test (daily 4 hr access, with hourly readings). Consistent with the hypothesis that high preference-drinking genotypes would consume more ethanol in the DID test, HAP-1 mice drank more ethanol than LAP-1 mice and had higher BECs (7.8 ± 1.1 vs. 2.5 ± 0.4 g/kg and 0.96 ± 0.15 vs. 0.15 ± 0.05 mg/ml, respectively). This relatively high intake of HAP mice in the DID procedure has been confirmed by others (N. Grahame, S. Boehm II, unpublished studies using only HAP genotypes). Therefore, long-term selective breeding for high vs. low preference in a 2-bottle choice continuous access procedure has produced lines of mice that differ in DID. There are other more technical reasons why estimates of genetic correlation may be found to be significant when selecting for Trait A and examining Trait B, but not vice versa, and why experiments with selected lines may not give the same result as those with inbred strains (for discussion, see Crabbe et al, 1990; Crabbe, 1999; Henderson, 1989). Overall, there is reasonable evidence for some genetic overlap between the two traits.
In extended tests with the range of low to moderate ethanol concentrations (e.g., 3% – 15%), g/kg intake tends to be maintained approximately stably at a genotype-specific value, and the animals appear to adjust their relative preference to maintain an approximate g/kg dose level of ethanol, although this level does vary modestly with concentration between 12 and 24% ethanol (McClearn, 1968a). In Experiment 1, mice were tested only using a series of ascending concentrations, so carryover effects could play a role. These results in fact tended to show a different pattern -- small and gradual increases in g/kg intake but relatively stable preference ratios as concentrations increased. As the highest concentration (≥ 30%) were introduced, HDID-1 mice showed the expected reductions in preference, while the HS/Npt mice did not. In fact, the HS mice showed increasing preference ratios as well as intakes over time. For an extended discussion of the role of concentration in absolute and relative alcohol intake, see (McClearn, 1968a).
The basis for the very high consumption of EtOH solutions ≥30% in HS/Npt control mice is unknown. This intake is in the range of C57BL/6J mice, a genotype known for high EtOH preference. A possibility suggested by an anonymous reviewer is that the earlier experience drinking lower ethanol concentrations affected the ability of the HS mice to discriminate among the offered flavors toward the end of the experiment. That is, they may have developed the taste indifference revealed in Experiment 2 (see discussion below) earlier than the HIDI-1 mice. On the other hand, they did show significant avoidance of these high ethanol concentrations relative to water. Preference ratios were 37, 31, and 22% for the 30, 35 and 40% ethanol concentrations, respectively. The HS mice were able to achieve very high intakes of the highest ethanol concentrations with low preference scores in Experiment 1 because they also drank significantly more water than did the HDID-1 mice (Table 2). When naïve mice of these genotypes were offered 15% ethanol vs water for two hrs/day, consumption, but not preference, was equivalent, at least across the first several weeks (Fig 4). Gradually, however, both genotypes increased their ethanol consumption and this increase from Block 7 over Block 1 was greater in HDID-1 mice than in HS mice (91% vs. 45%, respectively). Preference was clearly greater in HDID-1 than HS mice throughout Experiment 4 (74 – 89% vs. 51 – 71%, respectively), and in contrast to the results of Experiment 1, both genotypes showed significant preference for 15% ethanol during the 2 hr period (all blocks for HDID-1 mice but only 4/7 blocks for HS mice; see Fig. 4B).
HS mice drank significantly more water and total fluid than did HDID-1 mice during some experiments. In Experiment 1 with 24 hr access (Table 2), the line difference in water intake was about 1 ml on average (range 0.1 – 1.7 ml across the different ethanol concentrations). This accounted for the average difference in total fluid intake in this experiment. In Experiment 4 with 2 hr access periods (Table 4), the line difference in water intake was about 0.2 ml on average (range 0.12 – .26 ml) and that for total fluid was slightly less (0.15 ml). Interestingly, the genotypes did not differ in either water or total fluid intake during Experiment 3 (Table 3), and so this finding may be specific to two-bottle choice experiments for water vs ethanol but not water vs other tastants. We do not know the basis for this difference, but it appears to be a consistent correlated response, suggesting a genetic basis shared with high ethanol DID. The genetic relationship is inverse, with the HDID genotype drinking less water than the control HS stock. The difference does not appear to be because the HDID-1 line is drinking less water or fluid than do “normal” mice, as a survey of inbred strain data from the Mouse Phenome Database showed that mice of 7 of the 8 strains that make up the HS/Npt drink water in about the same range (1.82 – 7.68 ml/30 g body weight; Seburn, 2010, MPD:Seburn1:9238). Furthermore, data from our recent inbred strain survey using the DID test also shows that the range of 2 hr water intake in the HDID-1 line is consistent with that of the progenitor strains (0 – 1.45 ml/30 g body weight in 2 hr; Crabbe et al., unpublished data). The difference is not due to obvious differences in body size, as the genotypes do not differ in body weight and the intakes we report were indexed as ml/ 30 g. Some difference in body composition could be involved, or a difference in the numerous hypothalamic and extrahypothalamic peptides and hormones involved in water balance and fluid intake regulation. For example, in one report, deletion of the arginine vasopressin 1 receptor gene produced mice with high ethanol preference (Sanbe et al, 2008), although this was not seen by a different group that deleted the same gene (Caldwell et al., 2006).
Experiments 2 and 3 were undertaken to test the hypothesis that differences in taste preference were important for the differences in ethanol intake between genotypes. However, the original tastant data (Experiment 2) revealed a major problem, as they offered no evidence that either genotype either preferred or avoided any tastant at any concentration. Experiment 3, however, provided clear evidence that naive HDID-1 and HS/Npt mice did not differ in their preference for sweet solutions, nor in their avoidance of the bitter solutions of quinine. Because the outcomes for both a caloric (sucrose) and non-caloric (saccharin) solution were similar, the differences in ethanol intake between genotypes at high concentrations are not likely to be driven by calorie seeking.
Why did Experiment 2 fail completely? We considered two possible explanations. First, the concentrations chosen could have been inappropriate. If the tastants were imperceptible to both genotypes, they would have had no basis for discriminating between the two bottles and 50% preference ratios would be expected. A second possibility was that the exposure to very high ethanol concentrations may have affected in some way the subsequent tastant tests. For example, the ingested ethanol might have denatured proteins in the mouth or damaged taste bud cells. Some other carryover effects from the high ethanol concentration drinking may have affected results. For example, the taste of high concentration ethanol may have conditioned an aversion to sucrose and saccharin.
We deemed the first hypothesis unlikely given the use of these concentrations in the literature with multiple genotypes of mice (Blednov et al., 2005; Crabbe et al., 2006; Lush, 1981; Lush, 1984; Lush, 1989). We also consulted with Alex Bachmanov of the Monell Chemical Senses Institute, an expert on mouse taste, and he provided us with unpublished tastant preference drinking data that suggested we should have seen strong preference for sucrose and saccharin. Data for quinine were more sparse. We therefore pilot-tested some other concentrations for the three tastants, leading to our choices for Experiment 3.
The second hypothesis, suggested to us by our colleagues John Belknap and Aaron Janowsky, was conceded as a possibility by Alex Bachmanov, who pointed out that taste bud cells turn over in about 1–2 weeks. We have no physiological data to bring to bear on this hypothesis. Dr. Bachmanov also pointed out that exposure to drinking the highly-preferred tastant sucrose in particular could taint further taste experiments with the same mice. Based on this advice, we tested mice in Experiment 3 in the order deemed by Dr. Bachmanov least likely to lead to carryover effects, and we added a water-only washout between tastant tests. Carryover effects, however, could not easily explain the failure of the mice to avoid quinine solutions in Experiment 2. They also could not explain the fact that we saw no evidence of an effect of order of testing during Experiment 2.
There are substantial data in both mice and rats suggesting that there is some degree of coordinate genetic influence on preference for ethanol and sweet taste (Lemon et al., 2004; Kampov-Polevoy et al., 1999; Belknap et al., 2008). Our results suggest that two-bottle 24-hour access ethanol preference and preference for sweet solutions are not genetically correlated in the HDID-1 and HS genotypes. Genotypic differences in ethanol preference were clear throughout many weeks of limited access testing (Experiment 4), but the lack of a genotypic difference in preference for either sucrose or saccharin was clear at the outset of testing (Experiment 3). It is possible, however that long-duration tests for sucrose or saccharin preference could reveal the emergence of greater sweet preference in HDID-1.
Many studies have tested the effects of genetic engineering of either a knockout or an over expressing transgenic for a target gene on ethanol preference. When these studies were reviewed in 2006, 79 genes had been targeted and tested for ethanol preference, and most had also assessed the possibility that sucrose or saccharin or quinine preference had been affected (Crabbe et al., 2006). Many of those studies used the same mice tested for ethanol preference to assess preference for other tastants, much as we did for Experiments 1 and 2. We conclude that attempts to control EtOH preference studies for taste sensitivity and preference or avoidance of other tastants should be cautiously interpreted if tastants are offered after high EtOH concentrations. The safest procedure would be a completely between-subjects design for each tastant, starting with naive mice, or a serial test with naïve mice in which sucrose preference is left to last, as in our Experiment 3.
We doubt that any of the ethanol drinking data reported in this set of studies is of substantial pharmacological significance. By the end of Experiment 4, HDID mice were showing a high preference for 15% ethanol (90%, similar to that shown by C57BL/6J mice) and were ingesting about 1.7 g/kg during each 2 hr test. In a test of two-bottle DID with HDID-1 mice from the 9th selected generation, they drank about 3 g/kg in the 4 hr test but reached average BECs of only 0.12 mg/ml (Crabbe et al, 2009). Mice from the later generations reported here most likely drank more during their 2 hr tests than had the 9th generation animals reported earlier, but we doubt that they drank enough more to achieve pharmacologically significant blood levels. They were also only drinking 15% ethanol in Experiment 4 rather than 20% when tested in S9. Intakes of 1.5 – 2.0 g/kg ethanol in a 2 hr limited access two-bottle preference study resemble those reported for C57BL/6J (Rhodes et al., 2007) and are less than the 3 g/kg intakes seen in HAP mice (Grahame et al., 1999b), genotypes with known high preference for ethanol. Estimates of BEC in HAP mice after 2 hr access with water available also reveal modest BECs, averaging less than 0.5 mg/ml, although a few mice reached BECs greater than 1.0 mg/ml. While HDID mice are willing to ingest sufficient ethanol in the single bottle DID test to reach behavioral intoxication (Crabbe et al, 2009), it remains to be tested whether the current generation of mice will reach average BECs > 1.0 mg/ml when water is an alternative. On the other hand, BECs in the range between 0.5 and 1.0 mg/ml can have significant behavioral effects on mice. BECs in this range induce locomotor stimulation in FAST (Shen et al., 1995) and a genetically heterogeneous (HS) stock of mice (Lessov and Phillips, 1998) and facilitated performance on an accelerating rotarod task in some inbred strains (Rustay et al, 2003).
Finally, the difference in genetic outcomes between Experiment 1 and 4 may reflect the different exposure models employed. There was a clear difference in preference between genotypes during 2 hr daily limited access, with an absolute difference in intake developing after a few weeks. Perhaps the daily, but limited, access to alcohol across the circadian day led to greater intake in HDID-1. The temporal pattern in which alcohol is offered clearly affects intake in rodents. Early studies with rats showed that offering 10% ethanol every other day for 24 hr periods led to an escalation in intake (Wise, 1973), and this pattern has been employed more recently to increase intakes of higher concentrations as well (Simms et al., 2008). The role of frequency and duration of ethanol access in HDID mice remains to be explored.
Acknowledgements
We thank J.P. Schlumbohm, C.-H. Yu, and A.J. Cameron for assistance. We also thank Alex Bachmanov, John Belknap and Aaron Janowsky for their helpful suggestions, and Nick Grahame and Steve Boehm for sharing unpublished information. Supported by NIH-NIAAA INIA-West consortium grant AA13519, NIH grant AA10760, and the US Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Anderson SM, McClearn GE. Ethanol consumption: selective breeding in mice. Behav. Genet. 1981;11:291–301. doi: 10.1007/BF01070812. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk And LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin. Exp. Res. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Belknap ND, Berg JH, Coleman R. Preabsorptive vs. postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behav. Genet. 1977;7:413–425. doi: 10.1007/BF01066776. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Metten P, Beckley EH, Crabbe JC. Multivariate analyses reveal common and drug-specific genetic influences on responses to four drugs of abuse. Trends Pharmacol. Sci. 2008;29:537–543. doi: 10.1016/j.tips.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J × FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin. Exp. Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice as genetic models of high alcohol consumption. Behav. Genet. 2010;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Stewart J, Wiedholz LM, Millstein RA, Iacangelo A, Holmes A, Young WS, 3rd, Wersinger SR. The acute intoxicating effects of ethanol are not dependent on the vasopressing 1a or 1b receptors. Neuropeptides. 2006;40:325–337. doi: 10.1016/j.npep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Animal models in neurobehavioral genetics: Methods for estimating genetic correlation. In: Mormede P, Jones BC, editors. Neurobehavioral Genetics: Methods and Applications. Boca Raton, FL: CRC Press; 1999. pp. 121–138. [Google Scholar]
- Crabbe JC. Neurogenetic studies of alcohol addiction. Philos. Trans. R. Soc. Lond B Biol. Sci. 2008;363:3201–3211. doi: 10.1098/rstb.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu C-H, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol. Psychiat. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: Studies in rodent genetic models. Behav. Genet. 2010 doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict. Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin. Exp. Res. 1990;14(2):141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: Scale factors in the model. Proc. Natl. Acad. Sci. USA. 1984;81:3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Grose AM. Blood alcohol concentrations after scheduled access in high-alcohol- preferring mice. Alcohol. 2003;31:99–104. doi: 10.1016/j.alcohol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li T-K, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav. Genet. 1999a;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li T-K, Lumeng L. Limited access alcohol drinking in high- and low-alcohol preferruing selected lines of mice. Alcohol. Clin. Exp. Res. 1999b;23:1015–1022. [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin. Exp. Res. 2009;33:1892–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ND. Interpreting studies that compare high- and low-selected lines on new characters. Behav. Genet. 1989;19:473–502. doi: 10.1007/BF01066250. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Le AD, Israel Y, Juzytsch W, Quan B, Harding S. Genetic selection for high and low alcohol consumption in a limited- access paradigm. Alcohol Clin. Exp. Res. 2001;25:1613–1620. doi: 10.1111/j.1530-0277.2001.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J. Neurophysiol. 2004;92:536–544. doi: 10.1152/jn.00097.2004. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Phillips TJ. Duration of sensitization to the locomotor stimulant effects of ethanol in mice. Psychopharmacology. 1998;135:374–382. doi: 10.1007/s002130050525. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacol. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice. I. Sucrose octaacetate. Genet. Res. 1981;38:93–95. doi: 10.1017/s0016672300020425. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice III. Quinine. Genetic Research Cambridge. 1984;44:151–160. doi: 10.1017/s0016672300026355. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet. Res. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- Mardones J, Segovia-Riquelme N. Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol. Teratol. 1983;5:171–178. [PubMed] [Google Scholar]
- McClearn GE. Genetics and motivation of the mouse. In: Arnold WJ, editor. Nebraska Symposium on Motivation. Lincoln, Nebraska: University of Nebraska Press; 1968a. pp. 47–83. [Google Scholar]
- McClearn GE. The use of strain rank orders in assessing equivalence of techniques. Behav. Res. Meth. Instrument. 1968b;1:49–51. [Google Scholar]
- McClearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Quart. J. Stud. Alcohol. 1959;20:691–695. [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov Y, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc. Natl. Acad. Sci. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A, Grahame N. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav. Genet. 2010 Sep 19; doi: 10.1007/s10519-010-9394-5. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol. Clin. Exp. Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Reed C, Burkhart-Kasch S, Li N, Hitzemann R, Yu C-H, Brown LL, Helms ML, Crabbe JC, Belknap JK. A method for mapping intralocus interactions influencing excessive alcohol drinking. Mamm. Genome. 2010;21:39–51. doi: 10.1007/s00335-009-9239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Richter CP, Campbell KH. Alcohol taste thresholds and concentrations of solution preferred by rats. Science. 1940;91:507–508. doi: 10.1126/science.91.2369.507. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc. Natl. Acad. Sci. USA. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Sharpe AL, Tsivokovskaia NO, Weitemier AZ. Ethanol self-administration during the circadian dark phase. Alcohol Clin. Exper. Res. 2003;27:S183A. [Google Scholar]
- Sanbe A, Takagi N, Fujiwara Y, Yamauchi J, Endo T, Mizutani R, Takeo S, Tsujimoto G, Tanoue A. Alcohol preference in mice lacking the Avpr1a vasopressin receptor. Am. J. Phsyiol. Regul Integr. Comp. Physiol. 2008;294:R1482–R1490. doi: 10.1152/ajpregu.00708.2007. [DOI] [PubMed] [Google Scholar]
- Seburn KL. MPD:Seburn1. Mouse Phenome Database web site. Bar Harbor, Maine USA: The Jackson Laboratory; 2010. Comprehensive metabolic survey of 16 inbred strains of mice. http://phenome.jax.org, Nov, 2010. [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin. Exp. Res. 2005;29:1419–1426. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ. Bidirectional selective breeding for ethanol effects on locomotor activity: Characterization of FAST and SLOW mice through selection generation 35. Alcohol. Clin. Exp. Res. 1995;19:1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin. Exp. Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc. Natl. Acad. Sci. USA. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RJ, Berry LJ, Beerstecher E., Jr Biochemical individuality. III. Genetotrophic factors in the etiology of alcoholism. Arch. Biochem. 1949;23:275–290. [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]