Abstract
Background and Purpose
The Living Well With Stroke (LWWS) study has demonstrated effectiveness of a brief psychosocial treatment in reducing depressive symptoms after stroke. The purpose of this analysis was to determine whether key variables associated with prevalence of post-stroke depression (PSD) also predicted treatment response.
Methods
Response to a brief psychosocial/behavioral intervention for PSD was measured with the Hamilton Rating Scale for Depression (HRSD). ANCOVA models tested for interaction of potential predictor variables with treatment group on percent change in HRSD from pre to post-treatment as outcome.
Results
Initial depression severity, hemispheric location, level of social support, age, gender and antidepressant adherence did not interact with the treatment with respect to percent change in HRSD when considered one at a time. Participants who carried one or two s-alleles at the 5-HTTLPR serotonin transporter (SERT) polymorphism or one or two 9- or 12- repeats of the STin2 VNTR polymorphism had significantly better response to psychosocial treatment than those with no s- alleles or no 9- or 12- repeats.
Conclusions
Opposite to the effects of antidepressant drug treatment with selective serotonin reuptake inhibitors, the LWWS psychotherapy intervention was most effective in 5-HTTLPR s-allele carriers and STin2 VNTR 9- or 12- repeat carriers.
Clinical Trial Registration Information
This study was registered with the clinical trials identifier NCT00194454 at www.clinicaltrials.gov/ct/show/NCT00194454?order_1.
Keywords: behavioral therapy, behavioral genetics, depression, stroke
Introduction
Over 30% of stroke survivors experience post-stroke depression (PSD). There is evidence that factors such as female gender, history of depression, younger age, and serotonin transporter (SERT) genotype are predictive of PSD, but there are no published reports about factors influencing the response to any form of treatment for this condition.1, 2 We therefore queried data from our recent successful randomized controlled trial of a psychosocial treatment adjuvant to antidepressants (Living Well With Stroke, LWWS)3 to determine whether subsets of PSD patients respond better to treatment.
Methods
This analysis was a planned, exploratory aim from LWWS in which 101 clinically depressed patients with ischemic stroke were randomly assigned to a 9 session brief pleasant events, problem-solving intervention delivered by an advanced practice nurse therapist, plus antidepressant (intervention, n=48), or to usual care plus antidepressants (control, n=53). Investigated variables, chosen from those noted in prior literature to be predictive of PSD or predictive of response to pharmacotherapy in primary depression, were: age, gender, stroke severity as measured by the initial NIH Stroke Scale (NIHSS), stroke hemisphere location, baseline Hamilton Rating Scale for Depression (HRSD), depression history from the Diagnostic Interview and Structured Hamilton (DISH),4 level of social support measured by the ENRICHD Social Support Inventory (ESSI),5 antidepressant adherence, measured by a self-report medication log, and the 5-HTTLPR and STin2 VNTR polymorphisms of the serotonin transporter (SERT) genotyped in DNA extracted from blood as previously described.2 SERT polymorphisms were genotyped in only a subset of participants (n=61), because genotyping was done as part of an ancillary study which was initiated after LWWS was already well underway. As a result subjects enrolled earlier in the study were not given the opportunity to participate in genotyping. The 17 item HRSD was used to measure treatment response. Genotypes and outcome assessments were determined masked to treatment group. We examined the interaction of treatment group and each of the predictor variables on percent change in HRSD score using individual analysis of covariance (ANCOVA). Details of the parent study design, participant characteristics and measures are found in previous reports.3, 6
Results
There were no significant main effects or interactions with treatment group for gender, stroke severity, hemispheric location, baseline HRSD, prior history of depression, level of social support or antidepressant adherence (Table 1). We saw a significant main effect for age but no significant interaction with age; younger subjects had better mean percent improvement in HRSD in both intervention and control groups. There was a trend towards better treatment response in subjects with smaller strokes and those who were more adherent to antidepressant drug treatment. Because the SERT polymorphisms showed close to significant main and interaction effects, we further examined their impact on treatment outcome (Table 2). Among patients with the 5-HTTLPR s/s genotype or the STin2 VNTR 9/12 and 12/12 genotypes behavioral treatment had a large effect; there was no evidence of an effect of LWWS among l/l homozygotes (Figure). These results did not change substantially when the analysis was controlled for race as white versus non-white (not shown).
Table 1.
P values for the main effect of each variable and the interaction of that variable with intervention group.
| Main effect | Interaction | |
|---|---|---|
| HSRD baseline | .356 | .692 |
| Age | .015 | .351 |
| Gender | .700 | .352 |
| NIHSS | .725 | .163 |
| Hemisphere location | .909 | .296 |
| Hx of prior depression | .903 | .417 |
| ESSI | .858 | .566 |
| Regular anti-depressant use | .909 | .139 |
| 5-HTTLPR No. of of s alleles | .075 | .070 |
| STin2 VNTR No. of 9 or 12 alleles | .040 | .165 |
Note: HSRD baseline = Hamilton Depression Rating Scale, total score at baseline; NIHSS = NIH Stroke Scale, total score; ESSI = ENRICHD Social Support Inventory, total score; Regular antidepressant use is yes if 80% adherent, no if not taking or <80% adherent.
Table 2.
Percent reduction in HRSD score from baseline to end of treatment by genotype
| SERT Genotype | Randomization group | Number of participants | N (%) in remission | Mean % reduction in HDRS | Std. Deviation | Std. Error Mean | T (df) | P-value (two tailed) |
|---|---|---|---|---|---|---|---|---|
| 5-HTTLPR | ||||||||
| l/l | Intervention | 10 | 1 (10%) | −32.27 | 22.59 | 7.14 | − .622 (19) | 0.54 |
| Control | 11 | 1 (9%) | −25.70 | 25.46 | 7.68 | |||
| s/l | Intervention | 12 | 5 (42%) | −47.06 | 23.85 | 6.89 | −2.53 (20) | 0.02 |
| Control | 10 | 2 (20%) | −15.90 | 33.88 | 10.71 | |||
| s/s | Intervention | 8 | 7 (85%) | −62.78 | 11.18 | 3.95 | −3.8 (16) | 0.002 |
| Control | 10 | 3 (30%) | −27.20 | 24.32 | 7.69 | |||
| STin2 VNTR | ||||||||
| 10/10 | Intervention | 5 | 1 (20%) | −22.49 | 30.20 | 13.51 | −0.88 (4) | 0.43 |
| Control | 1 | 0 (0%) | 6.67 | . | . | |||
| 9/10 or 10/12 | Intervention | 11 | 3 (27%) | −44.99 | 10.49 | 3.16 | −2.2 (21) | 0.37 |
| Control | 12 | 2 (16%) | −26.85 | 25.11 | 7.25 | |||
| 9/12 or 12/12 | Intervention | 14 | 9 (64%) | −55.87 | 23.19 | 6.19 | −3.5 (30) | 0.001 |
| Control | 18 | 4 (22%) | −22.12 | 29.59 | 6.97 | |||
Note: For each SERT polymorphism (5-HTTLPR and STin2 VNTR) the number of participants in intervention and control groups are shown. The middle columns give the number and percentage of these subjects in remission after 9 weeks (Hamilton Depression Rating Scale, HDRS, score of <10 as a surrogate for remission), the mean % reduction in their HDRS scores over the same time span, as well as standard deviation and standard error of the mean HDRS change over time. Results of the statistical analysis are shown to the right (df = degrees of freedom).
Figure.
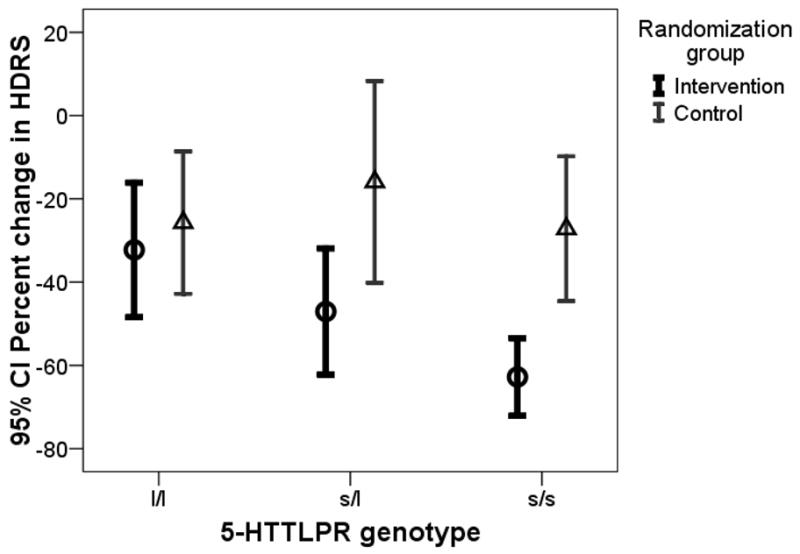
Interaction of 5 HTTLPR genotype (l/l, s/l, s/s) with treatment response (Percent reduction in Hamilton Depression Rating Scale (HRSD)
Discussion
5-HTTLPR and STin2 VNTR are functional length polymorphisms of the SERT gene, located in the promoter region (5-HTTLPR) and second intron (STin2 VNTR) where they act as regulators of SERT expression.7 The short (s) variant of 5-HTTLPR and the 9- or 12-repeat alleles of STin2 VNTR have previously been associated with co-morbid depression in medically ill populations. 2, 8–10 In addition, the s-allele of 5-HTTLPR has been associated with lower remission and response rates and a higher number of medication side effects in depressed patients treated with selective serotonin reuptake inhibitors (SSRIs).11 Our observation that SERT genotype is related to better treatment outcome with psychotherapy, in contrast with response to SSRIs, suggests the possibility of personalizing and tailoring both pharmacologic and psychosocial treatments for PSD. However, our study is limited by its small, ethnically heterogeneous sample, which allowed only for a limited detection of gene x treatment interactions. Our findings relate to reports in the literature showing 5-HTTLPR s-allele carriers to be more likely to view environmental stimuli with a negative bias, have heightened emotional reactivity, and develop negative information processing at an early age.12–14 Since the s/s genotype may confer an increased sensitivity to the social environment,15 subjects with this genotype could possibly derive particular benefit from an intervention like ours which aims to supply tools to enhance personal psychological resources.
Acknowledgments
Sources of Funding
This work was funded by National Institutes of Health, National Institute of Nursing R01NR07755.
Footnotes
Conflicts of Interest/Disclosures
None
Contributor Information
Ruth Kohen, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA.
Kevin C. Cain, Department of Biostatistics and Office for Nursing Research, University of Washington, Seattle, WA.
Ann Buzaitis, Department of Biobehavioral Nursing & Health Systems, University of Washington, Seattle, WA.
Vicki Johnson, Department of Neurology, University of Washington Stroke Center, University of Washington, Seattle, WA.
Kyra J Becker, Department of Neurology and Department of Neurological Surgery, University of Washington, Seattle, WA.
Linda Teri, Department of Psychosocial and Community Health, University of Washington, Seattle, WA.
David L Tirschwell, Department of Neurology, University of Washington, Seattle, WA.
Richard C Veith, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA.
Pamela H Mitchell, Department of Biobehavioral Nursing & Health Systems, Department of Health Services, University of Washington, Seattle, WA.
References
- 1.Hackett ML, Anderson CS. Predictors of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- 2.Kohen R, Cain KC, Mitchell PH, Becker K, Buzaitis A, Millard SP, Navaja GP, Teri L, Tirschwell D, Veith R. Association of serotonin transporter gene polymorphisms with poststroke depression. Arch Gen Psychiatry. 2008;65:1296–1302. doi: 10.1001/archpsyc.65.11.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell PH, Veith RC, Becker KJ, Buzaitis A, Cain KC, Fruin M, Tirschwell D, Teri L. Brief psychosocial-behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: Living well with stroke: Randomized, controlled trial. Stroke. 2009;40:3073–3078. doi: 10.1161/STROKEAHA.109.549808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedland KE, Skala JA, Carney RM, Raczynski JM, Taylor CB, Mendes de Leon CF, Ironson G, Youngblood ME, Krishnan KR, Veith RC. The depression interview and structured hamilton (dish): Rationale, development, characteristics, and clinical validity. Psychosom Med. 2002;64:897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, Froelicher ES, Czajkowski S, Youngblood M, Huber M, Berkman LF. A short social support measure for patients recovering from myocardial infarction: The enrichd social support inventory. J Cardiopulm Rehabil. 2003;23:398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell PH, Teri L, Veith R, Buzaitis A, Tirschwell D, Becker K, Fruin M, Kohen R, Cain KC. Living well with stroke: Design and methods for a randomized controlled trial of a psychosocial behavioral intervention for poststroke depression. J Stroke Cerebrovasc Dis. 2008;17:109–115. doi: 10.1016/j.jstrokecerebrovasdis.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali FR, Vasiliou SA, Haddley K, Paredes UM, Roberts JC, Miyajima F, Klenova E, Bubb VJ, Quinn JP. Combinatorial interaction between two human serotonin transporter gene variable number tandem repeats and their regulation by ctcf. J Neurochem. 2010;112:296–306. doi: 10.1111/j.1471-4159.2009.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-httlpr) with depression, perceived stress, and norepinephrine in patients with coronary disease: The heart and soul study. Am J Psychiatry. 2007;164:1379–1384. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarrett ME, Kohen R, Cain KC, Burr RL, Poppe A, Navaja GP, Heitkemper MM. Relationship of sert polymorphisms to depressive and anxiety symptoms in irritable bowel syndrome. Biol Res Nurs. 2007;9:161–169. doi: 10.1177/1099800407307822. [DOI] [PubMed] [Google Scholar]
- 10.Lenze EJ, Munin MC, Ferrell RE, Pollock BG, Skidmore E, Lotrich F, Rogers JC, Quear T, Houck P, Reynolds CF., 3rd Association of the serotonin transporter gene-linked polymorphic region (5-httlpr) genotype with depression in elderly persons after hip fracture. Am J Geriatr Psychiatry. 2005;13:428–432. doi: 10.1176/appi.ajgp.13.5.428. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 12.Fox E, Ridgewell A, Ashwin C. Looking on the bright side: Biased attention and the human serotonin transporter gene. Proc Biol Sci. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-httlpr) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden EP, Dougherty LR, Maloney B, Olino TM, Sheikh H, Durbin CE, Nurnberger JI, Jr, Lahiri DK, Klein DN. Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. J Affect Disord. 2008;107:227–230. doi: 10.1016/j.jad.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor SE. Inaugural article: Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]


