Abstract
Background
CSF analysis is often deferred in patients with cryptococcal disease, particularly in the absence of neurologic manifestations. We sought to determine if a subset of SOT recipients with high likelihood of CNS disease could be identified in whom CSF analysis must be performed.
Methods
Patients comprised a multicenter cohort of SOT recipients with cryptococcosis.
Results
Of 129 of 146 (88%) SOT recipients with cryptococcosis who underwent CSF analysis, 80 (62%) had CNS disease. In the overall study population, abnormal mental status, time to onset of cryptococcosis >24 months post-transplantation (late-onset disease), serum cryptococcal antigen titer >1:64, and fungemia were independently associated with an increased risk of CNS disease. Of patients with abnormal mental status, 95% had CNS cryptococcosis. When only patients with normal mental status were considered, three predictors (serum antigen titer >1:64, fungemia, and late-onset disease) independently identified patients with CNS cryptococcosis; the risk of CNS disease was 14% if none, 39% if one, and 94% if two of the aforementioned predictors existed (χ2 for trend p<0.001).
Conclusions
CSF analysis should be strongly considered in SOT recipients with cryptococcosis who have late-onset disease, fungemia, or serum cryptococcal antigen titer >1:64 even in the presence of normal mental status.
Keywords: cryptococcosis, solid organ transplant, central nervous system disease
Introduction
Cryptococcosis is a significant opportunistic mycosis in solid organ transplant (SOT) recipients (1–4). Currently, cryptococcal disease is the third most common mycosis following candidiasis and aspergillosis in SOT recipients, representing 9% of the invasive fungal diseases post-transplant (5). The overall incidence of cryptococcosis in SOT recipients is 1.6% (range: 0.5–4.1%) and central nervous system (CNS) disease accounts for 54–60% of all cryptococcal disease in these patients (3).
Prompt recognition of CNS disease is critical since it affects management, including the choice and duration of antifungal therapy and necessity of adjunctive therapy for alleviation of elevated intracranial pressure (6). The mortality rate approaches 30% in SOT recipients with CNS disease compared to 7% in those with cryptococcosis limited to the lungs (3). Given these prognostic and therapeutic implications, the current practice guidelines of the Infectious Diseases Society of America (IDSA) recommend CSF analysis in all patients with cryptococcosis (6). In the clinical setting however, CSF analysis is not always performed. Indeed, 14–27% of the non-HIV infected patients with cryptococcosis, including SOT recipients in previous studies did not undergo CSF analysis (7, 8). Thus, we sought to determine if a subset of SOT recipients with high likelihood of CNS disease could be identified in whom CSF analysis should be considered mandatory.
Materials and Methods
The study population comprised a multicenter cohort of SOT recipients with cryptococcosis at the participating sites. The study was conducted between 2001–07 and a detailed description of this cohort has been published elsewhere (9, 10). None of the patients were HIV infected. Cryptococcal disease was defined as per the European Organization for Research and Treatment in Cancer and the Mycoses Study Group (EORTC/MSG) criteria (11). CNS disease was diagnosed based on positive CSF culture or positive cryptococcal antigen in the CSF. Cryptococcal antigen testing was performed as part of standard clinical care at each institution. Variables assessed in this report included demographic characteristics, immunosuppressive regimen at the time of diagnosis, dose of prednisone, time elapsed from transplantation to onset, sites of infection, rejection episodes or retransplantation, renal dysfunction at baseline (defined as creatinine level ≥2.0 mg/dl), cytomegalovirus (CMV) infection and CMV disease, presenting symptoms (abnormal mental status, fever), serum cryptococcal antigen, fungemia, and mortality at 90 days. Serum cryptococcal antigen titer >1:64 was used to create a dichotomous variable since the overall median titer in the study cohort was 1:64. Likewise, late-onset cryptococcosis was considered as the time from transplantation to onset of cryptococcosis >24 months given that this time period approximated the median time to onset of cryptococcal disease in our patients (22 months). Mental status was assessed at presentation and categorized as alert, lethargic, stuporous, or comatose. Level of consciousness other than normal i.e., lethargy, stupor, or coma was considered as abnormal mental status. Only those patients who underwent CSF analysis were included in the study.
Statistical Analyses
Continuous data were compared using Mann-Whitney test. Categorical data were compared using chi-squared test or Fisher’s exact test when appropriate. Logistic regression models were constructed to calculate odds ratios (OR) and 95% confidence intervals (CIs) for factors associated with CNS cryptococcosis. Significant factors in univariable analyses (p<0.10) were entered into a multivariable model to assess for the effect of several factors as predictors of CNS disease. For these models, backward selection was used with factors removed at p>0.05. The final model was evaluated using the Hosmer-Lemeshow goodness of fit test. The power of the model’s predicted value to discriminate between the presence and absence of CNS disease was estimated using area under the receiver operating characteristic curve. The chi-squared test for trend was used to assess an increase in the risk of CNS disease associated with the number of predictors present. Intercooled Stata (version 10.1, StataCorp) was used for all analyses. A two-tail p<0.05 was considered statistically significant.
Results
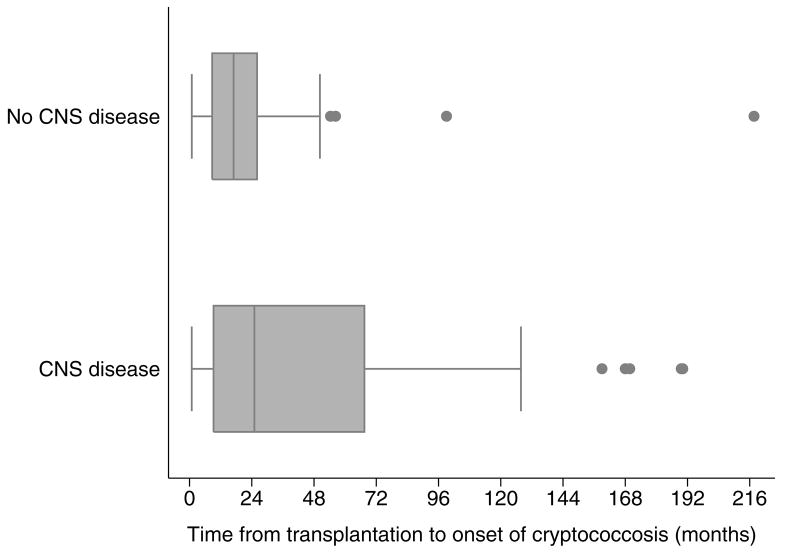
A total of 129 (88%) of the 146 SOT recipients underwent CSF analysis of whom 62% (80/129) had CNS cryptococcal disease (Table 1). The diagnosis of CNS disease was based on positive culture in 78% (62/80), and positive cryptococcal antigen in 22% (18/80) of the patients. Baseline characteristics of the patients with and without CNS cryptococcosis are shown in Table 2. Patients with abnormal mental status and fever at presentation were significantly more likely to have CNS disease (Table 2). A significantly greater number of the patients with CNS disease were receiving high-dose prednisone (≥10 mg/day) (p=0.009) and the median dose of prednisone was higher in patients with CNS disease compared to those without CNS disease (p=0.026). Overall, cryptococcosis was diagnosed at a median time of 22 months post-transplant (interquartile range [IQR] 8.0–50 months). Time elapsed from transplantation to the onset of cryptococcosis was a median of 25 months in patients with CNS disease and 17 months in those without CNS disease (p=0.051) (Figure 1) and proportionally fewer patients with calcineurin-inhibitor agent based immunosuppressive regimen had CNS disease (p=0.053) (Table 2).
Table 1.
Characteristics of the patients with cryptococcosis (N=129)
| Characteristic | N (%) |
|---|---|
| Age, median (IQR), year | 54 (44–60) |
| Male | 91/125 (73) |
| Type of transplant | |
| Liver | 30 (23) |
| Lung | 10 (8) |
| Kidney | 63 (49) |
| Heart | 11 (9) |
| Pancreas1 | 11 (9) |
| Combined2 | 4 (3) |
| Primary immunosuppressive agents | |
| Calcineurin inhibitors | 114 (88) |
| Tacrolimus | 95 (74) |
| Cyclosporine A | 19 (15) |
| Non-calcineurin inhibitor-based regimen3 | 15 (12) |
| T-cell antibody agent use4 | 7/79 (8) |
| Prednisone | 116 (90) |
| Median (IQR), mg | 10 (5–10) |
| Dose ≥10 mg/day | 64/116 (55) |
| Rejection | 30/128 (23) |
| Retransplantation | 16 (12) |
| Renal dysfunction (creatinine ≥2.0 mg/dl) | 35/107 (33) |
| CMV infection | 28/126 (22) |
| CMV disease | 15/122 (12) |
| Time to onset of cryptococcosis | |
| Median (IQR), month | 22 (8.9–50) |
| >12 months | 86 (67) |
| >24 months | 57 (44) |
| Symptoms | |
| Fever | 57/124 (46) |
| Abnormal mental status | 39/127 (31) |
| Site involved | |
| CNS | 80 (63) |
| Pulmonary | 71 (55) |
| Cutaneous | 23 (18) |
| Serum cryptococcal antigen | |
| Positive antigen | 80/98 (82) |
| Titer | |
| Median (IQR) | 64 (1–512) |
| >1:64 | 44/98 (45) |
| Fungemia | 31/122 (25) |
IQR, interquartile range; CMV, cytomegalovirus. Data are No. (%) of patients, unless otherwise indicated.
Denominators are shown when missing data exist.
Included 5 pancreas and 6 kidney-pancreas recipients.
Included 2 liver-kidney and 2 heart-kidney transplant recipients.
These patients received azathioprine (9), mycophenolate mofetil (3), mycophenolate mofetil and rapamycin (2), and prednisone (1) without a calcineurin-inhibitor agent.
Use within 6 months of onset of cryptococcosis.
Table 2.
Characteristics of the patients with and without central nervous system cryptococcal disease
| CNS disease |
||||
|---|---|---|---|---|
| Characteristic | Yes (n=80) | No (n=49) | OR (95% CI) | P-value |
| Age, median (IQR), year | 54 (43–60) | 54 (47–60) | … | 0.45 |
| Gender, Male | 59/79 (75) | 32/46 (70) | 1.29 (0.58–2.89) | 0.54 |
| Type of transplant | … | 0.092 | ||
| Liver | 17 (21) | 13 (27) | ||
| Lung | 4 (5) | 6 (12) | ||
| Kidney | 44 (55) | 19 (39) | ||
| Heart | 4 (5) | 7 (14) | ||
| Pancreas | 7 (9) | 4 (8) | ||
| Combined | 4 (5) | 0 (0) | ||
| Primary immunosuppressive agents | ||||
| Calcineurin inhibitors | 67 (84) | 47 (96) | 0.22 (0.047–1.02) | 0.053 |
| Tacrolimus | 55 (69) | 40 (82) | 0.50 (0.21–1.17) | 0.11 |
| Cyclosporine A | 12 (15) | 7 (14) | 1.06 (0.39–2.90) | 0.91 |
| Non-calcineurin inhibitor-based regimen1 | 13 (16) | 2 (4) | 4.56 (0.98–21.2) | 0.053 |
| T-cell antibody agent use2 | 5/79 (6) | 2 (4) | 1.59 (0.30–8.52) | 0.71 |
| Prednisone | 77 (96) | 39 (80) | 6.58 (1.71–25.3) | 0.002 |
| Median (IQR), mg | 10 (5–10) | 7 (4–10) | … | 0.026 |
| Dose ≥10 mg/day | 46/71 (65) | 18/45 (40) | 2.76 (1.28–5.96) | 0.009 |
| Rejection | 18/79 (23) | 12 (25) | 0.91 (0.39–2.10) | 0.82 |
| Retransplantation | 9 (11) | 7 (14) | 0.76 (0.26–2.2) | 0.61 |
| Renal dysfunction (creatinine ≥2.0 mg/dl) | 25/63 (40) | 10/44 (23) | 2.23 (0.94–5.32) | 0.066 |
| CMV infection | 16/78 (21) | 12/48 (25) | 0.77 (0.33–1.82) | 0.56 |
| CMV disease | 7/76 (9.2) | 8/46 (17) | 0.48 (0.16–1.43) | 0.18 |
| Time to onset of cryptococcosis | ||||
| Median (IQR), month | 25 (9.2–67) | 17 (8.7–26) | … | 0.051 |
| >12 months | 55 (69) | 31 (63) | 1.28 (0.60–2.70) | 0.52 |
| >24 months | 40 (50) | 17 (35) | 1.88 (0.90–3.92) | 0.089 |
| Symptoms | ||||
| Fever | 43/79 (54) | 14/45 (31) | 2.96 (1.38–6.38) | 0.005 |
| Abnormal mental status | 37/79 (47) | 2/48 (4.2) | 20.3 (4.60–89.3) | <0.001 |
| Site involved other than CNS | ||||
| Pulmonary | 33 (41) | 38 (78) | 0.20 (0.091–0.45) | <0.001 |
| Cutaneous | 15 (19) | 8 (16) | 1.18 (0.46–3.04) | 0.73 |
| Serum cryptococcal antigen | ||||
| Positive antigen | 53/55 (96) | 27/43 (63) | 15.7 (3.36–73.3) | <0.001 |
| Titer | ||||
| Median (IQR) | 512 (32–1024) | 8 (0–64) | … | <0.001 |
| >1:64 | 36/55 (66) | 8/43 (19) | 8.29 (3.21–21.4) | <0.001 |
| Fungemia | 29/76 (38) | 2/46 (4.3) | 13.6 (3.06–60.3) | <0.001 |
CNS, central nervous system; OR, odds ratio; CI, confidence interval; IQR, interquartile range. Data are No. (%) of patients, unless otherwise indicated. Denominators are shown when missing data exist.
Patients with CNS disease: these patients received azathioprine (9), mycophenolate mofetil (2), mycophenolate mofetil and rapamycin (1), and prednisone (1) without a calcineurin-inhibitor agent. Patients without CNS disease: these patients received mycophenolate mofetil (1), mycophenolate mofetil and rapamycin (1) without a calcineurin-inhibitor agent.
Use within 6 months of onset of cryptococcosis.
Figure 1.
Time elapsed from transplantation to onset of cryptococcosis was significantly longer in patients with CNS disease (median 25 months, IQR 9.2–67 months) than in those without CNS disease (median 17 months, IQR 8.7–26 months, p=0.051). CNS, central nervous system; IQR, interquartile range.
Results of the serum cryptococcal antigen test at the time of diagnosis were available in 98 (76%) of the 129 patients. A higher serum cryptococcal antigen titer correlated with CNS disease; the median titer was 1:512 in the patients with CNS disease as opposed to 1:8 in those without CNS disease (p<0.001). A majority (97%) of the patients with serum cryptococcal antigen titer >1:256 had CNS disease however, negative serum cryptococcal antigen did not appear to exclude CNS disease. Indeed, 11% (2/18) of those with negative serum antigen also had CNS disease. Presence of fungemia also significantly predicted CNS disease (p<0.001). Other variables such as age, gender, type of transplant, T-cell antibody agent use, prior rejection, retransplantation, renal dysfunction at baseline, and CMV infection or disease were not significantly different for patients with and without CNS disease.
A multivariable logistic regression model was constructed to identify predictors of CNS disease in SOT recipients with cryptococcosis as described in the methods. Late-onset cryptococcosis (p=0.009), abnormal mental status (p=0.033), serum cryptococcal antigen titer >1:64 (p=0.001), and fungemia (p=0.024) were significantly associated with CNS disease (Table 3). Prednisone dose, renal dysfunction, immunosuppressive agent, fever, and type of transplant were not statistically significant and were removed from the final model. Since the results of serum cryptococcal antigen test were available in 98/129 (76%) of the patients, another logistic regression model that excluded serum cryptococcal antigen was considered. The same factors i.e., abnormal mental status (OR 16, 95% CI 3.3–78; p=0.001), fungemia (OR 15, 95% CI 2.9–75; p=0.001), late-onset cryptococcosis (OR 4.3, 95% CI 1.7–11; p=0.003) remained significantly associated with CNS disease.
Table 3.
Variables independently associated with central nervous system disease in all solid organ transplant recipients with cryptococcocis
| Factor | OR (95% CI) | P-value |
|---|---|---|
| Late-onset disease (onset >24 months) | 5.0 (1.5–17) | 0.009 |
| Abnormal mental status | 7.1 (1.2–43) | 0.033 |
| Serum cryptococcal antigen titer >1:64 | 8.7 (2.5–30) | 0.001 |
| Fungemia | 7.2 (1.3–40) | 0.024 |
OR, odds ratio; CI, confidence interval.
The Hosmer-Lemeshow test for this model showed overall good fit with area under the receiver operating curve of 0.86.
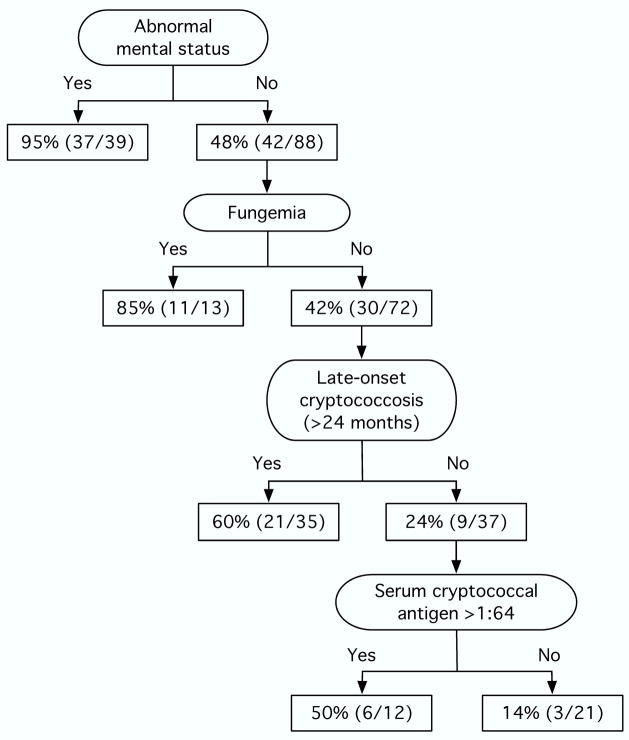
In all, 95% (37/39) of the patients with abnormal mental status had CNS disease. However, 48% (42/88) of those with normal mental status were also diagnosed with CNS disease. Given that abnormal mental status is an obvious sign indicating the need for CSF analysis, we investigated predictors of CNS disease in patients with normal mental status. Serum cryptococcal antigen titer >1:64 (OR 6.3, 95% CI 1.04–38; p=0.045), fungemia (OR 12.9, 95% CI 3.1–54; p<0.001), and late-onset cryptococcosis (OR 6.9, 95% CI 1.7–28; p=0.007) were independently associated with CNS disease in patients with normal mental status. The risk of CNS disease in SOT recipients with cryptococcosis who had normal mental status was 14% if none, 39% if one, and 94% if two of the aforementioned risk factors existed (X2 for trend p<0.001). The risk of CNS disease in SOT recipients with cryptococcosis is also depicted in a decision-tree fashion (Figure 2). The mortality rate at 90 days was 19% (15/80) in patients with CNS disease and 4.1% (2/49) in those without CNS disease (p=0.017).
Figure 2.
Risk of central nervous system disease in solid organ transplant recipients with cryptococcosis. The denominators depict the number of patients with data available in each cell.
Patients who did not undergo CSF analysis (n=17) did not differ from those who had CSF analysis performed (n=129) with regards to age, gender, type of transplant, immunosuppressive regimen, time to onset of cryptococcal disease, fungemia, or serum cryptococcal antigen titer (data not shown). Abnormal mental status was documented in 31% (39/127) of the patients who had CSF analysis versus 8.3% (1/12) of those who did not (p=0.18). Overall mortality rate at 90 days was 29% (5/17) in patients who did not have CNS analysis performed.
Discussion
Our prospective, multicenter study provided a unique opportunity to investigate the predictors of CNS disease specifically in SOT recipients. Overall, CNS disease was documented in 62% (80/129); 95% of the patients with cryptococcosis with abnormal mental status had CNS disease as did 48% of those with normal mental status. Serum cryptococcal antigen titer >1:64, fungemia, and onset of cryptococcosis >24 months after transplantation were significantly associated with CNS disease in patients with normal mental status. CNS disease was documented in 94% of the patients with normal mental status if any two of the aforementioned risk factors existed compared to 14% if none of these were present (p<0.001).
It is noteworthy that the patients who developed cryptococcosis >24 months after transplantation were more likely to have CNS disease than those with early-onset cryptococcosis. Two potential explanations exist for this observation. Patients in the late post-transplant period are typically cared for by local providers at sites remote from the transplant centers and subtle manifestations of early disease may not be recognized. Thus, it is possible that infrequent follow-up in the late post-transplant period may lead to delayed presentation with fungal burden reaching high levels before the diagnosis is established. Second, most cryptococcal disease in SOT recipients is considered to result from reactivation of latent infection (12). We have previously shown that patients with reactivation infection or prior seroreactivity against C. neoformans developed cryptococcal disease significantly earlier post-transplant than those with primary infection or without preexisting antibody (5.6 vs. 40.6 months; p=0.0011) (13). Thus, the absence of anticryptococcal antibody in the setting of primary infection in the late post-transplant period may lead to greater severity of disease such as dissemination and CNS disease. Indeed, a study in the pre-HIV era showed that the lack of cryptococcal antibody was associated with higher mortality in patients with cryptococcal meningitis (14). It is therefore plausible that SOT recipients with late-onset cryptococcosis were potentially more likely to develop primary infection with a higher attendant risk of disseminated or CNS disease.
Serum cryptococcal antigen >1:64 and fungemia are indicative of higher fungal burden and their association with CNS disease is therefore intuitively understandable. CSF cryptococcal antigen titers have been shown to correlate with inoculum size or cryptococcal colony-forming unit counts in the CSF (15). Positive serum cryptococcal antigen has also been associated with disseminated (7, 10) or more severe diseases (16–18). We have previously shown that serum cryptococcal antigen positivity in SOT recipients with pulmonary cryptococcosis correlated with extrapulmonary as well as more advanced radiographic disease (10). Moreover, serum cryptococcal antigen titer ≥1:64 was associated with disseminated disease in HIV-negative patients with pulmonary cryptococcosis (7). Fungemia likewise is an evidence for dissemination and severity of disease (17, 18). Indeed, 94% (29/31) of our patients with fungemia had CNS disease.
Precise reasons why CSF analysis is often deferred in patients with cryptococcosis are not fully understood. CSF analysis is an invasive procedure and the absence of overt CNS manifestations, perception that CSF analysis may not alter antifungal therapeutic plan, or relative contraindications to lumbar puncture, such as coagulopathy may preclude routine performance of this procedure in all patients. It should however, be noted that CSF analysis is critical not only for the diagnosis of CNS disease, but also for the assessment of intracranial pressure. The implications of measuring intracranial pressure were underscored by a recent study that evaluated adherence to the IDSA guidelines in patients with cryptococcosis (19). Of 14 of 26 patients (54%) who did not comply with the proposed guidelines, including assessment of the CSF opening pressure and/or measures to lower elevated intracranial pressure, 50% (7/14) developed new cranial nerve deficits or visual and auditory dysfunction (19).
Currently, a polyene (i.e., amphotericin B deoxycholate or lipid formulations of amphotericin B) is recommended for the treatment of CNS cryptococcosis in SOT recipients whereas mild to moderate cryptococcosis limited to the lungs can be treated with fluconazole (1, 20, 21). It may be argued that CSF analysis is expendable if a polyene is employed for the treatment of cryptococcosis in these patients. In this context, our group has recently shown that lipid formulations of amphotericin B appear to be associated with better outcome when compared to amphotericin B deoxycholate in SOT recipients with CNS cryptococcosis (22). Thus, CSF analysis may influence not only the class of antifungal agent but the type of polyene employed for the treatment of cryptococcosis.
Certain limitations of our study deserve to be acknowledged. A total of 12% (17/146) of the patients were excluded from this study because CSF analysis was not performed. While the precise reasons are not known, we speculate that lack of neurological symptoms in these patients may be the main reason for deferring CSF analysis. Ideally, our model identifying predictors of CNS disease should be confirmed in a validation study. That having been said, larger studies to validate these observations may be challenging, if not logistically infeasible given a relatively low frequency of cryptococcosis in SOT recipients.
In summary, we have identified readily assessable, previously unrecognized predictors of CNS disease in SOT recipients with cryptococcosis. While CSF analysis should be routinely performed in all patients with cryptococcosis, it should be a particular consideration in patients with serum cryptococcal antigen titer >1:64, fungemia, abnormal mental status, and development of cryptococcosis more than 24 months after transplantation.
Acknowledgments
National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI 054719-01 to NS)
Abbreviations
- CI
confidence interval
- CMV
cytomegalovirus
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EORTC/MSG
European Organization for Research and Treatment in Cancer and the Mycoses Study Group
- IDSA
Infectious Diseases Society of America
- IQR
interquartile range
- OR
odds ratio
- SOT
solid organ transplant
Footnotes
Conflicts of interest: Barbara D. Alexander has served on advisory board for Enzon, Basilea, Abbott, and Schering-Plough, served on the speaker’s bureau of Astellas and Pfizer, and received grant from Astellas, Enzon, and Pfizer; Graeme N. Forrest has received grant from Astellas. G. Marshall Lyon has served on advisory board for and received grant from Merck and Astellas, and on speaker’s bureau of Astellas, Schering-Plough, and Wyeth; Kenneth Pursell has served on speaker’s bureau of Merck; Michele I. Morris has served on advisory board for Astellas, Pfizer, and Merck, has received grant from Astellas, Basilea, and Pfizer, and has served on speaker’s bureau of Astellas and Pfizer; Patricia Muñoz has served on the speaker’s bureau of Merck and Novartis and on advisory board for Pfizer; Leonard B. Johnson has served on speaker’s bureau of Pfizer; Shahid Husain has served on consultant board for and received grant from Pfizer, Astellas, and Schering-Plough; Nina Singh has received grant from Pfizer; other authors have no conflicts.
Author contributions: RO participated in data analysis and writing of the paper. MMW participated in data analysis. NS participated in research design, performance of the research, data analysis, and writing of the paper. The other authors participated in performance of the research and writing of the paper.
References
- 1.Singh N, Dromer F, Perfect JR, Lortholary O. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis. 2008;47:1321. doi: 10.1086/592690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375. doi: 10.3201/eid0703.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566. doi: 10.1086/598936. [DOI] [PubMed] [Google Scholar]
- 4.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Alexander BD, et al. Prospective surveillance of invasive fungal infections (IFIs) among organ transplant recipients (OTRs) in the U.S. 2001–2006: review of TRANSNET [abstract M-1195]. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC: American Society for Microbiology; 2007. [Google Scholar]
- 6.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 7.Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis. 2008;27:937. doi: 10.1007/s10096-008-0529-z. [DOI] [PubMed] [Google Scholar]
- 8.Singh N, Lortholary O, Dromer F, et al. Central nervous system cryptococcosis in solid organ transplant recipients: clinical relevance of abnormal neuroimaging findings. Transplantation. 2008;86:647. doi: 10.1097/TP.0b013e3181814e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Alexander BD, Lortholary O, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195:756. doi: 10.1086/511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Alexander BD, Lortholary O, et al. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis. 2008;46:e12. doi: 10.1086/524738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37:3204. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha DC, Goldman DL, Shao X, et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin Vaccine Immunol. 2007;14:1550. doi: 10.1128/CVI.00242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974;80:176. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 15.Brouwer AE, Teparrukkul P, Pinpraphaporn S, et al. Baseline correlation and comparative kinetics of cerebrospinal fluid colony-forming unit counts and antigen titers in cryptococcal meningitis. J Infect Dis. 2005;192:681. doi: 10.1086/432073. [DOI] [PubMed] [Google Scholar]
- 16.Charlier C, Dromer F, Leveque C, et al. Cryptococcal neuroradiological lesions correlate with severity during cryptococcal meningoencephalitis in HIV-positive patients in the HAART era. PLoS One. 2008;3:e1950. doi: 10.1371/journal.pone.0001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dromer F, Mathoulin-Pelissier S, Launay O, Lortholary O. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4:e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS One. 2008;3:e2870. doi: 10.1371/journal.pone.0002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoham S, Cover C, Donegan N, Fulnecky E, Kumar P. Cryptococcus neoformans meningitis at 2 hospitals in Washington, D.C. : adherence of health care providers to published practice guidelines for the management of cryptococcal disease. Clin Infect Dis. 2005;40:477. doi: 10.1086/427213. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Lortholary O, Alexander BD, et al. Antifungal management practices and evolution of infection in organ transplant recipients with cryptococcus neoformans infection. Transplantation. 2005;80:1033. doi: 10.1097/01.tp.0000173774.74388.49. [DOI] [PubMed] [Google Scholar]
- 21.Dromer F, Mathoulin S, Dupont B, Brugiere O, Letenneur L. Comparison of the efficacy of amphotericin B and fluconazole in the treatment of cryptococcosis in human immunodeficiency virus-negative patients: retrospective analysis of 83 cases. French Cryptococcosis Study Group. Clin Infect Dis. 1996;22 (Suppl 2):S154. doi: 10.1093/clinids/22.supplement_2.s154. [DOI] [PubMed] [Google Scholar]
- 22.Sun HY, Alexander BD, Lortholary O, et al. Lipid formulations of amphotericin B significantly improve outcome in solid organ transplant recipients with central nervous system cryptococcosis. Clin Infect Dis. 2009 doi: 10.1086/647948. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]