Osteoarticular and soft tissue inflammation occur in subsequent infection with wild-type Brucella in mice deficient of IFN-γ, resembling aspects of human disease.
Keywords: arthritis, osteomyelitis, Brucella
Abstract
Human brucellosis exhibits diverse pathological manifestations that can affect almost any organ. In particular, osteoarticular complications are the most common focal manifestation of brucellosis and occur in 40–80% of patients. In immunocompetent mice, Brucella replication is generally restricted to the spleen, liver, and to a lesser extent, LNs, thereby limiting their use for study of focal inflammation often found in brucellosis. Here, we report that nasal, oral, or peritoneal infection of IFN-γ−/− mice with WT Brucella melitensis or Brucella abortus results in joint and periarticular tissue inflammation. Histological analysis of the affected joints revealed inflammatory infiltrates and debris within the joint space colocalizing with Brucella antigen. Osteoarthritis, necrosis, periarticular soft tissue inflammation, and substantial brucellae burdens were observed. Oral rifampicin was effective in clearing infection and halting further progression of focal inflammation from infected IFN-γ−/− mice, although some symptoms and swelling remained. Elevated IL-1β, but not TNF-α, IL-6, or IL-17, was detected in joint homogenates from infected IFN-γ−/− mice. Whereas more susceptible to systemic infection, IL-1R−/− mice depleted of IFN-γ were more resistant to focal inflammation than WT mice similarly depleted of IFN-γ. Collectively, these results show IFN-γ−/− mice represent a potential model for study of focal inflammation attributed to Brucella infection and will allow evaluation of intervention strategies targeting IL-1, IL-1R, or other inflammatory mediators, with the potential to complement antibiotic-based therapies.
Introduction
Brucellosis continues to be a global health problem resulting from zoonotic exposure [1]. Transmission of Brucella to humans occurs via inhalation of contaminated aerosols, skin abrasions, open wounds, and most commonly, via oral ingestion of unpasteurized dairy products [2, 3]. This disease remains problematic and results in an estimated 500,000 new cases annually [4], making brucellosis the most common zoonotic infection worldwide [1]. Although not life-threatening, brucellosis can cause disease with relapses of an undulating fever and lifelong complications, including arthritis, endocarditis, and possible neurological complications, despite antibiotic treatment [5, 6]. Osteoarticular complications are associated with prolonged illness in humans and are the most common localized manifestations of brucellosis occurring in 40–80% of cases [7, 8]. The osteoarticular structures affected by brucellosis vary depending on the age of the patient, and peripheral arthritis, sacroiliitis, and spondylitis are most common in children, young adults, and older adults, respectively [7–10]. Peripheral arthritis as a result of brucellosis most commonly affects weight-bearing joints, such as the hips, knees, and ankles [7, 11]. In peripheral arthritis, soft tissue swelling and periarticular osteoporosis are the most common clinical findings, whereas histological examination of synovial tissue reveals chronic lymphomononuclear or acute PMN leukocyte infiltrates [8, 12]. Cellulitis and myositis have also been reported in brucellosis patients; however, their manifestations are more rare [13–15].
Arthritis, as a result of Brucella, is thought to be septic in nature, resulting from hematogenous spreading to the joints and occurring without an obvious predisposition [16]. Whereas brucellar arthritis normally responds to antibiotic treatment, the resolution of inflammation is often prolonged [8, 16], and in some instances, the arthritis can progress to osteoarticular destruction [10]. In addition, cases refractive to antibiotic therapy may require arthroscopic debridement and drainage of the joint [12]. In earlier studies, others have isolated Brucella from the synovial fluid in only 27–66% of affected patients [8, 10, 17]. A more recent study has shown improved recovery rates of Brucella from synovial fluid by 50–100% when using a more advanced culture system designed to increase the isolation rate of intracellular bacteria [18]. In addition, the culture of synovial tissue, rather than synovial fluid, enhances the recovery of Brucella [19], presumably because brucellae are intracellular organisms that are freed only intermittently into bodily fluids [10].
IL-1 production has been implicated as a pathogenic immune response in rheumatoid and septic models of arthritis [20, 21]. This cytokine is present in the inflamed synovium of mice with antigen- or collagen-induced arthritis [21], and joint IL-1 levels correlate with arthritis severity in a mouse model of streptococcal arthritis [20, 22]. Injection of IL-1α or IL-1β into the knee joints of rabbits results in the accumulation of leukocytes in the synovial fluid, whereas therapies that block the effects of IL-1 have been shown to reduce the pathophysiological events of articular inflammation [21]. Importantly, IL-1 levels have been shown to be increased in the synovial fluid of humans with septic forms of bacterial arthritis [23–25]. To counter the role of IL-1, IL-1ra, which competitively binds to the IL-1R [21], has been suggested by a study evaluating the clinical course of arthritis in patients with Lyme disease. The authors found elevated IL-1ra/IL-1β ratios in the synovial fluid of patients correlated with recovery from disease [25]. IL-1 has also been associated with cutaneous and muscle inflammation following bacterial infections [26], including Mycobacterium tuberculosis [27].
Little information exists regarding the role of IL-1 in brucellosis. Stimulation of macrophages with IL-1 does not appear to affect intracellular brucellar colonization [28]; however, prophylactic, but not therapeutic, treatment of mice with IL-1 can enhance resistance to infection [29]. Interestingly, a recent publication has shown that supernatants from Brucella-infected human osteoblasts cause macrophages to produce IL-1β [30], whereas another publication has found elevated levels of IL-1 in the synovial fluid of a human brucellosis patient with bursitis [31]. Collectively, these results suggest a pathogenic role for IL-1 in human brucellar arthritis.
Whereas the immune response to Brucella has been studied extensively in murine models, to date, no murine model of brucellosis, which induces peripheral inflammation, has been documented. Herein, we report that infection of IFN-γ−/− mice with B. abortus or B. melitensis results in severe focal inflammation. Histopathological and immunological changes in affected paws and tails were characterized, along with the evaluation of the importance of the route of infection upon focal inflammation. IL-1β was found to be strikingly elevated in the paws of infected IFN-γ−/− mice. As a result of the extensive manifestations of this cytokine, the role of IL-1 in Brucella-induced inflammation was investigated.
MATERIALS AND METHODS
Brucella
All experiments with live brucellae were performed in BSL-3 facilities. B. abortus 2308 and the vaccine strain RB51 were obtained from the National Veterinary Services Laboratory, USDA (Ames, IA, USA). B. melitensis 16M was obtained from Louisiana State University (Baton Rouge, LA, USA). For generation of HKBA, B. abortus was cultured in BB (BD Diagnostic Systems, Franklin Lakes, NJ, USA), heat-killed by boiling for 20 min, washed five times with sPBS prior to being aliquoted, and stored at −70°C until use. Total absence of B. abortus viability, subsequent to heat killing, was verified by the absence of bacterial growth on BA (BD Diagnostic Systems).
Mouse infection studies
BALB/c mice (Frederick Cancer Research Facility, NCI, Frederick, MD, USA) and breeding colonies of IFN-γ−/− mice on a BALB/c background [32] and IRF-1−/− mice (The Jackson Laboratory, Bar Harbor, ME, USA) on a C57BL/6 (B6) background were maintained at the Montana State University Animal Resource Center (Bozeman, MT, USA). B6 mice, along with IL-1R−/− and IFN-γ−/− mice on a B6 background, were acquired from The Jackson Laboratory (Table 1). Mice were maintained in individually ventilated cages under high-efficiency particulate arresting-filtered barrier conditions of 12 h of light and 12 h of darkness in animal BSL-3 facilities and were provided with sterile food and water ad libitum. Experiments were conducted with 7- to 10-week-old, age-matched mice. WT B. abortus 2308 and B. melitensis 16M, ΔznuA B. melitensis [33], and B. abortus vaccine strain RB51 were grown overnight in BB at 37°C. Cells were pelleted, washed twice in sPBS, and spectrophotometrically adjusted to the appropriate concentration in sPBS prior to challenge of mice that were challenged by three different routes: i.p. with brucellae in 200 μl sPBS; oral gavage with brucellae in 200 μl sPBS (after neutralizing stomach's acidity by oral gavage with 200 μl of a 50% saturated sodium bicarbonate solution); or i.n. with brucellae in 30 μl sPBS. The actual viable inoculum was confirmed by serial dilution tests on BA. IFN-γ neutralization was performed by treating mice on Day −1 with 1 mg and on Days 7, 14, and 21 with 0.5 mg anti-IFN-γ antibody (Clone XMG1.2); they were then treated with 0.5 mg anti-IFN-γ antibody (Clone R4-6A2) on Days 28, 35, 42, and 49. Control animals received the same amount of rat IgG antibodies on the corresponding days. All antibodies were from Bio X Cell (Lebanon, NH, USA). All animal care and procedures were approved by Montana State University Institutional Animal Care and Use Committee.
Table 1. Mouse strains used in this study.
| Mouse strain | Immunodeficiency |
|---|---|
| BALB/c | WT |
| C57BL/6 | WT |
| IFN-γ−/− (on BALB/c or C57BL/6 backgound) | Do not produce IFN-γ |
| IRF-1−/− (C57BL/6 backgound) | Defects in CD8+, NK, and γδ T cell development, Th1 cell response |
| IL-1R−/− (C57BL/6 backgound) | Lack IL-1R |
Enumeration of brucellae in tissue homogenates
Individual spleens, along with one front and one rear paw (combined), were removed, cut into ∼1-cm pieces, and then mechanically homogenized in sPBS or RPMI 1640 (Invitrogen, Valencia, CA, USA) using disposable tissue grinders. Serial dilutions were plated onto BA plates that were incubated for 3–5 days at 37°C in 5% CO2, at which time, Brucella colonies were enumerated, and CFUs/tissue were calculated.
Histopathology
One hind paw, one front paw, and one tail from each mouse were fixed in neutral-buffered formalin for 7 days and then decalcified, ∼3 days in 15% formic acid, using decalcification endpoint determination, rinsed in running tap water, processed with alcohol gradient and clearing agent into paraffin on a VIP6 automated tissue processor (Sakura Finetek, Torrance, CA, USA), and embedded in paraffin. Paraffin sections (5 μm) were mounted on Plus Charge glass slides, and adjacent sections from each sample were stained with H&E and toluidine blue for cartilage.
Enzyme immunohistochemistry staining was done on deparaffinized sections using appropriate buffer and rinsing between each immunostaining step. Endogenous peroxidase was blocked with 3% H2O2 in methanol for 10 min during the deparaffinization procedure prior to the 70% alcohol step. Rehydrated tissue sections were immersed in the rinse buffer (DPBS, pH 7.4, 0.05% Tween 20). Antigen recovery with proteinase K (Dako USA, Carpinteria, CA, USA) for 10 min at room temperature was followed by a 10% donkey/2.5% mouse NSB in rinse buffer for 30 min and then streptavidin/biotin block (kit instructions; Vector Laboratories, Burlingame, CA, USA). Primary antibody, goat anti-Brucella (5.2 mg/ml, BEI Resources, Manassas, VA, USA), at 1.25 μg/ml in NSB, was incubated for 1 h at room temperature. The negative control was goat IgG in NSB (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at the same working concentration as primary antibody. Secondary antibody, biotinlyated F(ab′)2 fragment donkey anti-goat (1.3 mg/ml, Jackson ImmunoResearch Laboratories) at 1:500 in NSB, was incubated for 30 min at room temperature, followed by streptavidin-HRP (1 mg/ml; Invitrogen) at 1:1000 in rinse buffer for 20 min at room temperature. AEC+, ready-to-use (Dako USA), was developed microscopically for 5 min at room temperature, rinsed, lightly counterstained with hematoxylin 1 (Richard Allan, Thermo Scientific, Waltham, MA, USA), and coverslipped with aqueous mounting media.
Paw homogenization for cytokine measurement
One front and one rear paw from each mouse were removed, combined, and then homogenized in toto in 1.0 ml/200 mg paw weight of lysis medium (RPMI 1640 containing 2 mM PMSF and 1 μg/ml final concentration of aprotinin, leupeptin, and pepstatin A; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) [34]. The homogenized tissues were then centrifuged at 2000 g for 10 min, and supernatants were filter-sterilized (0.2 μm) and stored at −70° C until analyzed.
Cell culture and cytokine analysis
Mononuclear cells from spleens and axillary, popliteal, and inguinal LNs from Brucella-infected mice were isolated, as described [35–37], and cultured (5×106 cells/ml) for 3 days at 37°C/5% CO2. Some LN cultures were stimulated with 10 μg/ml CII (T cell proliferation grade; Chondrex, Redmond, WA, USA), as described previously [35, 37], or with 108 HKBA/ml. Supernatants were harvested, and 0.2 μM was filtered and stored at −70°C until evaluated by cytokine-specific capture ELISAs for TNF-α, IL-1β (using antibodies against the cleaved, bioactive peptide), IL-6, and IL-17 [35–39].
Clinical scoring and assessment of focal inflammation
Mice were scored using a scale of 0–3 for each paw for a maximal total score of 12: 0, no signs of inflammation; 1, mild redness or swelling of single digits; 2, significant swelling of ankle or wrist with erythema; and 3, severe swelling and erythema of multiple joints (see Figs. 2, 3, and 5). Severity of the disease was described by average clinical score. Paws of mice were scored as above (see Fig. 6); mice also received an additional score of 2 in cases of tail involvement. Swelling in all paws was measured with an electronic digital caliper (World Precision Instruments, Sarasota, FL, USA) in anesthetized mice, and cumulative swelling was calculated as a sum of all measurements/mouse minus the cumulative paw width of naïve mice (see Fig. 5) [35]. Paw swelling was scored as above (see Fig. 6) and combined with tail swelling, which was calculated by measuring peak diameter of tail inflammation and subtracting the diameter of the nearest ascending region of tail where there was no inflammation.
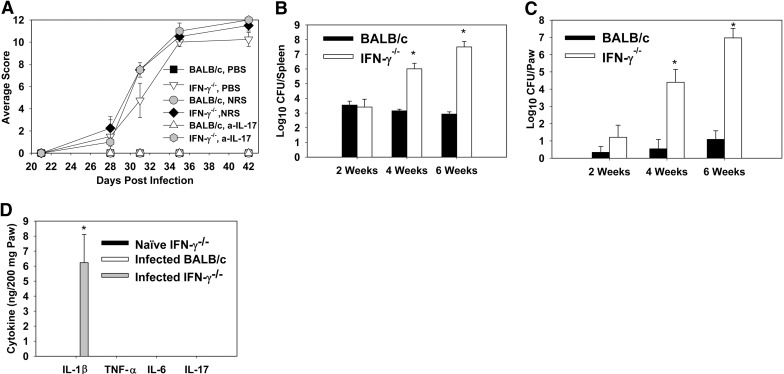
Figure 2. Brucella infection induces IL-17-independent inflammation in IFN-γ−/− but not in BALB/c mice.
BALB/c and IFN-γ−/− mice (on a BALB/c background) were infected i.n. with 2 × 104 CFUs of B. melitensis 16M. Starting on Day 14, mice were treated i.p. with PBS, NRS, or hyperimmune rabbit anti (a)-IL-17 serum once/week. (A) Mice were scored for clinical disease twice/week using a graded scale. (B) At various time-points, mice were killed and splenic brucellae burdens determined. At 28 and 42 days postinfection, but not at 14 days postinfection, spleens from IFN-γ−/− mice had significantly greater brucellae burdens than did WT mice. (C) A kinetic analysis of Brucella colonization in infected BALB/c and IFN-γ−/− mice paws (one front and one rear) was performed at 2, 4, and 6 weeks postinfection, as well as measuring (D) IL-1β, TNF-α, IL-6, and IL-17 levels at 6 weeks via cytokine ELISA. Each data point represents data from five to eight mice/group. Error bars represent sem; *P < 0.05 versus similarly treated BALB/c mice.
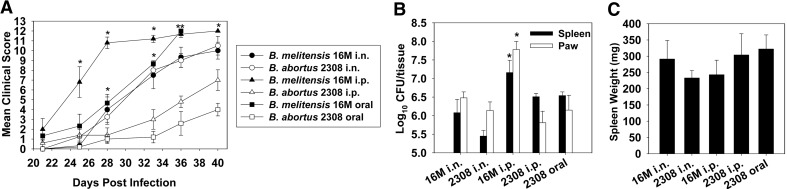
Figure 3. B. melitensis and B. abortus induce joint inflammation in IFN-γ−/− mice independent of route of infection.
IFN-γ−/− mice (BALB/c background, five to seven/group) were infected i.n. (2×104 CFUs), i.p. (2×104 CFUs), or orally (2×1010 CFUs) with B. melitensis 16M or B. abortus 2308. (A) Disease severity was monitored over time, as described, and colonization of the (B) spleens and paws and (C) splenic weights was determined on Day 40 postinfection (mice infected orally with B. melitensis became moribund and were euthanized on Day 36). Error bars represent sem; *P < 0.05, as compared with mice infected with B. abortus via the same route; at this time point, mean clinical scores in mice infected both orally and i.p. with B. melitensis were significantly greater (P < 0.05), than mice infected with B. abortus via the same route.
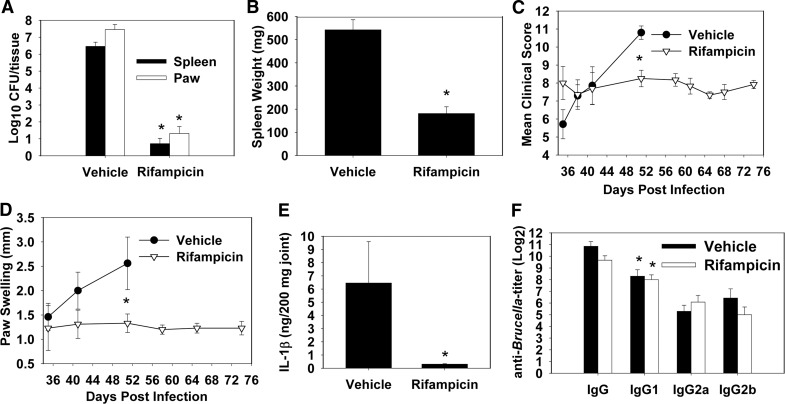
Figure 5. Oral rifampicin halts progression of established paw inflammation resolution in Brucella-infected IFN-γ−/− mice.
IFN-γ−/− mice (BALB/c background) were infected i.n. with 2 × 104 CFUs B. melitensis 16M. On Day 35 postinfection, some mice received 25 mg/kg rifampicin twice/week by oral gavage, while also receiving 0.4 mg/ml rifampicin in their drinking water, and vehicle-treated animals received sterile water. Mice receiving water only (n=7) became moribund and were killed on Day 51, whereas the remaining mice were killed on Day 74. (A) Tissue colonization and (B) splenic weights were decreased significantly in mice receiving rifampicin (n=12), which halted the progression of (C) clinical scores and (D) paw swelling. (E) Rifampicin treatment also decreased paws' IL-1β levels, (F) but did not affect the levels of anti-Brucella antibody in joint homogenates, which were predominantly of an IgG1 subclass. Error bars represent sem; *P < 0.05 as compared with mice receiving vehicle (A–E) and *P < 0.05 as compared with IgG2a and IgG2b levels within the same treatment group (F).
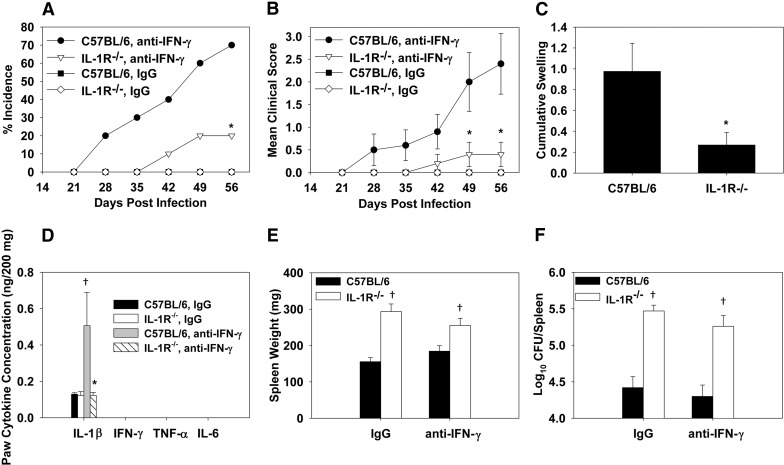
Figure 6. IL-1 controls systemic infection but is also required for focal inflammation during Brucella infection.
IL-1R−/− or WT C57BL/6 mice were treated i.p. with rat IgG (n=5/group) or IFN-γ-neutralizing mAb (n=10/group) prior to and after i.p. infection with 2 × 104 CFUs of B. melitensis 16M. (A) Incidence of peripheral inflammation (paws and/or tail) and (B) clinical scores were assessed over time. (C) Cumulative swelling (paws and tails) was measured on Day 56 postinfection. (D) Cytokine concentrations in paw homogenates, (E) spleen weights, and (F) splenic brucellae CFUs were also determined. Error bars represent sem; *P < 0.05 as compared with C57BL/6 mice depleted of IFN-γ; †P < 0.05 as compared with C57BL/6 mice receiving the same antibody treatment.
Antibiotic treatment
Mice were treated twice/week with 50 mg/kg rifampicin (Sigma-Aldrich Chemical Co.) via oral gavage, along with 0.4 mg/ml rifampicin in their drinking water, which was prepared fresh twice/week.
Hyperimmune anti-IL-17 rabbit sera
The entire coding region for IL-17A was amplified by RT-PCR and cloned into the BamHI-HindIII site of the pmal-c2 plasmid (New England BioLabs, Beverly, MA, USA). The IL-17A cDNA sequence was confirmed by DNA sequencing. The IL-17 gene was inserted downstream from the MBP, and murine rIL-17 was expressed as a fusion MBP. Expression of rMBP-IL-17 was induced by the addition of 0.6 mM IPTG to a midlog phase Escherichia coli culture. Cells were harvested 4 h later and disrupted by sonication; the rMBP-IL-17 fusion protein was purified on an amylose affinity column, according to the manufacturer's protocol. Rabbits were subsequently immunized, initially with 100 μg rMBP-IL-17 in CFA and boosted three times with 50 μg protein in IFA. Neutralization of IL-17 bioactivity was confirmed in vivo.
Antibody ELISA
Joint homogenates were added to microtiter plates coated with 2 μg/ml ELISA-grade chicken CII (Chondrex), as described previously [35, 37], or plates coated with 5 × 108/ml HKBA. Goat anti-mouse HRP-labeled IgG, IgG1, IgG2a, or IgG2b (Southern Biotechnology Associates, Birmingham, AL, USA) was used as detecting antibody. Enzymatic reaction was developed with ABTS (Moss, Pasadena, MD, USA). OD was read at 415 nm using an ELx808 microplate reader (Bio-Tek Instruments, Winooski, VT, USA). Endpoint titers represented a reciprocal logarithm of 2 for the joint homogenate dilution with an OD ≥0.1 above negative control.
Statistics
The Mann-Whitney U test was used for statistical evaluation of clinical scores, histology scores, and paw swelling. Focal inflammation incidence was analyzed by log-rank analysis on survival curves. Student's t test was performed to analyze cytokine concentration, tissue colonization, splenic weights, and antibody titers. Results were considered statistically significant if the P value were <0.05.
RESULTS
Brucella infection induces IL-17-independent inflammation in IFN-γ−/− but not in IFN-γ+/+ BALB/c mice
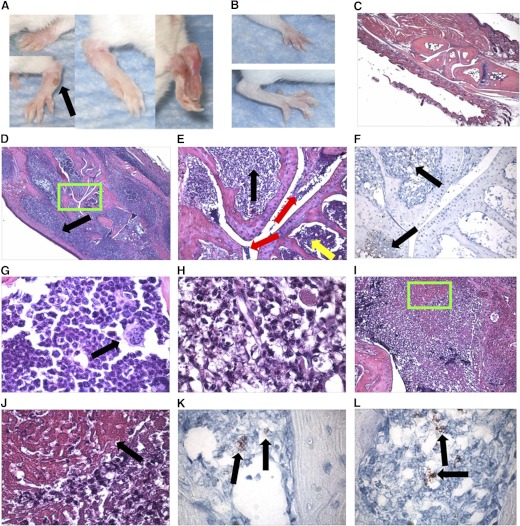
As part of an ongoing study assessing immunity to pulmonary Brucella infections, IFN-γ−/− mice (BALB/c background) treated with anti-IL-17 hyperimmune or NRS developed paw inflammation 6 weeks after i.n. infection with B. melitensis 16M (data not shown). To assess the induction of this inflammation, as well as the role for IL-17, which has been implicated in rodent models of rheumatoid and infectious arthritis [40, 41], a kinetic analysis was performed in IFN-γ+/+ and IFN-γ−/− BALB/c mice (Figs. 1 and 2). IFN-γ−/− (Fig. 1A), but not IFN-γ+/+ mice, developed redness and swelling of paws beginning between 3 and 4 weeks postinfection with B. melitensis (Fig. 1A), relative to naïve IFN-γ−/− mice (Fig. 1B). Histological examination of B. melitensis-infected IFN-γ−/− mouse joints revealed articular and periarticular inflammation. B. melitensis-infected IFN-γ−/− mice developed cutaneous and muscular swelling, myeloid cell infiltrates and necrosis (Fig. 1D, E, and G), bone marrow hyperplasia (Fig. 1G), and osteomyelitis (Fig. 1D, E, and G), relative to naïve IFN-γ−/− mice (Fig. 1C). Inflammatory debris and fibrin within the joint space of infected mice (Fig. 1E, I, and J) were also found. Brucella antigen was also detected via immunohistochemistry within the joint space (Fig. 1K) and bone (Fig. 1F and L). Brucella antigen appeared to colocalize more often with areas of inflammation and necrosis than with bone marrow hyperplasia (Fig. 1E and F). Treatment of mice with an anti-IL-17 hyperimmune serum or NRS on Days 14, 21, 28, and 35 postinfection had no effect on the induction of paw inflammation (Fig. 2A), indicating that the symptoms were IL-17-independent, and the treatment of IFN-γ−/− mice in our initial study with NRS or anti-IL-17 hyperimmune serum was not a cofactor in disease induction. Treatment of mice with anti-IL-17 hyperimmune serum prior to infection also had no impact on the induction of paw inflammation (data not shown).
Figure 1. Brucella infection induces paw inflammation in IFN-γ−/− mice.
IFN-γ−/− mice (on a BALB/c background) were infected i.n. with 2 × 104 CFUs of B. melitensis 16M. Depicted are photographs of (A) inflamed paws from IFN-γ−/− mice on Day 42 postinfection with B. melitensis compared with (B) normal paws from naïve IFN-γ−/− mice. The black arrow in A indicates an abscess that was occasionally observed on the paws of mice with severe disease. Paw joint sections were prepared from (C) naïve IFN-γ−/− mice and (D) B. melitensis-infected IFN-γ−/− mice, 42 days after infection, stained with H&E, and visualized at 40× original magnification The black arrow indicates periarticular soft tissue inflammation. (E) Magnified area (green box of D), visualized at 200× original magnification. Red arrows depict inflammatory debris in the joint space; black arrow indicates inflammatory and necrotic cells within bone; and yellow arrow indicates bone marrow hyperplasia. (F) Immunohistochemistry analysis corresponding to H&E slide depicted in E. Black arrows indicate Brucella antigen. (G) 1000× original magnification of region of cellular hyperplasia, such as that indicated by a yellow arrow in E; black arrow indicates megakaryocyte. (H) 1000× original magnification of area indicated by black arrow in E showing inflammatory and necrotic cells. (I) Fibrin (green box) was also found in the joint space of infected joints (200× original magnification) and magnified in J (1000× original magnification; black arrow). Intracellular Brucella antigen (black arrows) was also detected via immunohistochemistry (1000× original magnification) within (K) the joint space and (L) bone.
The onset of symptoms correlated with increased systemic and focal levels of Brucella. At 2 weeks postinfection, no differences in splenic colonization in IFN-γ+/+ and IFN-γ−/− BALB/c mice were observed; however, at 4–6 weeks postinfection, IFN-γ−/− mice harbored 103–104 more brucellae than WT mice (Fig. 2B). IFN-γ−/− mice also possessed significantly greater brucellae burdens in paw homogenates beginning at 4 weeks postinfection, with colony counts ∼106 times higher than in BALB/c mice at 6 weeks postinfection (Fig. 2C). The concentrations of various proinflammatory cytokines in paw homogenates from BALB/c and IFN-γ−/− mice were measured. TNF-α, IL-6, and IL-17A levels were below the limit of detection (∼100 pg/200 mg paw homogenate; Fig. 2D). However, elevated levels of IL-1β in paw homogenates from infected IFN-γ−/− mice, but not from infected IFN-γ+/+ BALB/c or naïve IFN-γ−/− mice, were observed (Fig. 2D). Thus, these findings show IFN-γ−/− mice are susceptible to Brucella-induced osteoarthritis with accompanying soft tissue inflammation, evident by the observed inflammation in the joints, bone, and periarticular soft tissue.
Multiple routes of infection with B. melitensis and B. abortus induce focal inflammation in IFN-γ−/− mice
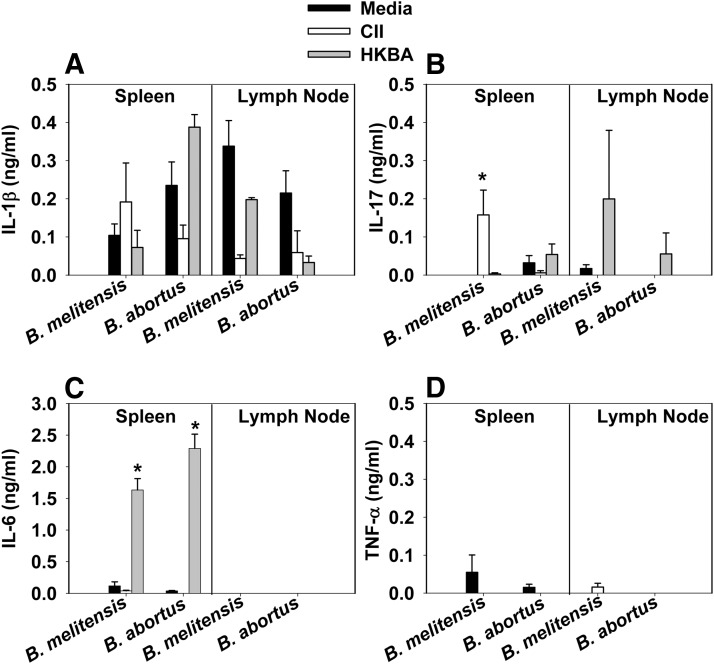
The pathogenesis of B. abortus in IFN-γ−/− mice infected by the i.p. route has been reported previously, but paw inflammation has not been reported in mice monitored for up to 10 weeks postinfection [42]. To determine whether the disease observed in IFN-γ−/− mice following i.n. infection with B. melitensis was influenced by the route of infection and the species of Brucella used (Fig. 3 and Table 2), IFN-γ−/− mice were infected with B. melitensis or B. abortus via i.p. (2×104 CFUs), i.n. (2×104 CFUs), or oral routes (2×1010 CFUs). Mice were monitored for paw inflammation over time. B. melitensis and B. abortus induced paw inflammation by all routes of infection; however, clinical scores as a result of infection with B. melitensis were enhanced significantly (P<0.05) compared with inflammation induced by B. abortus when mice were infected by the oral or parenteral route (Fig. 3A). Paws and spleens harvested from IFN-γ−/− mice bore substantial levels of brucellae, regardless of the route of infection or strain used (Fig. 3B; all mice orally infected with B. melitensis became moribund and were euthanized on Day 36, and CFU determinations were not performed). No significant differences in splenic weights were observed as a consequence of route of infection or Brucella species (Fig. 3C). To assess whether focal inflammation as a result of Brucella infection in IFN-γ−/− mice was a result of a host recall response to Brucella or collagen, cytokine production, by mononuclear cells isolated from the spleens and draining LNs from infected mice, was measured following restimulation with HKBA or CII. Whereas HKBA stimulation induced IL-6 production, CII stimulation resulted in minimal IL-1β, IL-6, IL-17, or TNF-α production (Fig. 4A–D). In addition, minimal anti-CII antibody titers were observed in joint homogenates (data not shown).
Table 2. B. abortus and B. melitensis induce joint inflammation in select mouse strainsa.
| Organism | Route | Challenge dose | Mouse (background) | Time monitored | Disease | Mean score |
|---|---|---|---|---|---|---|
| B. abortus RB51 | oral | 1 × 1011 CFUs | IFN-γ−/− (BALB/c) | 70 days | 0/6 | 0 |
| B. abortus RB51 | i.p. | 1 × 108 CFUs | IFN-γ−/− (BALB/c) | 84 days | 0/5 | 0 |
| B. abortus 2308 | i.n. | 2 × 104 CFUs | B6 | 84 days | 0/5 | 0 |
| B. abortus 2308 | i.n. | 2 × 104 CFUs | IRF-1−/− (B6) | 84 days | 0/6 | 0 |
| B. melitensis 16M | i.n. | 2 × 104 CFUs | B6 | 56 days | 0/6 | 0 |
| B. melitensis 16M | i.n. | 2 × 104 CFUs | IRF-1−/− (B6) | 100 days | 1/5 | 0.6 |
| B. abortus 2308 | i.p. | 2 × 104 CFUs | B6 | 28 days | 0/0 | 0 |
| B. abortus 2308 | i.p. | 2 × 104 CFUs | IFN-γ−/− (B6) | 28 days | 5/5 | 10.6 |
| ΔznuA B.melitensis 16M | oral | 1 × 1011 CFUs | IFN-γ−/− (BALB/c) | 70 days | 0/5 | 0 |
IFN-γ−/− (H-2d), C57BL/6 (B6), IRF-1−/−, and IFN-γ−/− (H-2b) mice were infected with B. abortus RB51 (vaccine strain), attenuated ΔznuA B. melitensis 16M, or WT strains B. abortus 2308 and B. melitensis 16M. Disease incidence and clinical scores were monitored over time as described in Materials and Methods.
Figure 4. Restimulation of mononuclear cells from Brucella-infected IFN-γ−/− mice with CII or HKBA induces a modest inflammatory cytokine response.
IFN-γ−/− mice (five to seven/group) were infected i.n. (2×104 CFUs) with B. melitensis 16M or B. abortus 2308. On Day 40 postinfection, mononuclear cells from the spleens and LNs were isolated and cultured with mouse CII (10 μg/ml), HKBA (108/ml), or media alone, and cell-free supernatants were assayed for (A) IL-1β, (B) IL-17, (C) IL-6, and (D) TNF-α via ELISA. IL-4 and IL-10 concentrations were below detection in all treatment groups. Error bars represent sem; *P < 0.05 depicts significant differences in cytokine production as compared with media-restimulated cultures.
Whereas WT B. melitensis and B. abortus strains induced paw inflammation by all routes examined (Fig. 3 and Table 2), we sought to determine if infection of IFN-γ−/− mice with the attenuated B. abortus RB51 vaccine strain or an attenuated ΔznuA mutant of B. melitensis [33] would result in disease. In this regard, no paw inflammation was observed for up to 10 weeks postinfection regardless of route of infection (i.p. or oral; Table 2). RB51 and ΔznuA B. melitensis were unable to persist, and infection was cleared readily in IFN-γ−/− mice (data not shown). To assess paw inflammation in another commonly used immunocompromised mouse model on a B6 background, IRF-1−/− mice (exhibit deficiencies in CD8+, NK, and γδ T cell development and Th1-type responses and are more susceptible to experimental brucellosis [43, 44]) and WT B6 mice were i.n.-infected with B. melitensis or B. abortus. No B6 mice infected with B. melitensis or B. abortus developed overt paw inflammation for up to 12 weeks postinfection (Table 2). Whereas B. abortus infection did not induce paw inflammation in IRF-1−/− mice, one of five IRF-1−/− mice infected i.n. with B. melitensis did develop inflammation in one hind paw (Table 2). To assess the influence of mouse background on IFN-γ deficiency, IFN-γ−/− mice on an H-2b (B6) background were infected i.p. with B. abortus and developed severe symptoms, suggesting a critical role of IFN-γ, rather than mouse haplotype, in the induction of paw inflammation (Table 2).
Oral antibiotics halt development of Brucella-induced paw inflammation in IFN-γ−/− mice
To assess the efficacy of antibiotics to treat focal inflammation as a result of experimental brucellosis, IFN-γ−/− mice (BALB/c background) were nasally infected with B. melitensis 16M and treated orally with rifampicin, starting 5 weeks after infection. Rifampicin was selected, as it can successfully treat experimental brucellosis [45, 46] and is commonly used in combination with doxycycline to treat human brucellosis [47, 48]. Doxycycline was not used in conjunction with rifampicin in this study, as it causes diarrhea and weight loss in mice [49].
A 6-week regimen of rifampicin was effective in treating established B. melitensis infection. Rifampicin-treated mice contained ∼106 fewer bacteria in their spleens and paws than untreated mice, which became moribund and were euthanized on Day 51 postinfection (Fig. 5A). Brucellae levels were below the limit of detection (20 CFU) in the spleens of eight of 12 mice treated with rifampicin, and paw colonization in six of 12 mice was below detectable levels (20 CFU) in rifampicin-treated mice. Upon initiation of treatment, mice had already exhibited weight loss and ruffling of their fur. Within 1–2 weeks of treatment, these symptoms were ameliorated rapidly by rifampicin (data not shown). Rifampicin also halted the progression of clinical scores (Fig. 5C) and paw swelling (Fig. 5D), but inflammation was not resolved completely, particularly in the redness of the paws. Rifampicin treatment also significantly decreased the amount of IL-1β found in the paws of treated mice (Fig. 5E), whereas it had little effect on IgG anti-Brucella levels present in paw homogenates (Fig. 5F).
IL-1R−/− mice are more susceptible to systemic infection but develop less focal inflammation than WT mice during experimental brucellosis
IL-1 is a proinflammatory cytokine involved in the pathogenesis of septic and rheumatoid forms of arthritis [20, 21, 23, 25]. As elevated levels of IL-1β were present in the paws of mice with paw inflammation as a result of experimental brucellosis (Fig. 2D), we assessed the role of IL-1 on focal inflammation. IL-1R−/− (B6 background) and WT mice were neutralized of IFN-γ, 1 day prior to and weekly after i.p. infection with 2 × 104 CFUs of B. melitensis. Inflammation of the paws or tails was then scored weekly, and on Day 56, after infection, swelling of the paws and tails was recorded, using a digital caliper (Fig. 6A–C). In contrast to IFN-γ−/− (BALB/c background) mice, in which the paws had the most commonly inflamed tissue, all B6 or IL-1R−/− mice, depleted of IFN-γ that developed focal inflammation (70% and 20%, respectively), presented with inflammation of the tails (Fig. 7), whereas B6 mice (40%), but not IL-1R−/− mice (0%) depleted of IFN-γ, also developed paw inflammation following B. melitensis infection. B6 and IL-1R−/− mice that were treated with IgG, were not found to develop focal inflammation (Fig. 6A). Brucellae counts in paw homogenates were below the limit of detection in IFN-γ-depleted mice (∼103 CFUs/tissue; data not shown), which could explain the muted paw inflammation in IFN-γ-depleted versus IFN-γ−/− mice; however, IL-1 levels in paw homogenates from B6 mice depleted of IFN-γ were still significantly higher than those observed in B6 mice treated with IgG (Fig. 6D). As seen previously in B. melitensis-infected IFN-γ−/− mice, IL-6 and TNF-α levels in paw homogenates were below the level of detection (∼100 pg/200 mg tissue). Similar to inflamed paws, inflamed tails displayed extensive neutrophil and mononuclear cell infiltration, along with fibrin and bone erosion (Fig. 7A and B). At termination of the study, IFN-γ neutralization had no impact on splenic weights or bacterial loads in WT or IL-1R−/− mice (Fig. 6E and F), indicating that the efficacy of our IFN-γ neutralization regimen may have waned over the extended duration of the experiment. Whereas we used two distinct IFN-γ-depleting mAb over the course of this study, in attempt to avoid the host immune response to these neutralizing antibody, both were of a rat IgG1 isotype, and therefore, it is possible that treated mice developed neutralizing antibodies against the anti-IFN-γ antibodies, leading to incomplete depletion of anti-IFN-γ. Nonetheless, it was found that IL-1R−/− mice (treated with IgG or anti-IFN-γ mAb) displayed significantly higher splenic weights and bacterial burdens than WT mice, showing the importance of IL-1 for resolution of systemic Brucella infection.
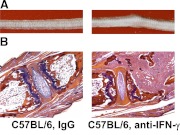
Figure 7. B. melitensis infection induces tail inflammation in mice lacking IFN-γ.
C57BL/6 mice were infected i.p. with 5 × 104 CFUs of B. melitensis 16M. One group of mice was neutralized of their IFN-γ by mAb treatments versus control mice given rat IgG. (A) Images depict Day 56 postinfection, showing that infected mice given rat IgG did not develop tail inflammation, whereas in contrast, mice depleted of IFN-γ developed gross swelling of the tail. (B) Tail sections (at Day 56 postinfection) were stained with H&E and visualized at 40× magnification. In contrast to IgG-treated mice, tails from mice depleted of IFN-γ displayed massive inflammation consisting of neutrophils, fibrin, and mononuclear cells, along with destruction of the bone.
DISCUSSION
With over 500,000 new, annual human cases [4], brucellosis is the most common zoonotic disease in the world [1]. Whereas the pathological manifestations of brucellosis are diverse, infection of the joints is the most common sequelae to brucellosis, and in fact, it is a common cause of infectious arthritis in endemic regions [50]. The study of osteoarticular manifestations of brucellosis has been hampered by the lack of relevant models, as mice are naturally resistant to Brucella and do not develop disease in the same way as humans or ruminants [51]. An earlier study has shown that direct intra-articular inoculation of high doses of the B. abortus S19 vaccine strain into young cattle could produce arthritis [52]. However, evaluation of host immune response and pathogenesis of disease in cattle is difficult as a result of the limited availability of genetically similar animals and feasibility of working with large animals in BSL-3 containment facilities, thereby, limiting the use of cattle as a model species to study brucellar arthritis.
Here, we report for the first time, to our knowledge, that IFN-γ−/− but not WT mice develop osteoarthritis, along with extensive periarticular soft-tissue inflammation following infection with Brucella. Arthritis, as a result of Brucella in humans, is thought to occur via a hematogenous route [16]; however, bacteremia, following experimental infection with B. suis or B. melitensis infection in WT mice, was found by others to be transient [53–55]. Although IFN-γ+/+ and IFN-γ−/− mice possessed similar splenic burdens of brucellae at 14, 28, and 42 days postinfection, IFN-γ−/− spleens contained 103–104 more brucellae than WT spleens. The timing of increased splenic and focal susceptibility of IFN-γ−/− mice correlated with the onset of paw inflammation, indicating that sustained, elevated levels of systemic brucellae may lead to hematogenous spread of brucellae to peripheral sites, resulting in osteoarthritis and soft tissue inflammation. Indeed, in a pilot study, we found that 4 weeks after nasal infection with B. melitensis, five of five IFN-γ−/− mice had detectable brucellae in their blood (30–400 CFUs/100 μl), whereas brucellae in blood from all five WT BALB/c mice tested were below our limit of detection (∼6 CFUs/100 μl).
A recent study has shown that human patients with focal complications of brucellosis are more likely to have an IFN-γ gene polymorphism that is associated with low IFN-γ production [56]. In addition, others have demonstrated that human patients with chronic brucellosis have an impaired IFN-γ response to Brucella antigen relative to patients at the onset of disease [57]. Therefore, suboptimal IFN-γ responses to brucellosis in mice and humans may result in peripheral inflammation. In addition, mice deficient in cytokine signaling have been used as models of human diseases to which mice are naturally resistant [58]. In particular, mice that lack IFN-α/βR and IFN-γR genes have been widely used to investigate vaccination and immunotherapeutic interventions to combat Dengue virus infection [59], thereby, demonstrating the use of cytokine-deficient animals to study and treat infection.
Paw homogenates from B. abortus- or B. melitensis-infected IFN-γ−/− mice were found to contain substantial brucellae burden. Histopathological examination of affected joints revealed swelling, fibrin, hypercellular bone marrow, osteomyelitis, myeloid infiltration, and necrosis. In humans, the osteoarticular structures affected by brucellosis vary depending on the age of the patient. In young patients, brucellosis causes arthritis of weight-bearing joints, such as the hip, knee, and ankle, and soft-tissue swelling and periarticular osteoporosis are the predominant clinical findings [7, 8, 10, 11], which resemble our findings in IFN-γ−/− mice. Whereas peripheral arthritis is most common in children, sacroiliitis and spondylitis are the most common manifestation in young adults and older adults, respectively [7–10]. In contrast to IFN-γ−/− BALB/c animals, the most commonly inflamed tissue in Brucella-infected B6 mice depleted of IFN-γ was the tail, which could resemble sacroiliitis as a result of Brucella infection in humans. The reason for the differing tropism of inflammation in BALB/c IFN-γ−/− versus IFN-γ-neutralized B6 mice is currently unknown; however, it may be a result of the incomplete abrogation of IFN-γ by our neutralization regimen. Whereas IFN-γ−/− mice infected with Brucella displayed ruffling of the fur and a hunched posture, no clinical symptoms, other than focal inflammation of the extremities, were observed in IFN-γ-neutralized B6 mice, indicating that the systemic loads of Brucella in IFN-γ-neutralized mice did not reach the level needed to induce systemic disease, as was observed in IFN-γ−/− mice. Studies by others, using an attenuated strain of bioluminescent Brucella, have found higher loads of bacteria in the tail rather than in the paws of IRF-1−/− mice, which have an impaired Th1 cell response during chronic infection [54]. Therefore, it is possible that a higher bacterial load of Brucella, as is seen in IFN-γ−/− mice, is needed to seed the paws rather than the tail for infection and inflammation. Spondylitis was not observed overtly in our studies; however, future histopathological studies will determine whether vertebrae are affected by Brucella infection in IFN-γ−/− mice.
Brucella antigen was also detected and colocalized with areas of inflammatory cells within the joint spaces, soft tissue, and bone of infected mice, indicating that Brucella infection of IFN-γ−/− mice induces a septic arthritis and osteomyelitis, which can be caused by cellular hyperplasia, along with inflammatory cell infiltrate, both of which were observed within the bone marrow of Brucella-infected IFN-γ−/− mice. Osteomyelitis, although not as common as arthritis, is also observed in human patients with brucellosis [9, 10, 60, 61], and the recovery of Brucella spp. from bone marrow in brucellosis patients is more common than recovery from the blood [62]. Minimal damage to cartilage was observed, and mice did not produce robust cytokine or antibody responses (data not shown) to CII collagen. In our study, considerable brucellae burdens (>106 CFU) were observed in the paws of Brucella-infected IFN-γ−/− mice, which is similar to the bacterial joint loads observed by others in murine models of Staphylococcus aureus- or Streptococcus group B-induced septic arthritis [22, 63]. Brucella is recovered frequently from the synovial fluid of affected patients, but the numbers of brucellae recovered are generally <500 CFU/ml [18]. However, the culture of synovial tissue, rather than synovial fluid, has been found to enhance the recovery of Brucella [19], although little quantitative data are available on the numbers of brucellae present in synovial tissue. Whereas paws from Brucella-infected IFN-γ−/− mice bore substantial levels of bacteria, brucellae colonization of the inflamed paws of B6 mice neutralized of their IFN-γ, was below the limit of detection (∼103 CFU/paw), indicating that prolonged infection of the joints with high levels of Brucella, such as seen in IFN-γ−/− mice, is not required for focal inflammation as a result of experimental brucellosis. Therefore, the incomplete neutralization of IFN-γ in our studies may mimic a defective Th1 cell response in humans, which has been hypothesized to lead to focal inflammation [57]. Histological examination of brucellosis patients reveals that myeloid cell infiltrates are detected in the affected joints of humans [8, 12], and brucellar arthritis generally does not lead to osteoarticular destruction [8]. Whereas the disease induced by Brucella infection in IFN-γ−/− mice does not completely resemble that seen in human brucellosis patients with osteoarticular symptoms, Brucella infection in humans is protean and can cause inflammation in myriad locations [5, 6, 55]. Therefore, IFN-γ−/− mice may be a useful alternative model to investigate Brucella-induced inflammation in sites not normally colonized by Brucella in murine models.
Leukocyte inflammation and Brucella antigen were also found in muscle and cutaneous tissues adjacent to and surrounding the joints in Brucella-infected IFN-γ−/− mice. Lesions were sporadically observed on the rear paws of some Brucella-infected IFN-γ−/− mice with severe inflammation. Bacterial infections of the bone and joint are known to spread to soft tissue [64], and some s.c. abscesses are manifestations of osteomyelitis [65]. Indeed, we observed areas of bone erosion that colocalized with inflammation and Brucella antigen in infected IFN-γ−/− mice, and it appeared that the infection could have led to inflammation and bone erosion. Therefore, possibly, Brucella infection of the bones and joints of IFN-γ−/− mice led to inflammation in surrounding soft tissue; however, future experimentation will be required to test this possibility.
Initially, mice were infected i.n. with B. melitensis to induce paw inflammation in IFN-γ−/− mice, as this route of infection led to our initial observation of paw inflammation. Others have i.p.-infected IFN-γ−/− mice on a BALB/c background with B. abortus and have not reported paw inflammation when mice were followed for ∼10 weeks postinfection [42]. Therefore, we queried whether paw inflammation as a result of Brucella in IFN-γ−/− mice was dependent on the species of Brucella and on the route of infection. B. melitensis and B. abortus induced paw inflammation in IFN-γ−/− mice, regardless of the route tested. In addition, substantial brucellae burden was found in the spleens and paws of infected IFN-γ−/− mice, regardless of the WT Brucella strain used or the route of infection, indicating that paw inflammation may have been present in earlier studies by others but went unnoticed. We also found that B. abortus infection induced paw inflammation in IFN-γ−/− mice on a B6 background, indicating that induction of localized inflammation was reliant on a lack of IFN-γ rather than on the genetic background of mice used. IFN-γ−/− mice, on a B6 (H-2b) background, actually developed more rapid and severe symptoms than IFN-γ−/− mice on a BALB/c (H-2d) background following i.p. infection with B. abortus (Fig. 3 and Table 2), which may have been a result of the enhanced importance of IFN-γ in the immune protection of B6 mice during Brucella infection [42]. Human brucellosis is normally attributed to the consumption of contaminated foods with B. melitensis [3]. Interestingly, B. melitensis was found to cause more severe inflammation than B. abortus via i.p. and oral routes of infection. CFU data from mice infected orally with B. melitensis were not available as mice became moribund; however, mice infected i.p. with B. melitensis possessed ∼100-fold more brucellae in their paws than mice infected i.p. with B. abortus, which may explain the enhanced paw inflammation as a result of B. melitensis infection. B. melitensis is also thought to be more virulent than B. abortus in humans [16] and more commonly associated with osteoarticular brucellosis than B. abortus [8], which together, may correspond to the increased paw inflammation following B. melitensis infection observed in this study.
Whereas others have found IL-17 to be required for induction of arthritis in infectious and autoimmune models [66, 67], paw inflammation as a result of B. melitensis infection in IFN-γ−/− mice was found not to be IL-17-dependent. TNF-α and IL-6 can also contribute to the pathogenesis of arthritis [20, 68, 69], and TNF-α is required for immunity to brucellosis [70]; however, TNF-α and IL-6 levels in joint homogenates in our study were below detection limits. Likewise, little TNF-α was generated upon restimulation with HKBA or CII by cultured, splenic, and LN mononuclear cells (Fig. 4). As TNF-α is required for immunity to brucellosis, immunotherapeutics that target TNF-α in an attempt to resolve joint inflammation, could actually render patients more susceptible to infection by Brucella spp. Indeed, anti-TNF therapies in humans have been linked to enhanced susceptibility to infections [71], and in some cases, treatment of rheumatoid arthritis patients with anti-TNF therapeutics may have actually led to infectious arthritis [72, 73]. Thus, TNF-α and IL-6 were not investigated further in our study. However, elevated levels of IL-1β were detected in the paws of mice with inflammation. IL-1 has been implicated as a pathogenic cytokine in septic and rheumatoid forms of arthritis [20, 21, 23, 25], and elevated IL-1 levels have been detected in the synovial fluid of humans with septic forms of bacterial arthritis [23–25]. In human cases of Lyme disease, elevated IL-1ra/IL-1β ratios in the synovial fluid of patients correlate with recovery from disease [25]. In addition, IL-1 has been associated with soft-tissue inflammation following bacterial infection [26, 27, 74]. Little is known about the role of IL-1 in Brucella infections. Treatment of Brucella-infected macrophages with IL-1 has no effect on intracellular bacteria survival [28], whereas administration of IL-1 to mice with established infection is unable to resolve brucellae burden in tissues [29]. IL-1β is generally not detected in the blood of human brucellosis patients [75]; however, human patients with other forms of arthritis have been shown to have elevated IL-1 in the synovial fluid but not in sera [76], indicating that the effect of IL-1β in arthritis may be local [20]. Along these lines, recent work has shown that supernatants from Brucella-infected human osteoblasts cause macrophages to produce IL-1β [30], whereas another report has found elevated levels of IL-1 in the synovial fluid from a human case of brucellar bursitis [31], which together, may support a role for IL-1 in the pathogenesis of human brucellar arthritis.
To investigate the role of IL-1 in inducing joint inflammation during experimental brucellosis, WT B6 and IL-1R−/− mice were neutralized of IFN-γ prior to and during infection with B. melitensis. Whereas IL-1R−/− mice were more susceptible to systemic B. melitensis colonization than WT mice, IL-1R−/− mice displayed less focal inflammation. A recent publication shows that IL-1R-associated kinase 4 is required for control of acute but not chronic B. abortus infection [77]. However, to our knowledge, this is the first report of IL-1 being required for control of chronic Brucella infection. Inflammasome-dependent IL-1 has been shown to be required for protection against several bacterial pathogens, including Bacillus anthracis [78], whereas induction of IL-1 and the inflammasome also contributes to the severity of experimental arthritis [79]. Therefore, future studies will assay the role of caspases and the inflammasome in immunity and focal inflammation during chronic brucellosis.
To assess the effects of antibiotic treatment to halt Brucella-induced arthritis, mice with established inflammation were treated with rifampicin, which is effective at treating experimental models of brucellosis [45, 46] and is used in conjunction with doxycycline for treatment of brucellosis in humans [9]. Rifampicin alone was effective at clearing infection, treating systemic symptoms of disease (weight loss/ruffling of the fur, etc.), and halting the progression of inflammation; however, resolution of established gross inflammation, particularly redness of the paws, remained incomplete, whereas leukocyte infiltration into osteoarticular structures was mostly resolved (data not shown), indicating that the residual gross swelling may have been a result of periarticular inflammation. Prolonged antibiotic therapy may be required to completely resolve experimental brucellosis, as human cases can require up to several months of therapy before symptoms of osteoarticular manifestations are resolved completely [16]. Elimination of brucellae by antibiotic treatment substantially decreased the levels of IL-1β present in paw homogenates, further implicating IL-1 in Brucella-induced focal inflammation.
Taken together, our data showed that IFN-γ−/− mice developed paw inflammation, characterized by periarticular soft-tissue swelling, osteomyelitis, and arthritis following infection with B. melitensis or B. abortus. The attenuated vaccine RB51 strain of B. abortus or ΔznuA B. melitensis did not induce paw inflammation and was cleared rapidly from IFN-γ−/− mice. As human brucellosis patients with focal complications of brucellosis may have an impaired ability to produce IFN-γ [56, 57], paw inflammation in IFN-γ−/− mice following infection with Brucella strains could be measured as a means to determine the safety of attenuated mutants of Brucella being studied as live vaccine strain candidates. This is the first time joint inflammation as a result of Brucella infection in mice has been documented, and the model characterized here could serve as a foundation for future studies investigating the efficacy of antibiotic and immunotherapeutic therapies on the resolution of paw inflammation as a result of brucellosis. In addition, elevated levels of IL-1β were detected in the affected paws of mice, whereas IL-1R−/− mice were more resistant to focal disease, suggesting a role of IL-1 in human brucellar arthritis, and potential therapies that target IL-1 or the IL-1R should be investigated.
ACKNOWLEDGMENTS
This work is supported by grants from U.S. Department of Agriculture, 2007-0612 and 2010-34397-21391, Montana Agricultural Experiment Station, U.S. Department of Agriculture Formula Funds, U.S. National Institutes of Health grant P20 RR020185, and an equipment grant from the M. J. Murdock Charitable Trust. We thank BEI Resources for providing polyclonal goat anti-Brucella antibody; Dr. Jerry R. McGhee for his helpful comments; and Ms. Nancy Kommers for her assistance in preparing this manuscript.
Footnotes
- −/−
- deficient
- BA
- Brucella agar
- BB
- Brucella broth
- BSL-3
- Biosafety Level 3
- CII
- collagen II
- HKBA
- heat-killed Brucella abortus
- IL-1ra
- IL-1R antagonist
- i.n.
- intranasal
- IRF
- IFN regulatory factor
- MBP
- maltose-binding protein
- NRS
- normal rabbit serum
- NSB
- normal serum block
- sPBS
- sterile PBS
- USDA
- U.S. Department of Agriculture
AUTHORSHIP
J.A.S. designed the study, performed laboratory experiments, analyzed the results, and wrote the paper. T.T., I.K., G.C., C.R., M.F.R., C.R., T.B., and S.G. performed laboratory experiments. I.K. and W.L. analyzed the results. D.W.P. designed the study, analyzed the results, and wrote the paper.
REFERENCES
- 1. Pappas G., Papadimitriou P., Akritidis N., Christou L., Tsianos E. V. (2006) The new global map of human brucellosis. Lancet Infect. Dis. 6, 91–99 [DOI] [PubMed] [Google Scholar]
- 2. Chomel B. B., DeBess E. E., Mangiamele D. M., Reilly K. F., Farver T. B., Sun R. K., Barrett L. R. (1994) Changing trends in the epidemiology of human brucellosis in California from 1973 to 1992: a shift toward foodborne transmission. J. Infect. Dis. 170, 1216–1223 [DOI] [PubMed] [Google Scholar]
- 3. Corbel M. J. (1997) Brucellosis: an overview. Emerg. Infect. Dis. 3, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colmenero J. D., Reguera J. M., Fernandez-Nebro A., Cabrera-Franquelo F. (1991) Osteoarticular complications of brucellosis. Ann. Rheum. Dis. 50, 23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ariza J., Corredoira J., Pallares R., Viladrich P. F., Rufi G., Pujol M., Gudiol F. (1995) Characteristics of and risk factors for relapse of brucellosis in humans. Clin. Infect. Dis. 20, 1241–1249 [DOI] [PubMed] [Google Scholar]
- 6. Sauret J. M., Vilissova N. (2002) Human brucellosis. J. Am. Board Fam. Pract. 15, 401–406 [PubMed] [Google Scholar]
- 7. Rajapakse C. N. (1995) Bacterial infections: osteoarticular brucellosis. Baillieres Clin. Rheumatol. 9, 161–177 [DOI] [PubMed] [Google Scholar]
- 8. Gotuzzo E., Alarcon G. S., Bocanegra T. S., Carrillo C., Guerra J. C., Rolando I., Espinoza L. R. (1982) Articular involvement in human brucellosis: a retrospective analysis of 304 cases. Semin. Arthritis Rheum. 12, 245–255 [DOI] [PubMed] [Google Scholar]
- 9. Shaalan M. A., Memish Z. A., Mahmoud S. A., Alomari A., Khan M. Y., Almuneef M., Alalola S. (2002) Brucellosis in children: clinical observations in 115 cases. Int. J. Infect. Dis. 6, 182–186 [DOI] [PubMed] [Google Scholar]
- 10. al Eissa Y. A., Kambal A. M., Alrabeeah A. A., Abdullah A.M., al Jurayyan N. A., al Jishi N. M. (1990) Osteoarticular brucellosis in children. Ann. Rheum. Dis. 49, 896–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosilkovski M., Krteva L., Caparoska S., Dimzova M. (2004) Osteoarticular involvement in brucellosis: study of 196 cases in the Republic of Macedonia. Croat. Med. J. 45, 727–733 [PubMed] [Google Scholar]
- 12. Martin-Hernandez C., Ballester-Jimerez J., Espallargas-Doñate T., Fuertes-Vallcorba A. (2009) Arthroscopic synovectomy, an alternative in the treatment of brucellar arthritis of the knee with prolonged course. A report of two cases. Int. J. Orthopedic Surg. 13 [Google Scholar]
- 13. Celik A. D., Celik Y., Yulugkural Z., Balci K., Utku U. (2008) Acute onset myositis associated with brucellosis, quite a rare diagnosis. Intern. Med. 47, 2091–2093 [DOI] [PubMed] [Google Scholar]
- 14. Akcali C., Savas L., Baba M., Turunc T., Seckin D. (2007) Cutaneous manifestations in brucellosis: a prospective study. Adv. Ther. 24, 706–711 [DOI] [PubMed] [Google Scholar]
- 15. Metin A., Akdeniz H., Buzgan T., Delice I. (2001) Cutaneous findings encountered in brucellosis and review of the literature. Int. J. Dermatol. 40, 434–438 [DOI] [PubMed] [Google Scholar]
- 16. Ayaslioglu E., Ozluk O., Kilic D., Kaygusuz S., Kara S., Aydin G., Cokca F., Tekeli E. (2005) A case of brucellar septic arthritis of the knee with a prolonged clinical course. Rheumatol. Int. 25, 69–71 [DOI] [PubMed] [Google Scholar]
- 17. Andonopoulos A. P., Asimakopoulos G., Anastasiou E., Bassaris H. P. (1986) Brucella arthritis. Scand. J. Rheumatol. 15, 377–380 [DOI] [PubMed] [Google Scholar]
- 18. Yagupsky P., Peled N. (2002) Use of the Isolator 1.5 microbial tube for detection of Brucella melitensis in synovial fluid. J. Clin. Microbiol. 40, 3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coventry M. B., Ivins J. C. (1949) Infection of the hip by Brucella suis. J. Am. Med. Assoc. 141, 320–325 [DOI] [PubMed] [Google Scholar]
- 20. Tissi L., Puliti M., Barluzzi R., Orefici G., von Hunolstein C., Bistoni F. (1999) Role of tumor necrosis factor α, interleukin-1β, and interleukin-6 in a mouse model of group B streptococcal arthritis. Infect. Immun. 67, 4545–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burger D., Dayer J. M., Palmer G., Gabay C. (2006) Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract. Res. Clin. Rheumatol. 20, 879–896 [DOI] [PubMed] [Google Scholar]
- 22. Puliti M., von Hunolstein C., Bistoni F., Orefici G., Tissi L. (2004) Inhibition of nitric oxide synthase exacerbates group B streptococcus sepsis and arthritis in mice. Infect. Immun. 72, 4891–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osiri M., Ruxrungtham K., Nookhai S., Ohmoto Y., Deesomchok U. (1998) IL-1β, IL-6 and TNF-α in synovial fluid of patients with non-gonococcal septic arthritis. Asian Pac. J. Allergy Immunol. 16, 155–160 [PubMed] [Google Scholar]
- 24. Beck G., Benach J. L., Habicht G. S. (1989) Isolation of interleukin 1 from joint fluids of patients with Lyme disease. J. Rheumatol. 16, 800–806 [PubMed] [Google Scholar]
- 25. Miller L. C., Lynch E. A., Isa S., Logan J. W., Dinarello C. A., Steere A. C. (1993) Balance of synovial fluid IL-1β and IL-1 receptor antagonist and recovery from Lyme arthritis. Lancet 341, 146–148 [DOI] [PubMed] [Google Scholar]
- 26. Norrby-Teglund A., Thulin P., Gan B. S., Kotb M., McGeer A., Andersson J., Low D. E. (2001) Evidence for superantigen involvement in severe group a streptococcal tissue infections. J. Infect. Dis. 184, 853–860 [DOI] [PubMed] [Google Scholar]
- 27. Marshall B. G., Wangoo A., Cook H. T., Shaw R. J. (1996) Increased inflammatory cytokines and new collagen formation in cutaneous tuberculosis and sarcoidosis. Thorax 51, 1253–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang X., Baldwin C. L. (1993) Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61, 124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhan Y. F., Stanley E. R., Cheers C. (1991) Prophylaxis or treatment of experimental brucellosis with interleukin-1. Infect. Immun. 59, 1790–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Delpino M. V., Fossati C. A., Baldi P. C. (2009) Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infect. Immun. 77, 984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallach J. C., Delpino M. V., Scian R., Deodato B., Fossati C. A., Baldi C. (2010) Prepatellar bursitis due to Brucella abortus: case report and analysis of the local immune response. J. Med. Microbiol. 59, 1514–1518 [DOI] [PubMed] [Google Scholar]
- 32. Wang X., Hone D. M., Haddad A., Shata M. T., Pascual D. W. (2003) M cell DNA vaccination for CTL immunity to HIV. J. Immunol. 171, 4717–4725 [DOI] [PubMed] [Google Scholar]
- 33. Clapp B., Skyberg J. A., Yang X., Thornburg T., Walters N., Pascual D. W. (2011) Protective live oral brucellosis vaccines stimulate Th1 and Th17 cell responses. Infect. Immun. 79, 4165–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tissi L., Bistoni F., Puliti M. (2009) IL-4 deficiency decreases mortality but increases severity of arthritis in experimental group B Streptococcus infection. Mediators Inflamm. 2009, 394021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kochetkova I., Golden S., Holderness K., Callis G., Pascual D. W. (2010) IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J. Immunol. 184, 7144–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ochoa-Repáraz J., Rynda A., Ascón M. A., Yang X., Kochetkova I., Riccardi C., Callis G., Trunkle T., Pascual D. W. (2008) IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J. Immunol. 181, 954–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kochetkova I., Trunkle T., Callis G., Pascual D. W. (2008) Vaccination without autoantigen protects against collagen II-induced arthritis via immune deviation and regulatory T cells. J. Immunol. 181, 2741–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ochoa-Repáraz J., Riccardi C., Rynda A., Jun S., Callis G., Pascual D. W. (2007) Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J. Immunol. 178, 1791–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pascual D. W., Trunkle T., Sura J. (2002) Fimbriated Salmonella enterica serovar Typhimurium abates initial inflammatory responses by macrophages. Infect. Immun. 70, 4273–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lubberts E., Joosten L. A., Oppers B., van den B. L., Coenen-de Roo C. J., Kolls J. K., Schwarzenberger P., van de Loo F. A., van den Berg W. B. (2001) IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J. Immunol. 167, 1004–1013 [DOI] [PubMed] [Google Scholar]
- 41. Burchill M. A., Nardelli D. T., England D. M., DeCoster D. J., Christopherson J. A., Callister S. M., Schell R. F. (2003) Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect. Immun. 71, 3437–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy E. A., Sathiyaseelan J., Parent M. A., Zou B., Baldwin C. L. (2001) Interferon-γ is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siegmund B., Sennello J. A., Lehr H. A., Senaldi G., Dinarello C. A., Fantuzzi G. (2004) Frontline: interferon regulatory factor-1 as a protective gene in intestinal inflammation: role of TCR γδ T cells and interleukin-18-binding protein. Eur. J. Immunol. 34, 2356–2364 [DOI] [PubMed] [Google Scholar]
- 44. Rajashekara G., Krepps M., Eskra L., Mathison A., Montgomery A., Ishii Y., Splitter G. (2005) Unraveling Brucella genomics and pathogenesis in immunocompromised IRF-1−/− mice. Am. J. Reprod. Immunol. 54, 358–368 [DOI] [PubMed] [Google Scholar]
- 45. Sezak N., Kuruuzum Z., Cakir N., Yuce A. (2008) Comparison of rifampicin and moxifloxacin efficacy in an experimental model of animal brucellosis. J. Chemother. 20, 58–62 [DOI] [PubMed] [Google Scholar]
- 46. De Rycke J. (1980) Effect of rifampicin and tetracycline alone and in combination against Brucella suis. Ann. Microbiol. (Paris) 131B, 277–287 [PubMed] [Google Scholar]
- 47. Agalar C., Usubutun S., Turkyilmaz R. (1999) Ciprofloxacin and rifampicin versus doxycycline and rifampicin in the treatment of brucellosis. Eur. J. Clin. Microbiol. Infect. Dis. 18, 535–538 [DOI] [PubMed] [Google Scholar]
- 48. Saltoglu N., Tasova Y., Inal A. S., Seki T., Aksu H. S. (2002) Efficacy of rifampicin plus doxycycline versus rifampicin plus quinolone in the treatment of brucellosis. Saudi Med. J. 23, 921–924 [PubMed] [Google Scholar]
- 49. Eger K., Hermes M., Uhlemann K., Rodewald S., Ortwein J., Brulport M., Bauer A. W., Schormann W., Lupatsch F., Schiffer I. B., Heimerdinger C. K., Gebhard S., Spangenberg C., Prawitt D., Trost T., Zabel B., Sauer C., Tanner B., Kolbl H., Krugel U., Franke H., Illes P., Madaj-Sterba P., Bockamp E. O., Beckers T., Hengstler J. G. (2004) 4-Epidoxycycline: an alternative to doxycycline to control gene expression in conditional mouse models. Biochem. Biophys. Res. Commun. 323, 979–986 [DOI] [PubMed] [Google Scholar]
- 50. Geyik M. F., Gur A., Nas K., Cevik R., Sarac J., Dikici B., Ayaz C. (2002) Musculoskeletal involvement of brucellosis in different age groups: a study of 195 cases. Swiss Med. Wkly. 132, 98–105 [DOI] [PubMed] [Google Scholar]
- 51. Maria-Pilar J. B., Dudal S., Dornand J., Gross A. (2005) Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation. Clin. Immunol. 114, 227–238 [DOI] [PubMed] [Google Scholar]
- 52. Johnson B., Mosier D. A., Morton R. J., Confer A. W. (1994) Experimental Brucella abortus strain 19 arthritis in young cattle. J. Vet. Diagn. Invest. 6, 56–61 [DOI] [PubMed] [Google Scholar]
- 53. Smither S. J., Perkins S. D., Davies C., Stagg A. J., Nelson M., Atkins H. S. (2009) Development and characterization of mouse models of infection with aerosolized Brucella melitensis and Brucella suis. Clin. Vaccine Immunol. 16, 779–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rajashekara G., Glover D. A., Krepps M., Splitter G. A. (2005) Temporal analysis of pathogenic events in virulent and avirulent Brucella melitensis infections. Cell. Microbiol. 7, 1459–1473 [DOI] [PubMed] [Google Scholar]
- 55. Paixao T. A., Roux C. M., den Hartigh A. B., Sankaran-Walters S., Dandekar S., Santos R. L., Tsolis R. M. (2009) Establishment of systemic Brucella melitensis infection through the digestive tract requires urease, the type IV secretion system, and lipopolysaccharide O antigen. Infect. Immun. 77, 4197–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hedayatizadeh-Omran A., Rafiei A., Hajilooi M., Haghshenas M. (2010) Interferon-γ low producer genotype +5644 over presented in patients with focal brucellosis. Pak. J. Biol. Sci. 13, 1036–1041 [DOI] [PubMed] [Google Scholar]
- 57. Rafiei A., Ardestani S. K., Kariminia A., Keyhani A., Mohraz M., Amirkhani A. (2006) Dominant Th1 cytokine production in early onset of human brucellosis followed by switching towards Th2 along prolongation of disease. J. Infect. 53, 315–324 [DOI] [PubMed] [Google Scholar]
- 58. Madsen K. L. (2001) Inflammatory bowel disease: lessons from the IL-10 gene-deficient mouse. Clin. Invest. Med. 24, 250–257 [PubMed] [Google Scholar]
- 59. Johnson A. J., Roehrig J. T. (1999) New mouse model for dengue virus vaccine testing. J. Virol. 73, 783–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Colmenero J. D., Ruiz-Mesa J. D., Plata A., Bermúdez P., Martin-Rico P., Queipo-Ortuño M. I., Reguera J. M. (2008) Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin. Infect. Dis. 46, 426–433 [DOI] [PubMed] [Google Scholar]
- 61. Al-Shahed M. S., Sharif H. S., Haddad M. C., Aabed M. Y., Sammak B. M., Mutairi M. A. (1994) Imaging features of musculoskeletal brucellosis. Radiographics 14, 333–348 [DOI] [PubMed] [Google Scholar]
- 62. Yagupsky P. (1999) Detection of brucellae in blood cultures. J. Clin. Microbiol. 37, 3437–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oh K. B., Nam K. W., Ahn H., Shin J., Kim S., Mar W. (2010) Therapeutic effect of (Z)-3-(2,5-dimethoxyphenyl)-2-(4-methoxyphenyl) acrylonitrile (DMMA) against Staphylococcus aureus infection in a murine model. Biochem. Biophys. Res. Commun. 396, 440–444 [DOI] [PubMed] [Google Scholar]
- 64. Wilson D. J. (2004) Soft tissue and joint infection. Eur. Radiol. 14 (Suppl 3), E64–E71 [DOI] [PubMed] [Google Scholar]
- 65. Brook I. (2008) Microbiology and management of soft tissue and muscle infections. Int. J. Surg. 6, 328–338 [DOI] [PubMed] [Google Scholar]
- 66. Nakae S., Nambu A., Sudo K., Iwakura Y. (2003) Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177 [DOI] [PubMed] [Google Scholar]
- 67. Nardelli D. T., Kevin Luk K. H., Kotloski N. J., Warner T. F., Torrealba J. R., Callister S. M., Schell R. F. (2008) Role of IL-17, transforming growth factor-β, and IL-6 in the development of arthritis and production of anti-outer surface protein A borreliacidal antibodies in Borrelia-vaccinated and -challenged mice. FEMS Immunol. Med. Microbiol. 53, 265–274 [DOI] [PubMed] [Google Scholar]
- 68. Van den Berg W. B., Joosten L. A., van de Loo F. A. (1999) TNF α and IL-1 β are separate targets in chronic arthritis. Clin. Exp. Rheumatol. 17, S105–S114 [PubMed] [Google Scholar]
- 69. Hultgren O., Eugster H. P., Sedgwick J. D., Korner H., Tarkowski A. (1998) TNF/lymphotoxin-α double-mutant mice resist septic arthritis but display increased mortality in response to Staphylococcus aureus. J. Immunol. 161, 5937–5942 [PubMed] [Google Scholar]
- 70. Skyberg J. A., Thornburg T., Rollins M., Huarte E., Jutila M. A., Pascual D. W. (2011) Murine and bovine γδ T cells enhance innate immunity against Brucella abortus infections. PLoS ONE 6, e21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bongartz T., Sutton A. J., Sweeting M. J., Buchan I., Matteson E. L., Montori V. (2006) Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295, 2275–2285 [DOI] [PubMed] [Google Scholar]
- 72. Choi S. W., Ahn J. J., Hwang Y. T., Koh S. H., Cho S. D. (2009) A case of tuberculous arthritis following the use of etanercept. Korean J. Intern. Med. 24, 397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Favero M., Raffeiner B., Cecchin D., Schiavon F. (2009) Septic arthritis caused by Rothia dentocariosa in a patient with rheumatoid arthritis receiving etanercept therapy. J. Rheumatol. 36, 2846–2847 [DOI] [PubMed] [Google Scholar]
- 74. Solis-Soto J. M., Quintanilla-Rodriguez L. E., Meester I., Segoviano-Ramirez J. C., Vazquez-Juarez J. L., Salinas Carmona M. C. (2008) In situ detection and distribution of inflammatory cytokines during the course of infection with Nocardia brasiliensis. Histol. Histopathol. 23, 573–581 [DOI] [PubMed] [Google Scholar]
- 75. Ahmed K., Al-Matrouk K. A., Martinez G., Oishi K., Rotimi V. O., Nagatake T. (1999) Increased serum levels of interferon-γ and interleukin-12 during human brucellosis. Am. J. Trop. Med. Hyg. 61, 425–427 [DOI] [PubMed] [Google Scholar]
- 76. Mannami K., Mitsuhashi T., Takeshita H., Okada K., Kuzuhara A., Yamashita F., Sakakida K. (1989) Concentration of interleukin-1 β in serum and synovial fluid in patients with rheumatoid arthritis and those with osteoarthritis. Nippon Seikeigeka Gakkai Zasshi 63, 1343–1352 [PubMed] [Google Scholar]
- 77. Oliveira F. S., Carvalho N. B., Brandao A. P., Gomes M. T., de Almeida L. A., Oliveira S. C. (2011) IL-1 receptor-associated kinase 4 (IRAK-4) is essential for initial host control of Brucella abortus infection. Infect. Immun. 79, 4688–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moayeri M., Crown D., Newman Z. L., Okugawa S., Eckhaus M., Cataisson C., Liu S., Sastalla I., Leppla S. H. (2010) Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 6, e1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Joosten L. A., Netea M. G., Mylona E., Koenders M. I., Malireddi R. K., Oosting M., Stienstra R., van de Veerdonk F. L., Stalenhoef A. F., Giamarellos-Bourboulis E. J., Kanneganti T. D., van der Meer J. W. (2010) Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 62, 3237–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]