PVL-mediated priming of PMNs enhances the host innate immune response.
Keywords: MRSA, priming, neutrophil
Abstract
CA-MRSA infections are often caused by strains encoding PVL, which can cause lysis of PMNs and other myeloid cells in vitro, a function considered widely as the primary means by which PVL might contribute to disease. However, at sublytic concentrations, PVL can function as a PMN agonist. To better understand this phenomenon, we investigated the ability of PVL to alter human PMN function. PMNs exposed to PVL had enhanced capacity to produce O2− in response to fMLF, but unlike priming by LPS, this response did not require TLR signal transduction. On the other hand, there was subcellular redistribution of NADPH oxidase components in PMNs following exposure of these cells to PVL—a finding consistent with priming. Importantly, PMNs primed with PVL had an enhanced ability to bind/ingest and kill Staphylococcus aureus. Priming of PMNs with other agonists, such as IL-8 or GM-CSF, altered the ability of PVL to cause formation of pores in the plasma membranes of these cells. Microarray analysis revealed significant changes in the human PMN transcriptome following exposure to PVL, including up-regulation of molecules that regulate the inflammatory response. Consistent with the microarray data, mediators of the inflammatory response were released from PMNs after stimulation with PVL. We conclude that exposure of human PMNs to sublytic concentrations of PVL elicits a proinflammatory response that is regulated in part at the level of gene expression. We propose that PVL-mediated priming of PMNs enhances the host innate immune response.
Introduction
S. aureus is a Gram-positive bacterium that causes a significant number of infections worldwide [1]. Indeed, MRSA is currently a leading contributor to HA infections [2, 3]. MRSA infections that occur outside of hospital settings, known as CA-MRSA infections, were reported in the early 1990s and have become widespread in the United States and Canada [4, 5]. In contrast to HA-MRSA, CA-MRSA causes infections in individuals with no known risk factors for infection. The ability of these strains to cause disease in otherwise healthy individuals suggests that they have enhanced virulence compared with traditional HA-MRSA strains. In vitro and in vivo work supports this hypothesis [6, 7]. Although progress has been made, the molecular basis of the enhanced virulence phenotype of CA-MRSA remains incompletely determined.
Genes encoding PVL are present in the genome of many CA-MRSA strains, including the epidemic USA300 strain [8, 9]. PVL consists of two subunits, LukS-PV and LukF-PV, whose genes are transcribed as an operon; it is a cytolytic toxin specific for myeloid cells, including PMNs [10]. The presence of both subunits is required for formation of pores within the PMN plasma membrane. Inasmuch as PMNs are the most prominent cellular component of the innate immune system and thus, the primary defense against S. aureus infections, it has been proposed that PVL contributes to virulence by causing lysis of PMNs and other myeloid cells. However, previous studies have shown that cytolysis in vitro requires a concentration of PVL that may not be achieved in vivo [11, 12].
Sublytic concentrations of PVL elicit numerous cellular responses, including release of MPO and chemotactic molecules, such as IL-8 and LTB4 [13–16]. PMNs exposed to PVL undergo granule exocytosis and produce ROS following stimulation with fMLF [17]. These observations suggest that sublytic levels of PVL prime PMNs for enhanced activation by a secondary stimulus, although the molecular basis for PVL-mediated PMN priming remains unknown.
To gain a better understanding of the molecular basis of PVL-mediated PMN priming, we investigated mobilization of the NADPH oxidase components to the plasma membrane and measured PMN gene expression following exposure of these cells to sublytic concentrations of PVL. In addition, we identified proinflammatory molecules secreted by PMNs following exposure to the leukotoxin. Our results provide new insight into a possible role played by PVL during human infection.
MATERIALS AND METHODS
Human PMN isolation
PMNs were isolated from venous whole blood of healthy individuals, as described previously [18]. Purity of PMNs was 99.7 ± 0.2%, and viability was 99.2 ± 1.1%, as determined from a sampling of >40 PMN preparations during the course of the studies. PMN preparations typically contain 95–98% neutrophils, and virtually all of the remaining cells are eosinophils. Each subject gave informed consent prior to participation in the study, and all work was approved by the Institutional Review Board for Human Subjects, NIAID, NIH (Hamilton, MT, USA), or by the Institutional Review Board for Human Subjects, Montana State University (Bozeman, MT, USA).
S. aureus culture conditions
USA300 strain LAC was cultured overnight in TSB (Difco, Detroit, MI, USA) from frozen bacterial stocks. Overnight cultures were diluted 1:200 in fresh TSB media and cultured to a midlogarithmic growth phase (OD600=0.75), as described [11]. Bacteria (108 CFUs) were centrifuged at 8000 rcf for 2 min, washed once with PBS, and centrifuged again to pellet bacteria. For assays that measured opsonophagocytic killing by PMNs, bacteria were resuspended in PBS and then opsonized with human serum as described below. To generate hkUSA300, the bacterial pellet was resuspended in RPMI-1640 medium, buffered with 10 mM HEPES, and boiled at 95°C for 10 min.
Purification of PVL subunits from USA300 culture supernatant
PVL subunits (LukF-PV and LukS-PV) were purified from culture supernatants of a USA300 hlgABC deletion strain (LACΔhlgABC), as described previously [11]. Purified LukF-PV and LukS-PV were evaluated by SDS-PAGE [gels were stained with GelCode Blue Safe Protein Stain (ThermoScientific Pierce, Rockford, IL, USA)], tested for pore-forming capacity toward human PMNs, aliquoted separately, and stored at −80°C in 0.2 M NaCl buffer 1 (30 mM sodium phosphate buffer, pH 6.5).
PMN membrane permeability and lysis assays
Formation of plasma membrane pores was measured by uptake of EtBr, as described by Gauduchon et al. [19], but with modifications [11]. Purified PVL subunits (LukF-PV and LukS-PV) were diluted at the desired concentrations in RPMI/H. PVL-mediated pore formation was evaluated by incubating human PMNs (1×106) with 4 μM EtBr and 1 nM, 2 nM, or 5 nM active PVL for 30 min. LukF-PV and LukS-PV were boiled at 95°C for 10 min to produce iPVL, which was used as a negative control for PMN assays where indicated. Alternatively, PMNs in RPMI/H ± 4 μM EtBr were electropermeabilized with a Gene Pulser II (Bio-Rad, Hercules, CA, USA) using a single pulse at 2 kV with the capacitor set at 25 μF (2 kV pulse in a 0.4-cm cuvette). EtBr uptake was analyzed by flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, USA).
Human IL-8 and GM-CSF were purchased from eBioscience (San Diego, CA, USA). fMLF, PMA, and LPS were purchased from Sigma-Aldrich (St. Louis, MO, USA). PMN agonists, 20 μM IL-8, 100 ng/ml GM-CSF, 1 μM fMLF, 1 μg/ml PMA, 100 ng/ml LPS, or hkLAC (2.5×105 cfu), were diluted in RPMI/H and added to human PMNs (5×105), suspended in RPMI/H, containing 4 μM EtBr. Samples were incubated for 30 min at 37°C. LPS was sonicated at a frequency of 40 kHz in ice water for 15 min before use (Branson 2200, Branson Ultrasonics, Danbury, CT, USA). PMNs were then incubated with 1 nM PVL (LukF-PV and LukS-PV) for the indicated times, and EtBr uptake was assessed by flow cytometry.
PMN lysis was determined by LDH release using the cytotoxicity detection kit (Roche Applied Sciences, Pleasanton, CA, USA), as described previously [6, 20]. Human PMNs (100 μl, 1×106) were combined with 1 nM, 2 nM, or 5 nM active PVL or iPVL in a 96-well plate (Costar, Corning, NY, USA) and incubated at 37°C for 3 h (total 200 μl/well). Plates were centrifuged at 1600 rpm for 7 min at 4°C, and 100 μl aliquots of each well were transferred to a new 96-well plate.
PMN assays for priming, activation, and bactericidal activity
Release of O2− was measured as described previously by DeLeo et al. [21] with modifications. PMNs (1×107/ml) were incubated at 37°C for 30 min with fMLF, LPS, IL-8, GM-CSF, active PVL (LukF-PV and LukS-PV), iPVL, or PVL subunits separately at concentrations described above. Electropermeabilized PMNs were used in some assays (see Fig. 3). Each agonist (1 μM fMLF, 20 μM IL-8, 100 ng/ml GM-CSF, or 1 nM PVL final concentration) was aliquoted into wells of a 96-well microtiter plate before the addition of primed PMNs (1×106). LPS is a known PMN-priming agent and was used as a positive control for this purpose. Peptidoglycan (InvivoGen, San Diego, CA, USA) is a known TLR2 agonist and was used as a positive control (at 10 μg/ml) for TLR2-priming assays that contained anti-TLR2-blocking antibody. Quiescent PMNs were activated with 1 μg/ml PMA as a positive control for production of O2−. All wells contained ferricytochrome c (Sigma-Aldrich) at a final concentration of 100 μM, and assays were performed in triplicate ± 40 μg/ml SOD (Sigma-Aldrich). O2− production was determined by measuring the SOD-inhibitable reduction of ferricytochrome c at 550 nm for 20 min using a microplate spectrophotometer (Synergy MX, BioTek, Winooski, VT, USA).
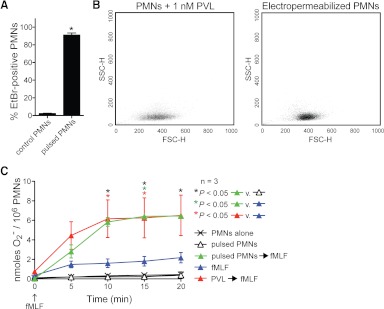
Figure 3. Pore formation primes PMNs for fMLF-stimulated production of O2−.
(A) Formation of plasma membrane pores by electropermeabilization of human PMNs. PMNs were electropermeabilized (pulsed PMNs), as described in Materials and Methods, and plasma membrane permeability was evaluated using an EtBr uptake assay. Results are the mean ± se of three separate experiments. *P = 0.0006 for the comparison with PMNs that were not electropermeabilized (control PMNs) using a paired t test. (B) Forward light-scatter-height (FCS-H) and side light-scatter-height (SSC-H) in PMNs incubated with 1 nM PVL for 30 min or PMNs that have been electropermeabilized. (C) Electropermeabilization primes PMNs for enhanced fMLF-mediated production of O2−. PMNs were electropermeabilized or incubated with 1 nM PVL for 30 min and then activated ± 1 μM fMLF for 20 min. Results are the mean ± se of three separate experiments. *P < 0.05 for the indicated comparisons using a one-way ANOVA and Tukey's post-test.
Neutralizing IgA2 mAb, specific for human TLR2 (clone B4H2), TLR4 (clone W7C11), and CD14 (clone D3B8), and an isotype control IgA2 mAb were obtained from InvivoGen. Rabbit polyclonal antibody specific for LukS-PV was generated by immunization of rabbits with purified, native LukS-PV using standard methods. Anti-LukS-PV IgG was purified using two HiTrap Protein A affinity columns (GE Healthcare, Piscataway, NJ, USA), connected in series. In brief, columns were equilibrated with 10 column vol of a 20-mM sodium phosphate-binding buffer (buffer 1, pH 8.0) at 4°C. Anti-PVL serum was diluted with Buffer 1 (v/v) and subsequently passed through the columns for 3 h using a flow rate of 1.0 mL/min. Columns were washed with Buffer 1 until the OD (OD280) of the flowthrough reached background levels. An additional wash with 10 mM sodium phosphate buffer (pH 8.0) was performed for 10 min at 1.0 mL/min. Bound polyclonal antibody was eluted with 0.1 M glycine buffer (pH 2.7) and collected in tubes containing 0.1 mL 1.0 M Tris-HCl. Total protein concentration was quantified with a protein assay from Bio-Rad. PMNs (107 cells/ml) were incubated with each mAb (5–10 μg/ml final concentration) at room temperature for 30 min with gentle agitation, prior to priming with LPS or PVL. O2− production was determined as described above.
Changes in intracellular Ca2+ were measured with a FlexStation II scanning fluorometer using fluorescent dye Fluo-4AM (Invitrogen, Life Technologies, Grand Island, NY, USA), as described previously [22]. PMNs, suspended in HBSS without Ca2+ and Mg2+, were loaded with Fluo-4AM dye (1.25 μg/ml final concentration) and incubated for 30 min in the dark at 37°C. After dye loading, the cells were washed with HBSS, resuspended in HBSS+, and aliquoted into the wells of flat-bottom, half-area-well black microtiter plates (2×105 cells/well). The source plate contained fMLF or dilutions of PVL (LukF-PV+LukS-PV) in HBSS+. Changes in fluorescence were monitored (excitation wavelength=485 nm; emission wavelegnth=538 nm) every 15 s for 15 min at room temperature after automated addition of control buffer, 100 nM fMLF (positive control), or PVL at the specified concentrations. Response curves are shown as relative fluorescence units measured over the 15-min monitoring period.
Surface expression of CD11b was determined after PMNs (1×106) were exposed to 1 nM PVL for 0, 15, and 30 min at 37°C. In some assays, PMNs were exposed to iPVL for 30 min or pretreated for 30 min at 37°C with 10 μM SB203580, a p38/reactivating kinase MAPK inhibitor (InvivoGen), and then incubated with PVL for an additional 30 min. After exposure to PVL or iPVL, cells were washed twice with stain buffer (BD Biosciences) and incubated on ice for 60 min with PE-conjugated anti-human CD11b primary antibody or PE-labeled mouse IgG1 isotype control (BD Biosciences). PMNs were washed three times with stain buffer and analyzed by flow cytometry.
Redistribution of gp91phox and p47phox from PMN granules and cytosol to the plasma membrane was determined as described [23]. In brief, proteins in human PMN plasma membrane-enriched fractions were resolved by 12.5% SDS-PAGE and then transferred to nitrocellulose membranes. Membranes were blocked overnight, and gp91phox and p47phox were detected using mAb specific for gp91phox (clone 54.1) and p47phox (clone 43.27) and peroxidase-conjugated anti-rabbit donkey IgG secondary antibody and ECL (SuperSignal West Pico, ThermoFisher Scientific, Pittsburgh, PA, USA).
Phagocytosis and killing of S. aureus by human PMNs were determined using a method described previously but with modifications [24]. Bacteria in PBS (108 CFUs) were diluted 1:1 in 100% serum (50% serum final concentration) and then opsonized for 30 min at 37°C. Opsonized S. aureus were centrifuged for 2 min at 8000 rcf, supernatant was aspirated, and bacteria were resuspended in RPMI/H at 107 CFUs/ml. S. aureus suspension (100 μl; 106 CFUs) was combined with 106 PMNs in a final volume of 200 μl, and the assay was rotated gently for 2 h at 37°C. Aliquots of the assays were evaluated by microscopy (cytospin followed by Wright-Giemsa stain) or mixed with saponin on ice for 15 min and subsequently plated on trypticase soy agar. CFUs were enumerated the following day and were used to determine percent S. aureus survival relative to a time-matched control assay that did not contain PMNs. To quantitate the percent PMNs with bound/ingested S. aureus, 250 PMNs from at least five fields of view were scored for associated bacteria (includes bound and ingested).
Identification of molecules released from human PMNs following incubation with PVL was performed by RBM (Austin, TX, USA). In brief, human PMNs from four different blood donors (1×107) were incubated ± 1 nM PVL at 37°C for 4 h. Cell suspensions were centrifuged at 1800 rpm for 10 min at 4°C. Supernatants were analyzed by RBM (HumanMAP v. 1.6), as described by the vendor http://www.myriadrbm.com/products-services/humanmap-services/humanmap/? (http://www.rulesbasedmedicine.com/productsservices/human-maps.aspx). Data were analyzed using a paired t test, and a zero was used for samples that were not measureable on the standard curve. A complete set of RBM data is provided as supporting information on the website (Supplemental Table 1).
Alternatively, the concentration of MPO and VEGF in PMN culture supernatants after a 4-h exposure to PVL or iPVL was determined using an EnzCheck MPO activity assay kit and VEGF ELISA kit, according to the manufacturer's instructions (Invitrogen, Life Technologies).
PMN apoptosis assays
PMNs were exposed to 1 nM PVL or individual PVL subunits (LukF-PV or LukS-PV) as described above, and apoptosis was determined using published methods [18, 25]. PMNs (1×104) were analyzed using a Cytospin 4 (Thermo Shandon, Waltham, MA, USA), as described by the manufacturer. PMNs were stained with Wright-Giemsa (Sigma-Aldrich), and condensed nuclei were visualized by light microscopy (Axioskop 2 Plus, Carl Zeiss, Thornwood, NY, USA) at ×100 magnification. A total of 250 cells was scored from five fields of view for each sample. Images were acquired with an AxioCam digital camera (Carl Zeiss). Alternatively, PMN apoptosis was assessed using a modified TUNEL assay (Apo-BrdU apoptosis detection kit, BD Biosciences), as described by Kobayashi et al. [18].
PMN microarray analysis
For the gene expression assays, purity of the PMNs (from three PMN donors) was 99.8 ± 0.2%, and viability was 99.6 ± 0.1%, as assessed by flow cytometry. PMNs (1×107) in RPMI/H were cultured with 1 nM PVL or iPVL at 37°C for 30, 60, or 180 min. At each indicated time-point, PMNs were lysed with RLT buffer, and RNA was purified and used to generate ≥12 μg biotin-labeled cRNA target, as described previously [18]. Samples from three different PMN donors were hybridized on HU133 + two GeneChips (Affymetrix, Santa Clara, CA, USA). cRNA labeling, GeneChip hybridization, and scanning were completed according to the manufacturer's protocols (http://media.affymetrix.com/support/downloads/manuals/expression_analysis_technical_manual.pdf). GeneChip hybridization and subsequent scanning were performed by the Genomics Unit, Rocky Mountain Laboratories, NIAID, NIH. Vehicle controls were analyzed to determine levels of background signal for each donor at every time-point.
Microarray data were normalized using GeneChip Operating Software (v1.4). At each time-point, data from PMNs, cultured with active PVL, were compared directly with those treated with iPVL using Partek Genomics Suite (Partek, St. Louis, MO, USA). Genes were defined as differentially expressed if they were significantly different from the iPVL control (P≤0.01, two-way ANOVA), changed twofold in expression, and had signal levels above background. The Venn diagram was generated by the Genomics Unit, Rocky Mountain Laboratories, NIAID, NIH. Analysis of signal transduction pathways was performed with Ingenuity Pathway Analysis (Redwood City, CA, USA). Microarray data have been posted online at http://www.ncbi.nlm.nih.gov/projects/geo/ under series number GSE33939 and are Minimum Information About a Microarray Experiment-compliant.
Statistical analyses
Data (see Figs. 1–6 and 9 and Supplemental Fig. 1) were compared using a one-way ANOVA and Dunnett's or Tukey's post-test to correct for multiple comparisons or a paired t test (GraphPad Prism 5, GraphPad Software, San Diego, CA, USA), as indicated in the legend. Data in Supplemental Table 1 were analyzed using a paired t test (GraphPad Prism 5).
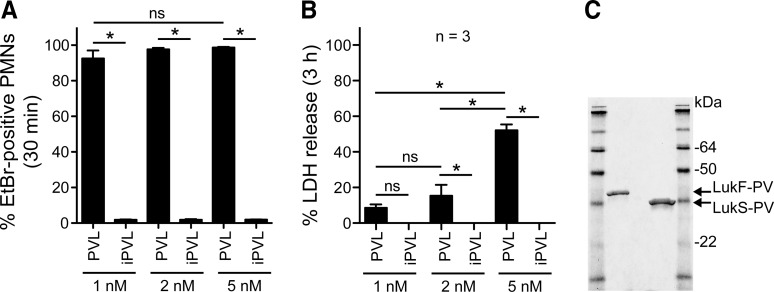
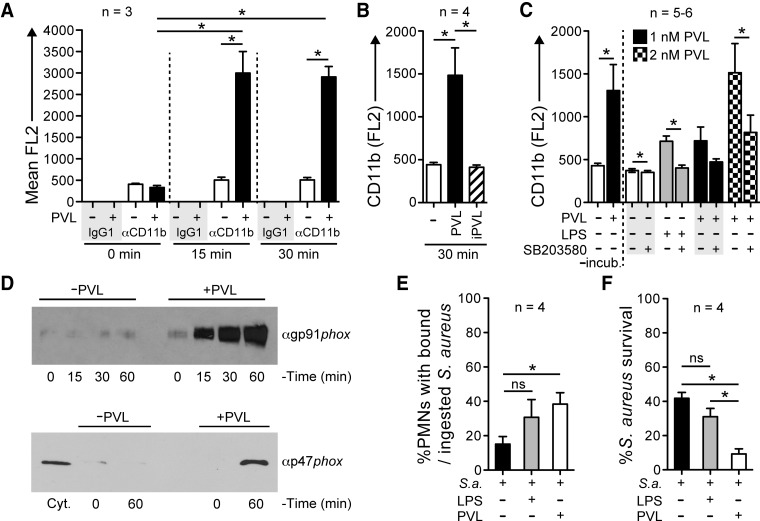
Figure 1. PVL-mediated PMN plasma membrane pore formation and cytolysis.
(A) Pore formation. PMNs (1×106) were incubated with 1 nM, 2 nM, or 5 nM native PVL or iPVL for 30 min, and plasma membrane permeability was evaluated using an EtBr uptake assay. (B) PMN lysis. PMNs (1×106) were incubated with 1 nM, 2 nM, or 5 nM native PVL or iPVL for 3 h. Lysis was determined by a standard assay that measures release of LDH. Bars in A and B indicate the mean ± se of three PMN donors. (C) SDS-PAGE of PVL subunits purified from USA300ΔhlgABC culture supernatants. *P < 0.05 for the indicated comparisons using a one-way ANOVA and Tukey's post-test.
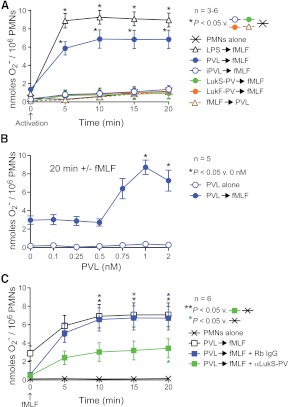
Figure 2. Sublytic concentrations of PVL prime human PMNs for fMLF-stimulated production of O2−.
(A) Priming for release of O2−. PMNs (1×106) were incubated with 1 nM PVL (LukF-PV+LukS-PV), 1 nM iPVL, 1 nM individual PVL subunits (LukF-PV or LukS-PV), or 100 ng/ml LPS for 30 min and then activated with 1 μM fMLF for 20 min, as indicated. Alternatively, PMNs were incubated first with fMLF for 30 min, followed by PVL (as indicated). Results are the mean ± se of three to six separate experiments. *P < 0.05 for the indicated comparisons using a one-way ANOVA and Tukey's post-test. Also, the difference between PVL-primed (closed blue circles) or LPS-primed (open black triangles) PMNs was significant at 5 min. (B) Concentration-dependent, PVL-mediated priming of PMNs for enhanced production of O2−. PMNs were incubated with the indicated concentrations of PVL for 30 min and then activated ± fMLF for 20 min. Results are the mean ± se of five separate experiments. *P < 0.05 versus 0 min using a one-way ANOVA and Dunnett's post-test. (C) Anti (α)-LukS-PV antibody inhibits PVL-mediated PMN priming. PMNs were incubated with 1 nM PVL ± 10 μg/ml anti-LukS-PV or rabbit IgG (Rb IgG) for 30 min and then activated ± 1 μM fMLF for 20 min as indicated. Results are the mean ± se of six separate experiments. *P < 0.05 for the indicated comparisons using a one-way ANOVA and Tukey's post-test. There was also a significant difference between PVL-primed PMNs ± rabbit IgG (open and blue boxes) and PMNs alone (Xs) at 5 min.
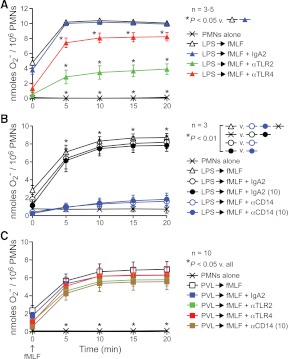
Figure 4. PVL-mediated priming of human PMNs does not involve TLR2 or TLR4.
(A–C) Inhibition of LPS or PVL-mediated priming by antibodies specific for TLR2, TL4, or CD14. PMNs were incubated with LPS (A and B) or PVL (C) as described above but after pretreatment with 5 μg/ml of the indicated antibodies. In some assays, antibodies specific for CD14 were used at 10 μg/ml (10), as indicated. Results are the mean ± se of three to 10 separate experiments or PMN donors. *P < 0.05 for the indicated comparisons using a one-way ANOVA and Tukey's post-test.
Figure 5. PVL enhances PMN bactericidal activity.
(A–C) Surface expression of CD11b. PMNs (1×106) were incubated with or without 1 nM PVL or iPVL (B), and surface expression of CD11b was measured by flow cytometry. Bars indicate the mean ± se of three or four separate experiments. *P < 0.05 for the indicated comparisons using a one-way ANOVA and Tukey's post-test. (C) PMNs pretreated for 30 min with 10 μM SB203580 were incubated ± 1 or 2 nM PVL or 100 ng/ml LPS for an additional 30 min at 37°C. Surface expression of CD11b was measured by flow cytometry. Bars indicate the mean ± se of five to six separate experiments. *P < 0.05 using a paired t test. −incub., PMNs not preincubated for 30 min, as needed for treatment with SB203580. (D) Redistribution of NADPH oxidase components following exposure of PMNs to 1 nM PVL. Immunoblots containing proteins from the plasma membrane-enriched fractions of PMNs, stimulated ± 1 nM PVL, were probed with antibodies specific for gp91phox (αgp91phox) or p47phox (αp47phox). Results shown are representative of three separate experiments. Cyt., Cytosol-enriched fraction. (E) Binding/ingestion of USA300 by human PMNs following priming by 1 nM PVL or 100 ng/mL LPS for 30 min. Following priming, bacteria were combined with PMNs at a 1:1 ratio and rotated gently for 2 h, at which time, an aliquot of the assay was used to determine the percent PMNs with bound/ingested USA300. (F) Bactericidal activity of PMNs toward USA300 following priming by 1 nM PVL or 100 ng/mL LPS, as described for E. Results for E and F are the mean ± se of four separate experiments as indicated. *P < 0.05 for the indicated comparisons using a one-way ANOVA and Tukey's post-test. S.a., S. aureus.
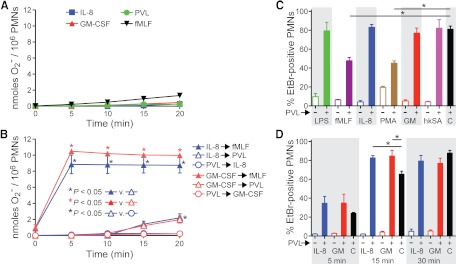
Figure 6. Proinflammatory molecules alter PMN susceptibility to PVL.
(A) Effects of PVL on PMN O2− release. O2− production was determined by reduction of ferricytochrome c, as described in Materials and Methods. (A) Assays performed without a secondary stimulus. (B) PMNs were primed with PVL and then stimulated with IL-8 (blue open circles) or GM-CSF (red open circles). Alternatively, PMNs were primed with IL-8 or GM-CSF and then stimulated with PVL (open triangles) or fMLF (closed triangles). Results are the mean ± se of three separate experiments. *P < 0.05 using an ANOVA with Tukey's post-test. (C) Pore formation. PMNs (5×105) were cultured with 100 ng/mL LPS, 1 μM fMLF, 20 μM IL-8, 1 μg/mL PMA, 100 ng/mL GM-CSF (GM), or hkUSA300 (hkSA) for 30 min in RPMI/H. PMNs were then incubated with 1 nM PVL and assayed for EtBr uptake. Bars indicate mean percent EtBr-positive PMNs. C, PMNs + 1 nM PVL (positive control). (D) Temporal increase in PMN susceptibility to PVL following exposure to IL-8 or GM-CSF. PMNs were treated with IL-8 or GM-CSF and then stimulated with PVL for the indicated times. For C and D, results are the mean ± se of four to 12 separate experiments. *P < 0.05 for the indicated comparisons in C and D using an ANOVA with Dunnett's post-test.
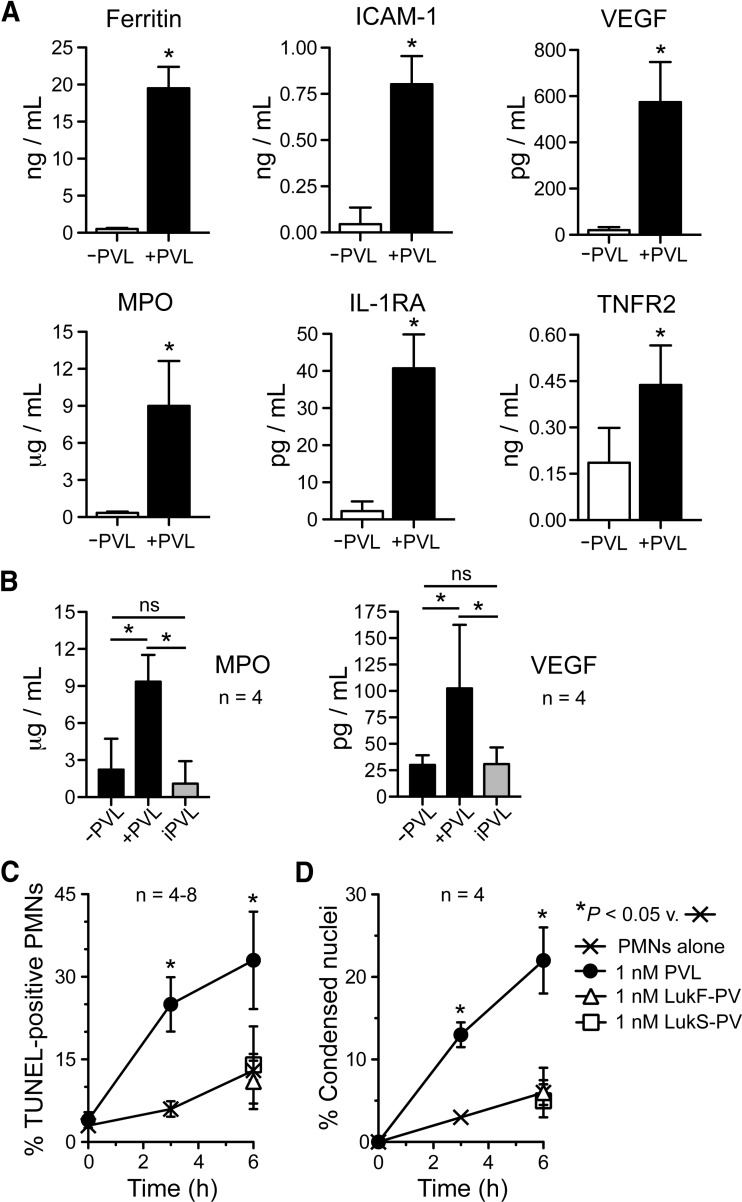
Figure 9. PVL-mediated release of proinflammatory molecules by PMNs.
(A) Accumulation of proinflammatory mediators. Human PMNs were cultured for 4 h in the ±1 nM PVL and proinflammatory molecules in RPMI/H culture medium were determined by RBM, as described in Materials and Methods. A complete set of results is provided in Supplemental Table 1. Results are the mean ± sd of four human PMN donors. *P < 0.05 versus samples minus PVL using a paired t test. (B) Verification of MPO and VEGF release using a MPO activity assay (left panel) and a VEGF ELISA (right panel). Results are the mean ± sd of four human PMN donors. *P < 0.05 for the indicated comparisons using an ANOVA and Tukey's post-test. (C and D) PMN apoptosis. PMNs were incubated with 1 nM PVL for 3 or 6 h, and TUNEL-positive PMNs were determined by flow cytometry (C) or by assessment of condensed nuclei (D). Control assays at 6 h contained either subunit alone (1 nM LukS-PV or LukF-PV). Results in C and D are the mean ± se of four to eight separate experiments. *P < 0.05 using a paired t test for assays at 3 h or an ANOVA with Dunnett's post-test at 6 h.
RESULTS
PVL-mediated pore formation and cytolysis
We first determined the concentration at which purified PVL from S. aureus culture supernatant (USA300 strain LAC) caused formation of PMN plasma membrane pores but limited cytolysis (Fig. 1A–C). By 30 min, >90% of PMNs were EtBr-positive at each of the PVL concentrations tested (pore formation was 93%, 98%, and 99% for 1 nM, 2 nM, and 5 nM PVL, respectively; Fig. 1A). PMN lysis was measured by release of LDH using conditions identical to the pore-formation assays, but incubation time was extended to 3 h (Fig. 1B). Despite high levels of pore formation with 1 nM and 2 nM PVL, subsequent cell lysis was limited (9±2% and 15±6%, respectively). We note that cell lysis increased significantly between 2 nM and 5 nM (e.g., lysis was 52±3% using 5 nM PVL). iPVL caused no pore formation and had no cytolytic capacity (Fig. 1A and B).
PVL primes human PMNs for enhanced production of O2−
In accordance with previous studies [13, 17, 26], 1 nM PVL caused an increase in free intracellular Ca2+ concentration and enhanced release of O2− after subsequent stimulation with fMLF (Supplemental Fig. 1A and Fig. 2A). The ability of PVL to prime for fMLF-mediated release of O2− was optimal at 1 nM PVL (Fig. 2B) and required both PVL subunits in their native form, as neither individual PVL subunit nor iPVL primed PMNs for fMLF-mediated O2− production (Fig. 2A). The ability of PVL to prime PMNs for an enhanced response to fMLF was not a result of an increase in surface-expressed FPR1, as surface expression of FPR1 was similar in the presence or absence of PVL priming (mean FL1 by flow cytometry was 24.2±7.7 and 25.9±7.0 for control and PVL-primed PMNs, respectively; n=4). As with other priming agents, such as LPS [27], PVL failed to elicit O2− production when added subsequently to fMLF (Fig. 2A). We note also that a polyclonal antibody specific for LukS-PV blocked PVL-mediated priming of PMNs in these assays—findings consistent with studies by Yoong and Pier [28] (Fig. 2C).
As a first step toward understanding the molecular basis of the PMN response to PVL, we tested whether formation of plasma membrane pores per se primes PMNs for fMLF-mediated release of O2− (Fig. 3A–C). With the use of electropermeabilization conditions that cause EtBr uptake (pore formation) in 91.1 ± 2.3% of the cells (comparable with pore formation caused by 1 nM PVL; see Fig. 1A), fMLF-mediated O2− production was similar to that in PMNs primed with PVL [e.g., O2− production at 20 min was 6.4±0.1 nmoles for electropermeabilized PMNs vs. 6.5±2.1 nmoles for PMNs primed with PVL (Fig. 3A–C)]. These findings indicate that formation of pores in the PMN plasma membrane can prime human PMNs for enhanced fMLF-mediated O2− production. Although these findings are consistent with the idea that formation of membrane pores by PVL contributes to PVL-mediated priming of PMNs (rather than—or in addition to—signal transduction through a surface receptor), there must still exist a link between formation of membrane pores and biochemical events that ultimately lead to the production of O2− after secondary stimulation with fMLF.
Inasmuch as LPS or PVL similarly primed human PMNs for fMLF-mediated release of O2−, it is possible that there is an overlap in signal transduction pathways following priming with these agents. In accordance with this notion, Zivkovic et al. [29] used a mouse alveolar macrophage cell line (MH-S) and transfected human embryonic kidney 293 cells to show that PVL binds to TLR2 and affects proinflammatory signal transduction through TLR2, CD14, and MyD88. This group also reported that LukS-PV alone was sufficient to elicit proinflammatory responses in vitro or in mice in vivo, which is at variance with our finding that both PVL subunits are required to prime human PMNs for fMLF-mediated O2− release. To determine whether PVL priming of human PMNs is mediated by a similar signal transduction process, we primed PMNs with LPS or PVL for 30 min and then measured fMLF-stimulated O2− release in the presence or absence of antibodies known to block binding of ligands to TLR2, TLR4, or CD14 (Fig. 4A–C). Anti-TLR2, anti-TLR4, and anti-CD14 antibodies inhibited the ability of LPS to prime human PMNs for enhanced fMLF-stimulated O2− release (e.g., at 20 min, fMLF-stimulated O2− generation was 8.2 nmoles/106 cells for LPS-primed PMNs in the presence an isotype control antibody vs. 1.6 nmoles/106 cells for those in the presence of anti-CD14 antibody; Fig. 4A and B). The inhibition of LPS priming by anti-TLR2 antibody is likely explained by the known contamination of commercial LPS preparations with lipopeptide, which signals through TLR2 [30, 31]. The anti-TLR2 antibody also inhibited PMN priming by a S. aureus peptidoglycan, a known TLR2 agonist (Supplemental Fig. 1B). By comparison, these antibodies failed to inhibit PVL-mediated priming of PMNs for production of O2− in response to fMLF (Fig. 4C). Taken together, these data suggest that PVL priming of human PMNs involves a process other than signal transduction through TLR2, TLR4, or CD14.
PVL causes granule exocytosis and primes human PMNs for enhanced microbicidal capacity
Inasmuch as priming of PMNs by LPS causes granule exocytosis and enrichment of NADPH oxidase components at the plasma membrane [23], which explains, in part, the ability of LPS to prime PMNs for enhanced production of O2− following stimulation with fMLF, we tested the ability of PVL to induce up-regulation of CD11b at the cell surface and subcellular redistribution of NADPH oxidase components (Fig. 5A–C). PMNs exposed to PVL had a significant increase in the surface expression of CD11b, a finding most compatible with the fusion of secretory vesicles and/or specific granules with the plasma membrane (Fig. 5A and B). These results are consistent with the known ability of priming agents, such as LPS, to up-regulate PMN surface expression of CD11b [32, 33]. SB203580, a widely used inhibitor of p38 MAPK that has been shown to block priming of PMNs by LPS [34], partially inhibited PVL-mediated up-regulation of CD11b surface expression (Fig. 5C).
There was time-dependent association of gp91phox and p47phox with the plasma membrane of human PMNs following exposure of these cells to 1 nM PVL (Fig. 5D). The ability of PVL to increase surface expression of CD11b (and other receptors present in specific granules) and cause partial assembly of the NADPH oxidase suggests that exposure to the leukocidin can prime for enhanced PMN phagocytosis and killing of S. aureus. To test this notion, we measured binding/uptake and killing of S. aureus (the USA300 epidemic clone) by PMNs (Fig. 5E and F). Priming of PMNs by PVL significantly increased binding/uptake and killing of S. aureus by PMNs (e.g., survival of USA300 was 41.8±3.5% and 9.3±3.1% in the presence and absence of PVL priming; P<0.05; Fig. 5E and F). Collectively, these results provide strong support for the idea that PVL can function as a PMN-priming agent.
Proinflammatory molecules alter PMN susceptibility to PVL
We next determined whether PVL primes PMNs for O2− generation in response to IL-8 or GM-CSF and whether these proinflammatory agents prime PMNs for PVL-mediated O2− production (Fig. 6A and B). IL-8, GM-CSF, or PVL alone had little or no capacity to elicit PMN O2− production (Fig. 6A). However, IL-8 and GM-CSF primed PMNs for enhanced fMLF-mediated O2− release, as described previously [35, 36] (Fig. 6B, solid triangles). By comparison, PVL elicited limited O2− release (2.0–2.2 nmoles/106 cells) from IL-8- and GM-CSF-primed PMNs (Fig. 6B, open triangles).
Inasmuch as proinflammatory factors typically prime PMNs for enhanced function, which includes reorganization and increase of receptors and other molecules at the plasma membrane, proinflammatory stimuli could influence the ability of PVL to interact with neutrophils. To test this hypothesis, we stimulated human PMNs with multiple proinflammatory factors/activating agents and evaluated PVL-mediated pore formation (Fig. 6C and D). Stimulation of PMNs with fMLF or PMA caused a significant decrease in the ability of PVL to form pores in the PMN plasma membrane (e.g., 48±3% of the fMLF-stimulated cells were EtBr-positive following exposure to PVL vs. 81±3% EtBr-positive cells following exposure to PVL alone; Fig. 6C). By comparison, neither LPS nor hkUSA300 altered the ability of PVL to cause formation of membrane pores. Priming of PMNs with IL-8 or GM-CSF caused a transient but significant increase in PVL-mediated pore formation (e.g., by 15 min, 83±2% of the PMNs were EtBr-positive after pre-exposure to IL-8 compared with 65±3% of those incubated with PVL alone; P≤0.01; Fig. 6D). These data indicate that remodeling of the PMN plasma membrane following exposure to specific proinflammatory agonists alters the ability of PVL to interact with PMNs. One possible explanation for these results is that PVL binds to a PMN receptor whose surface expression increases or decreases depending on the agonist, and this change in turn impacts the ability of PVL to form plasma membrane pores. Collectively, these data indicate that the ability of PVL to interact with human PMNs is influenced by the activation state of the cell.
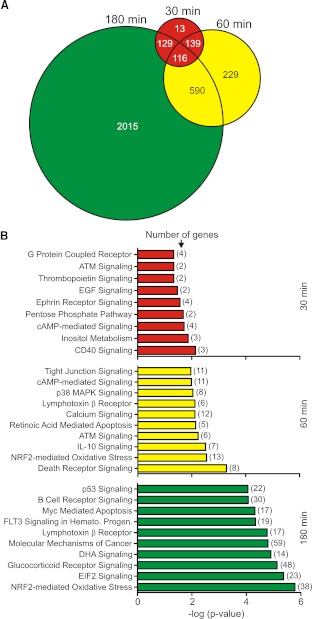
PVL induces global changes in PMN gene expression
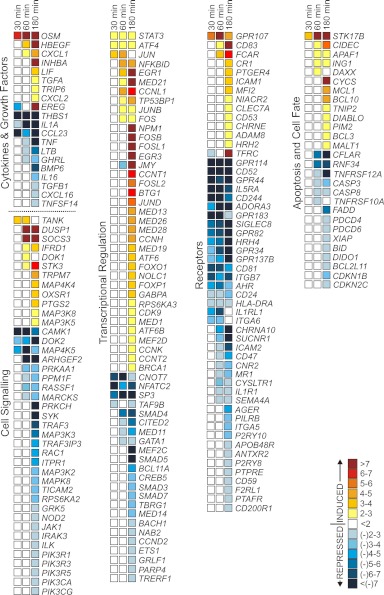
To gain further insight into the molecular basis of PVL-mediated PMN priming, we measured global changes in PMN gene expression following exposure to 1 nM PVL (Fig. 7). The number of differentially expressed genes increased in a time-dependent manner (i.e., there were 397 differentially expressed genes at 30 min, 1074 differentially expressed genes at 60 min, and 2850 differentially expressed genes at 180 min; Fig. 7A). Differentially expressed genes were categorized into biological pathways or grouped according to function (Fig. 7B). Only nine signal transduction pathways were significantly represented by differentially expressed genes at 30 min (Fig. 7B). However, 30 and 114 signal transduction pathways were significantly represented by differentially expressed genes at 60 min and 180 min, respectively (the top 10 are shown). In general, proinflammatory pathways, such as those mediated by CD40 and EGF, were significantly represented early following the PVL-PMN interaction (30 min). By comparison, cell-fate pathways, such as death receptor signaling and Myc-mediated apoptosis signaling, were significantly represented by differentially expressed genes, 60 and 180 min after exposure of PMNs to PVL (Fig. 7B).
Figure 7. PVL causes global changes in PMN gene expression.
PMNs (1×107) were cultured with 1 nM native PVL or iPVL, and changes in transcript levels between the two conditions were measured using Affymetrix HU133 + two GeneChips, as described in Materials and Methods. (A) Venn diagram depicting the total number of differentially expressed genes (i.e., as a result of exposure to PVL) at each time-point. (B) PMN signal transduction pathways represented by differentially expressed genes after cells were exposed to PVL. Pathways or processes were identified using Ingenuity Pathway Analysis, as described in Materials and Methods. The P value indicates the likelihood that genes are associated with a given pathway or process because of random chance. ATM, ataxia telangiectasia mutated; NRF2, NF-E2-related factor 2; FLT3, fms-like tyrosine-kinase 3; Hemato. Progen., hematopoietic progenitors; DHA, docosahexaenoic acid; EIF2, eukaryotic translation initiation factor 2.
Expression of PMN genes encoding major transcription regulators is increased following exposure to PVL
Genes encoding proteins known to mediate the inflammatory response, such as STAT3, SOCS3, JUNB, FOS, FOSB, FOSL1, JUN, and TANK, were up-regulated within 120 min after PMN exposure to 1 nM PVL (Fig. 8). Expression of SOCS3 is regulated by the transcription factor STAT3, and therefore, up-regulation of SOCS3 is consistent with activation of STAT3. JUNB, FOS, JUN, FOSB, and FOSL1 encode proteins that dimerize and form the AP-1 transcription factor, which is known to associate with NF-κB as a result of signal transduction through proinflammatory pathways (reviewed in ref. [37]). In addition, genes involved in NF-κB signal transduction, such as NFKBID, MAP3K3, MALT1, BCL10, and BCL3, were differentially expressed, 180 min after exposure of PMNs to PVL (Fig. 8).
Figure 8. PVL alters expression of genes encoding proteins involved in inflammation and cell fate.
Transcript levels were measured as described in Materials and Methods. Colors represent the mean increase or decrease (fold-change) in gene expression in PMNs treated with native PVL relative to those treated with iPVL. Results are the mean of three separate experiments or donors.
Priming of PMNs with PVL alters expression of transcripts encoding surface receptors and proinflammatory molecules
Genes encoding multiple PMN proinflammatory mediators, including CR1 (CD35), CXCL1 (GROα), CXCL2 (GROβ), FCAR (CD89) and OSM, were up-regulated 60 and/or 180 min after exposure to PVL (Fig. 8). FCAR encodes the IgA receptor CD89, CR1 encodes the serum CR CD35, and these molecules facilitate phagocytosis and PMN activation [38, 39]. GROα and GROβ are members of the IL-8SF of chemokines and facilitate recruitment and priming of PMNs for enhanced activation [40]. OSM, a member of the IL-6 family, is stored by mature PMNs as an active protein and released following priming or activation [41]. However, synthesis of OSM transcript by PMNs is known to occur under certain conditions, such as after culture with LPS and GM-CSF [41] or after phagocytosis [42].
Exposure of PMNs to PVL also caused down-regulation of a subset of genes encoding surface receptors and cytokines involved in the inflammatory response (Fig. 8). For example, IL5RA, IL1R1, IL1A, CCL23, TNFRSF12A, TNFRSF10A, and TNF transcripts were decreased, 60 min after exposure of human PMNs to PVL. Down-regulation of transcripts encoding TNFRs and TNF-α at later time-points (60 and 180 min) provides support to the idea that there is eventual moderation of the proinflammatory response induced by PVL, a phenomenon consistent with induction of PMN apoptosis by PVL.
Mediators of the inflammatory response are released from PMNs following PVL priming
Inasmuch as PVL primed PMNs for enhanced function and caused up-regulation of transcripts involved in the inflammatory response, we next measured release of molecules involved in the inflammatory response following exposure of PMNs to 1 nM PVL for 4 h (Fig. 9A and B, and Supplemental Table 1). PVL caused significant release of multiple molecules that are involved in (or are moderators of) the inflammatory response, including ferritin, ICAM-1, IL-1RA, MPO, TNFR2, and VEGF, from human PMNs (Fig. 9A and B), which are known to shed TNFR2 following stimulation with fMLF and GM-CSF, thereby decreasing the subsequent PMN response to TNF-α [43], and IL-1RA is a known inhibitor of IL-1α and IL-1β activity. Although these findings are consistent with the ability of PVL to function as a PMN-priming agent, we cannot exclude the possibility that some of the molecules present in culture media, especially those in low abundance, originated from some of the cells that underwent lysis [lysis was 13.1±3.1% (se) for PMNs exposed to 1 nM PVL for 4 h; n=6].
DISCUSSION
Recent multinational phase III clinical trials indicate that the presence of genes encoding PVL is not the primary determinant of outcome in patients with MRSA skin and soft-tissue infections [44, 45]. Rather, individuals with PVL-positive infections were more likely to be cured [44, 45]. It is also noteworthy that the concentration of PVL achieved during S. aureus infection in vivo may be insufficient to cause PMN lysis [12, 46]—findings that bring into question the role of PVL as a cytolytic toxin. Antibodies against PVL are present in individuals who have had previous S. aureus infections, caused by PVL-positive or -negative strains [47, 48]. These observations suggest that some of the antibodies were originally elicited by two-component toxins other than PVL, but the antibodies cross-reacted with PVL. Recurrent infections with PVL-positive S. aureus strains occur in patients that have anti-PVL antibodies, suggesting that PVL has little or no role in establishment of infection [49]. Furthermore, a previous study demonstrated that administration of anti-PVL antibodies prior to USA300 or USA400 infection (PVL-positive) hindered clearance of infection [28]. This observation seems at variance with the presumed role of PVL during infection (i.e., cytolytic toxin that enhances virulence) but is consistent with the ability of the molecule to function as a PMN-priming agent.
The cytolytic properties of PVL are well-known from extensive work in vitro. However, there is a paucity of evidence to indicate that the primary function of PVL in vivo is cytolysis of host leukocytes. For example, Diep et al. [50] used a rabbit model to show that PMNs and release of PMN molecules are required for PVL-mediated lung inflammation and injury, but it was not possible to determine whether the disease phenotype was caused by PMN lysis or priming and subsequent activation. It is possible that under certain conditions, PVL-mediated priming of PMNs contributes to enhanced inflammation and progression of disease, as PMNs are widely known to be major contributors to inflammatory syndromes. On the other hand, PVL-mediated lung inflammation and injury require relatively high concentrations of PVL or very high burden of PVL-positive S. aureus in the rabbit lung [50], and therefore, the relevance of this phenomenon to human disease remains unknown. It is noteworthy that in vitro S. aureus is ingested rapidly by PMNs, and these host phagocytes undergo rapid lysis, independent of PVL [6, 20].
PVL has been shown previously to cause release of IL-8, IL-6, and LTB4 and promote granule exocytosis by intact PMNs [13–16, 51], and Konig et al. [14] reported that inhibition of protein tyrosine kinases partially inhibits PVL-mediated release of IL-8 from PMNs. Consistent with these previous studies, we found that PMNs incubated with 1 nM PVL remained largely intact for at least 3 h, as there was little or no release of LDH using these assay conditions (Fig. 1B). Taken together, the data provide strong support to the idea that PVL-mediated release of PMN proinflammatory molecules is caused by activation of signal transduction pathways rather than cytolysis.
In accordance with previous work, PVL had proinflammatory effects on human PMNs, including priming for enhanced release of O2− and increased surface expression of CD11b. In addition, we discovered that PVL caused subcellular redistribution of NADPH oxidase components and promoted secretion of multiple inflammatory mediators (Figs. 5D and 9 and Supplemental Table 1). LPS priming of PMNs for enhanced production of O2− is known to involve redistribution of NADPH oxidase components [23]. Our finding that PVL caused a similar redistribution of NADPH oxidase components likely explains the enhanced release of O2− in PVL-treated PMNs that were subsequently stimulated with fMLF. Thus, at sublytic concentrations, PVL functions as a PMN-priming agent.
Despite the functional similarities of PVL to other priming agents, such as LPS, there are clear differences. For example, exposure of PMNs to sublytic concentrations of PVL caused release of MPO, a protein sequestered in azurophilic granules (Fig. 9A), and such a process is characteristic of PMN activation rather than priming by agents, such as GM-CSF, IL-8, or LPS. PVL also accelerated PMN apoptosis at a concentration (1 nM) that elicits a proinflammatory response (Fig. 9C and D), which is at variance with the ability of other priming agents to extend PMN survival in vitro. Thus, more work is needed to better understand the molecular basis of these PVL-mediated phenomena, and the microarray data presented here serve as a possible springboard for such work.
PMNs are exposed to a multitude of proinflammatory molecules during infection in vivo, and different combinations of stimuli are known to elicit different PMN responses. Application of this idea to PVL-positive S. aureus infections is perhaps reflected by our finding that fMLF, GM-CSF, and IL-8 alter the ability of PVL to interact with PMNs (Fig. 6C and D). These findings should not be interpreted to indicate that PVL binds to one of the receptors for these proinflammatory agents, although that is a possibility. Rather, many surface molecules, including receptors, are increased or decreased following exposure to activating agents or proinflammatory molecules, and expression of a putative PVL receptor on the PMN surface could thus be readily increased or decreased by activation or priming. Consistent with this idea, previous studies by Hensler et al. [52] suggested that heterotrimeric and low molecular-weight G-proteins are involved in PMN signal transduction following exposure to PVL. In addition, Gauduchon et al. [19] reported that PKC regulates the availability of the receptor for LukS-PV—findings that are in accordance with our data.
Importantly, our results demonstrate that sublytic concentrations of PVL prime PMNs for enhanced microbicidal capacity. This notion is also supported by Yoong and Pier [28], who reported anti-PVL rabbit sera inhibited PMN killing of several PVL-positive S. aureus strains in vitro. By comparison, killing of isogenic lukS/F-PV deletion mutants by PMNs was similar in the presence or absence of anti-PVL antibody [28]. Collectively, these observations suggest that PVL can enhance rather than hinder the host innate immune response to S. aureus infection. Nonetheless, the ability of PVL to enhance PMN bactericidal activity in vivo merits further investigation. Elucidation of signal transduction mechanisms following such stimulation with PVL may provide new insight into the ability of specific agonists to elicit differential responses from human PMNs.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIAID, U.S. National Institutes of Health, and by U.S. National Institutes of Health grants RR 020185 and GM 103500 (M.T.Q.).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- CA-MRSA
- community-associated methicillin-resistant Staphylococcus aureus
- CR1
- complement receptor 1
- EtBr
- ethidium bromide
- FCAR
- Fc fragment of IgAR
- FL1/2
- fluorescence 1/2
- GROα/β
- growth-related oncogene-α/β
- HA-MRSA
- hospital-acquired methicillin-resistant Staphylococcus aureus
- HBSS+
- HBSS with Ca2+ and Mg2+
- hk
- heat-killed
- IL-1RA
- IL-1R antagonist
- iPVL
- heat-inactivated Panton-Valentine leukocidin
- LTB4
- leukotriene B4
- NIAID
- National Institute of Allergy and Infectious Diseases
- O2−
- superoxide
- OSM
- oncostatin M
- PVL
- Panton-Valentine leukocidin
- RBM
- Rules-Based Medicine
- rcf
- relative centrifugal force
- RPMI/H
- Hepes-buffered RPMI
- SF
- superfamily
- SOCS
- suppressor of cytokine signaling
- TANK
- TRAF family member-associated NF-κB activator
- TSB
- trypticase soy broth
AUTHORSHIP
F.R.D. and S.D.K. conceived of and designed the study. S.F.G, K.R.B., A.R.W., D.E.S, D.L.R., and L.N.K. performed experiments. M.T.Q. contributed critical reagents. S.F.G., S.D.K., D.E.S, M.T.Q., and F.R.D. analyzed data. S.F.G., S.D.K., M.T.Q., and F.R.D. wrote the manuscript.
REFERENCES
- 1. Diekema D. J., Pfaller M. A., Schmitz F. J., Smayevsky J., Bell J., Jones R. N., Beach M. (2001) Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 32 (Suppl. 2), S114–S132 [DOI] [PubMed] [Google Scholar]
- 2. Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M., Craig A. S., Zell E. R., Fosheim G. E., McDougal L. K., Carey R. B., Fridkin S. K. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 [DOI] [PubMed] [Google Scholar]
- 3. DeLeo F. R., Chambers H. F. (2009) Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Invest. 119, 2464–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moran G. J., Krishnadasan A., Gorwitz R. J., Fosheim G. E., McDougal L. K., Carey R. B., Talan D. A. (2006) Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355, 666–674 [DOI] [PubMed] [Google Scholar]
- 5. Herold B. C., Immergluck L. C., Maranan M. C., Lauderdale D. S., Gaskin R. E., Boyle-Vavra S., Leitch C. D., Daum R. S. (1998) Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279, 593–598 [DOI] [PubMed] [Google Scholar]
- 6. Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Said-Salim B., Porcella S. F., Long R. D., Dorward D. W., Gardner D. J., Kreiswirth B. N., Musser J. M., DeLeo F. R. (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919 [DOI] [PubMed] [Google Scholar]
- 7. Li M., Diep B. A., Villaruz A. E., Braughton K. R., Jiang X., DeLeo F. R., Chambers H. F., Lu Y., Otto M. (2009) Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 106, 5883–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baba T., Takeuchi F., Kuroda M., Yuzawa H., Aoki K., Oguchi A., Nagai Y., Iwama N., Asano K., Naimi T., Kuroda H., Cui L., Yamamoto K., Hiramatsu K. (2002) Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359, 1819–1827 [DOI] [PubMed] [Google Scholar]
- 9. Diep B. A., Gill S. R., Chang R. F., Phan T. H., Chen J. H., Davidson M. G., Lin F., Lin J., Carleton H. A., Mongodin E. F., Sensabaugh G. F., Perdreau-Remington F. (2006) Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739 [DOI] [PubMed] [Google Scholar]
- 10. Woodin A. M. (1970) Staphylococcal leukocidin. In Microbial Toxins (Montje T., Kadis S., Ajl S., eds.), Academic, New York, NY, USA, and London, UK, 327–355 [Google Scholar]
- 11. Graves S. F., Kobayashi S. D., Braughton K. R., Diep B. A., Chambers H. F., Otto M., DeLeo F. R. (2010) Relative contribution of Panton-Valentine leukocidin to PMN plasma membrane permeability and lysis caused by USA300 and USA400 culture supernatants. Microbes Infect. 12, 446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badiou C., Dumitrescu O., Croze M., Gillet Y., Dohin B., Slayman D. H., Allaouchiche B., Etienne J., Vandenesch F., Lina G. (2008) Panton-Valentine leukocidin is expressed at toxic levels in human skin abscesses. Clin. Microbiol. Infect. 14, 1180–1183 [DOI] [PubMed] [Google Scholar]
- 13. Colin D. A., Mazurier I., Sire S., Finck-Barbancon V. (1994) Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: sequential binding and subsequent activation. Infect. Immun. 62, 3184–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konig B., Koller M., Prevost G., Piemont Y., Alouf J. E., Schreiner A., Konig W. (1994) Activation of human effector cells by different bacterial toxins (leukocidin, alveolysin, and erythrogenic toxin A): generation of interleukin-8. Infect. Immun. 62, 4831–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konig B., Prevost G., Piemont Y., Konig W. (1995) Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J. Infect. Dis. 171, 607–613 [DOI] [PubMed] [Google Scholar]
- 16. Hensler T., Konig B., Prevost G., Piemont Y., Koller M., Konig W. (1994) Leukotriene B4 generation and DNA fragmentation induced by leukocidin from Staphylococcus aureus: protective role of granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF for human neutrophils. Infect. Immun. 62, 2529–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colin D. A., Monteil H. (2003) Control of the oxidative burst of human neutrophils by staphylococcal leukotoxins. Infect. Immun. 71, 3724–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi S. D., Voyich J. M., Buhl C. L., Stahl R. M., DeLeo F. R. (2002) Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc. Natl. Acad. Sci. USA 99, 6901–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gauduchon V., Werner S., Prevost G., Monteil H., Colin D. A. (2001) Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect. Immun. 69, 2390–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voyich J. M., Otto M., Mathema B., Braughton K. R., Whitney A. R., Welty D., Long R. D., Dorward D. W., Gardner D. J., Lina G., Kreiswirth B. N., DeLeo F. R. (2006) Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194, 1761–1770 [DOI] [PubMed] [Google Scholar]
- 21. DeLeo F. R., Allen L. A., Apicella M., Nauseef W. M. (1999) NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163, 6732–6740 [PubMed] [Google Scholar]
- 22. Kirpotina L. N., Khlebnikov A. I., Schepetkin I. A., Ye R. D., Rabiet M. J., Jutila M. A., Quinn M. T. (2010) Identification of novel small-molecule agonists for human formyl peptide receptors and pharmacophore models of their recognition. Mol. Pharmacol. 77, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeLeo F. R., Renee J., McCormick S., Nakamura M., Apicella M., Weiss J. P., Nauseef W. M. (1998) Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 101, 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lei B., DeLeo F. R., Hoe N. P., Graham M. R., Mackie S. M., Cole R. L., Liu M., Hill H. R., Low D. E., Federle M. J., Scott J. R., Musser J. M. (2001) Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 7, 1298–1305 [DOI] [PubMed] [Google Scholar]
- 25. Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. (1989) Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finck-Barbancon V., Duportail G., Meunier O., Colin D. A. (1993) Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim. Biophys. Acta 1182, 275–282 [DOI] [PubMed] [Google Scholar]
- 27. Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr., (1984) Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J. Exp. Med. 160, 1656–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoong P., Pier G. B. (2010) Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc. Natl. Acad. Sci. USA 107, 2241–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zivkovic A., Sharif O., Stich K., Doninger B., Biaggio M., Colinge J., Bilban M., Mesteri I., Hazemi P., Lemmens-Gruber R., Knapp S. (2011) TLR 2 and CD14 mediate innate immunity and lung inflammation to staphylococcal Panton-Valentine leukocidin in vivo. J. Immunol. 186, 1608–1617 [DOI] [PubMed] [Google Scholar]
- 30. Sabroe I., Jones E. C., Usher L. R., Whyte M. K., Dower S. K. (2002) Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol. 168, 4701–4710 [DOI] [PubMed] [Google Scholar]
- 31. Sabroe I., Prince L. R., Jones E. C., Horsburgh M. J., Foster S. J., Vogel S. N., Dower S. K., Whyte M. K. (2003) Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J. Immunol. 170, 5268–5275 [DOI] [PubMed] [Google Scholar]
- 32. Condliffe A. M., Chilvers E. R., Haslett C., Dransfield I. (1996) Priming differentially regulates neutrophil adhesion molecule expression/function. Immunology 89, 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynam E. B., Simon S. I., Rochon Y. P., Sklar L. A. (1994) Lipopolysaccharide enhances CD11b/CD18 function but inhibits neutrophil aggregation. Blood 83, 3303–3311 [PubMed] [Google Scholar]
- 34. Yan S. R., Al-Hertani W., Byers D., Bortolussi R. (2002) Lipopolysaccharide-binding protein- and CD14-dependent activation of mitogen-activated protein kinase p38 by lipopolysaccharide in human neutrophils is associated with priming of respiratory burst. Infect. Immun. 70, 4068–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balazovich K. J., Almeida H. I., Boxer L. A. (1991) Recombinant human G-CSF and GM-CSF prime human neutrophils for superoxide production through different signal transduction mechanisms. J. Lab. Clin. Med. 118, 576–584 [PubMed] [Google Scholar]
- 36. Yuo A., Kitagawa S., Kasahara T., Matsushima K., Saito M., Takaku F. (1991) Stimulation and priming of human neutrophils by interleukin-8: cooperation with tumor necrosis factor and colony-stimulating factors. Blood 78, 2708–2714 [PubMed] [Google Scholar]
- 37. Tak P. P., Firestein G. S. (2001) NF-κB: a key role in inflammatory diseases. J. Clin. Invest. 107, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Monteiro R. C., Van De Winkel J. G. (2003) IgA Fc receptors. Ann. Rev. Immunol. 21, 177–204 [DOI] [PubMed] [Google Scholar]
- 39. Berger M., O'Shea J., Cross A. S., Folks T. M., Chused T. M., Brown E. J., Frank M. M. (1984) Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J. Clin. Invest. 74, 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haskill S., Peace A., Morris J., Sporn S. A., Anisowicz A., Lee S. W., Smith T., Martin G., Ralph P., Sager R. (1990) Identification of three related human GRO genes encoding cytokine functions. Proc. Natl. Acad. Sci. USA 87, 7732–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grenier A., Dehoux M., Boutten A., Arce-Vicioso M., Durand G., Gougerot-Pocidalo M. A., Chollet-Martin S. (1999) Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 93, 1413–1421 [PubMed] [Google Scholar]
- 42. Borjesson D. L., Kobayashi S. D., Whitney A. R., Voyich J. M., Argue C. M., DeLeo F. R. (2005) Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J. Immunol. 174, 6364–6372 [DOI] [PubMed] [Google Scholar]
- 43. Porteu F., Hieblot C. (1994) Tumor necrosis factor induces a selective shedding of its p75 receptor from human neutrophils. J. Biol. Chem. 269, 2834–2840 [PubMed] [Google Scholar]
- 44. Lalani T., Federspiel J. J., Boucher H. W., Rude T. H., Bae I. G., Rybak M. J., Tonthat G. T., Corey G. R., Stryjewski M. E., Sakoulas G., Chu V. H., Alder J., Steenbergen J. N., Luperchio S. A., Campion M., Woods C. W., Fowler V. G. (2008) Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J. Clin. Microbiol. 46, 2890–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bae I. G., Tonthat G. T., Stryjewski M. E., Rude T. H., Reilly L. F., Barriere S. L., Genter F. C., Corey G. R., Fowler V. G., Jr., (2009) Presence of genes encoding the Panton-Valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J. Clin. Microbiol. 47, 3952–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Badiou C., Dumitrescu O., George N., Forbes A. R., Drougka E., Chan K. S., Ramdani-Bouguessa N., Meugnier H., Bes M., Vandenesch F., Etienne J., Hsu L. Y., Tazir M., Spiliopoulou I., Nimmo G. R., Hulten K. G., Lina G. (2010) Rapid detection of Staphylococcus aureus Panton-Valentine leukocidin in clinical specimens by enzyme-linked immunosorbent assay and immunochromatographic tests. J. Clin. Microbiol. 48, 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lack C. H., Towers A. G. (1962) Serological tests for staphylococcal infection. Br. Med. J. 2, 1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Croze M., Dauwalder O., Dumitrescu O., Badiou C., Gillet Y., Genestier A. L., Vandenesch F., Etienne J., Lina G. (2009) Serum antibodies against Panton-Valentine leukocidin in a normal population and during Staphylococcus aureus infection. Clin. Microbiol. Infect. 15, 144–148 [DOI] [PubMed] [Google Scholar]
- 49. Hermos C. R., Yoong P., Pier G. B. (2010) High levels of antibody to Panton-Valentine leukocidin are not associated with resistance to Staphylococcus aureus-associated skin and soft-tissue infection. Clin. Infect. Dis. 51, 1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Diep B. A., Chan L., Tattevin P., Kajikawa O., Martin T. R., Basuino L., Maia T. T., Braughton K. R., Whitney A. R., Gardner D. J., Fan X., Tseung C. W., Lui G. Y., Badiou C., Etienne J., Lina G., Matthaya M. A., DeLeo F. R., Chambers H. F. (2010) Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. USA 107, 5587–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Woodin A. M. (1962) The extrusion of protein from the rabbit polymorphonuclear leucocyte treated with staphylococcal leucocidin. Biochem. J. 82, 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hensler T., Koller M., Prevost G., Piemont Y., Konig W. (1994) GTP-binding proteins are involved in the modulated activity of human neutrophils treated with the Panton-Valentine leukocidin from Staphylococcus aureus. Infect. Immun. 62, 5281–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.